Abstract
It has been well established that Clostridium difficile toxin A (TcdA) induces cell death in human epithelial cells. However, the mechanism of TcdA-induced cell death remains to be fully characterized. Here, we show that TcdA induces dose-dependent cell death in ovarian carcinoma and colonic carcinoma cell lines. TcdA-mediated cell death, as well as caspase 8 and caspase 3 activation, were specifically abrogated by anti-toxin antibodies. Although caspase 8 and caspase 3 were activated by TcdA in OVCAR3 ovarian carcinoma and T84 colonic cancer cells, pancaspase and caspase 8, 3, and 9 inhibitors did not block TcdA-induced cell death. In contrast, tumor necrosis factor-related apoptosis-inducing ligand-induced cell death was nearly completely blocked by caspase inhibitors in OVCAR3 cells. In these cells, TcdA induces the mitochondrial pathway of apoptosis, as demonstrated by changes in mitochondrial outer membrane permeabilization (MOMP). Furthermore, overexpression of the antiapoptotic proteins Bcl-2 and Bcl-XL significantly inhibited TcdA-induced cell death, as well as TcdA-induced MOMP. Conversely, small interfering RNA-mediated inhibition of Bcl-XL in TcdA-resistant SKOV3ip1 cells enhanced TcdA-induced cell death. Overexpression of the antiapoptotic proteins Bcl-2 and Bcl-XL in T84 cells also inhibited TcdA-induced cell death. Altogether, our data demonstrate that TcdA induces cell death in both ovarian and colonic cancer cells preferentially via the mitochondrial pathway of apoptosis by a death receptor-independent and a caspase-independent mechanism. This process is regulated by antiapoptotic members of the Bcl-2 family.
Apoptosis can be mediated by a variety of stimuli, including binding of ligands to death receptors, DNA-damaging agents, and growth factor withdrawal. Depending on the signal, apoptosis is initiated either by the death receptor pathway or by a mitochondrion-dependent pathway (31-33). In both pathways, however, effector caspases (caspases 3, 6, and 7) are activated and cleavage of cellular substrates occurs, leading to the morphological changes observed in apoptosis. In the mitochondrion-dependent pathway of apoptosis, effector caspase activation is triggered by an increase in mitochondrial outer membrane permeabilization (MOMP), resulting in the release of cytochrome c and the formation of the apoptosome (31, 33). Changes in MOMP are regulated by a balance between pro- and antiapoptotic members of the Bcl-2 family (31). The proapoptotic family members Bax and Bak form channels into the outer membrane of the mitochondria that allow the release of cytochrome c and other mitochondrial intermembrane proteins. Insertion of Bax and Bak into the outer mitochondrial membrane is regulated by antiapoptotic members of the Bcl-2 family. Antiapoptotic members, such as Bcl-2 and Bcl-XL, bind and neutralize Bax and/or Bak. Stimulation of death receptors by death ligands, such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), results in activation of initiator caspase 8. Upon binding to TRAIL, activated TRAIL receptors recruit the Fas-associated death domain (3). Via its death effector domain, the Fas-associated death domain recruits caspase 8 and assembles into a death-inducing signaling complex (16, 27). When recruited to the death-inducing signaling complex, pro-caspase 8 is activated and subsequently cleaves downstream effector caspases, leading to apoptosis. This process is efficiently blocked by the inhibition of caspases. An interconnection between cell surface death receptors and mitochondrion-initiated pathways of apoptosis has been found in many cellular systems. In this context, apoptosis can be inhibited by Bcl-XL or Bcl-2 (2, 10, 13). In contrast to the death receptor pathway, which is highly dependent on caspase activation, the inhibition of caspases fails to prevent apoptosis in caspase-independent cell death (32). Furthermore, as caspase-independent cell death often requires MOMP, this process can be blocked by Bcl-2 overexpression (2, 13).
C. difficile is the leading cause of hospital-acquired diarrhea and the etiological agent of pseudomembranous colitis. In humans, the intestinal damage is produced by the actions of toxin A (TcdA) and toxin B (TcdB), which are the major virulence determinants of C. difficile. The emergence in 2000, first in the United States and Canada and more recently in Western Europe, of a hypervirulent strain of C. difficile (NAP1/BI/027) has led to an increase in the incidence and the case-fatality ratio of hospital-acquired diarrhea, resulting, on average, in 10.7 additional days in the hospital (14, 23, 26, 28, 29, 35). This epidemic NAP1/B1/027 strain produces higher levels of TcdA and TcdB (35). TcdA is primarily responsible for the mucosal damage and the inflammatory response in animal models (24). TcdA was shown to induce apoptosis in many human cell types in vitro, including endothelial cells (11), monocytes (34), HeLa cells (30), and intestinal epithelial cells (4, 5, 9). The mechanisms by which TcdA induces apoptosis in the cells remain to be fully characterized. Brito et al. demonstrated that TcdA-induced intestinal cell death involves caspase 8, 3, and 9 activation, but the inhibition of these caspases only partially blocked TcdA-induced DNA laddering (4). Carneiro et al. have shown that TcdA induces caspase 6, 8, 9, and 3 and Bid (a proapoptotic member of the Bcl-2 family) cleavage, resulting in cell death in human intestinal epithelial cells (5). Bid cleavage, however, occurred by a caspase-independent mechanism. Gerhard et al. reported activation of caspases 3, 8, and 9 in colonic crypt cells treated with TcdA (9). Finally, Qa'Dan et al. showed that pancaspase inhibitor slowed but did not inhibit TcdB-induced cell death in HeLa cells, suggesting that cell death occurs by both caspase-dependent and caspase-independent mechanisms (30). In contrast to previous studies, apoptosis was completely blocked by the pancaspase inhibitor z-VAD-fmk. The role of caspase activation in TcdA-induced cell death thus remains controversial. In addition, the degree of mitochondrial contribution to TcdA-induced cell death has not been investigated.
In this study, we examined the mechanism of TcdA-induced cell death using two cell culture models involving human colonic carcinoma and ovarian carcinoma cell lines. The advantage of using the ovarian carcinoma cell model was our ability to compare the effect of caspase inhibitors on TcdA-induced cell death to that of a well-established caspase-dependent stimulus (TRAIL), which cannot be assessed in colonic cell lines because of their inherent resistance to TRAIL. In this way, we show that TcdA induces primarily caspase-independent cell death in both ovarian and colonic carcinoma cells. We further demonstrate that TcdA-induced cell death is death receptor pathway independent but strongly dependent on the activation of the mitochondrial pathway. Thus, our findings define a novel mechanism in which TcdA-induced cell death involves a mitochondrion-dependent, caspase- and death receptor-independent signaling pathway that contrasts with previous data.
MATERIALS AND METHODS
Cell lines.
The human ovarian cancer cell lines CaOV3 and OVCAR3 and the human epithelial colonic carcinoma cell lines caco-2 and T84 were obtained from the American Type Culture Collection (Manassas, VA). SKOV3ip1 ovarian cancer cells were kindly provided by J. Price (M. D. Anderson Cancer Center, Houston, TX). CaOV3 and SKOV3ip1 cells were cultured in Dulbecco's modified Eagle's medium-F-12 (Wisent, St-Bruno, QC, Canada), and T84 and caco-2 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B) at 37°C in 5% CO2. The OVCAR3 cells were maintained in RPMI 1640 (Wisent) with 20% FBS, insulin (10 mg/liter), 2 mM glutamine, and antibiotics at 37°C in 5% CO2.
TcdA.
TcdA was purified to homogeneity from culture supernatants of the reference C. difficile strain VPI 10463 by thyroglobulin affinity and anion-exchange chromatography as previously described (15). Purified TcdA migrated as a single band in a silver nitrate-stained sodium dodecyl sulfate-polyacrylamide gel (307 kDa). Specificity was assessed by Western blot analysis with mouse monoclonal anti-TcdA and anti-TcdB antibodies (Biodesign International) (data not shown).
Reagents.
Recombinant human TRAIL was purchased from PeproTech (Rocky Hill, NJ). The pancaspase inhibitor z-VAD-fmk, the caspase 8 inhibitor z-IETD-fmk, the caspase 3 inhibitor z-DEVD-fmk, the caspase 9 inhibitor z-LEHD-fmk, and the fluorogenic caspase substrates DEVD-AFC (caspase 3) and IETD-AFC (caspase 8) were from R&D Systems (Minneapolis, MN) (DEVD is aspartic acid-glutamic acid-valine-aspartic acid, AFC is 7-amino-4-trifluoromethyl coumarin, and IETD is isoleucine-glutamic acid-threonine-aspartic acid). Phenazine methosulfate and anti-tubulin antibody were obtained from Sigma. XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] reagents were from Invitrogen. Anti-Bcl-XL was purchased from Cell Signaling Technology (Danvers, MA) and anti-Bcl-2 from Dakocytomation (Glostruck, Danmark). Antibodies for phospho-Akt (Ser-473) were from Invitrogen (Biosource) and anti-Akt from Cell Signaling. Neutralizing anti-toxin polyclonal immunoglobulin G antibodies were from Bartels (Carlsbad, CA). Mouse monoclonal anti-TcdA antibody was from Meridian Life Sciences Inc. (Saco, ME). Lipofectamine 2000 was purchased from Invitrogen (Burlington, ON, Canada).
Viability assays.
Cell viability in the presence or absence of TcdA was determined by XTT assay. Briefly, cells were plated at 20,000/well (CaOV3, OVCAR3, T84, and caco-2) or 15,000/well (SKOV3ip1) in 96-well plates in complete medium. The next day, the cells (60 to 70% confluence) were treated with TcdA and incubated for 48 h. At the termination of the experiment, the culture medium was removed and a mixture of phosphate-buffered saline and fresh medium (without phenol red) containing phenazine methosulfate and XTT was added for 30 min at room temperature. The optical density was determined using a microplate reader at 450 nm (TecanSunrise, Research Triangle Park, NC). The percent cell survival was defined as the relative absorbance of untreated versus TcdA-treated cells. In some experiments, cells were preincubated for 1 h with anti-toxin antibodies (polyclonal antibodies from Bartels) or caspase inhibitors before TcdA or TRAIL was added. All assays were performed in triplicate and repeated three times.
Caspase assays.
Caspase 3 and caspase 8 fluorogenic-protease assays were performed according the manufacturer's protocol (R&D Systems). In brief, 3 × 106 cells were lysed in 250 μl of cell lysis buffer (142 mM KCl, 5 mM MgCl2, 25 mM HEPES, 2 mM EGTA, 0.3% Nonidet P-40), and total cell lysates were incubated with 50 μM of DEVD-AFC (caspase 3) or IETD-AFC (caspase 8) substrate for 1 h at 37°C. Caspase 3 and caspase 8 activities were measured on a Versa Fluor fluorimeter (Bio-Rad, Hercules, CA). The protein concentrations of the lysates were measured with a Bio-Rad protein assay kit according to the manufacturer's recommendations.
The release of nucleosomal DNA into the cytoplasm as a measure of apoptosis was determined using the Cell Death Detection ELISA kit (Roche, Laval, Quebec, Canada) according to the manufacturer's instructions. Briefly, cells were treated with TcdA for 24 h. The cells were then lysed, and the extracted cytoplasmic nucleosomal DNA was captured in ELISA wells containing anti-histone antibodies. The nucleosomal DNA was detected with an anti-DNA-peroxidase-conjugated antibody. The absorbance of each well was determined using a microplate reader at 410 nm (TecanSunrise).
Immunoblot analysis.
Cells were harvested and washed with ice-cold phosphate-buffered saline. Whole-cell extracts were prepared in lysing buffer containing protease inhibitors [0.1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), 5 μg/ml pepstatin, 0.5 μg/ml leupeptin], and 2 μg/ml aprotinin), and the proteins were separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. The proteins were transferred to polyvinylidene difluoride membranes (Roche, Laval, Québec, Canada) by electroblotting, and immunoblot analysis was performed as previously described (8). All primary antibodies were incubated overnight at 4°C. The proteins were visualized by enhanced chemiluminescence (GE Healthcare, Baie d'Urfé, Québec, Canada).
Vector construction and transfection with small interfering RNA (siRNA) oligonucleotides.
The construction of the pcDNA3/Bcl-XL expression vector has been described previously (8). The expression of Bcl-XL is under the control of the cytomegalovirus promoter. The pRC/CMV-Bcl-2 vector was kindly provided by J. C. Reed (Burnham Institute). T84 and OVCAR3 cells stably expressing Bcl-XL or Bcl-2 were generated as follows. Cells (6 × 105) were transfected with 6 μg of linearized pcDNA3/Bcl-XL or pRC/CMV-Bcl-2 by using Fugene (Roche Diagnostic). The transfected cells were then selected in medium containing 100 μg/ml of G418 (Wisent) for 14 days. Stable transfectants were expanded, and expression of Bcl-XL and Bcl-2 was determined by Western blotting.
The siRNA oligonucleotide duplexes were purchased from Integrated DNA Technologies (Coralville, IA). The antisense strand of the siRNAs corresponded to AA(N)19 sequences in the coding region of the Bcl-XL mRNA (GenBank accession no. NM 138578). The GC content of the duplexes was kept within 40 to 55%. The siRNA oligonucleotides were targeted to nucleotides 469 to 489. The fluorescein-labeled luciferase GL2 duplex (siGLO) was purchased from Dharmacon Research, Inc. The cells were transfected with a mixture containing Lipofectamine (Invitrogen Life Technologies), optiMem, and siRNA oligonucleotides (50 μM) according to the protocol suggested by the manufacturer. The siRNA-Lipofectamine complex was then added to SKOV3ip1 cells in suspension and plated on 96-well plates (15,000 cells/well). After 24 h, medium containing FBS was added, along with TcdA (25 ng/ml). The cells were incubated for 48 h, and XTT assays were performed to determine cell viability. For Western blotting, the cells were lysed 24 to 72 h posttransfection, depending on the experiment, and total cell lysates were analyzed by immunoblotting.
MOMP.
Cells were seeded to 70 to 80% confluence. The next day, 20 ng/ml of TcdA was added for 5 h, and MOMP was assayed using the MitoLight apoptosis detection kit according to the manufacture's protocol (Chemicon).
Statistical analysis.
The data are presented as the means ± standard errors of the mean (SEM) from three independent experiments. Statistical comparisons between groups were performed using Student's t test. Statistical significance was indicated by a P value of <0.05.
RESULTS
C. difficile TcdA induces cell death in ovarian and colonic carcinoma cells.
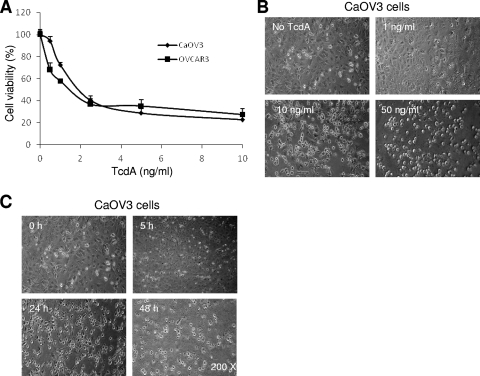
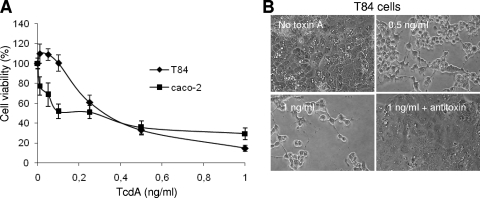
Human ovarian and colonic carcinoma cell lines were used to investigate TcdA-induced cell death. We have previously characterized the mechanisms of TRAIL- and drug-induced cell death in the ovarian carcinoma cell lines CaOV3 and OVCAR3 (8, 19-21). Efficient TRAIL-induced cell death in these cell lines requires interconnection between the cell surface death receptor and the mitochondrion-dependent apoptotic pathway and is highly dependent on caspase activation. We therefore used CaOV3 and OVCAR3 cell lines as models to investigate the mechanisms of TcdA-induced cell death. TcdA induced dose-dependent cell death, as well as cell rounding, in OVCAR3 and CaOV3 cells (Fig. 1A and B). CaOV3 cell rounding up was seen as early as 5 h and increased for up to 48 h after treatment with TcdA (10 ng/ml) (Fig. 1C). To confirm these results in more physiologically relevant cells, the human colonic cell lines T84 and caco-2 were treated with increasing concentrations of TcdA. As observed with ovarian cell lines, TcdA induced dose-dependent cell death and rounding up of T84 and caco-2 cells (Fig. 2A and B). TcdA-induced cell death in both ovarian and colonic cells was completely inhibited by coincubation with C. difficile antitoxins (Fig. 2B, bottom right [data are shown only for T84 cells]).
FIG. 1.
Sensitivity of human ovarian epithelial cells to TcdA-induced cell death. (A) The ovarian cancer cell lines CaOV3 and OVCAR3 were treated with increasing concentrations of TcdA for 48 h. Cell viability was determined by XTT assay. The data are expressed as percentages of the viability of untreated cells. (B and C) Morphological changes assessed by phase-contrast microscopy in CaOV3 cells treated with different doses of TcdA for 24 h (B) or with 10 ng/ml of TcdA for the indicated times (C). All cell viability assay data are means ± SEM of three independent experiments performed in triplicate.
FIG. 2.
Sensitivity of human colonic epithelial cells to TcdA-induced cell death. (A) The colonic cancer cell lines caco-2 and T84 were treated with increasing concentrations of TcdA for 48 h. Cell viability was determined by XTT assay. The data are expressed as percentages of the viability of untreated cells. The data are means ± SEM of three independent experiments performed in triplicate. (B) Morphological changes in T84 cells treated with different doses of TcdA for 24 h. The cytotoxic effect of TcdA was blocked by C. difficile antitoxins (bottom right).
TcdA induces caspase activation and MOMP.
Oligonucleosomal DNA fragmentation is one of the biochemical feature that occur during apoptosis (17). To determine whether apoptosis occurs in ovarian and colonic cancer cells during TcdA treatment, oligonucleosomal DNA fragmentation was measured in the presence or absence of TcdA in OVCAR3 and T84 cells. As shown in Fig. 3A, TcdA induced DNA fragmentation in both OVCAR3 and T84 cells at 24 h compared to untreated cells (P < 0.001). Next, CaOV3, OVCAR3, caco-2, and T84 cells were treated with TcdA, and caspase 8 and caspase 3 activation was determined at 24 h using fluorogenic substrates. TcdA induced the activation of caspase 8 and caspase 3 in the T84 and OVCAR3 cell lines (Fig. 3B). Similar results were seen in CaOV3 and caco-2 cells (data not shown). Caspase activation was completely blocked by coincubation of TcdA with antitoxins (Fig. 3B). Induction of apoptosis via the mitochondria leads to a loss of mitochondrial-membrane potential, followed by release of cytochrome c and activation of caspase 9. To evaluate whether TcdA activates the mitochondria, mitochondrial-membrane integrity was assessed by the uptake of a lipophilic cationic dye. Compared to untreated cells, TcdA-treated OVCAR3 cells displayed substantially more apoptotic mitochondria, as indicated by a greater number of green-labeled cells, indicating the inability of mitochondria to concentrate the dye within their membranes, leaving it in its monomeric form in the cytosol (green) (Fig. 3C). Red fluorescence represented dimeric dye that had accumulated in the intact mitochondrial membrane (Fig. 3C, left). Green fluorescence represented cytoplasmic pools of monomeric lipophilic cationic dye, indicating the inability of mitochondria to concentrate the dye, and consequently showed apoptotic cells (Fig. 3C, middle). Overlays clearly showed an increased number of apoptotic cells when OVCAR3 cells were treated with TcdA (Fig. 3C, right). These results confirmed that TcdA induces oligonucleosomal DNA fragmentation and activation of caspases and the mitochondria to induce cell death.
FIG. 3.
DNA fragmentation, caspase activation, and MOMP induced by TcdA. (A) OVCAR3 and T84 cells were treated with TcdA (5 ng/ml and 0.5 ng/ml, respectively) for 24 h. Lysates were obtained, and oligosomal DNA fragmentation was assessed using a Cell Death ELISA kit. *, P < 0.001 versus untreated cells. (B) Caspase 8 and caspase 3 activation was detected 24 h after incubation with TcdA (1 ng/ml) in the presence or absence of antitoxin in extracts from CaOV3 and OVCAR3 cells. The data are means ± SEM of three independent experiments performed in triplicate. *, P < 0.001 versus no toxin or antitoxin. RFU, relative fluorescence units. (C) Fluorescence microscopic analysis of mitochondrial-membrane potential in OVCAR3 cells treated with TcdA. OVCAR3 cells were cultured for 24 h without TcdA, and the mitochondrial-membrane integrity was assessed using the MitoLight apoptosis detection kit. In treated cells, fresh culture medium containing 20 ng/ml of TcdA was added for 5 h prior to MitoLight staining. In each overlay, the red fluorescence represents dimeric dye that accumulated in the intact mitochondrial membrane. The green fluorescence represents cytoplasmic pools of monomeric lipophilic cationic dye, indicating the inability of mitochondria to concentrate the dye, and consequently shows apoptotic cells. Cells containing green-labeled cytoplasm were scored as apoptotic cells.
Caspase inhibition only partially prevents TcdA-induced cell death.
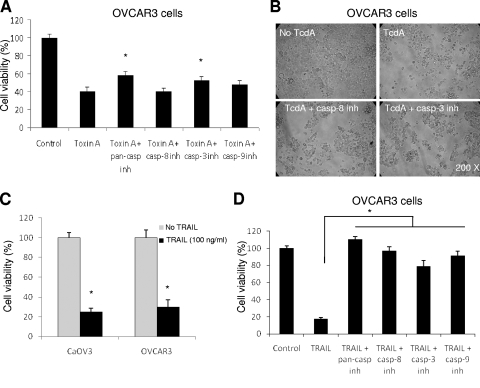
To clarify whether the activation of caspases is required for TcdA-induced cell death, we measured cell death in the presence of a pancaspase inhibitor or with specific caspase inhibitors. Caspase inhibition by the pancaspase inhibitor zVAD-fmk resulted in only a partial increase in cell viability in the presence of TcdA in OVCAR3 cells (40% versus 52%; P < 0.01) (Fig. 4A). Similarly, caspase 3 and caspase 9 inhibitors had very limited effects on TcdA-induced cell death (40% versus 51% and 40% versus 47%, respectively; P < 0.05), whereas caspase 8 had no effect (40% versus 40%). Of note, a relatively low dose of caspase inhibitors (10 μM) was used to reduce off-target effects, such as inhibition of calpains and cathepsins. Importantly, while caspases were efficiently inhibited at 10 μM of inhibitors, minimal cell toxicity was observed (data not shown). Although some of these differences in cell viability are statistically significant (pancaspase and caspase 3 inhibitors), their biological significance is probably limited. Consistent with this idea, caspase 3 and caspase 8 inhibitors did not inhibit the cell rounding mediated by TcdA in CaOV3 and OVCAR3 cells (Fig. 4B and data not shown).
FIG. 4.
Effects of caspase inhibitors on TcdA- and TRAIL-induced cell death in ovarian cells. (A) OVCAR3 cells were incubated with various caspase inhibitors (casp inh) (all at 10 μM) for 1 h, and then TcdA (5 ng/ml) was added for 48 h. Cell viability was assessed by XTT assay. (B) Morphological changes in OVCAR3 cells treated with TcdA (5 ng/ml) in the presence or absence of caspase 8 or caspase 3 (10 μM) inhibitor at 48 h. (C) OVCAR3 and CaOV3 cells were seeded in 96-well plates and treated with 100 ng/ml of TRAIL for 48 h, and cell viability was assessed. (D) OVCAR3 cells were treated as described for panel A with caspase inhibitors, and TRAIL (100 ng/ml) was added for 48 h. The data are means ± SEM of three independent experiments performed in triplicate. *, P < 0.05.
Apoptosis can be induced through death receptors or through the mitochondria. An interconnection exists between cell surface death receptors and the mitochondrion-initiated pathway of apoptosis, where death receptor ligands induce caspase 8-mediated Bid cleavage to activate the mitochondria. While TcdA induces MOMP in ovarian carcinoma cells, this process can be initiated by activation of the death receptors. To evaluate this possibility, we treated CaOV3 and OVCAR3 cells with TRAIL. As we have previously shown (19), TRAIL, a death receptor ligand, induced substantial cell death in CaOV3 and OVCAR3 cells (Fig. 4C), a process completely blocked by the pancaspase inhibitor and almost completely blocked by the specific caspase inhibitors, clearly indicating that the dose of inhibitors used is sufficient to efficiently block caspases and that TRAIL-induced cell death is dependent on caspase activation (Fig. 4D).
Similar experiments could not be performed in T84 and caco-2 cells because these cells are resistant to TRAIL-induced cell death (Fig. 5A). Nonetheless, incubation of caco-2 and T84 cells with pancaspase or specific caspase inhibitors had little effect on TcdA-induced cell death, as shown in Fig. 5B. Taken together, these results and those obtained with ovarian cancer cells suggest that TcdA induces caspase-independent cell death. In addition, the fact that both caco-2 and T84 cells are resistant to TRAIL-induced cell death, despite expressing TRAIL receptors, suggests that TcdA-induced cell death is also death receptor independent.
FIG. 5.
Effects of caspase inhibitors on TcdA- and TRAIL-induced cell death in colonic cells. (A) T84 and caco-2 cells were treated with increasing concentrations of TRAIL for 48 h. Cell viability was assessed by XTT assay. (B) T84 and caco-2 cells were incubated with various caspase inhibitors (casp inh) (all at 10 μM) for 1 h and then treated with TcdA (0.5 ng/ml) for 48 h. Cell viability was assessed by XTT assay. The data are means ± SEM of three independent experiments performed in triplicate.
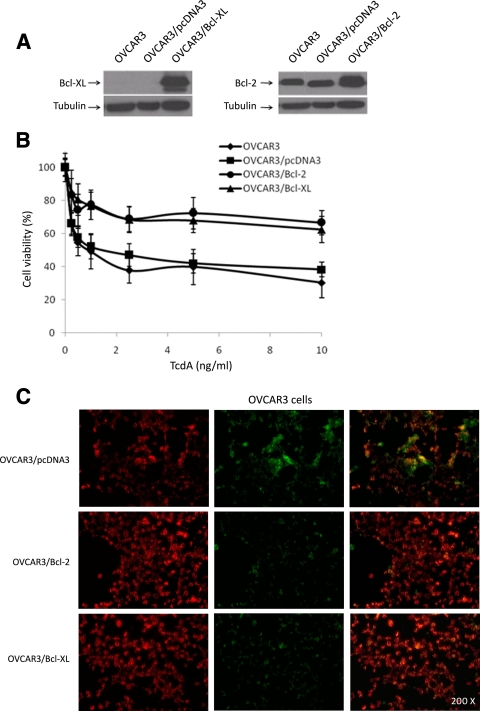
Overexpression of the antiapoptotic proteins Bcl-2 and Bcl-XL significantly inhibits TcdA-induced cell death.
Because caspase-independent cell death usually involves activation of the mitochondria, we examined whether the inhibition of the mitochondrion-dependent apoptotic pathway affects TcdA-induced cell death. To this end, we generated Bcl-2- and Bcl-XL-overexpressing OVCAR3 cell lines (Fig. 6A), treated these cells with TcdA, and evaluated cell viability. Consistent with the results shown in Fig. 1, we observed that TcdA induced dose-dependent cell death in parental and empty-vector-expressing OVCAR3 cells, where 50% of the cells were killed at a TcdA concentration of 1 ng/ml (50% inhibitory concentration [IC50]) (Fig. 6B). However, TcdA treatment of Bcl-2- and Bcl-XL-overexpressing OVCAR3 cells induced significantly less cell death. At the highest dose (10 ng/ml) of TcdA used, less than 50% of the cells were killed, implying that the TcdA IC50 exceeded 10 ng/ml for Bcl-2- and Bcl-XL-overexpressing OVCAR3 cells, which represents at least a 10-fold increase in the IC50 relative to the controls. Preincubation of Bcl-2- and Bcl-XL-overexpressing OVCAR3 cells with the different caspase inhibitors had no effect on TcdA-induced cell death (data not shown).
FIG. 6.
Effects of Bcl-2 and Bcl-XL overexpression on TcdA-induced cell death in OVCAR3 cells. (A) Immunoblot analysis of OVCAR3 cells stably transfected with either empty vector (OVCAR3/pcDNA3), Bcl-2 (OVCAR3/Bcl-2), or Bcl-XL (OVCAR3/Bcl-XL). (B) Effects of enforced expression of Bcl-2 and Bcl-XL on the viability of OVCAR3 cells in response to a 48-h treatment with increasing concentrations of TcdA. The data are means ± SEM of three independent experiments performed in triplicate. P < 0.001 relative to parental OVCAR3 and OVCAR3/pcDNA3. (C) OVCAR3/pcDNA3 (control), OVCAR3/Bcl-2, and OVCAR3/Bcl-XL cells were cultured for 24 h without TcdA, and the mitochondrial-membrane integrity was assessed using the MitoLight apoptosis detection kit. For treated cells, fresh culture medium containing 20 ng/ml of TcdA was added for 5 h prior to MitoLight staining. The red fluorescence (left column) represents dimeric dye that accumulated in the intact mitochondrial membrane, indicating nonapoptotic cells. The green fluorescence (middle column) represents cytoplasmic pools of monomeric lipophilic cationic dye, indicating the inability of mitochondria to concentrate the dye, and consequently shows apoptotic cells. The right column contains overlays of the left and middle columns.
To confirm the role of the mitochondria in TcdA-induced cell death, we treated Bcl-2- and Bcl-XL-overexpressing OVCAR3 cells and empty-vector-expressing OVCAR3 cells (control) with TcdA (20 ng/ml) for 5 h. As shown in Fig. 6C, fewer green-labeled cells (representing apoptotic mitochondria [middle]) were observed in TcdA-treated Bcl-2- and Bcl-XL-overexpressing OVCAR3 cells than in control cells, indicating that TcdA-mediated MOMP is inhibited by the expression of Bcl-2 or Bcl-XL.
To confirm these results in colonic carcinoma cells, Bcl-2- and Bcl-XL-overexpressing T84 cell lines were generated (Fig. 7A). TcdA-induced cell death was strongly attenuated in T84 cells expressing Bcl-2 or Bcl-XL compared to vector-transfected cells or the parental cell line (Fig. 7B). More than 70% cell viability was observed at concentrations as high as 5 ng/ml of TcdA in Bcl-2/Bcl-XL-expressing T84 cells, whereas the control and parental cell lines displayed <20% cell viability at this concentration. Taken together, the results demonstrate that the antiapoptotic proteins Bcl-2 and Bcl-XL regulate TcdA-induced cell death and MOMP.
FIG. 7.
Effects of Bcl-2 and Bcl-XL overexpression on TcdA-induced cell death in T84 cells. (A) Immunoblot analysis of T84 cells stably transfected with either empty vector (T84/pcDNA3), Bcl-2 (T84/Bcl-2), or Bcl-XL (T84/Bcl-XL). (B) Effects of enforced expression of Bcl-2 and Bcl-XL on the viability of T84 cells in response to a 48-h treatment with increasing concentrations of TcdA. The data are means ± SEM of three independent experiments performed in triplicate. *, P < 0.001 relative to parental T84 cells and T84/pcDNA3 cells.
Specific inhibition of Bcl-XL enhances TcdA-induced cell death.
The mitochondria appear to play an important role in TcdA-induced cell death. When we tested the susceptibilities of different cell lines to TcdA-induced cell death, we found that the ovarian cancer cell line SKOV3ip1 was resistant to TcdA (Fig. 8A). This provided us with a unique model to confirm the role of the mitochondria in TcdA-induced cell death. Interestingly, SKOV3ip1 cells expressed higher levels of Bcl-XL protein than the TcdA-sensitive cell lines CaOV3 and OVCAR3 (Fig. 8B). We then investigated the effects of Bcl-XL downregulation in the Bcl-XL-overexpressing, TcdA-resistant SKOV3ip1 cell line. To inhibit Bcl-XL expression, Bcl-XL-overexpressing and TcdA-resistant SKOV3ip1 cells were transfected with control siGLO or Bcl-XL siRNA, which have previously been shown to effectively suppress Bcl-XL protein levels in ovarian cancer cells (6). SKOV3ip1 cells transfected with Bcl-XL siRNAs showed a marked reduction of Bcl-XL protein levels at 24 h (Fig. 8C) and for up to 72 h (data not shown). Inhibition of Bcl-XL expression in SKOV3ip1 cells led to a significant (P < 0.001) decrease in survival (70% to 36% cell viability) after TcdA treatment (Fig. 8D) compared to mock-transfected cells or cells transfected with the control siRNA (siGLO) (P < 0.001). These data suggest that resistance to TcdA-induced cell death may be mediated by antiapoptotic members of the Bcl-2 family and further support the central role of the mitochondria in TcdA-induced cell death.
FIG. 8.
Downregulation of Bcl-XL enhances TcdA-induced cell death. (A) SKOV3ip1 epithelial ovarian cancer cells were treated with increasing concentrations of TcdA, and cell viability was assessed by XTT assay after 48 h. (B) Immunoblot analysis of Bcl-XL expression in SKOV3ip1, OVCAR3, and CaOV3 cells. (C) SKOV3ip1 cells were left untreated, treated with siGLO (control siRNA), or treated with Bcl-XL siRNA for 24 h. The expression of Bcl-XL was assessed by immunoblotting. (D) SKOV3ip1 cells were left untreated or treated with siGLO or with siRNA Bcl-XL for 24 h. The data are means ± SEM of three independent experiments performed in triplicate. The cells were then treated with TcdA (25 ng/ml) for 48 h, and cell viability was determined. *, P < 0.001 relative to mock-transfected or siGLO-transfected SKOV3ip1 cells.
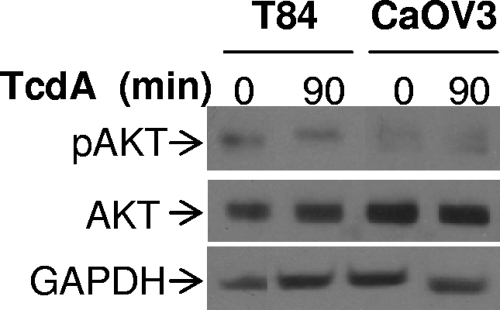
TcdA does not affect the PI3K/Akt pathway.
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is a well-established cell survival pathway. The downstream target of PI3K, Akt, regulates the expression and/or the activity of pro- and antiapoptotic members of the Bcl-2 family (7). In this context, it is conceivable that inhibition of this pathway by TcdA would promote TcdA-induced cell death. We thus assessed the activation of Akt by determining the levels of Akt phosphorylation in the presence or absence of TcdA in CaOV3 and T84 cells. As shown in Fig. 9, Akt phosphorylation was not affected by TcdA, suggesting that the PI3K/Akt pathway is not involved in TcdA-mediated cell death.
FIG. 9.
Effect of TcdA on Akt activation. Colonic T84 and ovarian CaOV3 cells were plated and incubated for 24 h in complete medium. The medium was removed, and TcdA (0.5 ng/ml for T84 cells; 5 ng/ml for CaOV3 cells) was added for 90 min, after which the lysates were obtained and examined by Western blotting with antibodies for phospho-Ser473 Akt (p-Akt) and Akt antibody (AKT). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used to ensure equal loading.
DISCUSSION
It has been well established that C. difficile TcdA induces cell death in human epithelial cells in vitro with typical apoptotic alterations, such as DNA fragmentation and caspase activation (4, 5, 9). Epithelial cell death is also observed in the mucosa of ligated ileal loops injected with TcdA from experimental animal models (12) and results in a significant increase in epithelial cell apoptosis in ileal loops, as assessed by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay and caspase 3 activity (6). While epithelial cell death caused by TcdA may play an important role in the pathogenicity of C. difficile in humans, some conflicting data exist as to the involvement of caspases and mitochondria in TcdA-mediated cell death. The contribution of TcdA-induced apoptosis to mucosal damage also remains unclear. In this study, we have examined the mechanisms of TcdA-induced cell death. We found that TcdA induces caspase activation and oligosomal DNA fragmentation in both ovarian and colonic epithelial cells. These findings are consistent with the current concept that TcdA induces apoptosis in epithelial cells. In addition, our data clearly demonstrate an important role for Bcl-2 and Bcl-XL in modulating TcdA-induced cell death in ovarian and colonic epithelial cells, whereas caspase activation appears to be nonessential. Bcl-2 and Bcl-XL can almost completely inhibit cell death mediated by TcdA at the level of the mitochondria, likely by neutralizing the proapoptotic proteins Bax and Bak, which form channels in the outer mitochondrial membrane, allowing the release of cytochrome c and subsequent activation of effector caspases. This is supported by the fact that Bcl-2 and Bcl-XL overexpression can prevent TcdA-induced cell death in ovarian and colonic cells and prevent MOMP in ovarian cells. Conversely, Bcl-XL downregulation by RNA interference in TcdA-resistant SKOV3ip1 cells enhanced TcdA-induced cell death, not only supporting the central role of the mitochondria, but also suggesting that resistance to TcdA may be mediated by antiapoptotic members of the Bcl-2 family.
The fact that expression of Bcl-2 and Bcl-XL almost completely blocks TcdA-induced cell death in T84 cells, as assessed by XTT assay, is also important with regard to how cells died. For instance, although we found evidence that TcdA induces apoptosis in T84 cells, this does not rule out the possibility that necrosis, a form of cell death, plays a role in TcdA-induced cell toxicity. However, it is well established that Bcl-2/Bcl-XL expression does not block necrosis (17). This is important because, as mentioned above, expression of these antiapoptotic proteins efficiently blocks TcdA-induced death, suggesting that necrosis plays a minor role, if any, in TcdA-induced cytotoxicity. Furthermore, MOMP changes are typical features of apoptosis and are not usually observed in necrosis (17). As can be seen from Fig. 3C, most TcdA-treated cells showed increased mitochondrial permeability, further supporting the concept that most cells died by apoptosis.
Both caspase 8 and caspase 3 were activated by TcdA in ovarian and colonic cells, as determined by fluorimetric assays, suggesting possible involvement of the death receptor pathway of apoptosis in TcdA-mediated cell death. However, we demonstrated that TRAIL-induced cell death was nearly completely inhibited by the caspase 8 inhibitor z-IETD-fmk, whereas the inhibitor did not block TcdA-induced cell death, indicating that the death receptor pathway is not involved in TcdA-induced cell death. The activation of caspase 8 is not essential for the cell death induced by TcdA. This is consistent with data from Brito et al., which showed that Bid cleavage, an event associated with activation of the death receptor pathway of apoptosis, occurred before caspase 8 activation and was not blocked by caspase 8 inhibitors, also suggesting a death receptor-independent mechanism (4). Toxin-mediated death receptor-independent cell death has also been reported for Staphylococcus aureus alpha toxin (1) and Shiga toxin 1 (22).
To adequately appreciate the role of caspase activation in TcdA-induced cell death, we used a well-characterized epithelial ovarian cancer model in which the death receptor pathway of apoptosis has been defined. We knew from previous studies (19, 20) that TRAIL-induced apoptosis is dependent on caspase activation in these cells. This allowed us to compare the extents of TcdA- and TRAIL-induced cell death inhibition mediated by various caspase inhibitors. In agreement with previous reports (19, 20), TRAIL-induced cell death was nearly completely inhibited by the various caspases inhibitors. In contrast, caspase inhibitors had limited or no impact on TcdA-induced cell death. It should be noted that we used a low dose of caspase inhibitor (10 μM) to limit off-target effects, which may be observed with doses of >20 μM. The difference between our study and other studies regarding the role of caspase in TcdA-induced cell death may be explained by the use of a higher concentration (50 μM) of the caspase inhibitor z-VAD-fmk (9) and the lack of appropriate controls to which to compare the effects of caspase inhibitors and the use of different cell types. However, our analysis demonstrated that caspase inhibitors had similar effects on ovarian and colonic cells. Alternatively, it is possible that although TcdA-induced cell death occurs mostly through a caspase-independent pathway, the bystander activation of caspases by TcdA may still contribute to a limited extent to the cell death observed in different systems.
Because TcdA-induced cell death occurred by a caspase- and death receptor-independent pathway, we examined the role of the mitochondria. Apoptosis mediated by the mitochondria involves the translocation of the proapoptotic proteins Bax and Bak to the mitochondria, where they can form channels and lead to MOMP (31). This process is inhibited by antiapoptotic members of the Bcl-2 family (2). Our data support the central role of the mitochondria in TcdA-induced cell death: (i) in Bcl-2- or Bcl-XL-transfected OVCAR3 cells, TcdA-induced cell death and MOMP were significantly attenuated compared to empty vector-transfected cells; (ii) in contrast, downregulation of Bcl-XL in TcdA-resistant SKOV3ip1 cells significantly enhanced TcdA-induced cell death. Consistent with these results, overexpression of Bcl-2 and Bcl-XL in T84 cells also strongly attenuated TcdA-induced cell death. Altogether, the data suggest that TcdA targets the mitochondria, possibly by upregulating the expression of BH3-only proapoptotic proteins, such as Bik and Puma (I. Matte, unpublished data), that can promote MOMP. The ability of TcdA to activate the mitochondria appears to be controlled by antiapoptotic members of the Bcl-2 family in our models. The involvement of the mitochondria in toxin-mediated cell death has also been described recently for S. aureus alpha toxin (1) and Shiga toxin 1 (22), further supporting our data. In addition, pneumococcal infection triggers apoptosis in vivo by decreasing the antiapoptotic protein Mcl-1, a member of the Bcl-2 family. Conversely, overexpression of Mcl-1 in a transgenic-mouse model blocks pneumococcus-induced apoptosis, also supporting the concept that Bcl-2 family members are important regulators of pathogen-induced apoptosis (25).
Although the physiological significance of apoptosis in vivo in C. difficile pathogenicity remains unclear at this point, there are several examples where the mechanism of pathogen-induced cell death involves the modulation of the apoptotic response (reviewed in reference 18). Pathogens may trigger apoptosis through different mechanisms, including production of bacterial toxins and virulence factors and interference with cell survival pathways. By inducing apoptosis, the bacteria may eliminate host immune cells, decrease the immune response, and prevent the host inflammatory response. Alternatively, intestinal epithelial cell apoptosis may promote pathogen clearance (16). The role of apoptosis in C. difficile infection could be assessed using Bcl-2- or Bcl-XL-overexpressing transgenic mice, for example.
In summary, our data demonstrate that C. difficile TcdA induces cell death in epithelial ovarian and colonic cancer cells through the activation of the mitochondria independently of the death receptor signaling cascade and caspase activation. This process is regulated by antiapoptotic proteins of the Bcl-2 family.
Acknowledgments
These studies were supported by an internal grant from the Centre de Recherche Clinique Étienne-Lebel from the Centre Hospitalier Universitaire de Sherbrooke. Élodie Côté received a scholarship from the Fonds de Recherche en Santé du Québec.
We thank Louis Valiquette and Claudine Rancourt for careful reading of the manuscript.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 21 September 2009.
REFERENCES
- 1.Bantel, H., B. Sinha, W. Domschke, G. Peters, K. Schulze-Osthoff, and R. U. Jänicke. 2001. α-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signalling. J. Cell Biol. 155:637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boise, L. H., and C. B. Thompson. 1997. Bcl-XL can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc. Natl. Acad. Sci. USA 94:3759-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodmer, J. L., N. Holler, S. Reynard, P. Vinciguerra, P. Schneider, P. Juo, J. Blenis, and J. Tschopp. 2000. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat. Cell Biol. 2:241-243. [DOI] [PubMed] [Google Scholar]
- 4.Brito, G. A. C., J. Fujii, B. A. Carneiro-Filho, A. A. M. Lima, T. Obrig, and R. L. Guerrant. 2002. Mechanisms of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 186:1438-1447. [DOI] [PubMed] [Google Scholar]
- 5.Carneiro, B. A., J. Fujii, G. A. C. Brito, C. Alcantara, R. B. Oria, A. A. M. Lima, T. Obrig, and R. L. Guerrant. 2006. Caspase and Bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro. Infect. Immun. 74:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chae, S., L. Eckmann, Y. Miyamoto, C. Pothoulakis, M. Karin, and M. F. Kagnoff. 2006. Epithelial cell IκB-kinase has an important protective role in Clostridium difficile toxin A-induced mucosal injury. J. Immunol. 177:1214-1220. [DOI] [PubMed] [Google Scholar]
- 7.Datta, S. C., A. Brunnet, and M. E. Greenberg. 2007. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 8.Dodier, P., and A. Piché. 2006. Bcl-XL is functionally non-equivalent for the regulation of growth and survival in human ovarian cancer cells. Gynecol. Oncol. 100:254-263. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard, R., S. Nottrott, J. Schoentaube, H. Tatge, A. Olling, and I. Just. 2008. Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J. Med. Microbiol. 57:765-770. [DOI] [PubMed] [Google Scholar]
- 10.Hinz, S., A. Trauzold, L. Boenicke, C. Sandberg, S. Beckmann, E. Bayer, H. Walczak, H. Kalthoff, and H. Ungefroren. 2000. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene 19:5477-5486. [DOI] [PubMed] [Google Scholar]
- 11.Hippenstiel, S., B. Schmeck, P. D. N′Guessan, J. Seybold, M. Krull, K. Preissner, C. Von Eichel-Streiber, and N. Suttorp. 2002. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. 238:L830-L838. [DOI] [PubMed] [Google Scholar]
- 12.Ishida, Y., T. Maegawa, T. Kondo, A. Kimura, Y. Iwakura, S. Nakamura, and N. Mukaida. 2004. Essential involvement of IFN-γ in Clostridium diffiicile toxin A-induced enteritis. J. Immunol. 172:3018-3025. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara, A., T. Kobayashi, and S. Nagata. 1998. Inhibition of Fas-induced apoptosis by Bcl-2. Oncogene 17:2549-2554. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, C. P., and J. T. LaMont. 2008. Clostridium difficile—more difficult than ever. N. Engl. J. Med. 359:1932-1940. [DOI] [PubMed] [Google Scholar]
- 15.Krivan, H. C., and T. D. Wilkins. 1987. Purification of Clostridium difficile toxin A by affinity chromatography on immobilized thyroglobulin. Infect. Immun. 55:1873-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kischkel, F. C., S. Hellbardt, I. Behrmann, M. Germer, M. Pawlita, P. H. Krammer, and M. E. Peter. 1995. Cytotoxicity-dependent APO-1 (FAS/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroemer, G., L. Galluzzi, P. Vandenabeele, J. Abrams, E. S. Alnemri, E. H. Baehrecke, M. V. Blagosklonny, W. S. El-Deiry, P. Golstein, D. R. Green, M. Hengartner, R. A. Knight, S. Kumar, S. A. Lipton, W. Malorni, G. Nunez, M. E. Peter, J. Tschopp, J. Yuan, M. Piacentini, B. Zhivotovsky, and G. Melino. 2009. Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 16:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labbé, K., and M. Saleh. 2008. Cell death in the host response to infection. Cell Death Differ. 15:1339-1349. [DOI] [PubMed] [Google Scholar]
- 19.Lane, D., A. Cartier, S. L'Espérance, M. Côté, C. Rancourt, and A. Piché. 2004. Differential induction of apoptosis by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in human ovarian carcinoma cells. Gynecol. Oncol. 93:594-604. [DOI] [PubMed] [Google Scholar]
- 20.Lane, D., V. Robert, R. Grondin, C. Rancourt, and A. Piché. 2007. Malignant ascites protect against TRAIL-induced apoptosis by activating the PI3K/Akt pathway in human ovarian carcinoma cells. Int. J. Cancer 121:1227-1237. [DOI] [PubMed] [Google Scholar]
- 21.Lane, D., M. Côté, R. Grondin, M. C. Couture, and A. Piché. 2006. Acquired resistance to TRAIL-induced apoptosis in human ovarian cancer cells is conferred by increased turnover of mature caspase-3. Mol. Cancer Ther. 5:509-521. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S.-Y., R. P. Cherla, I. Caliskan, and V. L. Tesh. 2005. Shiga toxin 1 induces apoptosis in the human myelogenous leukemia cell line THP-1 by a caspase-8-dependent, tumor necrosis factor receptor-independent mechanism. Infect. Immun. 73:5115-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo, V. G., M. D. Libman, M. A. Miller, A. M. Bourgault, C. H. Frenette, M. Kelly, S. Michaud, T. Nguyen, L. Poirier, A. Vibien, R. Horn, P. J. Laflamme, and P. René. 2004. Clostridium difficile: a formidable foe. Can. Med. Assoc. J. 171:47-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyverly, D. M., K. E. Saum, D. K. MacDonald, and T. D. Wilkins. 1985. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 47:349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marriott, H. M., C. D. Bingle, R. C. Read, K. E. Braley, G. Kroemer, P. G. Hellewell, et al. 2005. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J. Clin. Investig. 115:359-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owen, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridum difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 27.Muzio, M., B. R. Stockwel, H. R. Stennicke, G. S. Salvesen, and V. M. Dixit. 1998. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273:2926-2930. [DOI] [PubMed] [Google Scholar]
- 28.Pépin, J., L. Valiquette, M. E. Alary, P. Villemure, A. Pelletier, K. Forget, K. Pépin, and D. Chouinard. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Can. Med. Assoc. J. 171:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pépin, J., L. Valiquette, and B. Cossette. 2005. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can. Med. Assoc. J. 173:1037-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qa'Dan, M., M. Ramsey, J. Daniel, L. M. Spyres, B. Safiejko-Mroczka, W. Ortiz-Leduc, and J. D. Ballard. 2002. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell Microbiol. 4:425-434. [DOI] [PubMed] [Google Scholar]
- 31.Reed, J. C. 1998. Bcl-2 family proteins. Oncogene 17:3225-3236. [DOI] [PubMed] [Google Scholar]
- 32.Tait, S. W., and D. R. Green. 2008. Caspase-independent cell death: leaving the set without the final cut. Oncogene 27:6452-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor, R. C., S. P. Cullen, and S. J. Martin. 2008. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 9:231-241. [DOI] [PubMed] [Google Scholar]
- 34.Warny, M., and C. P. Kelly. 1999. Monocytic cell necrosis is mediated by potassium depletion and caspase-like proteases. Am. J. Physiol. 276:C717-C724. [DOI] [PubMed] [Google Scholar]
- 35.Warny, M., J. Pépin, A. Fang, G. Killgore, A. Thompson, J. Brazier, E. Frost, and L. C. McDonald. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079-1084. [DOI] [PubMed] [Google Scholar]