Abstract
Sakacin P is a class IIa bacteriocin that is active against the food-borne pathogen Listeria monocytogenes, and use of this compound as a biopreservative in foods has been suggested. In the present study, we characterized 30 spontaneous sakacin P-resistant mutants of L. monocytogenes obtained after single exposure to sakacin P. The frequency of development of sakacin P resistance for all strains was in the range from 10−8 to 10−9. Using the 50% inhibitory concentration (IC50) of sakacin P, the strains could be grouped into strains with high levels of resistance (IC50, ≥104 ng ml−1) and strains with low levels of resistance (IC50, <104 ng ml−1). Resistant strains belonging to the same IC50 group also had similar physiological and genetic characteristics. Generally, the resistant strains showed substantial variations in many parameters, such as differences in the stability of the acquired resistance to sakacin P, growth fitness, food-related stress tolerance, and biofilm-forming ability. Fourier transform infrared spectroscopy revealed differences between wild-type and resistant strains in polysaccharide, fatty acid, and, protein regions. A mannose-specific phosphotransferase (PTS) operon has been described for class IIa bacteriocin resistance, and the sakacin P-resistant strains displayed both up- and downregulation of the expression of the mptA gene encoding the PTS system. This is the first comprehensive study of the diversity of a large number of spontaneous resistant mutants obtained after one exposure to a class IIa bacteriocin, particularly to sakacin P. The great diversity among the resistant strains exposed to the same stress conditions suggests that there are different resistance mechanisms.
Listeria monocytogenes is a gram-positive human and animal pathogen, and nearly all human L. monocytogenes infections are due to ingestion of contaminated food (29, 30, 47). Listeriosis is a severe disease and has high hospitalization and fatality rates (30). L. monocytogenes is ubiquitous in nature (49), and it is relatively resistant to harsh environments, as well as to a broad range of food processing and storage conditions (16).
Bacteriocins produced by lactic acid bacteria are antimicrobial peptides and are usually active against gram-positive species with low G+C contents (5). The bacteriocins can be grouped into different classes (12, 26), and the class IIa bacteriocins are characterized by conserved sequence motifs and high specific activity against L. monocytogenes (9, 13). So far, nisin (class Ia) is the only bacteriocin approved for use as a food preservative (E234). If more bacteriocins are to be approved for various industrial applications, class IIa bacteriocins are considered the next in line (15). Sakacin P is a class IIa bacteriocin produced by several strains of Lactobacillus sakei (22, 26, 42, 43). Sakacin P is active against L. monocytogenes (23-25), and unlike other class IIa bacteriocins, it has modest activity against lactic acid bacteria (13).
The presence of isolates that are resistant to bacteriocins is of great concern for use of bacteriocins as biopreservatives (48). L. monocytogenes isolates that are naturally resistant to class IIa bacteriocins have been reported (14, 37). Susceptible strains can acquire resistance to class IIa bacteriocins through exposure to bacteriocins (19, 25). Depending on the conditions, the frequency of development of resistance to class IIa bacteriocin ranges from 10−3 to 10−6 (11, 19, 25, 38). The reported stability of the acquired resistance varies considerably (10, 11, 19, 36, 38). Class IIa bacteriocin-resistant mutants of L. monocytogenes have been grouped into mutants with high levels of resistance and mutants with intermediate levels of resistance (2, 10, 20, 21, 25, 44, 45).
It has been reported that in L. monocytogenes downregulation of either the mpt (mannose permease two) operon, (7, 20, 21, 36), the mpo (mannose permease one) operon (2), the rpoN gene (encoding the σ54 factor), or the manR gene (transcriptional activator for σ54) (2, 6, 7, 39) led to resistance to class IIa bacteriocins. This is in contrast to results reported by Gravesen et al., who showed that there was upregulation of expression of the mpt and manR genes in class IIa bacteriocin-resistant mutants (20). Alterations in cell envelope fatty acid composition (33, 45) and changes in the cell surface charge (44) have also been reported to be involved in mechanisms of resistance to class IIa bacteriocins.
In numerous studies of mutants of L. monocytogenes strains resistant to class IIa bacteriocins only one or a few wild-type or mutant strains were included, or the mutants with acquired resistance were derived using different experimental conditions; either the strains were exposed to different types and concentrations of bacteriocins, or they were exposed successively many times (10, 11, 19, 20, 33, 38, 46).
The purpose of this study was to characterize a large number of spontaneous mutant strains of L. monocytogenes that acquired resistance after a single exposure to sakacin P. To our knowledge, this is the first comprehensive study of the diversity of a large number of spontaneous resistant mutants obtained after a single exposure to class IIa bacteriocins, particularly to sakacin P.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The L. monocytogenes wild-type strains were selected based on their natural susceptibility to sakacin P (25). L. monocytogenes L502 and L1037, for which the 50% inhibitory concentrations (IC50) of sakacin P are high, and L. monocytogenes L40 and L688, for which the IC50 of sakacin P are low (25), as well as laboratory reference strain EGDe, were used (18). L. sakei Lb790(pMLS114) was used to produce cell-free supernatant (CFS) containing sakacin P (32) (Table 1).
TABLE 1.
Wild-type strains used in this study
| Strain | Description | Reference(s) |
|---|---|---|
| L. monocytogenes L40 | Chicken isolate, serotype 3b | 25, 35; Liv M. Rørvik, personal communication |
| L. monocytogenes L688 | Sheep brain isolate, serotype 4b | 25, 35; Liv M. Rørvik, personal communication |
| L. monocytogenes L502 | Cheese isolate, serotype 1/2a | 25, 35; Liv M. Rørvik, personal communication |
| L. monocytogenes L1037 | Seawater isolate, serotype 1/2a | 25, 35; Liv M. Rørvik, personal communication |
| L. monocytogenes EGD-e | Laboratory reference strain, serotype 1/2a | 18 |
| Lactobacillus sakei Lb790(pMLS114) | Strain producing sakacin P from plasmid pMLS114 | 22, 32 |
Depending on the experiment, the L. monocytogenes strains were grown in tryptone soy broth (TSB) (Oxoid Ltd., Basingstoke, England), on tryptone soy agar (TSA) (Oxoid Ltd., Basingstoke, England), in brain heart infusion (BHI) broth (Oxoid Ltd., Basingstoke, England), on BHI agar (Oxoid Ltd., Basingstoke, England), in Listeria enrichment broth (Oxoid, Ltd., Basingstoke, England) with 0.1% Tween 80 (Sigma), or in Luria-Bertani broth (LB) (1% tryptone [Oxoid], 0.5% yeast extract [Oxoid], and 0.5% NaCl [Merck]). For L. sakei Lb790, deMan-Rogosa-Sharpe medium (Oxoid Ltd., Basingstoke, England) was used. Unless otherwise stated, cultivation in broth media was performed without shaking, and the sakacin P-resistant mutants were characterized in the absence of sakacin P. The L. monocytogenes and the L. sakei Lb790 stock cultures were stored frozen (−80°C) in BHI broth and deMan-Rogosa-Sharpe medium, respectively, in the presence of 15% glycerol.
Production of CFS containing sakacin P.
CFS containing sakacin P was produced by heterologous expression in L. sakei Lb790 as previously described, with a slight modification (25). L. sakei Lb790 was grown overnight at 20°C (32) with shaking at 200 rpm, and the CFS was subjected to heat treatment (85°C for 20 min). Aliquots of the CFS and pure sakacin P (stock concentration, 490 mg liter−1; kindly donated by Inga M. Aasen, SINTEF, Norway) were stored at −20°C.
Development of spontaneous mutants with resistance to sakacin P.
Spontaneous mutants of L. monocytogenes that were resistant to sakacin P were selected after a single exposure to sakacin P as previously described (19, 25). For each strain, a total of six spontaneous mutants resistant to sakacin P (five mutants obtained after exposure to the CFS and one mutant obtained after exposure to pure sakacin P at a final concentration of 4.9 mg liter−1) were selected. The sakacin P-resistant strains were designated by using the suffixes 1 to 6 following the wild-type strain designations (see Table 3).
TABLE 3.
IC50 of sakacin P for wild-type strains and spontaneous mutants of L. monocytogenes strains
| Straina | IC50 (ng ml−1)b |
|---|---|
| L502 | 0.32 |
| L502-1c | >105 |
| L502-2 | >105 |
| L502-3 | >105 |
| L502-4 | 103 |
| L502-5 | 102 |
| L502-6 | 102 |
| L1037 | 0.22 |
| L1037-1c | >105 |
| L1037-2 | 103 |
| L1037-3 | 102 |
| L1037-4 | 102 |
| L1037-5 | 102 |
| L1037-6 | 102 |
| EGDe | 0.27 |
| EGDe-1 | >105 |
| EGDe-2c | 102 |
| EGDe-3 | 10 |
| EGDe-4 | <10 |
| EGDe-5 | <10 |
| EGDe-6 | <10 |
| L40 | 0.12 |
| L40-1 | 104 |
| L40-2 | 103 |
| L40-3 | 103 |
| L40-4 | 103 |
| L40-5c | 102 |
| L40-6 | 102 |
| L688 | 0.06 |
| L688-1c | 104 |
| L688-2 | 103 |
| L688-3 | 103 |
| L688-4 | 103 |
| L688-5 | 103 |
| L688-6 | 103 |
The designations of the sakacin P-resistant strains end with the suffixes 1 to 6.
>105 represents concentrations higher than the highest sakacin P concentration used.
Sakacin P-resistant strains derived from exposure to pure sakacin P.
Serotyping of L. monocytogenes strains.
The serotypes of the wild-type and selected sakacin P-resistant strains were determined using a Listeria antiserum kit (Denka Seiken, Japan) by following the manufacturer's instructions.
Sakacin P susceptibility test.
The susceptibility of the wild-type strains and the spontaneous mutants to sakacin P was determined using a pure sakacin P solution and a slight modification of a previously described method (25). The inoculum for the IC50 test was prepared by diluting a 24-h culture (0.001%, vol/vol) in Listeria enrichment broth. The IC50 determination was done twice on different days. Spontaneous mutants for which the IC50 was higher than the IC50 for the wild-type strain were considered resistant to sakacin P.
Stability of sakacin P resistance.
The stability of the acquired sakacin P resistance of the spontaneous mutants of L. monocytogenes was assessed by serial cultivation in TSB in the absence of sakacin P. Every 12 h for 10 days, the cultures were transferred (1%, vol/vol) to fresh prewarmed TSB at 30°C. The stability was checked after the first 2 h of incubation (day 0) and then every other day. The frequency of retention of resistance was computed by dividing the number of CFU that grew on TSA with 30% CFS by the number of CFU that grew on TSA without CFS. The wild-type strains were included as controls.
Growth fitness in the presence of different sugars.
LB broth supplemented with different sugars (0.5%, wt/vol; glucose, mannose, rhamnose, cellobiose, and lactose) was prepared and sterilized using 0.2-μm polyethersulfone membrane filters (Nalge Nunc International, United States). Growth was monitored using a Bioscreen instrument (Oy Growth Curves Ab Ltd., Helsinki, Finland) at 30°C for 18 h. The growth study experiment was done at least three times on different days.
The growth rate was computed from the slope of a linear regression line of the growth curve using the logarithmic region and was expressed in h−1 (31). The maximum cell density was estimated from the highest optical density at 600 nm obtained after 18 h of incubation (46). The relative fitness of a resistant strain was calculated by comparison with the wild type (resistant strain/wild-type strain). Analysis of variance in conjunction with Tukey's multiple-comparison tests was performed using Statistix 8.1 (Analytical Software Tallahassee, United States), and P values less than 0.05 were considered significant.
Growth fitness in the presence of food-related stresses.
The protocol described above was used, with slight modifications. BHI broth media were prepared by adding hydrochloric acid (pH 4.0 to 5.5), lactic acid (pH 5; total concentration, 5 mM to 50 mM), acetic acid (pH 5; total concentration, 2 mM to 50 mM), or sodium chloride (total concentration, 0.5% to 10% [wt/vol]). Plain BHI broth was used as a control, and the growth was monitored at different temperatures (10°C, 20°C, and 30°C).
Biofilm formation.
The wild-type and resistant strains of L. monocytogenes were screened for biofilm formation essentially as described by Borucki et al. (4). The experiment was done twice on different days.
Electron microscopy.
The wild-type and resistant strains of L. monocytogenes were grown in BHI broth at 30°C, and for a study of flagellum formation the strains were grown at 20°C. The cells were harvested at mid-exponential and late stationary phases and were fixed as described by Giotis et al. (17). Two independent biological samples were collected, and the cells were examined by scanning electron microscopy using the standard protocol at the Molecular Imaging Center of the University of Bergen, Bergen, Norway.
FT-IR spectroscopy.
Preparation of samples for Fourier transform infrared (FT-IR) spectroscopy was performed as described by Oust et al. (35). A bacterial suspension was uniformly dispensed in duplicate into 96 wells of a transmittance Si microplate (Bruker, Optics, Germany), and FT-IR measurement was performed using an HTS-XT spectrometer (Bruker Optics, Germany). For each strain, three independent biological samples were measured, and this gave a total of six spectra.
The spectra were preprocessed by taking the second derivative and applying extended multiplicative signal correction (27). The preprocessed spectra were analyzed by principal component analysis (PCA) using The Unscrambler software (The Unscrambler v9.6; CAMO AS, Norway). The PCA was performed using different biochemical fingerprint regions of the FT-IR spectra (34). The three biological samples were included in the PCA. Inspections of score plots for the first four principal components (usually PC1 and PC2) were used to determine if the wild-type strains were different from the resistant strains. Loading and line plots were used to identify chemical bands that were important for the separation of the wild-type strains from the resistant strains.
qRT-PCR.
A quantitative real-time reverse transcriptase PCR (qRT-PCR) experiment was performed to quantify the level of transcription of the mptA gene, and spontaneous mutants of L. monocytogenes representing organisms with high and low levels of resistance to sakacin P were included. The strains were grown at 30°C in plain LB broth or LB broth supplemented with glucose, mannose, or cellobiose (0.5%, wt/vol) with shaking. A sample used for isolation of total RNA was taken at mid-exponential phase. RNA extraction was done using an RNeasy Protect bacterial mini kit (Qiagen) according to the manufacturer's recommendation with “on-column” digestion of genomic DNA. First-strand cDNA was synthesized from 300 ng of total RNA using random hexamers and SuperScript III reverse transcriptase as suggested by the manufacturer (Invitrogen). In order to assess DNA contamination, a control sample with all components of the reaction mixtures except SuperScript III was included.
The primers and probes for mptA (lmo0096) and the 16S rRNA gene (Table 2) were designed using L. monocytogenes EGDe genome sequences available in the ListiList database (http://genolist.pasteur.fr/ListiList) using Primer Express 2.0 (Applied Biosystems). Amplification and detection of the cDNA were done as described previously (40). Data analysis was done as described by Schmittgen and Livak (41). For each gene, two biological replicates were included, and each biological replicate was measured four times. Changes were calculated relative to corresponding wild-type strains grown on glucose. P values less than 0.05 and changes that were ≥3-fold were considered significant.
TABLE 2.
Primers and probes used for qRT-PCR
| Primer | Sequencea |
|---|---|
| MptAF | 5′-CCTCGCAACTCACGGTGAAT-3′ |
| MptAR | 5′-TCTTGCTCGCCGAAAATCA-3′ |
| MptA-Taq | 5′-(6-FAM)TGCTGAAGGTATTTTGCAGTCCGGAACA(TAMRA)-3′ |
| 16S rRNAF | 5′-GCGCAGGCGGTCTTTTAAG-3′ |
| 16S rRNAR | 5′-CAATGACCCTCCCCGGTTA-3′ |
| 16S rRNA-Taq | 5′-(6-FAM)CTGATGTGAAAGCCCCCGGC (TAMRA)-3′ |
6-FAM, 6-carboxyfluorescein (fluorophore); TAMRA, 6-carboxytetramethylrhodamine (quencher).
RESULTS
Development of sakacin P resistance.
The natural IC50 of sakacin P for L. monocytogenes wild-type strain EGDe was high (Table 3), and this strain was assigned to the group containing L. monocytogenes L502 and L1037 (25). The frequency of development of sakacin P resistance for all strains ranged from 10−8 to 10−9. The sakacin P-resistant strains displayed a wide range of levels of resistance to sakacin P (the IC50 were 20 to >105 times the IC50 for the wild-type strains). Based on the IC50 of sakacin P, the spontaneous mutants could be grouped into strains with high levels of resistance (IC50, ≥104 ng/ml) and strains with low levels of resistance (IC50, <104 ng/ml). The available stock solution of sakacin P was not concentrated enough to determine the IC50 for some of the strains with high levels of resistance to sakacin P. The resistant mutants had the same serotype as the wild-type strains from which they were derived.
Characteristics of the resistant strains grown in the absence of sakacin P. (i) Stability of sakacin P resistance phenotype.
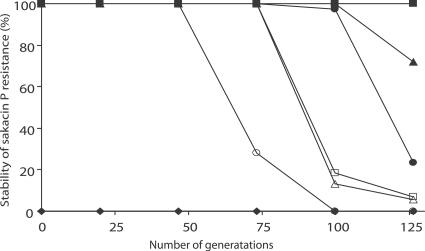
The stability of the resistance phenotype differed substantially when the resistant strains were grown in the absence of selective pressure. As shown in Fig. 1, L. monocytogenes L40-6 started to revert after 50 generations, and L. monocytogenes L40-1 was stable for more than 125 generations.
FIG. 1.
Stability of sakacin P resistance phenotype in the absence of sakacin P. Symbols: ⧫, L40; ▪, L40-1; ▴, L40-2; □, L40-3; ▵, L40-4; •, L40-5; ○, L40-6. Wild-type strain L. monocytogenes L40 was included as a control.
(ii) Growth fitness on different carbon sources.
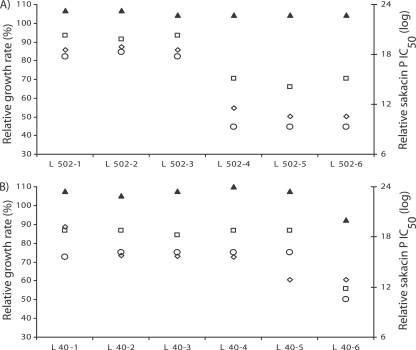
Generally, the specific growth rates of the resistant strains on glucose, mannose, and lactose were lower than the growth rates of the corresponding wild-type strains. However, in LB broth supplemented with cellobiose, the growth of the wild-type strains and the growth of the resistant strains were similar (Fig. 2; see Table S1 in the supplemental material).
FIG. 2.
Growth rates and levels of resistance to sakacin P of spontaneous bacteriocin-resistant mutants of L. monocytogenes L502 (A) and L40 (B). Symbols: ⋄, sakacin P; □, glucose; ○, mannose; ▴, cellobiose. Values are expressed relative to the value for the wild-type strain (mutant/wild-type strain).
In the presence of mannose or glucose spontaneous mutants of L. monocytogenes L40 and L502 could be placed in groups of resistant strains that grew fast or slow (P < 0.05) (Fig. 2). A positive correlation between the levels of sakacin P resistance and growth rates on glucose and mannose was observed for resistant strains derived from L. monocytogenes L502 (r = 0.99 and P < 0.05) (Fig. 2A). However, as shown for a representative strain in Fig. 2B, no correlation was observed for resistant strains derived from the other strains (r = 0.3 and P > 0.05). In general, the resistant strains derived from L. monocytogenes EGDe behaved differently than the other resistant strains (see Table S1 in the supplemental material).
The resistant and wild-type strains had similar maximum optical densities (total growth) on glucose and cellobiose (see Table S2 in the supplemental material). On mannose, with the exception of the slowly growing resistant strains of L. monocytogenes L502 (Fig. 2A), the total growth of the wild-type strains and the total growth of the resistant strains were similar (P > 0.05). Some of the resistant strains had unique growth fitness, particularly on rhamnose and lactose (see Tables S1 and S2 in the supplemental material).
(iii) Food-related stresses.
Generally, the resistant strains were affected more by food-related stresses than the wild-type strains. When L. monocytogenes L502-6 was grown at room temperature in the presence of 2 mM acetic acid, the lag phase was prolonged by approximately 15 h and the specific growth rate was reduced by 50% compared to the lag phase and specific growth rate of L monocytogenes wild-type strain L502. Under extreme stress conditions (i.e., low pH, low temperature, and high concentration of organic acids), the difference in tolerance between the wild-type strains and the resistant strains was minimal.
Variation in tolerance among the resistant strains was observed; for instance, the lengths of the lag phase for L. monocytogenes L502-1 and L502-6 in BHI broth at 20°C (no-stress controls) were 40 and 80 min, respectively. In the control experiment, L. monocytogenes L502-1 grew 15% slower than L. monocytogenes L502-6. Addition of 5% NaCl prolonged the lag phases of L. monocytogenes L502-1 and L502-6 by 2 and 20 h, respectively. This salt also reduced the growth rate of L. monocytogenes L502-6 by 70% compared to that of L. monocytogenes L502-1. Generally, sakacin P-resistant strains showed less tolerance to salt and organic acid stresses than to stresses resulting from inorganic acid and low temperatures.
(iv) Biofilm formation.
The resistant strains produced less biofilm than the corresponding wild-type strains, and substantial variation among the resistant strains was also observed. For example, the biofilm formation by L. monocytogenes L502-1 was greater than that by L. monocytogenes L502-6 (P < 0.05), and no difference was observed among resistant strains of L. monocytogenes L40 (data not shown).
(v) Electron microscopy.
No significant difference in morphology was observed between the resistant and wild-type strains at either the exponential or stationary phase (data not shown).
(vi) FT-IR spectroscopy.
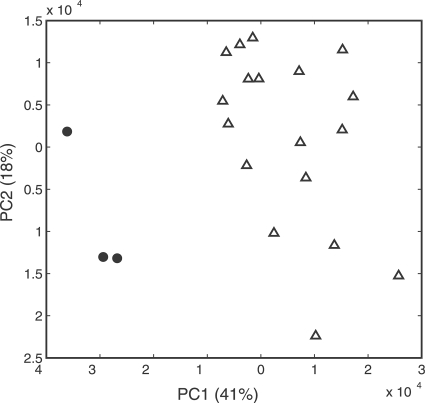
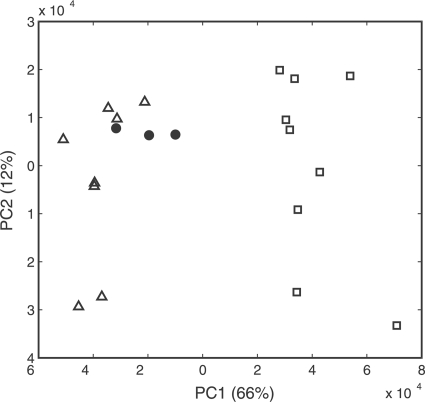
PCA of the FT-IR spectra showed that the wild-type strains could be separated clearly from the corresponding mutants, with few exceptions (Table 4). Figure 3 shows the separation of L. monocytogenes wild-type strain L688 from the resistant strains in the polysaccharide region (1,200 to 900 cm−1). The discriminatory power of the polysaccharide region was greater than those of the fatty acid and protein regions, in that order, and also varied from strain to strain. Surprisingly, the resistant strains derived from L. monocytogenes L502 clustered according to the level of resistance to sakacin P (high or low) (Fig. 4). The wild-type strain L. monocytogenes L502 clustered together with the strains resistant to high levels of sakacin P, and all the spectral regions confirmed the separation (Table 4).
TABLE 4.
FT-IR spectral regions that differentiate wild-type strains from sakacin P-resistant strains of L. monocytogenes
| Spectral regiona | L688 | L502c | EGDe | L1037 | L40d |
|---|---|---|---|---|---|
| Fatty acid (3,100-2,800 cm−1) | +b | + | + | − | + |
| Protein (1,800-1,500 cm−1) | + | + | − | − | − |
| Fatty acid and protein mixed (1,500-1,200 cm−1) | + | + | + | + | − |
| Polysaccharide (1,200-900 cm−1) | + | + | + | + | + |
| Fingerprint (900-700 cm−1) | + | + | + | + | − |
The assignment of bands is the assignment described by Naumann (34).
+, wild-type strain forms a cluster distinct and separate from that formed by sakacin P-resistant strains (see Fig. 3); −, no visible separation.
For L. monocytogenes L502, the clustering was according to the level of sakacin P resistance (see Fig. 4).
L. monocytogenes L40-6 was frequently separated from the other strains (see text).
FIG. 3.
PCA score plot using spectra in the polysaccharide region (1,200 to 900 cm−1) as variables for wild-type and sakacin P-resistant strains of L. monocytogenes L688. The sakacin P-resistant strains (▵) clustered separately from the wild-type (•). Three biological replicate samples were included in the PCA.
FIG. 4.
PCA score plot using spectra in the polysaccharide region (1,200 to 900 cm−1) as variables for wild-type and sakacin P-resistant strains of L. monocytogenes L502. The sakacin P-resistant strains of L. monocytogenes L502 clustered according to their levels of resistance to sakacin P as follows: low levels of resistance (□) and high levels of resistance (▵). Wild-type strain L502 (•) clustered with the strains with high levels of resistance. Three biological replicate samples were included in the PCA.
The resistant strains had FT-IR spectral band intensities distinct from those of the wild-type strains. For example, the intensities of bands at ∼2,922 cm−1, ∼2,852 cm−1, and ∼1,230 cm−1 were higher for the resistant strains than for the wild-type strains (data not shown).
(vii) qRT-PCR.
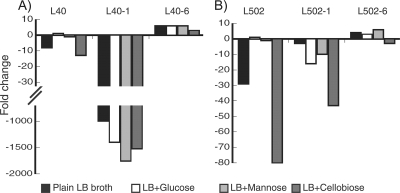
The expression of the mptA gene in L. monocytogenes strain L40-1 with a high level of resistance was downregulated at least 1,000-fold on all sugars tested compared to the expression in the corresponding wild-type strain grown on glucose (P < 0.001) (Fig. 5A). Interestingly, mptA gene expression was upregulated in L. monocytogenes strain L40-6 with a low level of resistance compared to the expression in the wild type (P < 0.001 and ≥3-fold change). Resistant strains derived from wild-type strain L. monocytogenes L502 (L502-1 and L502-6) showed patterns of mptA gene regulation with respect to the resistance level similar to those observed for L. monocytogenes L40 (Fig. 5B).
FIG. 5.
Changes in mptA gene expression in wild-type and sakacin P-resistant strains of L. monocytogenes L40 (A) and L502 (B). The calculated changes are relative to the expression in the corresponding wild-type strains grown on glucose.
(viii) Spontaneous mutants obtained with pure sakacin P and CFS.
Resistant strains obtained after exposure to pure sakacin P had phenotypic and gene expression profiles similar to those of the resistant strains obtained after exposure to L. sakei fermentate.
DISCUSSION
Spontaneous mutants of L. monocytogenes with acquired resistance to class IIa bacteriocins restrict the potential use of these bacteriocins as food biopreservatives. The present study is a continuation of our previous work (25, 35), in which two wild-type strains with high levels of natural susceptibility to sakacin P and two strains with low levels of natural susceptibility to sakacin P (25), as well as strain EGDe, were further characterized. In order to obtain insight into the mechanisms and effects of acquired resistance to sakacin P, we included 30 spontaneous mutants derived from the five wild-type strains.
The frequency of development of resistance for all strains in the present study ranged from 10−8 to 10−9. For class IIa bacteriocins frequencies between 10−3 and 10−6 have been reported (10, 11, 19, 25, 37, 38, 48). The observed divergence between the present study and previous studies could be due to strain variation (19, 48) and methodological differences. However, Gravesen et al. reported that the frequency of mutation to resistance to class IIa bacteriocins was not dependent on temperature, pH, salt, or the concentration of bacteriocin used (19).
In the present study, wide variation in the levels of acquired resistance was observed among the sakacin P-resistant strains (Table 3), and we divided the spontaneous mutants into strains with high levels of resistance and strains with low levels of resistance. The strains with high levels of resistance were over 105 times more resistant than the corresponding wild-type strains, and the strains with low levels of resistance were 20 to 104 times more resistant. Previous studies grouped mutants of L. monocytogenes that were resistant to class IIa bacteriocins into mutants with high levels of resistance (500 to 106 times more resistant than the wild-type strain) and mutants with intermediate levels of resistance (2 to 8 times more resistant than the wild-type strain) (2, 10, 20, 21, 44, 45).
In the present study, the sakacin P resistance phenotype was stable for at least for 50 generations (Fig. 1), and a class IIa bacteriocin-resistant strain with a stable phenotype for 20 generations has been described as a mutant (36). In addition, the resistant strains had different phenotypic and genotypic characteristics than the wild-type strains when they were tested in the absence of sakacin P (see below). Membrane adaptation has been described as a mechanism of resistance to class IIa bacteriocins, and the authors also highlighted the presence of extra resistance mechanisms among the mutants (44, 45). Taken together, these findings strongly suggest that the resistant strains obtained in the present study are mutants and that resistance due only to phenotypic adaptation is an unlikely explanation.
Sakacin P-resistant strains derived from the same strain showed differences in the stability of the sakacin P resistance phenotype. The strains derived from L. monocytogenes L40 (Fig. 1) and EGDe (data not shown) with high levels of sakacin P resistance had a more stable resistance phenotype than the strains with low levels of resistance. In contrast to previous studies (10, 11, 19, 33, 36, 38), the present study included several resistant strains, and the strains were grown for more than 120 generations. To our knowledge, this is the first evidence that links levels of resistance to sakacin P with the stability of the resistance phenotype in spontaneous mutants derived from the same wild-type strain. The observed stability and other differences (see below) in the two groups (high and low levels of resistance) derived from same strain may indicate that there are different modes of resistance to class IIa bacteriocins, as suggested previously (10, 36).
Resistant strains of L. monocytogenes L40 and L502 were the most thoroughly studied strains, and the spontaneous mutants derived from these strains with the two different levels of resistance also had different physiological and genetic characteristics. L. monocytogenes L502 strains, with a low level of resistance to sakacin P, generally grew slower (Fig. 2A), had reduced tolerance to stresses, and produced less biofilm than the strains with a high level of resistance to sakacin P. Representatives of the two groups of L. monocytogenes L40 strains showed similar tendencies. L. monocytogenes L40-6 was found to be the least stable strain (Fig. 1) and grew slower than L. monocytogenes L40-1 (Fig. 2B). In addition, the expression of the mptA gene was upregulated in L. monocytogenes L40-6 and L502-6 with low levels of resistance and downregulated in L. monocytogenes L40-1 and L502-1 with high levels of resistance compared to the expression in the wild-type strain (Fig. 5). Our FT-IR spectroscopy results also confirmed the grouping according to the level of resistance (Table 4 and Fig. 4).
Generally, the resistant strains had a reduced growth rate when they were grown on mannose and glucose compared to the growth on cellobiose (see Table S1 in the supplemental material). This is consistent with studies that showed the involvement of the mannose phosphotransferase (PTS) system in class IIa bacteriocin resistance (2, 21). In contrast, some of the resistant strains had growth rates on glucose (and to some extent growth rates on mannose) that were similar to those of the corresponding wild-type strains, and resistant strains with unique growth fitness on cellobiose, lactose, or rhamnose were observed (see Table S1 in the supplemental material). The relationship between the level of resistance to sakacin P and growth fitness was strain specific and sugar source dependent (Fig. 2). For example, spontaneous mutants derived from L. monocytogenes L502 with high levels of resistance to sakacin P grew significantly faster on glucose and mannose than the strains with low levels of resistance (Fig. 2A). However, no apparent relationship was observed among the resistant strains derived from the other wild-type strains. This substantial variation among the spontaneous mutants derived from the same strain may show that there are complex responses of the resistant strains due to alteration of the cell metabolism and/or different targets of the bacteriocin on the bacterial cell.
The results for reference strain EGDe, the only fully sequenced strain of L. monocytogenes (18), were often different from the results for the other four strains used. Strain Scott A, another laboratory reference strain, showed similar aberrant results (28). The EGDe strain may not be representative under the conditions of this study, and the generalizations that we made were based mainly on the results obtained with the other four strains.
In stress conditions that mimic the food environment, the resistant strains had less chance to grow, particularly in the presence of acids (organic and inorganic), salt, and low temperatures. We also found that the resistant strains had reduced abilities to form biofilms. This may suggest that the resistant strains do not compete well during harsh food processing and under storage conditions in foods when the bacteriocin is not present. More research is necessary to evaluate if resistant mutants are outcompeted in food or if their presence represents a bottleneck for use of bacteriocins in food.
Data obtained from FT-IR spectroscopy confirmed the grouping of the wild-type strains in the previously established groups (35) (data not shown). For both wild-type and resistant strains, the serotypes were accurately predicted by FT-IR spectroscopy irrespective of the observed high IC50 of sakacin P. Resistance to sakacin P introduced distinct changes mainly in the polysaccharide, fatty acid, and protein regions, which represented the major cell membrane components (Table 4). The variations among the resistant strains observed by FT-IR spectroscopy also may suggest that there are pleiotropic effects of resistance to sakacin P in the intact cells.
Differences in band intensity between the wild-type and resistant strains were observed. All of the resistant strains had higher band intensities at ∼2,922 cm−1, ∼2,852 cm−1, and 1,230 cm−1 than the wild-type strains. Bands at ∼2,922 cm−1 and ∼2,852 cm−1 are assigned to carbon-hydrogen stretching of CH2 in fatty acids, and bands around 1,230 cm−1 are due to double-bond stretching of the head group of phospholipids or phosphorus-containing carbohydrates, such as teichoic and lipoteichoic acids (34). It is often reported that strains of L. monocytogenes that are resistant to class IIa bacteriocins have alterations in the cell envelope, such as alterations in the fatty acid composition and cell surface charge (33, 44, 45). Taken together, FT-IR spectroscopy results mentioned above also strongly reflect the overall destabilized state of the cell envelope in the resistant strains.
With regard to expression of the mptA gene, upregulation and downregulation have previously been described for class IIa bacteriocin-resistant mutants (2, 20, 44). It has also been shown that components of the mannose PTS act as receptors for class IIa bacteriocins, thus explaining the mutant downregulation of mptA (8). This is consistent with our data for the resistant strains in the group with a high level of resistance to sakacin P (L. monocytogenes L40-1 and L502-1). However, in the characterized resistant strains belonging to the group of strains with low levels of resistance to sakacin P (L. monocytogenes L40-6 and L502-6), the mptA gene was upregulated. Gravesen et al. reported similar observations for a mutant with an intermediate level of resistance (20). In the present study, compared to the wild-type strains, the resistant strains with induced upregulation of the mptA gene were approximately 1,000 times more resistant, had reduced growth fitness, and had different FT-IR spectroscopy profiles. The present study and the report of Gravesen et al. (20) clearly showed that the level of mptA gene expression was not correlated to the level of resistance, in contrast to other findings (2, 44).
It is not clear whether the upregulation of mptA indicates a new mechanism of resistance. But since the growth in the presence of mannose and glucose still was significantly reduced in the resistant strains, it indicates that the mannose PTS system is somehow involved, although in a different manner than previously described. In bacteria, the PTS system in general (3) and the mannose PTS in particular (1) have a central role in a variety of regulatory events controlling carbon metabolism. Whether the observed upregulation of the mptA gene represents a new resistance mechanism itself or is due to indirect effects of an altered mannose PTS system remains to be investigated.
In conclusion, the present study showed substantial diversity among the spontaneous mutants. This finding emphasizes that care has to be taken when generalizations based on studies of a few strains and mutants are made. Spontaneous mutants with high levels of resistance to sakacin P tended to be more stable, had better growth fitness, and were more tolerant to food-related stresses than mutants with low levels of resistance. Previously, we showed that spontaneous mutants that were resistant to sakacin P were also resistant to other members of the class IIa bacteriocin group (25). In this respect, the results obtained in the present study may apply to other related class IIa bacteriocins, and this should create significant concern with regard to application of class IIa bacteriocins to food.
Supplementary Material
Acknowledgments
This work was supported by The Fund for the Research Levy on Agricultural Products.
We thank Tove Maugesten for technical assistance and Per Lea for helpful comments and statistical analysis.
Footnotes
Published ahead of print on 18 September 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abranches, J., M. M. Candella, Z. T. Wen, H. V. Baker, and R. A. Burne. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J. Bacteriol. 188:3748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arous, S., K. Dalet, and Y. Hechard. 2004. Involvement of the mpo operon in resistance to class IIa bacteriocins in Listeria monocytogenes. FEMS Microbiol. Lett. 238:37-41. [DOI] [PubMed] [Google Scholar]
- 3.Barabote, R. D., and M. H. Saier, Jr. 2005. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol. Mol. Biol. Rev. 69:608-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borucki, M. K., J. D. Peppin, D. White, F. Loge, and D. R. Call. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7336-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 6.Dalet, K., S. Arous, Y. Cenatiempo, and Y. Hechard. 2003. Characterization of a unique σ54-dependent PTS operon of the lactose family in Listeria monocytogenes. Biochimie 85:633-638. [DOI] [PubMed] [Google Scholar]
- 7.Dalet, K., Y. Cenatiempo, P. Cossart, and Y. Hechard. 2001. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 8.Diep, D. B., M. Skaugen, Z. Salehian, H. Holo, and I. F. Nes. 2007. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. USA 104:2384-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drider, D., G. Fimland, Y. Hechard, L. M. McMullen, and H. Prevost. 2006. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffes, F., P. Jenoe, and P. Boyaval. 2000. Use of two-dimensional electrophoresis to study differential protein expression in divercin V41-resistant and wild-type strains of Listeria monocytogenes. Appl. Environ. Microbiol. 66:4318-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dykes, G. A., and J. W. Hastings. 1998. Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett. Appl. Microbiol. 26:5-8. [DOI] [PubMed] [Google Scholar]
- 12.Eijsink, V. G. H., L. Axelsson, D. B. Diep, L. S. Håvarstein, H. Holo, and I. F. Nes. 2002. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie van Leeuwenhoek 81:639-654. [DOI] [PubMed] [Google Scholar]
- 13.Eijsink, V. G. H., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ennahar, S., N. Deschamps, and J. Richard. 2000. Natural variation in susceptibility of Listeria strains to class IIa bacteriocins. Curr. Microbiol. 41:1-4. [DOI] [PubMed] [Google Scholar]
- 15.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi, M., and M. L. Chikindas. 2007. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 113:1-15. [DOI] [PubMed] [Google Scholar]
- 17.Giotis, E. S., I. S. Blair, and D. A. McDowell. 2007. Morphological changes in Listeria monocytogenes subjected to sublethal alkaline stress. Int. J. Food Microbiol. 120:250-258. [DOI] [PubMed] [Google Scholar]
- 18.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K.-D. Entian, H. Fsihi, F. G.-D. Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L.-M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J.-C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J.-A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 19.Gravesen, A., A.-M. Jydegaard Axelsen, J. Mendes da Silva, T. B. Hansen, and S. Knochel. 2002. Frequency of bacteriocin resistance development and associated fitness costs in Listeria monocytogenes. Appl. Environ. Microbiol. 68:756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gravesen, A., B. Kallipolitis, K. Holmstrom, P. E. Hoiby, M. Ramnath, and S. Knochel. 2004. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 70:1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravesen, A., M. Ramnath, K. B. Rechinger, N. Andersen, L. Jansch, Y. Hechard, J. W. Hastings, and S. Knochel. 2002. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148:2361-2369. [DOI] [PubMed] [Google Scholar]
- 22.Holck, A. L., L. Axelsson, K. Hühne, and L. Kröckel. 1994. Purification and cloning of sakacin 674, a bacteriocin from Lactobacillus sake Lb674. FEMS Microbiol. Lett. 115:143-150. [DOI] [PubMed] [Google Scholar]
- 23.Katla, T., T. Moretro, I. Sveen, I. M. Aasen, L. Axelsson, L. M. Rorvik, and K. Naterstad. 2002. Inhibition of Listeria monocytogenes in chicken cold cuts by addition of sakacin P and sakacin P-producing Lactobacillus sakei. J. Appl. Microbiol. 93:191-196. [DOI] [PubMed] [Google Scholar]
- 24.Katla, T., T. Moretro, I. M. Aasen, A. Holck, L. Axelsson, and K. Naterstad. 2001. Inhibition of Listeria monocytogenes in cold smoked salmon by addition of sakacin P and/or live Lactobacillus sakei cultures. Food Microbiol. 18:431-439. [Google Scholar]
- 25.Katla, T., K. Naterstad, M. Vancanneyt, J. Swings, and L. Axelsson. 2003. Differences in susceptibility of Listeria monocytogenes strains to sakacin P, sakacin A, pediocin PA-1, and nisin. Appl. Environ. Microbiol. 69:4431-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-86. [DOI] [PubMed] [Google Scholar]
- 27.Kohler, A., U. Böcker, J. Warringer, A. Blomberg, S. Omholt, E. Stark, and H. Martens. 2009. Reducing inter-replicate variation in Fourier-transform infrared spectroscopy by extended multiplicative signal correction. Appl. Spectrosc. 63:296-305. [DOI] [PubMed] [Google Scholar]
- 28.Lianou, A., J. D. Stopforth, Y. Yoon, M. Wiedmann, and J. N. Sofos. 2006. Growth and stress resistance variation in culture broth among Listeria monocytogenes strains of various serotypes and origins. J. Food Prot. 69:2640-2647. [DOI] [PubMed] [Google Scholar]
- 29.Lunden, J., R. Tolvanen, and H. Korkeala. 2004. Human listeriosis outbreaks linked to dairy products in Europe. J. Dairy Sci. 87:E6-E12. [Google Scholar]
- 30.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medircio, S. N., V. A. Leao, and M. C. Teixeira. 2007. Specific growth rate of sulfate reducing bacteria in the presence of manganese and cadmium. J. Hazard. Mater. 143:593-596. [DOI] [PubMed] [Google Scholar]
- 32.Moretro, T., K. Naterstad, E. Wang, I. M. Aasen, S. Chaillou, M. Zagorec, and L. Axelsson. 2005. Sakacin P non-producing Lactobacillus sakei strains contain homologues of the sakacin P gene cluster. Res. Microbiol. 156:949-960. [DOI] [PubMed] [Google Scholar]
- 33.Naghmouchi, K., E. Kheadr, C. Lacroix, and I. Fliss. 2007. Class I/class IIa bacteriocin cross-resistance phenomenon in Listeria monocytogenes. Food Microbiol. 24:718-727. [DOI] [PubMed] [Google Scholar]
- 34.Naumann, D. 2000. Infrared spectroscopy in microbiology, p. 102-131. In R. A. Meyers (ed.), Encyclopedia of analytical chemistry. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 35.Oust, A., T. Moretro, K. Naterstad, G. D. Sockalingum, I. Adt, M. Manfait, and A. Kohler. 2006. Fourier transform infrared and Raman spectroscopy for characterization of Listeria monocytogenes strains. Appl. Environ. Microbiol. 72:228-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramnath, M., M. Beukes, K. Tamura, and J. W. Hastings. 2000. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66:3098-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasch, M., and S. Knochel. 1998. Variations in tolerance of Listeria monocytogenes to nisin, pediocin PA-1 and bavaricin A. Lett. Appl. Microbiol. 27:275-278. [DOI] [PubMed] [Google Scholar]
- 38.Rekhif, N., A. Atrih, and G. Lefebvre. 1994. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr. Microbiol. 28:237-241. [Google Scholar]
- 39.Robichon, D., E. Gouin, M. Debarbouille, P. Cossart, Y. Cenatiempo, and Y. Hechard. 1997. The rpoN (σ54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J. Bacteriol. 179:7591-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rode, T. M., S. Langsrud, A. Holck, and T. Møretrø. 2007. Different patterns of biofilm formation in Staphylococcus aureus under food-related stress conditions. Int. J. Food Microbiol. 116:372-383. [DOI] [PubMed] [Google Scholar]
- 41.Schmittgen, T. D., and K. J. Livak. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3:1101-1108. [DOI] [PubMed] [Google Scholar]
- 42.Tichaczek, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH1174 and sakacin P from L. sake LTH673. Syst. Appl. Microbiol. 15:460-468. [Google Scholar]
- 43.Tichaczek, P. S., R. F. Vogel, and W. P. Hammes. 1994. Cloning and sequencing of sakP encoding sakacin P, the bacteriocin produced by Lactobacillus sake LTH673. Microbiology 140:361-367. [DOI] [PubMed] [Google Scholar]
- 44.Vadyvaloo, V., S. Arous, A. Gravesen, Y. Hechard, R. Chauhan-Haubrock, J. W. Hastings, and M. Rautenbach. 2004. Cell-surface alterations in class IIa bacteriocin-resistant Listeria monocytogenes strains. Microbiology 150:3025-3033. [DOI] [PubMed] [Google Scholar]
- 45.Vadyvaloo, V., J. W. Hastings, M. J. van der Merwe, and M. Rautenbach. 2002. Membranes of class IIa bacteriocin-resistant Listeria monocytogenes cells contain increased levels of desaturated and short-acyl-chain phosphatidylglycerols. Appl. Environ. Microbiol. 68:5223-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vadyvaloo, V., J. L. Snoep, J. W. Hastings, and M. Rautenbach. 2004. Physiological implications of class IIa bacteriocin resistance in Listeria monocytogenes strains. Microbiology 150:335-340. [DOI] [PubMed] [Google Scholar]
- 47.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vignolo, G., J. Palacios, M. E. Farias, F. Sesma, U. Schillinger, W. Holzapfel, and G. Oliver. 2000. Combined effect of bacteriocins on the survival of various Listeria species in broth and meat system. Curr. Microbiol. 41:410-416. [DOI] [PubMed] [Google Scholar]
- 49.Welshimer, H. J., and J. Donker-Voet. 1971. Listeria monocytogenes in nature. Appl. Environ. Microbiol. 21:516-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.