Abstract
Two new compounds, a cyclopenta[bc]benzopyran, ponapensin (1), and an aglaialactone, 5,6-desmethylenedioxy-5-methoxy aglalactone (2), together with nine known compounds were isolated from the CHCl3 soluble extract of the leaves and twigs of Aglaia ponapensis. Their structures were established by spectroscopic data interpretation. Ponapensin (1) exhibited significant NF-κB inhibitory activity in an Elisa assay, and was found to be more potent than the positive control rocaglamide. All of the compounds isolated were also tested in a panel of human cancer cell lines, with the known sterol E-volkendousin (3) and methyl rocaglate (aglafoline) found to be the only active substances.
Keywords: Ponapensin, Cyclopenta[bc]benzopyran, NF-κB, Aglaia ponapensis
The genus Aglaia Lour. (Meliaceae) consists of about 130 species, which are distributed mainly in the Indo-Malayan region, southern mainland China, and the Pacific Islands.1 Since rocaglamide, the first cyclopenta[b]benzofuran, was isolated as a constituent of Aglaia elliptifolia, and was found to be active in an in vivo P388 model,2 the phytochemical investigation of the genus Aglaia has led to the isolation of many related compounds. To date, about 60 naturally occurring cyclopenta[b]benzofuran type compounds, many of which exhibit insecticidal and antiproliferative activities, have been isolated from over 30 Aglaia species.3,4 The cyclopenta[b]benzofurans and two structurally related groups, the cyclopenta[bc]-benzopyrans and benzo[b]oxepines, are considered characteristic secondary metabolites of the genus Aglaia, because they have been isolated only from this taxon.3 The collective name “flavagline” has been proposed for these compounds because their mutual biogenetic origin has been postulated to be a flavonoid nucleus linked to a cinnamic acid moiety.5,6 Among the flavaglines, many members of the cyclopenta[b]-benzofuran-type compounds have shown pronounced inhibitory activity against cancer cell lines at nanomolar concentrations, while the cyclopenta[bc]benzopyrans and benzo[b]oxepines evaluated so far were not active.4 Cyclopenta[b]benzofurans have also been shown to inhibit TNF-α or PMA-induced NF-κB activity in different mouse and human T-lymphocyte cell lines.7 NF-κB has been shown to be crucial for inducing genes involved in inflammation and in a wide range of diseases originating from chronic activation of the immune system, including colon cancer.8 Thus, NF-κB may play a key role in regulating the expression of pro-inflammatory and/or apoptotic genes in cancer, making it an attractive target for therapeutic intervention.
In a preliminary study of the stems of Aglaia ponapensis Kaneh. (syn. A. mariannensis Merr.) carried out in our laboratories, it was discovered that this species is a good source of methyl rocaglate (aglafoline).9 Methyl rocaglate is the most frequently tested compound among the cyclopenta[b]benzofurans, and overall it has been found to exhibit slightly more potent inhibitory activity compared to rocaglamide against the several cancer cell lines in which it has been examined.4 Therefore, the large-scale isolation of methyl rocaglate was carried out from the leaves and stems of A. ponapensis (8 kg) collected in Ponape Island, the Federated States of Micronesia, so that this compound could be utilized for chemical transformation and biological studies.10 The air-dried plant material of A. ponapensis was extracted with methanol, and partitioned in turn with n-hexane, CHCl3, and EtOAc. The CHCl3-soluble fraction was subjected to fractionation using silica gel, Diaion HP-20, Sephadex LH-20, and reversed-phase HPLC to afford two new (1 and 2) and nine known compounds.11 The new compound 1, ponapensin, was found to be a potent inhibitor of NF-κB in an Elisa assay carried out in our laboratory. In addition, all compounds isolated were also tested against a small panel of cancer cell lines.
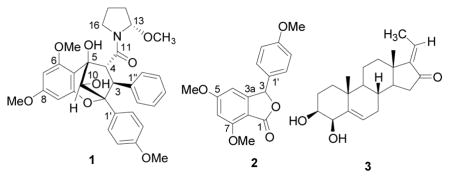
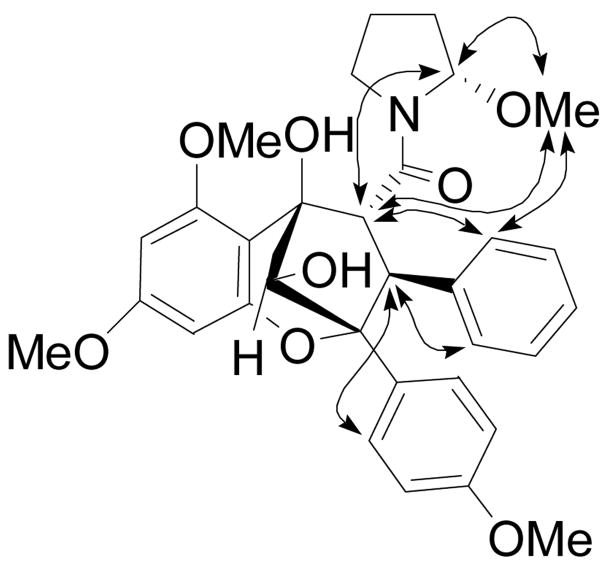
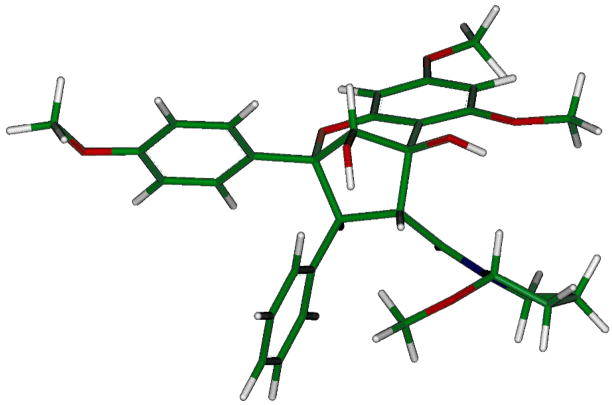
Compound 1 was obtained as yellow oil, [α]22D 167 (c 0.4, CHCl3), and gave a sodiated molecular ion peak at m/z 584.2266 (calcd for C32H35NO8Na, 584.2260) in the HRESIMS.12 The 1H NMR spectrum showed signals for three aromatic rings: a monosubstituted benzene ring at δH 7.18 (2H, d, J = 6.8 Hz, H-2″,6″) and 6.96 (3H, m, H-3″– H-5″), a para-disubstituted benzene ring at δH 7.40 (2H, d, J = 8.9 Hz, H-2′,6′) and 6.61 (2H, d, J = 8.9 Hz, H-3′,5′), and two meta-coupled aromatic protons at δH 6.12 (1H, d, J = 2.2 Hz, H-7) and 6.03 (1H, d, J = 2.2 Hz, H-9). In addition, signals belonging to three methoxy groups [δH 3.79 (3H, s, OMe-6), 3.73 (3H, s, OMe-8) and 3.67 (3H, s, OMe-4′)], an oxygenated methine [δH 4.62 (1H, s, H-10)], and two vicinal methines [δH 4.40 (1H, d, J = 9.4 Hz, H-3) and 4.36 (1H, d, J = 9.4 Hz, H-4)] were observed, suggesting the presence of a cyclopenta[bc]benzopyran moiety.6 Analysis of the remaining signals by DQF-COSY revealed the presence of a pyrrolidine ring. Consistent with the 1H NMR spectrum of compound 1, its 13C NMR spectrum also displayed signals characteristics of a cyclopenta[bc]benzopyran skeleton. The signal for C- 13 at δC 89.6 suggested the presence of an oxygen atom next to this methine carbon, and an HMBC cross-peak between δH 5.80 (H-13) and δC 54.3 (OMe-13) confirmed the location of the methoxy group at C-13. HMBC correlations from δH 4.40 (H-3) to δC 131.4 (C-2″,6″) and 90.0 (C-2), and from δH 4.36 (H-4) to δC 172.4 (C-11), 143.1 (C-1″), 107.3 (C-5a), and 83.1 (C-5) established the locations of the aromatic ring and the pyrrolidine ring at C-3 and C-4, respectively. The relative configuration of 1 was determined from analysis of the 1H NMR coupling constant and the 2D NOESY data (Fig. 1). The configuration at C-3 and C-4 was determined as H-3α and H-4β, based on the 3J(H-3,H-4) coupling constant (J = 9.4 Hz).13 In the 2D NOESY spectrum, a correlation between H-10 and H-4 was not observed, suggesting an exo relationship between the two protons. The configuration at C-13 was S, as a result of the NOE correlations observed between H-13/H-4, H-13/OMe-13, OMe-13/H-4, and OMe-13/H2″,6″, and examination using Dreiding models. The Insight II molecular modeling program was used to generate a 3D model of 1 (Fig. 2); the optimized model showed that OMe-13 was placed in the shielding zone of the monosubstituted benzene ring, which is in agreement with the NOEs observed and the upfield proton chemical shift of OMe-13 (δ 2.80). Accordingly, compound 1 was assigned as (−) rel-(2R,3S,4R,5R, 10S,2′S)-1-[2,3,4,5,-tetrahydro-5,10-dihydroxy-2-(4-methoxyphenyl)-6,8-dimethoxy-3-phenyl-2,5-methano-1-benzoxepin-4-carbonyl]-2-methoxypyrrolidine, to which we have given the trivial name, ponapensin.
Figure 1.
Selected 2D NOE correlations for ponapensin (1)
Figure 2.
3D structure of ponapensin (1)
Compound 2 was isolated as a white amorphous powder, [α]22D –13 (c 1.1, CHCl3). Its HRESIMS exhibited a sodiated molecular ion peak at m/z 323.0895, indicating an elemental formula of C17H16O5Na (calcd 323.0890).14 The 1H NMR spectrum showed signals for two aromatic rings, constituted by two meta-coupled aromatic protons at δH 6.25 (1H, d, J = 0.9 Hz, H-4) and 6.43 (1H, d, J = 1.4 Hz, H-6), and a characteristic AA′BB′ system of a p-disubstituted benzene ring at δH 7.17 (2H, d, J = 8.5 Hz, H-2′,6′) and 6.88 (2H, d, J = 8.5 Hz, H-3′,5′). In addition, two singlets characteristic of OMe groups [δH 3.96 (3H, s, OMe-7), 3.79 (6H, s, OMe-5, OMe-4′)] and a downfield methine signal at δH 6.17 (H-3) were observed. The 13C NMR spectrum displayed the signals for a tetrasubsituted and a disubstituted benzene ring, and three methoxy carbons, consistent with the 1H NMR data. Furthermore, an unsaturated carbonyl (δC 168.4, C-1) and an oxygenated methine carbon (δC 81.4, C-3) were also observed, suggesting the presence of a benzofuranone moiety. In the HMBC spectrum, correlations were observed from H-3 to C-1, C-3a, C-4, C-1′, and C-2′,6′, confirming the position of the lactonic proton as ortho relative to the ring fusion position C-3a. The structure of 2 is related to that of aglalactone,15 except for the absence of a 5,6-methylenedioxy group and the presence of an extra methoxy group in C-5. Accordingly, this new compound was assigned as 5,7-dimethoxy-3-(4-methoxyphenyl)-1,3-dihydrobenzo[c]-furan-1-one, or 5,6-desmethylenedioxy-5-methoxy-aglalactone.
Nine known compounds, a cyclopenta[b]benzofuran (methyl rocaglate16), four cyclopenta[bc]benzopyrans (4-epi-aglain A,17 aglain B,18 10-O-acetylaglain B,17 and aglain C18) and four pregnane steroids [(E)-volkendousin (3),19 (Z)-volkendousin,19 2β,3β-dihydroxy-5-pregn-17(20)-(E)-en-16-one,20 and 2β,3β-dihydroxy-5-pregn-17(20)-(Z)-en-16-one20] were also isolated from A. ponapensis. All of these compounds, except methyl rocaglate, are reported for the first time from this species. The occurrence of pregnane steroids as isolated in this study is quite rare in the plant kingdom, and such compounds have only been isolated from two species in the Meliaceae family (Melia volkensii and Aglaia grandis).19,20
In an enzyme-based Elisa NF-κB assay,21,22 ponapensin (1) showed a potent NF-κB inhibitory activity with an IC50 of 0.06μM, while rocaglamide (positive control) and methyl rocaglate exhibited IC50 values of 2.0 and 2.3 μM, respectively. The NF-κB inhibitory activity of 2 (IC50 = 1.9μM), was comparable to that of the positive control. The other cyclopenta[bc]benzopyran-type compounds isolated in this study (4-epi-aglain A, aglain B, 10-O-acetylaglain B, and aglain C) were not active in the NF-κB assay (IC50 > 5μM). Only one cyclopenta[bc]benzopyran-type compound has been tested previously for NF-κB inhibitory activity, using Jurkat T cells, and was found to be inactive at concentrations up to 2 mM.7 It is interesting that a change in the pyrrolidine side chain of the cyclopenta[bc]benzopyran-type compounds, from a methylbutanoylamino group as found in aglain C, to a methoxy group as found in ponapensin, drastically enhanced the NF-κB inhibitory activity. This finding may be significant for the structure-activity-relationship (SAR) study of this type of compound. Further biological studies need to be performed to uncover the specific mechanism of NF-κB inhibition of the potent compound 1, such as finding whether the IκB kinase complex is a target.
All compounds isolated from this study, except methyl rocaglate, were also tested in a panel of cancer cell lines.23 Only one compound, E-volkendousin (3), was found to be cytotoxic, with ED50 values of 4.6, 4.7, and 4.2 μg/mL against Lu1, LNCaP, and MCF-7 cell lines, respectively. Methyl rocaglate is a known cytotoxic agent, and has been tested previously in a panel of cancer cell lines in our laboratory.24
In conclusion, two new (1 and 2) and nine known compounds have been isolated from the stems and leaves of A. ponapensis in the present study. The relative configuration of new compound 1, ponapensin, has been confirmed by 2D NOESY data, observation using Dreiding models, and molecular modeling.
Acknowledgments
This investigation was supported by a new faculty start up package to E.J.C.-B. and, in part, by grant U19-CA52956 from NCI/NIH (A.D.K.). We thank Dr. Y. Sagawa and S. F. Glassman, for the collection and identification of the plant material, respectively, with financial support from the NCI. We also thank Mr. J. Fowble, College of Pharmacy, The Ohio State University, for facilitating the running of the 400 MHz NMR instrument, and Dr. Christopher M. Haddad and Ms. Susan Fletcher, Department of Chemistry, The Ohio State University, for the mass spectrometric data.
References and notes
- 1.Pannell CM. Kew Bulletin Additional Series XVI. HMSO; Kew, Richmond, Surrey, UK: 1992. A Taxonomic Monograph of the Genus Aglaia Lour. (Meliaceae) [Google Scholar]
- 2.King ML, Chiang CC, Ling HC, Fujita E, Ochiai M, McPhail AT. J Chem Soc, Chem Commun. 1982:1150. [Google Scholar]
- 3.Proksch P, Edrada R, Ebel R, Bohnenstengel FI, Nugroho BW. Curr Org Chem. 2001;5:923. [Google Scholar]
- 4.Kim S, Salim AA, Swanson SM, Kinghorn AD. Anticancer Agents Med Chem. 2006;6:319. doi: 10.2174/187152006777698123. [DOI] [PubMed] [Google Scholar]
- 5.Nugroho BW, Edrada RA, Wray V, Witte L, Bringmann G, Gehling M, Proksch P. Phytochemistry. 1999;51:367. [Google Scholar]
- 6.Bacher M, Hofer O, Brader G, Vajrodaya S, Greger H. Phytochemistry. 1999;52:253. [Google Scholar]
- 7.Baumann B, Bohnenstengel F, Siegmund D, Wajant H, Weber C, Herr I, Debatin KM, Proksch P, Wirth T. J Biol Chem. 2002;277:44791. doi: 10.1074/jbc.M208003200. [DOI] [PubMed] [Google Scholar]
- 8.(a) Tanos D, Maniatis T. Cell. 1995;80:529. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]; (b) Haefner B. Drug Discovery Today. 2002;7:653. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 9.Pawlus AD, Choi JK, Kang YH, Farnsworth NR, Pezzuto JM, Mehta RG, Kinghorn AD. Abstracts of Papers. 44th Annual meeting of the American Society of Pharmacognosy; Chapel Hill, NC. July 12–16, 2003; American Society of Pharmacognosy; 2003. p. P-196. [Google Scholar]
- 10.The leaves and stem of A. ponapensis Kaneh. (Meliaceae) were collected in rainforest at 600 ft. elevation on Mt. Poaipoai, Ponape Island, one of the Eastern Caroline Islands, the Federated States of Micronesia. The plant was collected by Y. Sagawa, University of Hawaii, Honolulu, HI and identified by S. F. Glassman. A voucher specimen (accession number 2264217) has been deposited at the Economic Botany Laboratory, USDA, Beltsville, MD, USA.
- 11.The dried and milled leaves and stems of A. ponapensis (8 kg) were extracted using MeOH (3 × 28 L) at rt, for 48 h each. The combined extracts were evaporated in vacuo, and water was added to give a 10% methanolic extract (4 L), followed by subsequent partition with hexane (3×4 L), CHCl3 (3×4 L), and EtOAc (3×4 L). The CHCl3-soluble extract (115 g) was chromatographed over a silica gel column (12 ×33 cm, 70–230 mesh) and eluted with CHCl3–MeOH (99:1→9:1). Fractions eluting in 5–7.5% MeOH were combined (20 g) and further chromatographed over a silica gel column (7.5×43 cm, 230–400 mesh) using mixtures of hexane-EtOAc-MeOH (10:10:0.1→10:10:2) as solvents to give 11 sub-fractions (Ap01 – Ap11). Sub-fraction Ap07 (1.2 g, eluted with hexane-EtOAc-MeOH, 10:10:1), was chromatographed over Diaion HP-20 gel (eluted with 90% MeOH) to remove the chlorophylls, followed by Sephadex LH-20 gel (2.5×75 cm, in MeOH) to afford pure methyl rocaglate (270 mg). Sub-fraction Ap08 (0.9 g, eluted with hexane-EtOAc-MeOH, 10:10:1.2), was processed similarly to the previous fraction to afford an additional quantity of methyl rocaglate (30 mg) and 5,6-desmethylenedioxy-5-methoxyaglalactone (2) (15 mg). Sub-fraction Ap09 (2 g, eluted with hexane-EtOAc-MeOH, 10:10:1.4), was chromatographed sequentially on Diaion HP-20, Sephadex LH-20 and C18 RP-HPLC (MeOH-H2O, 50→70% in 20 min, then isocratic at 70%) to furnish ponapensin (1) (5 mg, tR 29.4 min), 2β,3β-dihydroxy-5-pregn-17(20)-(Z)-en-16-one (5 mg, tR 32.4 min), (Z)-volkendousin (1 mg, tR 34.5 min), 2β,3β-dihydroxy-5-pregn-17(20)-(E)-en-16-one (2 mg, tR 39.0 min), and (E)-volkendousin (3) (1 mg, tR 41.5 min). Aglain C (300 mg) precipitated from sub-fraction Ap11 (4 g, eluted with hexane-EtOAc-MeOH, 10:10:1.8) as a white amorphous powder, and the filtrate was treated in a similar fashion to sub-fraction Ap09 (C18 RP-HPLC, MeOH-H2O, isocratic at 60% for 15 min, 60→70% in 5 min, then isocratic at 70%) to afford 10-O-acetylaglain B (40 mg, tR 41.8 min), aglain B (21 mg, tR 45.9 min), and 4-epi-aglain A (65 mg, tR 49.5 min).
- 12.Yellow oil, [α]22D –167 (c 0.4, CHCl3); UV (MeOH) λmax (log ε) 224 (3.84) nm; IR (film) νmax 3474, 2936, 1618, 1590, 1518, 1437, 1251, 1214, 1148, 1082 cm−1; 1H NMR (400 MHz, MeOH- d4, calibrated at δH 3.30) δ 7.40 (2H, d, J = 8.9 Hz, H-2′,6′), 7.18 (2H, d, J = 6.8 Hz, H-2″,6″), 6.96 (3H, m, H-3″–H-5″), 6.61 (2H, d, J = 8.9 Hz, H-3′,5′), 6.12 (1H, d, J = 2.2 Hz, H-7), 6.03 (1H, d, J = 2.2 Hz, H-9), 5.80 (1H, br d, J = 3.3 Hz, H-13), 4.62 (1H, s, H-10), 4.40 (1H, d, J = 9.4 Hz, H-3), 4.46 (1H, d, J = 9.4 Hz, H-4), 3.79 (3H, s, OMe-6), 3.73 (3H, s, OMe-8), 3.67 (3H, s, OMe-4′), 3.34 (2H, m, H2-16, overlap with MeOH-d4), 2.80 (3H, s, OMe-13), 2.02 (1H, m, H-14a), 1.90 (2H, m, H2-15), 1.88 (1H, m, H-14b); 13C NMR (100 MHz, MeOH-d4, calibrated at δC 49.0) δ 172.4 (C-11), 162.7 (C-8), 160.0 (C-4′), 159.8 (C-6), 155.0 (C-1a), 143.1 (C-1″), 132.0 (C-1′), 131.7 (2C, C-2′,6′), 131.4 (2C, C-2″,6″), 128.6, (2C, C-3″,5″), 127.0 (C-4″), 113.4 (2C, C-3′/5′), 107.3 (C-5a), 95.2 (C-9), 92.8 (C-7), 90.0 (C-2), 89.6 (C-13), 83.1 (C-5), 80.9 (C-10), 63.6 (C-4), 58.5 (C-3), 56.5 (OCH3-6), 55.8 (OCH3-8), 55.5 (OCH3-4′), 54.3 (OCH3-13), 46.3 (C-16), 32.7 (C-14), 21.7 (C-15); HRESIMS m/z 584.2266 [M + Na]+ (calcd for C32H35NO6Na, 584.2260).
- 13.Previous studies of other cyclopenta[bc]benzopyran-type compounds have shown that 3J(H-3,H-4) of 5-6 Hz is compatible with the H-3β,H-4α configuration, while the vicinal coupling constant of 9-10 Hz is compatible with the H-3α,H-4β configuration.3,6
- 14.White amorphous powder; [α]22D –13 (c 1.1, CHCl3); UV (MeOH) λmax (log ε) 257 (3.99), 220 (4.37), 208 (4.26) nm; IR (film) νmax 1755, 1612, 1514, 1462, 1330, 1259, 1157, 1058 cm−1; 1H NMR (400 MHz, CDCl3, TMS) δ 7.17 (2H, d, J = 8.5 Hz, H-2′,6′), 6.88 (2H, d, J = 8.5 Hz, H-3′,5′), 6.43 (1H, d, J = 1.4 Hz, H-6), 6.25 (1H, d, J = 0.9 Hz, H-4), 6.17 (1H, s, H-3), 3.96 (3H, s, OMe-7), 3.79 (6H, s, OMe-5, OMe-4′); 13C NMR (100 MHz, CDCl3, TMS) δ 168.4 (C-1), 166.8 (C-5), 160.2 (C-4′), 159.4 (C-7), 154.9 (C-3a), 128.7 (2C, C-2′,6′), 128.6 (C-1′), 114.2 (2C, C-3′,5′), 106.6 (C-7a), 99.0 (C-6), 98.3 (C-4), 81.4 (C-3), 56.0 (OCH3-7), 55.9 (OCH3-5 or OCH3-4′), 55.3 (OCH3-5 or OCH3-4′); HRESIMS m/z 323.0895 [M + Na]+ (calcd for C17H16O5Na, 323.0890).
- 15.(a) Brader G, Vajrodaya S, Greger H, Bacher M, Kalchhauser H, Hofer O. J Nat Prod. 1998;61:1482. doi: 10.1021/np9801965. [DOI] [PubMed] [Google Scholar]; (b) Seger C, Hofer O, Greger H. Monatsh Chem. 2000;131:1161. [Google Scholar]
- 16.(a) Ko FN, Wu TS, Liou MJ, Huang TF, Teng CM. Eur J Pharmacol. 1992;218:129. doi: 10.1016/0014-2999(92)90156-x. [DOI] [PubMed] [Google Scholar]; (b) Ishibashi F, Satasook C, Ismant MB, Towers GHN. Phytochemistry. 1993;32:307. [Google Scholar]
- 17.Inada A, Sorano T, Murata H, Inatomi Y, Darnaedi D, Nakanishi T. Chem Pharm Bull. 2001;49:1226. doi: 10.1248/cpb.49.1226. [DOI] [PubMed] [Google Scholar]
- 18.Dumontet V, Thoison O, Omobuwajo OR, Martin MT, Perromat G, Chiaroni A, Riche C, Pais M, Sevenet T. Tetrahedron. 1996;52:6931. [Google Scholar]
- 19.Rogers LL, Zeng L, McLaughlin JL. J Org Chem. 1998;63:3781. [Google Scholar]
- 20.Inada A, Murata H, Inatomi Y, Nakanishi T, Darnaedi D. Phytochemistry. 1997;45:1225. doi: 10.1016/s0031-9422(99)00519-1. [DOI] [PubMed] [Google Scholar]
- 21.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Nucleic Acids Res. 2001;29:e21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The NF-κB assay was carried out according to ref. 21. An Elisa assay (EZ-Detect™ Transcription Factor Assay System) available from Pierce Biotechnology (Rockford, IL) was used to assess the ability of all compounds to interfere with the specific binding between the biotinylated-consensus sequence for the respective factor and the active form of NF-κB transcription factor. Nuclear extract of HeLa cells (purchased from the American Type Culture Collection, ATCC # CCL-2) treated with TNF-α and test compound was used for evaluation of specific binding. The detection of NF-κB activity was based on the measurement of chemiluminescent signal in plate reader Fluostar Optima from BMG. TNF-α stimulated nuclear extract was used as a control in the chemiluminescent assay. Rocaglamide was used as a positive control in the NF-κB assay.
- 23.The cytotoxicity assay was carried out according to established protocol: Seo EK, Kim NC, Mi Q, Chai H, Wall ME, Wani MC, Navarro HA, Burgess JP, Graham JG, Cabieses F, Tan GT, Farnsworth NR, Pezzuto JM, Kinghorn AD. J Nat Prod. 2001;64:1483. doi: 10.1021/np0103158.
- 24.(a) Lee SK, Cui B, Mehta RR, Kinghorn AD, Pezzuto JM. Chem Biol Interact. 1998;115:215. doi: 10.1016/s0009-2797(98)00073-8. [DOI] [PubMed] [Google Scholar]; (b) Rivero-Cruz JF, Chai H-B, Kardono LBS, Afriastini JJ, Riswan S, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. J Nat Prod. 2004;67:343. doi: 10.1021/np0304417. [DOI] [PubMed] [Google Scholar]