Abstract
Four point-of-use disinfection technologies for treating sewage-contaminated well water were compared. Three systems, based on flocculant-disinfectant packets and N-halamine chlorine and bromine contact disinfectants, provided a range of 4.0 to >6.6 log10 reductions (LR) of naturally occurring fecal indicator and heterotrophic bacteria and a range of 0.9 to >1.9 LR of coliphage.
Disasters and flooding can overwhelm sanitation infrastructure, leading to sewage contamination of potable waters. This may be routine during the wet season in many parts of the world and spreads numerous waterborne diseases (21). Point-of-use (POU) water treatment has reduced the incidence of diarrheal disease when used for household drinking water (3, 4, 6, 13) and is now being promoted for disaster relief. While POU systems have recently been reviewed (14), to our knowledge there has been no direct, experimental comparison for treating actual sewage-contaminated waters. In this study, the efficacies of four POU disinfection systems (based on sodium dichloroisocyanurate [NaDCC] tablets, a flocculent-disinfectant powder, and chlorine and bromine contact disinfectant cartridges) in reducing the concentrations of six microbial indicators in well water contaminated with raw sewage were compared.
The NaDCC tablets (67 mg; Aquatabs; Medentech, Wexford, Ireland), used for disinfection in low-turbidity water, have shown preliminary efficacy for routine household drinking water treatment (3, 4). The flocculant-disinfectant packet (4 g; PUR; Procter & Gamble Co., Cincinnati, OH) includes Fe2(SO4)3, bentonite, Na2CO3, chitosan, polyacrylamide, KMnO4, and Ca(OCl)2 (13). It achieved >7.3 log10 reductions (LR) of 24 bacteria species; >4.6 LR of poliovirus and rotavirus in EPA no. 2 test water (turbidity, >30 nephelometric turbidity units [NTU]) (15); and reduced diarrheal illness in Guatemala, Liberia, Kenya, and Pakistan (6, 7, 11, 13).
HaloPure canisters (Eureka Forbes, Mumbai, India) contain N-halamine polymer disinfectant beads, poly[1,2-dichloro-5-methyl-5-(4′-vinylphenyl)hydrantoin] for chlorine canisters, and poly[1,2-dibromo-5-methyl-5-(4′-vinylphenyl)hydrantoin] for bromine canisters. Seeded laboratory trials achieved >6.8 LR for Escherichia coli and Staphylococcus aureus as water was passed through the canisters (2). The Cl-contact (producing residuals ranging from 0 to 0.6 mg/liter) and Br-contact (with residuals of 0.68 to 1.8 mg/liter) disinfectants achieved 2.9 LR and 5.0 LR of the bacteriophage MS2, respectively, and 27.5% and 88.5% reductions of the algal toxin microcystin, respectively (5).
Sewage-contaminated water was prepared by mixing 9 liters of potable, nonchlorinated well water (pH 7.8; turbidity, 0.33 NTU; Williamston, MI) with 1 liter of raw sewage (City of East Lansing Wastewater Treatment Plant, MI) with an average pH of 6.6 ± 0.1, a biochemical oxygen demand of 144 ± 36 mg/liter, a concentration of total suspended solids of 146 ± 31 mg/liter, and a turbidity of 132 ± 12 NTU. Three disinfection trials were conducted at room temperature for each POU system on three different days to allow for variance in sewage strength. The turbidities of 1:10 dilutions of raw sewage averaged 7.5 ± 2.0 NTU. Table 1 lists the indicator microorganism concentrations in the influent and effluent for each system.
TABLE 1.
Concentrations of influent and 30-min-effluent microorganisms for POU disinfectant systems treating sewage-contaminated water
| Microorganism group | Geometric mean concn (range) [% of samples below detection limit]a |
|||||||
|---|---|---|---|---|---|---|---|---|
| NaDCC |
Flocculant-disinfectant |
Cl-contact |
Br-contact |
|||||
| Influent | Effluent at 30 min | Influent | Effluent at 30 min | Influent | Effluent at 30 min | Influent | Effluent at 30 min | |
| Total coliforms | 2.7 × 104 (6.7 × 103 to 7.6 × 104) | 4.3 (4.0 × 10−2 to 1.6 × 102) | 1.7 × 104 (1.2 × 104 to 2.7 × 104) | 4.0 × 10−2 (<1.0 × 10−2 to 2.4 × 10−1) [33] | 2.9 × 104 (2.3 × 104 to 4.0 × 104) | <1.0 × 10−2 [100] | 4.5 × 104 (1.9 × 104 to 7.2 × 104) | 1.1 × 10−2 (<1.0 × 10−2 to 1.3 × 10−2) [66] |
| Heterotrophic plate counts | 8.7 × 104 (2.7 × 104 to 1.8 × 105) | 6.4 × 101 (2.1 × 101 to 4.5 × 102) | 8.9 × 104 (2.9 × 104 to 4.3 × 105) | 8.5 (4.7 to 2.7 × 101) | 6.6 × 104 (3.5 × 104 to 1.1 × 105) | 3.9 (3.5 to 4.2) | 8.3 × 104 (2.4 × 104 to 2.0 × 105) | 4.6 (2.2 to 7.7) |
| E. coli | 3.3 × 103 (7.7 × 102 to 1.1 × 104) | 1.8 × 101 (9.0 × 10−1 to 5.3 × 102) | 6.7 × 103 (2.3 × 103 to 4.3 × 104) | 1.1 × 10−2 (<1.0 × 10−2 to 1.3 × 10−2) [66] | 4.7 × 103 (2.3 × 103 to 1.1 × 104) | <1.0 × 10−2 [100] | 1.5 × 104 (6.3 × 103 to 4.6 × 104) | <1.0 × 10−2 [100] |
| Enterococci | 8.8 × 102 (5.7 × 102 to 1.3 × 103) | 2.3 (<1.0 × 10−2 to 4.9 × 101) [33] | 6.3 × 102 (5.0 × 102 to 8.7 × 102) | <1.0 × 10−2 [100] | 9.9 × 102 (5.3 × 102 to 1.7 × 103) | <1.0 × 10−2 [100] | 1.3 × 103 (7.3 × 102 to 2.3 × 103) | <1.0 × 10−2 [100] |
| Clostridia | 1.6 × 102 (6.0 × 101 to 3.0 × 102) | 6.4 (6.7 × 10−1 to 7.7 × 101) | 2.0 × 102 (7.0 × 101 to 6.0 × 102) | 7.9 × 10−1 (4.5 × 10−1 to 1.4) | 3.4 × 101 (2.0 × 101 to 6.3 × 101) | 2.4 × 10−2 (<1.0 × 10−2 to 6.0 × 10−2) [33] | 4.4 × 101 (2.7 × 101 to 9.3 × 101) | 7.4 × 10−2 (<1.0 × 10−2 to 3.6 × 10−1) [33] |
| Coliphage | 1.5 × 102 (1.2 × 102 to 2.2 × 102) | 3.1 × 101 (<1.0 to 1.8 × 102) [33] | 1.4 × 102 (1.3 × 102 to 1.4 × 102) | 1.9 × 101 (<1.0 to 1.1 × 102) [33] | 9.4 × 101 (4.3 × 101 to 1.6 × 102) | 7.3 (1.3 to 4.7 × 101) | 7.7 × 101 (4.0 × 101 to 1.2 × 102) | <1.0 [100] |
Values shown are numbers of CFU/ml except those for coliphage, which are numbers of PFU/ml. The percentage of samples below the detection limit (n = 3 for all systems) is 0% if not shown.
All systems were used in accordance with the manufacturer's directions for 10 liters of water. For NaDCC trials, one tablet was added and allowed 30 min of contact time (total dose of 3.2 mg/liter of hypochlorite; in deionized water, one tablet produced 2.1 mg/liter free Cl residual). For flocculant-disinfectant trials, one packet was added, stirred vigorously for 5 min, strained through cheesecloth after 10 min, and allowed 20 min of further contact time. The amount of hypochlorite included in one packet was not indicated, but one packet provided 1.5 mg/liter free Cl residual in 10 liters of deionized water. Samples were taken at 1, 3, 5, 10, 15, and 30 min for both systems.
For the Cl-contact and Br-contact trials, disinfectant cartridges were installed in AquaSure housings consisting of an upper reservoir for influent, which flows by gravity through the disinfectant cartridge to a lower reservoir with a tap for dispensing (Fig. 1). The housings usually include cloth and activated charcoal prefilters, but these were removed in order to directly evaluate the disinfectant. With the tap open, 10 liters of influent was added and samples were collected at first flow (6 to 12 min) and after 15 and 30 min of flow. A single chlorine canister was used for all trials; the bromine canister was replaced for the third trial because the original clogged.
FIG. 1.
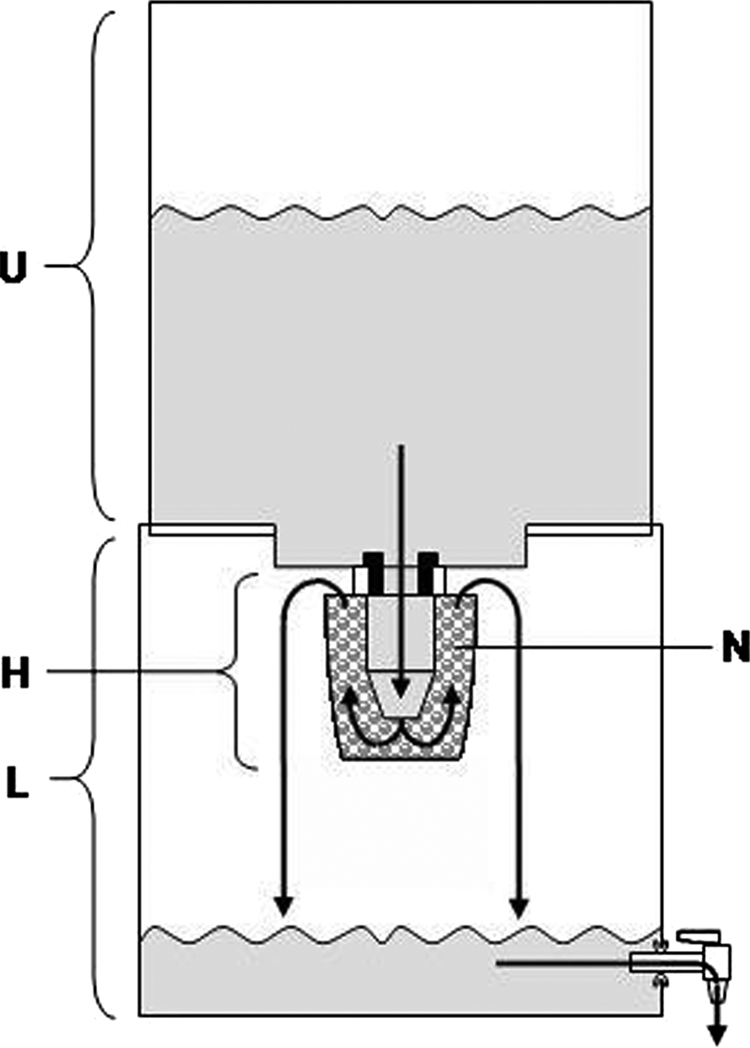
Flow schematic for contact disinfectant cartridges. Arrows indicate the directions of water flow from the upper reservoir (U), through the halogen (chlorine or bromine) disinfectant cartridge (H) containing packed N-halamine beads (N), to the lower reservoir (L) and out through the open tap.
Microbial indicators in the influent and effluent (collection tubes contained sodium thiosulfate) in triplicate were quantified as numbers of CFU/ml by using mENDO agar for total coliforms (9), mHPC agar for heterotrophic plate counts (8), mTEC medium for E. coli (19), mEI agar for the genus Enterococcus (18), and mCP agar for the genus Clostridium (1) (Becton, Dickinson and Co., Franklin Lakes, NJ). Coliphage (PFU/ml) were measured with a double agar overlay assay, EPA method 1601 (17). Residuals (mg/liter) were measured using a Hach chlorine (free and total) test kit, model CN66 (Hach Co., Loveland, CO) (used for bromine in accordance with Hach method 8016 [10], with the instrument reading multiplied by 2.25 [the ratio of the atomic weights of bromine and chlorine], as advised by Hach Co. technical support).
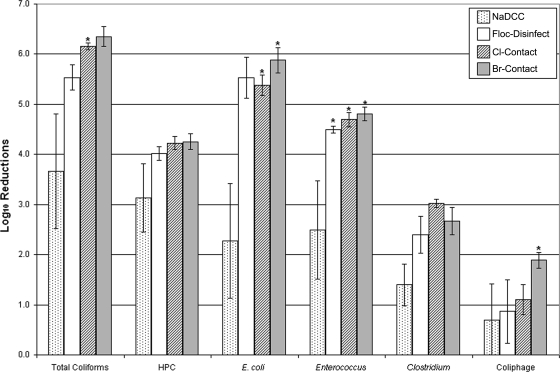
Comparison of water quality levels was done at 30 minutes. LR were calculated, with zeros replaced with the detection limits (Fig. 2). All POU systems reduced microbial concentrations below the detection limit in some trials (Table 1), making the calculated reductions the lower bound for those trials.
FIG. 2.
Average LR of naturally occurring microorganisms at 30 min for sewage-contaminated well water (1:10 dilution of raw sewage in well water) with the use of four POU disinfection systems (error bars represent 1 standard error). * indicates that effluent was below the limit of detection for all samples. Limit of detection was substituted to calculate LR and actual reductions may be greater than shown.
Average LR for each POU system were compared using two-way analysis of variance with post hoc least-significant-difference (LSD) tests, performed with SPSS 11.0.1 (SPSS, Inc.). LR at 30 min differed significantly between systems (analysis of variance; F3,5 = 20.6; P < 0.001). There was no significant difference between the LR achieved by flocculant-disinfectant and contact disinfectants (LSD; mean difference, 0.2 to 0.5 LR; P > 0.05), while the NaDCC tablets induced significantly lower reductions (LSD; mean difference, 1.5 to 2.0 LR; P < 0.001).
There was detectable residual free chlorine after 30 min for one NaDCC trial (0.4 mg/liter) and two flocculant-disinfectant trials (0.1 and 0.4 mg/liter). No contact disinfectant trial produced a measurable residual.
No system in this study reliably produced residuals for safe storage after POU treatment or ideal virus reduction. Except for the NaDCC system, the POU systems achieved approximately 5.5 LR for E. coli and coliforms, 4.5 LR for enterococci, 4.0 LR for heterotrophs, 2.5 LR for clostridia, and 1.0 LR for coliphage. Coliphage was reduced below detection limits in all trials with Br-contact, similar to what was found in previous research (5). Bromine disinfection has proved safe and effective for large-scale maritime applications, like U.S. Navy vessels (20), and appears promising for household treatment. Further assessment of the Br-contact system is warranted, as is field comparison of POU systems in disaster relief.
Acknowledgments
We thank Srikanth Aravamuthan, who was funded as part of a Department of Homeland Security summer internship, for his technical support in the laboratory phase of this study. Eureka Forbes (Mumbai, India) produced the HaloPure and AquaSure units, and we have no relationship with this company; however, the units were provided to MSU by Halosource, Inc. (Bothell, WA), which designed the contact disinfectants. Halosource, Inc., was not involved in the design of the study goals. PUR packets and Aquatabs were generously provided by the manufacturers, with assistance from Jeffrey Sloan of the Chlorine Chemistry Council.
Halosource, Inc., provided financial support to MSU for the testing of the HaloPure and AquaSure units. The Center for Advancing Microbial Risk Assessment (a center of excellence funded by EPA and DHS [EPA STAR grant no. R83236201]) provided additional funding.
Footnotes
Published ahead of print on 18 September 2009.
REFERENCES
- 1.Bisson, J. W., and V. J. Cabelli. 1979. Membrane filter enumeration method for Clostridium perfringens. Appl. Environ. Microbiol. 37:55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, Y., S. D. Worley, J. Kim, C.-I. Wei, T.-Y. Chen, J. I. Santiago, J. F. Williams, and G. Sun. 2003. Biocidal poly(styrenehydantoin) beads for disinfection of water. Ind. Eng. Chem. Res. 42:280-284. [Google Scholar]
- 3.Clasen, T., and P. Edmondson. 2006. Sodium dichloroisocyanurate (NaDCC) tablets as an alternative to sodium hypochlorite for the routine treatment of drinking water at a household level. Int. J. Hyg. Environ. Health 209:173-181. [DOI] [PubMed] [Google Scholar]
- 4.Clasen, T., T. F. Saeed, S. Boisson, P. Edmondson, and O. Shipin. 2007. Household water treatment using sodium dichloroisocyanurate (NaDCC) tablets: a randomized, controlled trial to assess microbiological effectiveness in Bangladesh. Am. J. Trop. Med. Hyg. 76:187-192. [PubMed] [Google Scholar]
- 5.Coulliette, A. D., L. A. Peterson, J. Mosberg, and J. B. Rose. Evaluation of a new approach for delivering disinfection: efficacy of chlorine and bromine halogenated contact disinfection units for the reduction of viruses and microcystin. Am. J. Trop. Med. Hyg., in press. [DOI] [PMC free article] [PubMed]
- 6.Crump, J. A., G. O. Okoth, L. Slutsker, D. O. Ogaja, B. H. Keswick, and S. P. Luby. 2004. Effect of point-of-use disinfection, flocculation, and combined flocculation-disinfection on drinking water quality in western Kenya. J. Appl. Microbiol. 97:225-231. [DOI] [PubMed] [Google Scholar]
- 7.Doocy, S., and G. Burnham. 2006. Point-of-use water treatment and diarrhoea reduction in the emergency context: an effectiveness trial in Liberia. Trop. Med. Int. Health 11:1542-1552. [DOI] [PubMed] [Google Scholar]
- 8.Eaton, A. D., L. S. Clesceri, E. W. Rice, A. E. Greenberg, and M. H. Franson (ed.). 2005. Standard methods for the examination of water and wastewater, 21st ed. Section 9215. American Public Health Association, Washington, DC.
- 9.Eaton, A. D., L. S. Clesceri, E. W. Rice, A. E. Greenberg, and M. H. Franson (ed.). 2005. Standard methods for the examination of water and wastewater, 21st ed. Section 9222B. American Public Health Association, Washington, DC.
- 10.Hach Company. 2008. Method 8016: bromine, 5th ed. DOC316.53.01011. Hach Company, Loveland, CO.
- 11.Luby, S. P., M. Agboatwalla, J. Painter, A. Altaf, W. Billhimer, B. Keswick, and R. M. Hoekstra. 2006. Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomized controlled trial. Trop. Med. Int. Health 11:479-489. [DOI] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Reller, M. E., C. E. Mendoza, M. Beatriz Lopez, M. Alvarez, R. M. Hoekstra, C. A. Olson, K. G. Baier, B. H. Keswick, and S. P. Luby. 2003. A randomized controlled trial of household-based flocculant-disinfectant drinking water treatment for diarrhoea prevention in rural Guatemala. Am. J. Trop. Med. Hyg. 69:411-419. [PubMed] [Google Scholar]
- 14.Sobsey, M. D., C. E. Stauber, L. M. Cassanova, J. M. Brown, and M. A. Elliott. 2008. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 42:4261-4267. [DOI] [PubMed] [Google Scholar]
- 15.Souter, P. F., G. D. Cruikshank, M. Z. Tankerville, B. H. Keswick, B. D. Ellis, D. E. Langworthy, K. A. Metz, M. R. Appleby, N. Hamilton, A. L. Jones, and J. D. Perry. 2003. Evaluation of a new water treatment for point-of-use household applications to remove microorganisms and arsenic from drinking water. J. Water Health 1:73-84. [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.U.S. EPA. 2001. Method 1601: male-specific (F+) and somatic coliphage in water by two-step enrichment procedure. EPA-821-R-01-030. U.S. EPA Office of Water, Washington, DC.
- 18.U.S. EPA. 2002. Method 1600: enterococci in water by membrane filter using membrane Enterococcus indoxyl-B-D-glucoside agar (mEI). U.S. EPA Office of Water, Washington, DC.
- 19.U.S. EPA. 2005. Method 1603: Escherichia coli (E. coli) in water by membrane filtration using modified membrane-thermotolerant Escherichia coli agar (modified mTEC). EPA 821-R-04-025. U.S. EPA Office of Water, Washington, DC.
- 20.U.S. Navy Medical Corps. 2005. Water supply afloat. Manual of naval preventative medicine. NAVMED P-5010-6. Navy Medicine Publications, Washington, DC.
- 21.Wisner, B., and J. Adams (ed.). 2003. Environmental health in emergencies and disasters: a practical guide, p. 92-147. World Health Organization, Geneva, Switzerland.