Abstract
Background
Albuminuria, a kidney marker of microvascular disease, may herald microvascular disease elsewhere, including in the brain.
Study Design
Cross sectional.
Setting and Participants
Boston, MA (USA) elders receiving home health services to maintain independent living who consented to brain magnetic resonance imaging.
Predictor
Urine albumin to creatinine ratio (ACR).
Outcome
Performance on a cognitive battery assessing executive function and memory using principal components analysis and white matter hyperintensity volume on brain imaging, evaluated in logistic and linear regression models.
Results
Of 335 participants, mean age was 73.4 ± 8.1 years; 123 participants had microalbuminuria or macroalbuminuria. Each doubling of ACR was associated with worse executive function [β=-0.05 (p=0.005) in univariate and β=-0.07 (p=0.004) in multivariable analyses controlling for age, sex, race, education, diabetes, cardiovascular disease, hypertension, medications, and estimated glomerular filtration rate] but not with worse memory or working memory. Individuals with microalbuminuria or macroalbuminuria were more likely to be in the lower versus the highest tertile of executive functioning [Odds ratio =1.18 (1.06 to 1.32) and 1.19 (1.05 to 1.35) per doubling of ACR in univariate and multivariable analyses, respectively]. Albuminuria was associated with qualitative white matter hyperintensity grade [Odds ratio =1.13 (1.02 to 1.25) and 1.15 (1.02 to 1.29) per doubling of ACR] in univariate and multivariable analyses, and with quantitative white matter hyperintensity volume [β=0.11 (p=0.007) and β=0.10 (p=0.01)] in univariate and multivariable analyses of log-transformed data, respectively. Results were similar when excluding individuals with macroalbuminuria.
Limitations
Single measurement of ACR, indirect creatinine calibration and reliance on participant recall for elements of medical history
Conclusions
Albuminuria is associated with worse cognitive performance, particularly in executive functioning, as well as increased white matter hyperintensity volume. Albuminuria likely identifies greater brain microvascular disease burden.
Index Words: Chronic kidney disease, dementia, cognitive impairment, albuminuria, stroke
Introduction
Cognitive impairment may decrease quality of life and increase healthcare utilization. Cognitive impairment is often classified according to the anatomic correlate; cortical impairment is characterized by deficits in memory and is typified by Alzheimer's Disease, while frontal/subcortical impairment is characterized by deficits in processing and executive functioning and is typified by vascular dementia.1 Typically, cognitive impairment secondary to vascular causes is a chronic process resulting from subclinical vascular insults.1 The summation of multiple insults, often in the absence of clinically appreciated stroke, may manifest as subtle cognitive impairment, particularly in executive functioning, and presage subsequent development of dementia.2
Several studies have shown a high prevalence of cognitive impairment in dialysis patients,3-5 with recent investigations suggesting that small vessel cerebrovascular disease, often manifest as white matter hyperintensities on brain imaging, contributes to cognitive impairment in this population.6 It is likely that the cerebrovascular disease and subsequent cognitive impairment manifest in dialysis patients begins during earlier stages of chronic kidney disease (CKD).7-11
CKD is an independent risk state for incident and prevalent cerebrovascular disease.12 With the rising prevalence of CKD, this relationship increases in public health importance.13 Microalbuminuria, which defines most stage 1 and 2 CKD and is often a feature of later stages of CKD, likely represents chronic glomerular and tubular endothelial damage resulting from hypertension, diabetes mellitus and other diseases of the vasculature. Accordingly, the presence of albuminuria may herald diseases of other vascular beds, including cerebrovascular disease. This has been appreciated in several epidemiologic studies, where microalbuminuria has a strong, independent association with coronary heart disease and clinical stroke.12, 14, 15 However, there are limited data on the relationship between microalbuminuria and cognitive impairment, with currently published reports examining the National Health and Nutrition Examination Survey (NHANES) 1999-2002 population and noting poorer performance on the digit symbol substitution test (a measure of attention and processing speed) in individuals with both microalbuminuria and peripheral vascular disease.16
In this study, we evaluate the association between albuminuria and both cognitive impairment and white matter hyperintensity volume in an elderly population, qualifying the specific cognitive performance metrics impacted in individuals with albuminuria. As microalbuminuria may serve as an early indicator of endothelial disease, we hypothesized that albuminuria would identify individuals with a higher burden of cerebrovascular disease and therefore a higher burden of impairment in executive function.
Methods
Study Population
Detailed methodology for the Nutrition, Aging, and Memory in Elders (NAME) study has been described.17 Between 2003 and 2007, participants were recruited at four Boston-area homecare agencies that provide services to make home living possible for elders. Eligible individuals were at least 60-years-old, had low income (determined by the state), diminished ability to perform activities of daily living, and an unmet need in a critical area including food or personal care. NAME exclusion criteria were non-English fluency, severe auditory or visual impairment, mental retardation, and known brain tumor. The Institutional Review Board at Tufts Medical Center approved the protocol.
All 1,248 NAME participants agreed to 3 home evaluations over several weeks: evaluation 1 included neuropsychological testing; evaluation 2 included fasting phlebotomy; evaluation 3 included history, medication ascertainment, and physical assessment. In a planned subset analysis targeting 25% of the total population, 366 participants underwent examination at Tufts Medical Center including brain magnetic resonance imaging (MRI); 361 were approached for an ancillary study that included urine collection (5 excluded for investigator unavailability). Excluding 7 individuals receiving kidney replacement therapy, 3 who refused consent, 1 unable to consent, 2 missing urine samples, and 13 unable to produce urine, the final population comprised 335 participants.
Data Collection
Information related to demographics and medical history was obtained through interviewer-administered questionnaires. Seated blood pressure was measured after 5 minutes of rest and defined by the average of two measurements. Interviewers examined medication bottles and participants were queried regarding current medication use. Fasting blood was obtained at the participant's home, immediately aliquoted, centrifuged, and transported to the laboratory on ice.
Serum chemistries were initially measured on a Roche Cobas Mira (F. Hoffmann-La Roche Ltd, Basel Switzerland) until an equipment change in the NAME central laboratory to the Olympus AU 400e (Olympus America Inc, Center Valley, PA, USA). Serum creatinine was assessed on both instruments using an alkaline picrate kinetic method. Values were calibrated to each other and to an isotope dilution mass spectrometry (IDMS) standard as follows: 1) Published data demonstrates that the Roche Cobas Mira overestimates an IDMS methodology by 0.18 mg/dL (14 μmol/L) while the Olympus overestimates the an IDMS standard by 0.09 mg/dL (7 μmol/L), implying a differential of approximately +0.09 mg/dL (7 μmol/L);18 and 2) At the time of equipment change, 89 fresh samples with creatinine level between 0.4 mg/dL (31 μmol/L) and 5 mg/dL (381 μmol/L) were concurrently analyzed on both the Cobas Mira and Olympus AU 400e, revealing a differential of +0.09 mg/dL (7 μmol/L). Accordingly, 0.18 mg/dL (14 μmol/L) was subtracted from all serum creatinine levels obtained using the Cobas Mira and 0.09 mg/dL (7 μmol/L) was subtracted from Olympus values to indirectly calibrate to an IDMS standard. Estimated glomerular filtration rate (eGFR) was calculated using the 4-variable MDRD equation re-expressed for the IDMS standard.19
Random morning urine was obtained at the hospital visit by a mid-stream clean-catch technique. Grossly cloudy and hematuric urine was not analyzed (n=0). Urine was assayed at the Tufts Medical Center clinical laboratory within 2 hours of collection for albumin and creatinine with the Beckman UniCel® DxC 800 (Beckman Coulter, Inc., Fullerton, CA, USA) using turbidimetric and alkaline picrate methods, respectively.
The Mini Mental State Examination (MMSE) and the North American Adult Reading Test (NAART) were administered to determine study eligibility. Individuals with MMSE ≤10 or NAART <75 were excluded in order to prevent floor effects. The neuropsychological test battery included multiple tests to assess a broad range of cognitive functioning (Table S1, provided as online supplementary material available with this article at www.ajkd.org).17 All individuals completed the majority of neuropsychiatric testing, with 309 (92.2%) participants completing all testing.
Brain MRI series obtained on a 1.5 T Symphony Siemens' scanner include: T1-weighted axial images; intermediate and T2-weighted conventional spin-echo axial images; and Fluid Attenuation Inversion Recovery (FLAIR) Turbo SE axial images. Trained readers blinded to participants' characteristics analyzed images under the supervision of a neuroradiologist. Analyze™ image software (Biomedical Imaging Resource, Versions 6.0 and 7.0) was used for quantitative measurements of intracranial, brain, and cerebrospinal fluid (CSF) volume.17 Using transaxial sections, brain volume was delineated by manually outlining the intracranial vault above the tentorium to determine total intracranial volume. Next, skull, other non-brain tissues and CSF were removed from the image, followed by mathematical modeling to determine total brain volume (the sum of supratentorial gray and white matter volume).17 The ratio of total brain volume to total intracranial volume was used to account for differences in head size.20 White matter hyperintensities (WMH) are defined as hyperintense changes on intermediate intensity/FLAIR and T2 weighted images with no corresponding T1 abnormality. Quantitative WMH volume was normalized by dividing WMH by the ratio of total brain to total intracranial volume. A single neuroradiologist qualitatively graded WMH severity on a 10-point scale, successively increasing from no or barely detectable change (grades 0 and 1, respectively) to almost all white matter involved (grade 9).21, 22
Complete brain imaging was obtained in 305 participants; of the 30 without sufficient MRI data, 10 either had metal present or withdrew consent, 11 had excessive movement, 6 requested early discontinuation, and 3 had insufficient imaging for determination of brain volume, yielding 305 (91.1%) with WMH, total brain and total intracranial volumes assessed.
Definitions
Albumin to creatinine ratio (ACR) was defined as (urine albumin/urine creatinine)*100, with units of mg/g creatinine. Microalbuminuria was defined as an ACR of 17-250 mg/g in men and 25-350 mg/g in women.23 Macroalbuminuria comprised values >250 and 350 mg/g, respectively. In cases where urine albumin level was below the threshold for detection, the ACR was assigned a value of 1 mg/g to allow log-transformation. Diabetes was defined by fasting plasma glucose ≥126 mg/dl (7 mmol/L) or glycemic therapy. In 2 individuals without serum, diabetes was classified by medication use and patient recall. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or medication use. Cardiovascular disease was defined by patient recall of myocardial infarction or coronary revascularization. Stroke was defined by patient recall. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. When standing height was unobtainable, knee height was used to calculate BMI.24 Education was classified on four levels based on highest achievement. Activities of daily living were assessed by questionnaire.
Statistical Analysis
All analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC). Variable distributions were evaluated; ACR, normalized WMH volume and C-reactive protein (CRP) were log-transformed to meet statistical test assumptions. Clinical and demographic characteristics, stratified by normoalbuminuria, microalbuminuria, and macroalbuminuria, were compared using ANOVA and the Cochran-Armitage test for trend for continuous and categorical variables, respectively. The Kruskal-Wallis test was used for comparing skewed variables. All testing was 2-sided.
Univariate and multiple linear regression models were constructed to examine associations of ACR with cognitive function and with normalized quantitative WMH volume. Principal components analysis was used as a data reduction technique for cognitive performance. The analysis of cognitive components was completed with an orthogonal rotational procedure. The adequacy of the correlation was assessed with Bartlett's test and Kaiser-Meyer-Olkin measure. Components with Eigen values >1.0 were defined by items with component loading scores >0.4 as representing executive functioning, memory and working memory. Working memory refers to temporarily storing and manipulating information, while memory refers to longer-term recall. Components are standardized to mean values of 0 with standard deviations of 1. Component scores (Table S2, provided as online supplementary material available with this article at www.ajkd.org) were incorporated into univariate regression models as the dependent variable with albuminuria as the predictor. A priori, model 2 adjusted for age, center and education, while model 3 further adjusted for sex, race, diabetes, cardiovascular disease, hypertension, current use of angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers, and eGFR. In confirmatory analyses, representative tests that were specified from each component where ACR was significant were explored as the dependent variable. The same modeling process was used to explore the relationship between albuminuria and WMH volume. To better conceptualize these associations, unadjusted and adjusted logistic regression was performed with the independent term being presence or absence of micro/macroalbuminuria and dichotomous dependant terms as highest cognition versus lower two tertiles or WMH grade dichotomized as grades 0 or 1 versus all other grades in separate models.
In sensitivity analyses, backward elimination (p<0.1 for retention) was used for selection among candidate variables in table 1 after age, education and center were forced into models. Further analyses examined the relationship between urine albumin levels and cognitive tests in planned interaction analyses with diabetes, race, cardiovascular disease and eGFR (stratified at 60 mL/min/1.73m2 (1 mL/sec/1.73m2)). To maintain power to appreciate interactions, these models controlled for age, center and education only. In two additional analyses, we excluded individuals with macroalbuminuria and excluded participants with a clinical history of stroke.
Table 1.
Baseline characteristics stratified by sex-specific level of albuminuria
| Total N=335 |
Normoalbuminuria N=212 |
Microalbuminuria N=103 |
Macroalbuminuria N=20 |
p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 73.4 ± 8.1 | 72.4 ± 8.1 | 75.3 ± 8.1 | 73.6 ± 6.3 | 0.01 |
| Sex (% Women) | 73.4 | 77.8 | 70.9 | 45.0 | 0.004 |
| African American | 34.0 | 35.4 | 33.0 | 25.0 | 0.39 |
| Education | |||||
| <9th grade | 10.8 | 11.4 | 10.7 | 5.0 | 0.38 |
| 9th-11th grade | 17.4 | 15.2 | 18.5 | 35.0 | -- |
| High School Graduate | 34.5 | 35.2 | 30.1 | 50.0 | -- |
| Post-graduate | 37.2 | 38.1 | 40.8 | 10.0 | -- |
| Home Care Agency | |||||
| Boston Senior | 29.0 | 26.4 | 32.0 | 40.0 | 0.12 |
| Boston Central | 30.5 | 30.7 | 29.1 | 35.0 | -- |
| Ethos | 18.2 | 18.9 | 19.4 | 5.0 | -- |
| Somerville/Cambridge | 22.4 | 24.6 | 19.4 | 20.0 | -- |
| Medical History | |||||
| Diabetes | 31.3 | 25.9 | 37.9 | 55.0 | 0.002 |
| Hypertension | 83.6 | 79.3 | 90.3 | 95.0 | 0.007 |
| Stroke | 19.8 | 17.7 | 23.0 | 25.0 | 0.25 |
| Coronary Disease | 30.7 | 25.9 | 37.8 | 45.0 | 0.01 |
| RAAS Use | 47.2 | 41.0 | 55.3 | 70.0 | 0.002 |
| Statin Use | 42.7 | 41.0 | 46.6 | 40.0 | 0.65 |
| Current Smoker | 23.7 | 24.6 | 19.6 | 35.0 | 0.95 |
| Functional Status | |||||
| No Difficulty | 28.7 | 30.7 | 25.2 | 25.0 | 0.26 |
| Some Difficulty | 33.1 | 33.0 | 33.0 | 35.0 | |
| Much Difficulty/Unable | 38.2 | 36.3 | 33.6 | 40.0 | |
| Exam and Laboratories | |||||
| Systolic Blood Pressure | 136.3 ± 20.8 | 133.0 ± 19.8 | 142.0 ± 21.6 | 141.6 ± 21.6 | <0.001 |
| Diastolic Blood Pressure | 76.3 ± 11.3 | 75.1 ± 10.8 | 78.0 ± 11.7 | 81.7 ± 12.2 | 0.01 |
| Body Mass Index | 31.1 ± 7.9 | 31.3 ± 7.3 | 30.4 ± 8.9 | 32.6 ± 8.5 | 0.45 |
| Creatinine | 0.93 ± 0.39 | 0.84 ± 0.24 | 1.01 ± 0.40 | 1.46 ± 0.86 | <0.0001 |
| Estimated GFR | 78.2 ± 27.2 | 82.8 ± 24.8 | 72.4 ± 28.2 | 66.0 ± 50.4 | <0.0001 |
| Total Cholesterol | 189.3 ± 42.6 | 193.2 ± 42.5 | 182.4 ± 44.2 | 182.6 ± 30.7 | 0.09 |
| HDL Cholesterol | 50.1 ± 14.9 | 50.8 ± 14.3 | 48.9 ± 16.4 | 48.1 ± 12.6 | 0.48 |
| Serum Albumin | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.4 | 4.0 ± 0.3 | 0.001 |
| C-reactive protein | 3.0 [1.3,7.9] | 2.9 [1.5,6.9] | 2.9 [1.0,8.0] | 8.4 [3.5,17.3] | 0.79 |
| Hemoglobin | 13.2 ± 1.5 | 13.4 ± 1.4 | 13.1 ± 1.7 | 12.8 ± 1.8 | 0.11 |
| Urine ACR | 11.7 [5.9,46.3]) | 6.9 [3.9,10.6] | 56.4 [33.2,118.9] | 646.2 [429.7,1408.9] | <0.0001 |
All p-values are 2-sided and test for trend across albuminuria groups.
Categorical variables are % and continuous variables are mean ± standard deviation except ACR (albumin-creatinine ratio) and C-reactive protein which are presented as median (25th and 75th percentile). Age is in years, blood pressures are in mm Hg, body mass index in kg/m2, creatinine and cholesterol in mg/dL, estimated GFR (glomerular filtration rate) in mL/min/1.73m2, albumin and hemoglobin in g/dL, C-reactive protein in mg/L, and ACR in mg/g.
To convert creatinine to μmol/L, multiply by 88.4. To convert total and high density lipoprotein (HDL) cholesterol to mmol/L, multiply by 0.02586. To convert hemoglobin and albumin to g/L, multiply by 10.
Functional status refers to performance on Activities of Daily Living, with categories defined by degree of impairment in one or more of 5 activities (mobility, eating, dressing, bathing, toileting).
Results
Among 335 participants, the mean age was 73.4 ± 8.1 years (Table 1). Median ACR was 11.6 mg/g (25-75%: 5.9- 46.3 mg/g). There were 212 (63.3%), 103 (30.8%), and 20 (6.0%) with ACR falling within ranges consistent with normoalbuminuria, microalbuminuria and macroalbuminuria. Overall, participants had substantial physical impairment, with 71.3% having at least “some difficulty” on one or more activity of daily living.
Cognition
There was no difference in baseline ACR level among individuals with partial versus complete neuropsychological testing (p=0.7). Individuals classified as having micro- or macroalbuminuria had poorer performance on tests of cognitive functioning that assess attention and mental processing speed, verbal fluency, visual construction and fluid reasoning, and supraspan learning, while there was no difference in MMSE scores or tests of intelligence, auditory retention and memory (Table 2). The relationship between ACR and word recall was fully attenuated after adjustment for age (data not shown).
Table 2.
Performance on Cognitive Testing
| Total (n=335) |
No Albuminuria (n=212) |
Albuminuria (n=123) |
p-value | |
|---|---|---|---|---|
| Screening Tests | ||||
| MMSE | 25.5 ± 3.2 | 25.6 ± 3.3 | 25.3 ± 3.2 | 0.37 |
| Verbal IQ | 99.4 ± 13.4 | 99.4 ± 13.6 | 99.5 ± 13.2 | 0.93 |
| Simple and Complex Attention & Processing Speed | ||||
| WAIS-III Digit Span: Backwards | 5.3 ± 2.2 | 5.5 ± 2.3 | 5.0 ± 1.9 | 0.02 |
| WAIS-III Digit Span: Forwards | 9.1 ± 2.2 | 9.2 ± 2.1 | 8.8 ± 2.2 | 0.13 |
| WAIS-III Digit Symbol-Coding | 36.5 ± 15.1 | 38.3 ± 15.9 | 33.5 ± 13.1 | 0.003 |
| Mental Alternation Test | 8.4 ± 4.3 | 8.6 ± 4.3 | 8.1 ± 4.3 | 0.32 |
| Trail Making Test A | 77.5 ± 49.7 | 74.7 ± 48.6 | 82.3 ± 51.4 | 0.02 |
| Trail Making Test B | 197.0 ± 84.3 | 187.0 ± 84.9 | 215.5 ± 80.7 | 0.008 |
| Verbal Fluency | ||||
| Controlled Oral Word Association Test | 29.1 ± 13.2 | 30.1 ± 13.9 | 27.3 ± 11.7 | 0.05 |
| Visual Construction & Fluid Reasoning | ||||
| WAIS-III Block Design | 21.7 ± 10.1 | 22.4 ± 10.4 | 20.5 ± 9.3 | 0.10 |
| Matrix Reasoning | 8.3 ± 4.9 | 8.7 ± 5.1 | 7.7 ± 4.5 | 0.06 |
| Story Recall: Retention of New Information | ||||
| WMS-III Logical Memory: Recognition | 23.0 ± 4.3 | 23.2 ± 4.2 | 22.7 ± 4.5 | 0.35 |
| WMS-III Logical Memory: Immediate Recall | 35.3 ± 11.9 | 35.8 ± 11.7 | 34.3 ± 12.2 | 0.29 |
| WMS-III Logical Memory: Percent Retention | 70.0 ± 25.0 | 69.6 ± 25.5 | 70.7 ± 24.2 | 0.71 |
| Naming | ||||
| ADAS-cognitive subscale: Object Naming | 15.0 ± 1.8 | 15.1 ± 1.8 | 14.8 ± 1.9 | 0.12 |
| Wordlist Learning: Retention of Unrelated Items | ||||
| WMS-III Word List Learning: Recall Total | 24.1 ± 7.4 | 25.0 ± 7.3 | 22.6 ± 7.3 | 0.004 |
| WMS-III Word List Learning: Delayed Recognition | 20.6 ± 3.1 | 20.8 ± 3.1 | 20.2 ± 3.0 | 0.09 |
| WMS-III Word List Learning: Percent Retention | 49.1 ± 29.4 | 52.7 ± 28.8 | 42.8 ± 29.5 | 0.003 |
MMSE, Mini-Mental State Exam; IQ, Intelligence Quotient; WAIS-III, Weschler Adult Intelligence Scale-3rd edition; ADAS, Alzheimer's Disease Assessment Scale; WMS-III, Weschler Memory Scale-3rd edition. Albuminuria includes both micro- and macroalbuminuria and is defined by urine albumin to creatinine ratio ≥25 mg/g in women and ≥17 mg/g in men. P-value is for comparisons of albuminuria versus no albuminuria. For all tests except Trails A and Trails B, a higher score is consistent with better performance. Results for Trails A and Trails B were compared using the Wilcoxon Rank Sum Test.
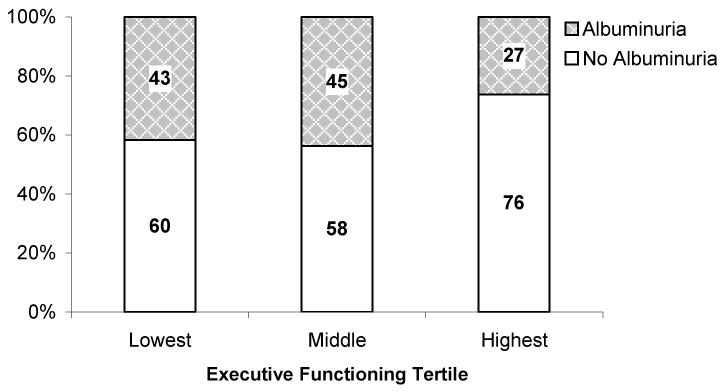
In univariate analysis, increasing ACR was associated with poorer executive functioning (Component 1, p=0.005) but was not associated with tests focusing on memory (Component 2) or attention and working memory (Component 3). In multivariable analysis this persisted (Table 3). The association between ACR and individual cognitive tests that assess executive functioning was most notable on the Digit Symbol Substitution Test and the Trail Making Test B (Table 3). In the digit symbol test, each doubling of the ACR was associated with approximately one fewer successful coding in univariate and multivariable analyses, while, in the Trail Making Test B, each doubling of the ACR was associated with a 6 second increase in time to complete the task in univariate analysis and a 3 second increase in multivariable analyses (maximum 300 seconds allowed). There were no significant associations between ACR and performance on individual cognitive tests primarily assessing memory after adjustment for age, education and center (data not shown). Models that used selection methodology rather than a priori fitting to adjust for potential covariates were similar (data not shown). When evaluated in logistic regression, higher urine ACR was associated with executive functioning in the lowest two tertiles versus the highest tertile in univariate and adjusted analyses [Odds ratio = 1.18 (95% confidence interval: 1.06 to 1.32) and 1.19 (1.05 to 1.35), respectively, per each doubling of the urine ACR] (Figure 1).
Table 3.
The association between log-transformed Albumin-Creatinine Ratio (ACR) and cognitive components, including cognitive tests comprising Component 1 (Executive Functioning)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Doubling | p-value | Doubling | p-value | Doubling | p-value | |
|
Component 1: Executive Functioning |
-0.07 | 0.005 | -0.05 | 0.01 | -0.06 | 0.006 |
| Digit Symbol Coding | -1.2 | <0.001 | -0.9 | 0.005 | -0.9 | 0.007 |
| Mental Alternation Test | -0.2 | 0.1 | n/a | 0.3 | n/a | 0.4 |
| Trail Making Test A | 2.4 | 0.02 | 1.6 | 0.1 | 1.7 | 0.1 |
| Trail Making Test B | 5.6 | 0.003 | 3.6 | 0.04 | 2.6 | 0.2 |
| COWAT | -0.6 | 0.05 | -0.5 | 0.09 | -0.6 | 0.08 |
| Block Design | -0.4 | 0.09 | n/a | 0.4 | n/a | 0.4 |
| Matrix Reasoning | -0.2 | 0.04 | n/a | 0.2 | -0.2 | 0.09 |
|
Component 2: Memory |
-0.01 | 0.6 | n/a | 0.99 | n/a | 0.6 |
|
Component 3: Attention/ Working Memory |
-0.02 | 0.3 | n/a | 0.3 | n/a | 0.6 |
COWAT, Controlled Oral Word Association Test.
Doubling was determined by multiplying the beta coefficient for log-transformed ACR by the natural log of 2. Component scores have a mean of 0 and a standard deviation of 1. For tests comprising Component 1, scores reflect the specific test metric.
Model 1 is a univariate analysis. Model 2 adjusts for age, center and education. Model 3 adjusts for model 2 components as well as diabetes, cardiovascular disease, use of ACE inhibitors or angiotensin receptor blockers, sex, race, hypertension and eGFR.
For all tests except Trails A and Trails B, a higher score is consistent with better performance.
N/A: Not applicable as p-value >0.2.
Figure 1.
Executive functioning in tertiles from poorest to best stratified by the presence of micro- or macroalbuminuria as compared to non-albuminuric participants. Numbers within bars represent the number of participants within each group. P for trend = 0.02.
In analyses of multivariable models after adjustment for age, center and education, increasing ACR had similar associations with executive function regardless of diabetes, sex, race, cardiovascular disease and eGFR <60 mL/min/1.73m2 (p for interaction = 0.6, 0.2, 0.6, 0.9, and 0.8, respectively). Notably, in univariate and multivariable models examining executive functioning, reduced eGFR was not associated with poorer cognitive performance (data not shown). In analyses excluding participants with macroalbuminuria, ACR remained associated with worse executive functioning in univariate, partially adjusted and fully adjusted models (p=0.006, 0.005, and 0.004, respectively) but was not associated with other cognitive domains. In analyses excluding participants with previously diagnosed stroke, ACR was associated with worse executive functioning in univariate models (p=0.04); however, this relationship was slightly attenuated in multivariable models (p=0.1 in models 2 and 3).
Brain Imaging
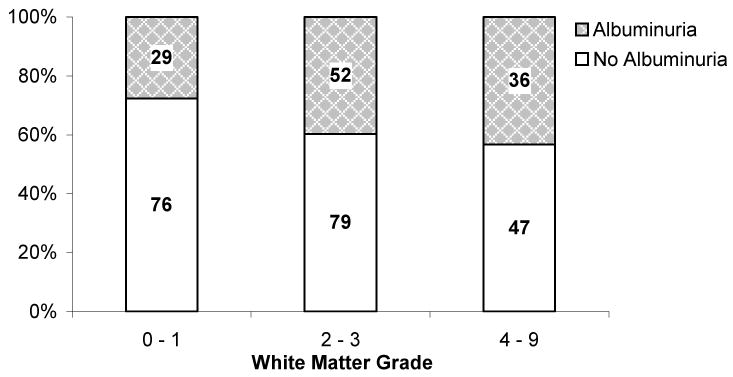
Qualitative WMH grade was greater in participants with micro- and macroalbuminuria than in those without albuminuria (Figure 2). Using logistic regression, the presence of albuminuria was associated with a WMH grade of ≥2 [Odds ratio = 1.13 (1.02 to 1.25) and 1.15 (1.02 to 1.29) for each doubling of urine ACR in univariate and fully adjusted models, respectively]. In univariate and multivariable analysis of quantitative WMH volume, increasing ACR was associated with increasing normalized WMH volume (Table 4). In models excluding participants with macroalbuminuria, higher ACR remained associated with increased WMH volume in univariate and approached significance in partially and fully adjusted models (Table 4).
Figure 2.
Distribution of white matter hyperintensity grades by the presence of micro- or macroalbuminuria as compared to non-albuminuric participants. Numbers within bars represent the number of participants within each group. P for trend = 0.02.
Table 4.
The association between log-transformed Albumin-Creatinine Ratio and log-transformed white matter hyperintensity volume on brain magnetic resonance imaging
| All Participants n=305 |
Excluding Macroalbuminuria n=287 |
|||
|---|---|---|---|---|
| Log (ACR) β-Coefficient | p-value | Log (ACR) β-Coefficient | p-value | |
| Model 1 | 0.11 | 0.007 | 0.13 | 0.006 |
| Model 2 | 0.10 | 0.009 | 0.09 | 0.06 |
| Model 3 | 0.10 | 0.01 | 0.10 | 0.05 |
ACR, albumin to creatinine ratio in mg/g. White matter hyperintensity volume was normalized to brain and intracranial volume and was analyzed as mL.
Model 1 is a univariate analysis. Model 2 adjusts for age, center and education. Model 3 adjusts for model 2 components as well as diabetes, cardiovascular disease, use of angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers, sex, race, hypertension and eGFR.
Discussion
The findings in the current study demonstrate that the presence of albumin in the urine, even at levels consistent with microalbuminuria, is independently associated with cognitive impairment. Specifically, albuminuria is associated with impaired executive functioning, manifest by impaired mental processing speed, planning and sequencing of events, and ability to complete unstructured tasks. This type of deficit is consistent with pathology in subcortical brain pathways and may result from cerebrovascular disease.25, 26 Supporting this association, albuminuria was independently associated with increased white matter hyperintensity volume on brain imaging.
The association between albuminuria and cognitive impairment is important for patient management as it may identify a large at-risk population. Estimates using NHANES 1999-2004 data suggest that up to 19% of individuals age 60 years and older in the US have microalbuminuria.27 The association of albuminuria with executive function and white matter hyperintensities is likely not causal; rather it probably reflects long-term vascular disease burden manifest in two disparate end-organs – kidney and brain – resulting from factors including hypertension, diabetes and dyslipidemia. This hypothesis is supported by the findings in the current study, specifically that there was a significant association with cognitive factors typically associated with vascular dementia (executive functioning) in the absence of an association with cognitive factors typically associated with Alzheimer's dementia (memory). Further supporting this hypothesis, other studies have also demonstrated an association between urine albumin excretion and vascular disease.14, 28, 29
There is little literature evaluating albuminuria, CKD, cerebrovascular disease and cognition, and the findings of the current study add substantially to current knowledge. To date, two studies have explored the association between albuminuria and cognitive impairment. In the Cardiovascular Health Study, Barzilay et al. found that for each doubling of albuminuria the prevalence of dementia increased by 12%.30 This study benefits from a large and relatively generalizable population but is limited by detailed cognitive testing in only the highest risk subset as well as brain imaging that preceded albuminuria assessment and cognitive testing by several years. In NHANES 1999–2002, cross-sectional analyses of 2,386 individuals over age 60 who underwent limited cognitive testing revealed significantly decreased performance on the digit symbol substitution test when microalbuminuria was accompanied by peripheral vascular disease but not in the absence of peripheral vascular disease.16 This report is limited by only 80% completion of the single cognitive test and missing data on peripheral vascular disease in another 24% of participants. Our study also differs from a recent study by Kurella-Tamura et al in that reduced eGFR was not associated with poorer cognitive performance.11 This may reflect the NAME Study population, comprised of elders with substantial physical comorbidity in the setting of frequent obesity, rendering serum creatinine-based estimation of GFR less precise.
There are limitations to the current study. First, causality cannot be determined from this cross-sectional study. Second, as participants in this study were enrolled through home healthcare agencies, the population may not be generalizable. However, the elderly population with multiple comorbid conditions is increasingly common and efforts to maintain semi-independent living are critical for the solvency of the healthcare system; accordingly this is an important population to characterize, particularly as intact cognitive functioning is critical for aging success. Third, we have a disproportionate number of women in the study. Fourth, although we have detailed medication ascertainment supplementing participant recall for the identification of comorbid conditions like diabetes and hypertension, we rely on participant recall to identify prior clinically recognized cerebrovascular and cardiovascular disease, potentially biasing results. Fifth, by adjusting for characteristics like hypertension and diabetes in multivariable models, we likely have included factors preceding albuminuria on a potential causal pathway between cerebrovascular disease and cognitive function; however, as demonstrated in our results, the association of albuminuria with cognitive and cerebrovascular findings remains robust, demonstrating the utility of albuminuria as a potential marker of the duration and severity of cerebrovascular risk. Finally, we are classifying albuminuria using a single urine sample; however, it is likely that this will bias our results toward the null as repeat testing generally reveals decreased albuminuria on subsequent assessments.31 Importantly, in clinical practice, many risk stratification decisions are based on a single assessment and previous studies have shown that even if one out of three random urine tests shows the presence of albuminuria it may increase risk for overall morbidity.32
This study has several strengths, most notably concurrent assessment of albuminuria, cognitive functioning and brain imaging. Additionally, we use a large battery of tests to assess cognitive function, allowing differentiation of cognitive deficit subtypes associated with albuminuria.
In summary, the presence of albuminuria, even at levels consistent with microalbuminuria, is significantly associated with worse cognitive performance in an elderly population with a high prevalence of physical impairment, particularly in executive functioning. Our finding that albuminuria is also associated with WMH volume on brain imaging suggests that the underlying pathology may be subcortical small vessel cerebrovascular disease. Future studies could utilize a longitudinal format to further characterize the association between albuminuria and cognitive decline to better improve prediction of incident cognitive impairment, distribute resources for detailed cognitive evaluation to higher risk individuals, and intervene at an earlier stage in vulnerable elders.
Supplementary Material
Table S1. Descriptions of cognitive tests performed in NAME with standardized scores that reflect reported norms for a 70 to 75-year-old woman with a high school education.
Table S2. Loading of principal components based on cognitive function assessed.
Acknowledgments
Preliminary data from this study was presented in abstract form at the 2007 Annual Meeting of the American Society of Nephrology, November 1-5, in San Francisco, CA. The authors would like to thank the entire NAME team, particularly the researchers who visited Boston elders in their homes to perform the cognitive testing, the homecare agencies that allowed us access to their clients, as well as the contributions of Arema Pereira MD, Panagiotis Vlagopolous MD MS and Jennifer Buell PhD, without whom the ancillary study could not have occurred.
Disclosure:
This research was supported by US National Institutes of Health grants K23 DK71636 (DEW), K24 DK078204 (MJS), R01 AG021790 (MFF), and the Clinical Research Center at Tufts Medical Center. Dr. Weiner and Dr. Sarnak have received research funding from Amgen, Inc. Dr. Weiner received research funding from Covidien. Dr. Sarnak has received support from Tap Pharmaceauticals. Other investigators have no disclosures.
References
- 1.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 2.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 3.Pereira AA, Weiner DE, Scott T, et al. Subcortical cognitive impairment in dialysis patients. Hemodial Int. 2007;11:309–314. doi: 10.1111/j.1542-4758.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 4.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 5.Fazekas G, Fazekas F, Schmidt R, et al. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134:83–88. doi: 10.1016/0022-510x(95)00226-7. [DOI] [PubMed] [Google Scholar]
- 6.Kim CD, Lee HJ, Kim DJ, et al. High prevalence of leukoaraiosis in cerebral magnetic resonance images of patients on peritoneal dialysis. Am J Kidney Dis. 2007;50:98–107. doi: 10.1053/j.ajkd.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 8.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate Renal Impairment and Risk of Dementia among Older Adults: The Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15:1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 9.Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS) Stroke. 2007;38:3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seliger SL, Longstreth WT, Jr, Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16:3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 11.Kurella-Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: The REGARDS study. Am J Kidney Dis. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 14.Yuyun MF, Khaw KT, Luben R, et al. Microalbuminuria and stroke in a British population: the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. J Intern Med. 2004;255:247–256. doi: 10.1046/j.1365-2796.2003.01264.x. [DOI] [PubMed] [Google Scholar]
- 15.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 16.Kuo HK, Lin LY, Yu YH. Microalbuminuria is a negative correlate for cognitive function in older adults with peripheral arterial disease: results from the U.S. National Health and Nutrition Examination Survey 1999-2002. J Intern Med. 2007;262:562–570. doi: 10.1111/j.1365-2796.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- 17.Scott TM, Peter I, Tucker KL, et al. The Nutrition, Aging, and Memory in Elders (NAME) study: design and methods for a study of micronutrients and cognitive function in a homebound elderly population. Int J Geriatr Psychiatry. 2006;21:519–528. doi: 10.1002/gps.1503. [DOI] [PubMed] [Google Scholar]
- 18.Miller WG, Myers GL, Ashwood ER, et al. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129:297–304. doi: 10.5858/2005-129-297-CMSOTA. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 20.Seshadri S, DeStefano AL, Au R, et al. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet. 2007;8 Suppl 1:S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 22.Yue NC, Arnold AM, Longstreth WT, Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 23.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 24.Chumlea WC, Roche AF, Steinbaugh ML. Estimating stature from knee height for persons 60 to 90 years of age. J Am Geriatr Soc. 1985;33:116–120. doi: 10.1111/j.1532-5415.1985.tb02276.x. [DOI] [PubMed] [Google Scholar]
- 25.Desmond DW. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci. 2004;226:3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Roman GC. Vascular dementia: distinguishing characteristics, treatment, and prevention. J Am Geriatr Soc. 2003;51(5 Suppl Dementia):S296–304. doi: 10.1046/j.1532-5415.5155.x. [DOI] [PubMed] [Google Scholar]
- 27.Castro A, Coresh J. CKD Surveillance Using Laboratory Data and Population-Based Surveys (NHANES) Am J Kidney Dis. 2008;52(3 Suppl 1) doi: 10.1053/j.ajkd.2008.07.054. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer H, Jacobs DR, Jr, Bild D, et al. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 29.Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 30.Barzilay JI, Fitzpatrick AL, Luchsinger J, et al. Albuminuria and Dementia in the Elderly: A Community Study. Am J Kidney Dis. 2008;52:216–226. doi: 10.1053/j.ajkd.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 32.Romundstad S, Holmen J, Kvenild K, Hallan H, Ellekjaer H. Microalbuminuria and all-cause mortality in 2,089 apparently healthy individuals: a 4.4-year follow-up study. The Nord-Trondelag Health Study (HUNT), Norway. Am J Kidney Dis. 2003;42:466–473. doi: 10.1016/s0272-6386(03)00742-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Descriptions of cognitive tests performed in NAME with standardized scores that reflect reported norms for a 70 to 75-year-old woman with a high school education.
Table S2. Loading of principal components based on cognitive function assessed.