Abstract
Outbreaks of Escherichia coli infections linked to fermented meats have prompted much research into the kinetics of E. coli inactivation during fermented meat manufacture. A meta-analysis of data from 44 independent studies was undertaken that allowed the relative influences of pH, water activity (aw), and temperature on E. coli survival during fermented meat processing to be investigated. Data were reevaluated to determine rates of inactivation, providing 484 rate data points with various pH (2.8 to 6.14), aw (0.75 to 0.986), and temperature (−20 to 66°C) values, product formulations, and E. coli strains and serotypes. When the data were presented as an Arrhenius model, temperature (0 to 47°C) accounted for 61% of the variance in the ln(inactivation rate) data. In contrast, the pH or aw measured accounted for less than 8% of variability in the data, and the effects of other pH- and aw-based variables (i.e., total decrease and rates of reduction of those factors) were largely dependent on the temperature of the process. These findings indicate that although temperatures typically used in fermented meat manufacture are not lethal to E. coli per se, when other factors prevent E. coli growth (e.g., low pH and aw), the rate of inactivation of E. coli is dominated by temperature. In contrast, inactivation rates at temperatures above ∼50°C were characterized by smaller z values than those at 0 to 47°C, suggesting that the mechanisms of inactivation are different in these temperature ranges. The Arrhenius model developed can be used to improve product safety by quantifying the effects of changes in temperature and/or time on E. coli inactivation during fermented meat manufacture.
Fermented meats encompass a diverse range of product styles in which raw, ground meat is preserved by the processes of fermentation and drying (or maturation). These products are typically manufactured without a bactericidal heat treatment, and instead, inhibition of growth and inactivation of contaminating pathogens rely upon the collective effects of acid pH, reduced water activity (aw), and the presence of lactic acid and, potentially, curing salts (nitrate and/or nitrite) and spices. However, pathogenic Escherichia coli can contaminate and survive in fermented meat products at levels sufficient to cause serious illness in consumers, as evidenced by numerous outbreaks of E. coli infections epidemiologically linked to uncooked fermented meat products (8, 9, 54, 64). Knowledge of the kinetics of nonthermal inactivation of E. coli, and the factors affecting it, is important to be able to optimize the safety of fermented meat processes.
Several research groups have conducted studies on the survival of pathogenic E. coli during the processing of specific fermented meat products (4, 6, 14, 16, 23, 25). In some cases, the effects of alternative ingredients or processing parameters have also been determined (12, 21, 24, 30). Such investigations have allowed the lethality of a specific fermented meat process to be determined and the microbiological safety of that product, in regards to E. coli, to be described. However, most of these studies have been essentially empirical and product/process specific, i.e., they have not sought to discern key variables or their interactions that influence the extent of inactivation of E. coli or other pathogens during meat fermentation and maturation. Thus, while they are very important and useful, it has been difficult to extrapolate the results of those studies to different fermented meat processes, confounding efforts to assess product safety without the requirement for challenge tests or to give manufacturers the confidence to develop new or modified processes that remain safe.
In an attempt to identify the main factors that influence the inactivation of E. coli in fermented meat products, we utilized data that already existed in the scientific literature and reassessed that information via a process similar to meta-analysis. Meta-analysis is a statistical technique that involves amalgamating, summarizing, and reviewing previous quantitative research to identify trends. It is used, albeit rarely in the area of microbiology, as a means to address a wide variety of questions where a reasonable body of primary research studies exists. In a preliminary investigation, based on an analysis of limited published and other data, Ross and Shadbolt (51) observed that inactivation of E. coli in fermented meat processes was dominated by temperature and that pH and aw levels appeared to be less influential, except insofar as creating conditions inimical to E. coli growth. The objective of the present investigation was to rigorously test that observation by collation and analysis of a large data set describing the inactivation of E. coli during manufacture of fermented meats and to attempt to identify underlying patterns in the responses of E. coli to conditions encountered during fermented meat processes. From the observations of Ross and Shadbolt (51), and because the inactivation of E. coli in fermented meat is not instantaneous, we based our analyses on the rate of E. coli inactivation, calculated from viable count data and processing times reported by a variety of published and unpublished sources. We sought to relate the inactivation rate to reported environmental conditions, including pH, aw, and temperature, and to summarize the observations in a predictive mathematical model.
MATERIALS AND METHODS
Data search strategy and selection criteria.
Studies published before January 2008 on the survival of E. coli in fermented meat products or in aqueous systems (broth or peptone water) with levels of pH and/or aw that prevented growth of E. coli were sought by computer-based searches of the U.S. National Library of Medicine's PubMed and ISI Web of Knowledge literature databases. Searches were done by topic and used the keyword “Escherichia coli” with words related to bacterial inactivation (“death,” “inactivation,” “survival,” and “viability”) and fermented meat (“fermented meat” and “salami”). Reference lists of relevant publications were also reviewed to identify additional studies. Other data, obtained from Ph.D. theses authored by members of the University of Tasmania food microbiology research group, reports prepared for industry organizations within Australia, and our own unpublished results, were also included. For pragmatic reasons, only studies that (i) were written in English and (ii) contained data that enabled an estimate of inactivation rate at one or more temperatures were included in analyses.
Data abstraction and determination of inactivation rates.
As far as possible, and where relevant, the following information was collated from each study: E. coli serotype(s) and strain(s), food or broth type, process (e.g., fermentation, maturation, or heating) and process duration, temperature, aw, pH, lactic acid concentration, presence and concentrations of other additives (e.g., glucono-δ-lactone or nitrite), and E. coli viability data. For each source that included data describing a set of conditions for an interval of time for which E. coli was enumerated at least at the beginning and end of that interval, and during which at least the temperature was constant, a rate of inactivation of E. coli was estimated by simple linear regression (Microsoft Excel) of E. coli viability (log10 CFU/unit, where “unit” is a gram or milliliter) versus time data. Where semilogarithmic inactivation curves were apparently multiphasic, i.e., were not able to be well described by a straight line, and there was no reported evidence of environmental change in the product during the process, the rate of inactivation in the slowest stage (whether a “shoulder” or “tail”) was calculated as described above. This strategy was adopted to be consistent with a “worst-case” approach for model development.
Statistical analysis.
To identify possible predictor variables for the inactivation of E. coli, inactivation rates were collated and fitted to simple regression models based on various predictor variables (e.g., temperature, pH, and aw).
To investigate the influence of temperature, the data were transformed to ln(inactivation rate) and the reciprocal of absolute temperature. The transformed data were then analyzed by simple linear regression, i.e., fitted to an Arrhenius model, and the strength of the relationship was determined by calculation of the correlation coefficient (R2). The root mean square error (RMSE) and bias and accuracy factors (50) were also calculated. A number of other, empirical equations were also investigated to assess the goodness of fit that could be obtained without being constrained to an Arrhenius model.
Many of the studies used enabled inactivation rates to be determined at several temperatures. Thus, data for individual studies for temperatures in the range of 0 to 47°C were individually fitted to an Arrhenius model and compared to the model fitted to the entire data set for the same temperature range. This was done to determine whether the analysis of pooled data obscured differences in responses between individual strains or processes.
To assess possible differences in the effects of lethal (i.e., >∼47°C) and sublethal temperatures, inactivation rate data for temperatures above 47°C were analyzed separately. Due to a paucity of studies of the change in viability of E. coli in fermented meats at lethal temperatures, inactivation data for E. coli in fresh meat in the temperature range of 54.4 to 70°C were also included to augment the data set. Five studies, each enabling ≥14 inactivation rate estimates, were selected from a list of publications identified by a computer-based search of the ISI Web of Knowledge, using the keywords “Escherichia coli,” “thermal inactivation,” and “ground beef.” For each temperature range, z values were calculated.
The effect of pH on E. coli inactivation in fermented meats and analogous aqueous systems was analyzed by plotting, where available, the log10-transformed rate or total amount of E. coli inactivation against a range of pH-based predictor variables. Predictor variables investigated included the final pH of an aqueous system or of a fermented meat product after a given manufacturing process and the total reduction in pH or rate of reduction in pH during fermentation (i.e., the processing stage where significant decreases in pH occur). Using regression analysis, a straight line was fitted to each log10-transformed inactivation rate or total inactivation data set versus the various proposed predictor variables, and R2 was determined. To remove the confounding effect of the influence of temperature, the relationship was further investigated by normalizing the inactivation rate data for the effect of temperature. Specifically, the residuals of the data compared to the inactivation rate predicted from the Arrhenius model fitted to the data were plotted against the selected pH variable. Simple linear regression analysis of the residuals against the pH predictor variable was then performed by fitting straight lines to identify any relationship between the rate data normalized for the effect of temperature and the pH variable under investigation.
Similarly, the influence of aw on the decline of E. coli in fermented meats and analogous aqueous systems was examined by plotting the log10-transformed rate or the total amount of E. coli inactivation against the final aw of an aqueous system or of fermented meat at the end of a manufacturing process and the total reduction in aw or rate of reduction in aw during drying of meat or other postfermentation processes (i.e., processing stages where significant decreases in aw occur). The reduction in aw during drying, storage, or heating was determined as the difference in aw measured at the end of the process and that at the end of fermentation. Three studies (4, 5, 25) reported aw at the end of drying or another postfermentation process but not at the end of fermentation. For those studies, aw at the completion of fermentation was assumed to be 0.96, which allowed additional data to be included in analyses of the total reduction in aw (19 data points) and the rate of reduction in aw (7 data points) during drying, storage, or heating. For one study (28), the aw of broth was estimated from the salt (NaCl) concentration reported, using the tables of Chirife and Resnik (13), for two inactivation data. Simple linear regression was applied to each data set, and R2 values were determined. The inactivation rate data normalized for the effect of temperature, as described above, were also plotted against aw variables and analyzed by simple linear regression.
RESULTS
Data set.
In total, 44 relevant studies relating to the inactivation of E. coli in fermented meats or analogous aqueous systems were identified and are summarized in Table 1. The majority of studies enabled multiple inactivation rate estimates to be determined, providing a data set of 484 inactivation rates, encompassing over 50 E. coli strains and temperatures ranging from −20 to 66°C (mean = 25.5°C), pHs ranging from 2.8 to 6.14 (mean = 4.72), and aw values ranging from 0.75 to 0.986 (mean = 0.901). Twenty-five (25) of the 484 inactivation rates were derived from biphasic inactivation curves and were based on the slowest phase of inactivation. The remaining 459 rates were from inactivation curves that were well described by single-phase inactivation models. Those data were compiled and analyzed to discern and quantify patterns of E. coli inactivation in response to environmental factors relevant to fermented meat products and processes.
TABLE 1.
Studies included in meta-analysis of E. coli inactivation in fermented meats
| Reference | No. of strains | Food or broth type | No. of rates | Temp. (°C)a | pHa | awa |
|---|---|---|---|---|---|---|
| 2 | 2 | Dry fermented sausage | 10 | 13-21.8 (2) | 5.1 (1) | 0.87-0.94 (2) |
| 4 | 5 | Semidry beef summer sausage | 7 | 4-66 (5) | 4.6-5 (2) | 0.94 (1) |
| 5 | 5 | Soudjouk | 6 | 4-23 (3) | 4.48-5.48 (4) | 0.88-0.91 (2) |
| 6 | 5 | Soudjouk | 10 | 4-27.7 (5) | 4.81-6.14 (8) | — |
| 7 | 1 | Fermented sausage | 8 | 15-37 (2) | 4.7 (1) | — |
| 10 | 5 | Fermented dry sausage | 6 | 13-22.67 (2) | 4.8-4.9 (3) | 0.88-0.94 (2) |
| 11 | 4 | Lebanon bologna | 27 | 31-40 (3) | 4.3-5.6 (8) | — |
| 12 | 4 | Sliced, vacuum-packed Lebanon bologna | 2 | 3.6-13 (2) | — | — |
| 14 | 6 | Salami slices, tryptone soy broth (TSB) | 13 | 5-30 (3) | 4.63-6 (5) | 0.9-0.95 (2) |
| 16 | 1 | Soudjouk | 4 | 4-24 (3) | 4.8-4.9 (3) | — |
| 17 | 1 | Pepperoni | 16 | 55-62 (4) | 4.4-4.8 (2) | — |
| 18 | 3 | Salami | 3 | 50-60 (3) | 4.9 (1) | — |
| 19 | 5 | Fermented sausage, brain heart infusion (BHI) broth | 35 | 14-26 (4) | 4.5-5.5 (5) | 0.89-0.97 (6) |
| 20 | 5 | Fermented sausage | 4 | 26-42 (4) | — | — |
| 21 | 4 | Lebanon bologna mix in tubes | 2 | 43.3-46.1 (2) | 4.7 (1) | — |
| 22 | 1 | Dry fermented sausage | 8 | 17-20.1 (2) | 4.9-5.1 (3) | 0.90-0.96 (2) |
| 23 | 5 | Salami | 18 | 4-24 (4) | 4.8 (1) | 0.90-0.93 (3) |
| 24 | 5 | Pepperoni | 9 | −20-36 (5) | 4.67-5.1 (8) | 0.881-0.96 (6) |
| 25 | 5 | Pepperoni | 18 | 4-36 (4) | 4.68-4.85 (3) | 0.87-0.89 (3) |
| 27 | 5 | Lebanon bologna | 4 | 27.3-44.3 (4) | — | — |
| 28 | 5 | Fermented dry sausage, TSB | 10 | 4-37 (3) | 3.5-4.7 (6) | 0.87-0.94 (3) |
| 29 | 2 | Minisalami, salami, BHI broth | 85 | 15-37 (5) | 4-5.6 (14) | 0.84-0.96 (9) |
| 30 | 5 | Pepperoni | 4 | 13-36 (2) | 4.7-4.9 (4) | 0.87-0.96 (3) |
| 35 | 1 | Salami | 5 | 40 (1) | 4.78 (1) | — |
| 36 | 1 | Salami | 5 | 40 (1) | 4.2-5.8 (5) | — |
| 37 | 1 | Salami | 3 | 40 (1) | 4.25-5.8 (2) | — |
| 38 | 1 | Dry fermented sausage | 12 | 16-22.5 (2) | 4.7-5.3 (3) | 0.76-0.90 (3) |
| 39 | 5 | Fermented sausage, shredded salami | 4 | 5-32 (2) | 4.4-5 (2) | — |
| 41 | 8 | TSB | 18 | 37 (1) | — | — |
| 42 | 1 | Luria-Bertani broth, nutrient broth, TSB | 3 | 25 (1) | — | 0.90 (1) |
| 44 | 5 | Dry fermented sausage | 4 | 13-22.67 (2) | 4.9 (1) | 0.87-0.986 (3) |
| 45 | 1 | Norwegian, fermented dry sausage | 2 | 14-27 (2) | 4.8-4.9 (2) | 0.89-0.93 (2) |
| 46 | 1 | Short-ripened fermented sausage | 4 | 12 (1) | 5.1 (1) | 0.81 (1) |
| 47 | 1 | Hungarian fermented sausage | 6 | 25 (1) | 4.4-4.7 (3) | — |
| 49 | 1 | Pepperoni | 4 | 15-37.8 (3) | 4.4-4.8 (2) | 0.76-0.93 (4) |
| 53 | 1 | Greek, dry fermented sausage | 3 | 15-20.7 (2) | 4.5 (1) | — |
| 55 | 3 | 0.1% Peptone water | 12 | 25 (1) | 2.8-3.2 (2) | — |
| 56 | 1 | Cooked meat medium, TSB | 26 | 15-37 (3) | 4.6-5.5 (10) | 0.82-0.93 (5) |
| C. T. Shadbolt, unpublished results | 1 | Unspecified broth | 16 | 4-50 (6) | — | 0.75-0.90 (3) |
| 57 | 1 | Nutrient broth | 9 | 4-50 (5) | — | 0.75-0.95 (5) |
| 58 | 1 | TSB | 1 | 25 (1) | — | 0.90 (1) |
| 60 | 1 | BHI broth | 2 | 10 (1) | 4.5 (1) | — |
| 62 | 3 | Fermented sausage | 4 | 7-22 (2) | — | — |
| 63 | 7 | Fermented sausage | 32 | 14-43 (11) | — | 0.837-0.962 (16) |
| Total for 44 references, with overall ranges | >50 unique strainsb | 484 | −20-66 | 2.8-6.14 | 0.75-0.986 |
The number of distinct temperature, pH, or aw values for which an inactivation rate was determined is given in parentheses. —, not described.
The exact number of unique strains is unknown because the strain was unspecified in some studies.
Effect of temperature.
The effect of temperature on the inactivation of E. coli was determined by fitting data transformed to ln(inactivation rate) and the reciprocal of absolute temperature. Equation 1, as follows, is the best fitting of a range of parsimonious equations fitted to all 484 inactivation rate data (i.e., irrespective of pH, aw, strain, etc., and with a temperature range of −20 to 66°C): ln[inactivation rate (log10 CFU/unit/h)] = 5.33 × 10−22 × [1/temperature (K)]−8.8455 − 8.5115. The RMSE for equation 1 is 1.19.
Analysis of the same data by simple linear regression suggested two distinct Arrhenius relationships for temperatures in the approximate ranges of 0 to 47°C and >47°C (Fig. 1). Equation 2, as follows, is the simple Arrhenius model fitted to all the data (i.e., irrespective of pH, aw, strain, etc.) for temperatures in the range of 0 to 47°C (n = 456): ln[inactivation rate (log10 CFU/unit/h)] = 30.974 − 10,483 × [1/temperature (K)]. The R2 for equation 2 is 0.607, indicating that temperature alone explains 61% of the variability in the data. The RMSE is 1.01.
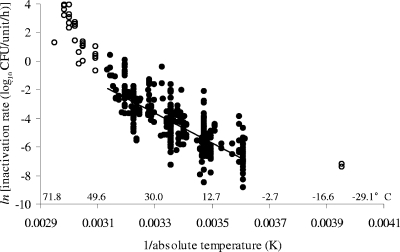
FIG. 1.
Effect of temperature on rate of inactivation of E. coli in fermented meats or analogous aqueous systems. The data are shown as an Arrhenius plot [ln(inactivation rate) versus 1/absolute temperature]. The line shown is a simple linear regression fitted to the data for temperatures of 0 to 47°C (•) and fits the equation y = −10,483x + 30.974 (R2 = 0.607). Inactivation rate data for temperatures of <0°C and >47°C are given by open symbols. Equivalent Celsius temperatures are shown for convenience.
Inactivation rate observations were compared to those predicted by equation 2, once transformed into equivalent inactivation rates. The goodness of fit of equation 2 was determined by calculating bias and accuracy factors (50). The bias factor is a measure of the average ratio of the observed values and the corresponding modeled values. It is a multiplicative factor by which the model prediction must be divided, on average, to equal the observed value. A bias factor of 1 is interpreted as indicating no systematic over- or underprediction, while a bias factor of 1.1 means that, on average, the model overpredicts the observed inactivation rate by 10%. The bias factor for equation 2 is 0.999, indicating that, on average, the model very slightly underpredicts the observed rates. The accuracy factor measures the average deviation between observation and model predictions. It is similar in concept to the variance of the data compared to corresponding model predictions. Thus, an accuracy factor of 2 means that, on average, the predicted value differs from the observed value by a factor of 2 (i.e., it is either twice as large or half as large). An accuracy factor of 1 means perfect agreement between observed and modeled responses. For equation 2, the accuracy factor was determined to be 2.17.
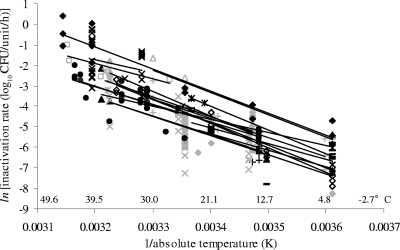
To further test the strength of the apparent influence of temperature on E. coli inactivation in fermented meats held at 0 to 47°C, the entire inactivation rate data set was disaggregated into the individual studies from which the rate data were derived. For studies that provided inactivation rates at three or more temperatures in the range of 0 to 47°C, these data were fitted individually to Arrhenius models. Thus, 19 submodels were generated (Fig. 2). The average slope and y intercept of linear regressions fitted to the models are −10,012 (standard deviation = 3,110) and 29.557 (standard deviation = 10.68), respectively, which are quite similar to the slope (−10,483) and y intercept (30.974) for the combined data set shown in Fig. 1.
FIG. 2.
Effect of temperature on rate of inactivation of E. coli for 19 individual studies with rate data for three or more temperatures in the range of 0 to 47°C. The data are shown as Arrhenius plots [ln(inactivation rate) versus 1/absolute temperature]. The lines shown are simple linear regressions fitted to the data for each individual study. Equivalent Celsius temperatures are shown for convenience.
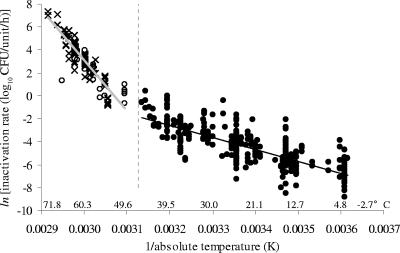
At temperatures greater than approximately 47°C, the slope of the linear regression line fitted to the data appears to increase (Fig. 1). This is demonstrated by the different z values for inactivation rate data for fermented meats and analogous aqueous systems held at 0 to 47°C (n = 456) and 50 to 66°C (n = 26), which are 19.2°C and 9.7°C, respectively. However, this change was difficult to assess because only 26 pieces of inactivation rate data were obtained for temperatures above 47°C for fermented meats and analogous aqueous systems. Therefore, to investigate the apparent change in temperature effect more rigorously, 115 additional inactivation rate data for temperatures of >47°C were obtained from nonfermented meat-based studies (15, 31, 34, 43, 65).
A comparison of the rates of inactivation at 0 to 47°C and 50 to 70°C (Fig. 3) more clearly illustrates the changing effect of temperature on the inactivation of E. coli at temperatures greater than approximately 47°C. The Arrhenius equation (equation 3) fitted to the data for temperatures above 47°C is as follows: ln[inactivation rate (log10 CFU/unit/h)] = 134.11 − 43,665 × [1/temperature (K)]. The R2 for equation 3 is 0.811. The z value for the combined inactivation rate data in the range of 50 to 70°C (n = 141) is 5.5°C. Similarly, the effect of temperature on E. coli inactivation at temperatures below 0°C appears to differ from that at 0 to 47°C (Fig. 1), although this could not be tested rigorously due to a lack of data in the accessible scientific literature. Using equations 2 and 3, the RMSE for the 484 data points is 1.06, lower than that for the empirical equation 1 but requiring two more parameters.
FIG. 3.
Changing effect of temperature on rate of inactivation of E. coli for temperatures above and below 47°C (shown by the dashed line), which is the maximum temperature for growth of most E. coli strains. The data are shown as an Arrhenius plot [ln(inactivation rate) versus 1/absolute temperature]. Inactivation rate data for temperatures above 47°C include data derived from fermented meats and analogous broth systems (○), previously shown in Fig. 1, and from fresh meat (×). Inactivation rate data for temperatures of 0 to 47°C are from fermented meats and analogous aqueous systems (•) and are also shown in Fig. 1. A simple linear regression line fitted to the data for temperatures above 47°C is given by the gray line (y = −43,665x + 134.11; R2 = 0.8110), and that for 0 to 47°C is given by the black line (y = −10,483x + 30.974; R2 = 0.607). Equivalent Celsius temperatures are shown for convenience.
Effect of pH.
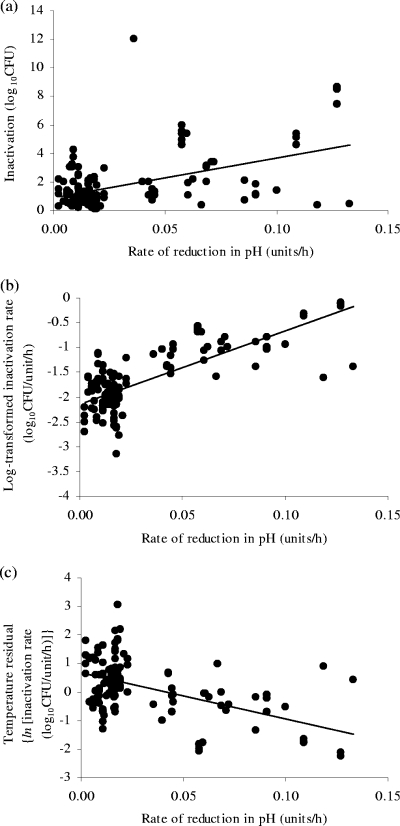
Where available, the total amount or log10-transformed rate of E. coli inactivation in fermented meats or analogous aqueous systems was plotted against the final pH, the reduction in pH during fermentation, or the rate of pH decline during fermentation. A simple linear regression fitted to each plot indicated that the final pH and the reduction in pH during fermentation explained less than 10% of the inactivation data (R2 < 0.10) (Table 2). A better correlation was observed between E. coli inactivation and the rate of reduction in pH during fermentation, where a greater rate of pH decline was linked to an increased total amount and log10-transformed rate of inactivation (R2 = 0.234 and 0.585, respectively). However, when the rate of inactivation of E. coli was normalized for the effect of temperature (0 to 47°C), using the Arrhenius model given by equation 2, and plotted against the rate of reduction in pH during fermentation, the correlation between the two parameters was greatly reduced (R2 = 0.266). Furthermore, the relationship between the two parameters was reversed (i.e., an increasing rate of reduction in pH correlated with a decreasing rate of E. coli inactivation after normalizing data for the effect of temperature). These three relationships are shown in Fig. 4. Whether data analysis used the rate of inactivation or log10-transformed rates, the results were qualitatively similar.
TABLE 2.
Effect of pH on inactivation of E. coli in fermented meats and analogous aqueous systems from linear regression analyses of inactivation versus pH variables
| y axis | x axis | No. of data points | Linear regression equation | R2 |
|---|---|---|---|---|
| Total inactivation (log10 CFU/unit) | Final pH | 360 | y = −0.8647x + 5.9447 | 0.0698 |
| Reduction in pHa | 163 | y = 1.6092x − 0.0217 | 0.0965 | |
| Rate of reduction in pHa,b | 163 | y = 27.8847x + 0.8466 | 0.2343 | |
| Log10-transformed inactivation rate (log10 CFU/unit/h) | Final pH | 360 | y = −0.3123x − 0.3410 | 0.0265 |
| Reduction in pHa | 149 | y = 0.3346x − 2.0538 | 0.0374 | |
| Rate of reduction in pHa,b | 149 | y = 14.7078x − 2.1350 | 0.5851 | |
| Temperature residualsc | Final pH | 352 | y = 0.2306x − 1.0065 | 0.0127 |
| Reduction in pHa | 149 | y = −0.6221x − 0.8302 | 0.0483 | |
| Rate of reduction in pHa,b | 149 | y = −16.2437x − 1.0065 | 0.2663 |
During fermentation.
pH units/h.
Data were normalized for the effect of temperature by using the Arrhenius model: ln[inactivation rate (log10 CFU/unit/h)] = 30.974 − 10,483 × [1/absolute temperature (K)] (equation 2).
FIG. 4.
Effect of rate of reduction in pH during fermentation on total amount (a) and rate of inactivation (b) of E. coli and on inactivation rate normalized for temperature in the range of 0 to 47°C (c), using equation 2, to assess the cryptic effect of temperature in this analysis. Simple linear regression fitted to the data is given by the lines, which fit the equations y = 27.8847x + 0.8466 (R2 = 0.234), y = 14.7078x − 2.1350 (R2 = 0.585), and y = −16.2437x − 1.0065 (R2 = 0.266), respectively.
Effect of aw.
The effect of aw on the inactivation of E. coli in fermented meats and analogous aqueous systems was analyzed by plotting the total amount or log10-transformed rate of inactivation of E. coli against various aw variables (final aw of any process, the reduction in aw during drying or another postfermentation process, and the rate of reduction in aw during drying or another postfermentation process). As shown in Table 3, simple linear regression analysis illustrated a correlation between the log10-transformed rate of reduction in aw and the rate of E. coli inactivation that occurred during drying, storage, or heating (R2 = 0.482), whereas the same analysis of all other plots showed that the effect of aw accounted for less than 8% of the E. coli inactivation data (R2 < 0.08). However, the apparent effect of the rate of aw reduction during drying or another postfermentation process was found to be highly dependent on the temperature of the process, since normalizing the inactivation rate data for the effect of temperature (0 to 47°C), using the method described above, reduced the R2 value to 0.169 based on analysis of 56 data. As with the pH data, normalizing for the effect of temperature revealed the inverse relationship between the rate of reduction in aw and the rate of inactivation (Table 3). Again, linear regression analyses of the inactivation rate or log10-transformed inactivation rate were qualitatively similar.
TABLE 3.
Effect of aw on inactivation of E. coli in fermented meats and analogous aqueous systems from linear regression analyses of inactivation versus aw variables
| y axis | x axis | No. of data points | Linear regression equation | R2 |
|---|---|---|---|---|
| Total inactivation (log10 CFU/unit) | Final aw | 278 | y = −10.1371x + 11.0802 | 0.0789 |
| Reduction in awa | 102 | y = 0.0537x + 2.2396 | 0.00004 | |
| Rate of reduction in awa,b | 64 | y = 6.2464x + 2.1500 | 0.0005 | |
| Log10-transformed inactivation rate (log10 CFU/unit/h) | Final aw | 294 | y = −2.0387x − 0.2363 | 0.0206 |
| Reduction in awa | 102 | y = 0.3418x − 2.5608 | 0.0123 | |
| Rate of reduction in awa,b | 64 | y = 87.4534x − 2.5630 | 0.4823 | |
| Temperature residualsc | Final aw | 286 | y = 7.1086x − 6.2635 | 0.1422 |
| Reduction in awa | 94 | y = −1.1016x + 0.4178 | 0.0753 | |
| Rate of reduction in awa,b | 56 | y = −744.3522x + 0.3722 | 0.1685 |
During drying or another postfermentation process (i.e., storage and heating).
aw units/h.
Data were normalized for the effect of temperature by using the Arrhenius model: ln[inactivation rate (log10 CFU/unit/h)] = 30.974 − 10,483 × [1/absolute temperature (K)] (equation 2).
DISCUSSION
The studies analyzed in this investigation for temperatures in the range of 0 to 47°C are based on combinations of pH, aw, and other factors that alone or in combination cause the inactivation of E. coli in fermented meats, irrespective of temperature. For example, the lower pH and aw limits and upper undissociated lactic acid limit for growth of E. coli are approximately 4, 0.95, and 11 mM, respectively (33, 48, 52). Combinations of these and other factors that together preclude E. coli growth can be estimated by growth/no-growth models (e.g., see the work of Presser et al. [48]). At temperatures above approximately 47°C and below 0°C, additional lethal factors (i.e., heat and freeze-thaw injury, respectively) may act upon E. coli cells to cause their more rapid inactivation.
In this study, a model based on two different Arrhenius equations (equations 2 and 3) for the effect of temperature on E. coli inactivation rate in the ranges above 47°C and from 0 to 47°C provided a better description of the data than did equation 1, a purely empirical, parsimonious model generated to describe all the data. This was despite the fact that equation 1 was both fitted to and evaluated against the 484 inactivation rate data first collated (Fig. 1), while equations 2 and 3 were fitted to a reduced and an expanded data set, respectively (Fig. 3), but were evaluated against the 484 data originally collated. Use of the Arrhenius model in this approach is consistent with established principles of thermal inactivation kinetics. The quite different “(in)activation energies” predicted from equations 2 and 3 and the different z values estimated from the data reiterate that different mechanisms of inactivation are involved at temperatures above 47°C, presumed to be “thermal” inactivation involving macromolecular denaturation, and those in the range of 0 to 47°C. In this modeling, the effect of freeze-thaw injury on apparent inactivation rate was not specifically considered.
In many of the studies included in this meta-analysis, it was observed that lower levels of pH (4-7, 11, 14, 49) and aw (49, 57) are associated with greater reductions in E. coli in fermented meats. However, from our analyses of the collated data, while inactivation of E. coli ensues when environmental factors preclude growth, further decreases in pH or aw do not appear to greatly hasten inactivation. Instead, temperature has the dominant effect on inactivation rate, with higher temperatures associated with faster inactivation. Others have reported a similar correlation between increased temperature and enhanced inactivation (5, 12, 14, 23-25, 45, 57, 61, 62), implying that although temperatures of fermented meat processes do not necessarily cause the destruction of E. coli cells, the temperatures of fermentation, maturation, and storage are important explanatory variables of the inactivation rate, and thus of the total inactivation observed.
From our analyses involving observations at pHs ranging from 2.8 to 6.14, at aw values ranging from 0.75 to 0.986, and with various bacterial serotypes, strains, and product formulations, temperature (in the range of 0 to 47°C) accounted for 61% of the variance in the inactivation rate data. In contrast, the pH or aw measured in broth or at the end of a given fermented meat manufacturing process accounted for less than 8% of the variability in the inactivation data. The apparent influence of other pH and aw variables tested (i.e., the total decrease during fermentation or drying and other postfermentation processes, and also the rate of reduction) appeared to be largely dependent on the temperature of the process and its influence on those variables, and when the effect of temperature was accounted for, these pH and aw variables were relatively poor explanatory variables. The remaining variability in the inactivation rate data is likely due to differences between studies, such as specific strains, ingredients, and processing conditions. The fact that this background noise in the collated data is greater than any systematic effect of pH or aw suggests that differences due to such “uncontrolled” factors are as influential, or more so, than the effect of pH or aw on the inactivation of E. coli in fermented meats.
The above conclusion seems counter to the expectation, and reported experience, that pH and aw are significant effectors of inactivation of pathogens in nonthermally processed foods. To further investigate this, we examined the relationship between inactivation rate and pH or aw at the end of a specified process (or in an aqueous system) for the individual studies collated. Data were derived from individual studies that provided multiple inactivation rate estimates at a single temperature, but with various pH or aw values, where variance was greater than the assumed measurement error of 0.1 pH unit and 0.01 aw unit. Linear regression fitted to the data indicated that the inactivation rate increased with decreasing pH and decreasing aw, as previously described, for only 73% (n = 15) and 62% (n = 13) of the data sets analyzed, respectively. This suggests that while more stringent levels of pH and aw may often be associated with faster inactivation of E. coli in fermented meats, their effect is, in some cases, overshadowed by other factors in the system, even when temperature is constant. Therefore, any effect of pH and aw in the combined data sets that we analyzed was presumably masked by the diversity in E. coli serotypes and strains, food and broth types, and processing conditions employed within the different studies. This was not the case, however, for the effect of temperature. Twenty studies included in the data set provided inactivation rate estimates for three or more temperatures in the range of −20 to 66°C, and in all cases, linear regression fitted to the data revealed that a higher temperature was associated with a greater rate of inactivation of E. coli.
Differences in the physiological state of the cell, which can affect survival time in lethal environments, might also account for the apparent insensitivity of E. coli to increasingly severe levels of pH and aw, revealed in the current investigation, and the variability in responses observed in individual studies described above. Shadbolt et al. (57) showed that inactivation of E. coli by aw values in the range of 0.75 to 0.95, adjusted with NaCl, was biphasic. While the rate of inactivation and the total inactivation observed during the first phase were strongly dependent on the severity of the aw stress, the rate of inactivation during the second phase was essentially the same for all aw levels. The same effect was observed in exponentially growing E. coli cells exposed to inimical pHs, set by HCl, in the range of 2.5 to 3.5 (3), and other researchers have demonstrated an insensitivity to levels of nonthermal environmental stresses in E. coli, Listeria monocytogenes, and Yersinia enterocolitica (40). These findings suggest that, with time, cells become increasingly resistant to unfavorable conditions, which is reflected in a reduced inactivation rate. This supports a considerable body of scientific knowledge pertaining to bacterial stress responses that has been reviewed extensively (1, 26, 59).
In fermented meat batters, initial pH and aw are not usually sufficient to inactivate E. coli cells. Rather, they decline over time as the temperature of the batter increases and fermentation proceeds, with pH, lactic acid, and/or aw eventually attaining levels that prevent pathogen growth. During this time, at the commencement of fermentation, it is possible that the cells respond to the increasingly harsh environment by initiating stress responses. This is supported by the data of Leyer et al. (39) for the inactivation of acid-adapted and nonadapted E. coli during sausage fermentation. Initially, the nonadapted cells were inactivated more rapidly than the acid-adapted cells. However, at later stages of fermentation, the rates of inactivation of both populations were highly comparable. The ability of cells to adapt to an increasingly stringent environment during fermented meat manufacture is further supported by the predominance of single-phase, log-linear inactivation curves seen in the current study. We hypothesize that the observed rate of inactivation is that which is characteristic of the second, slower phase associated with biphasic kinetics, whose rate is likely to be largely unaffected by the severity of the pH and aw stress applied. The exact effect of pH or aw on E. coli inactivation in this case would be dependent on the rate of change of these factors during processing, specifically, the time taken for inimical conditions to be reached and for inactivation to commence. This will, in turn, depend on the specific growth limits and stress responses of bacterial serotypes and strains and on other environmental factors that affect this. We are currently conducting further investigations to quantify the effects of pH and aw on E. coli inactivation in fermented meats and broth model systems, with reference to the hypotheses described here.
To meet the intrinsic limitations of starter cultures and to avoid undesirable textural damage, temperatures used in the manufacture of fermented meats rarely exceed 50°C, despite the fact that high-temperature processing has been shown empirically to be a powerful means of reducing pathogen numbers in some products (30). From our analysis, the relationship between temperature and E. coli inactivation appears to be altered at temperatures above approximately 47°C, with increasing temperature more substantially accelerating inactivation in the higher temperature range, as evidenced by the change in z value from 19.2°C for temperatures in the range of 0 to 47°C to 9.7°C for temperatures above 50°C. Although this observation is based on a limited number of fermented meat data for temperatures above 50°C, reflecting the infrequent use of high temperatures in fermented meat processes, our analysis of thermal inactivation (i.e., >50°C) of E. coli in fresh meats supports this conclusion and is consistent with the effect of temperatures above 47°C on the inactivation rate of E. coli in fermented meats (Fig. 3). We propose that at temperatures above the maximum for growth of E. coli, thermal injury (e.g., denaturation of macromolecules) becomes significant and becomes the principle mechanism causing E. coli inactivation. The exact delineation between growth-permissive and lethal temperatures could not be discerned from the current analysis and is likely to reflect the growth-no-growth boundaries for individual strains. However, based on reports of growth of most E. coli strains at temperatures up to 47°C (33) and the demonstration of growth at temperatures up to approximately 50°C (32, 52), the changing effect of temperature on the E. coli inactivation rate is likely to occur somewhere between 45 and 50°C. Similarly, at temperatures below freezing, the growth of E. coli is prevented and freeze injury may function as a further barrier to cell survival. This might account for the higher rate of inactivation at −20°C observed in the study of Faith et al. (24) than that predicted by the Arrhenius model (equation 2) based on temperatures in the growth-permissive range.
By this meta-analysis, the relative influences of temperature, pH, and aw on the inactivation of E. coli in fermented meats were determined. Although other characteristics of fermented meats, such as lactic acid and nitrite concentrations, also affect the survival of E. coli, their influence could not be assessed in this study due to a lack of relevant information provided in the scientific literature used. For example, in a fermented meat product, acidification is due to the formation of organic acids, particularly lactic acid. In the presence of organic acid, the inhibitory effects of pH on microorganisms are accentuated in a manner related to the concentration of undissociated acid, which is itself a function of pH (as described by the Henderson-Hasselbalch equation). Although we recorded the concentration of lactic acid where it was reported, this information was provided only rarely. Those data were insufficient to enable reliable differentiation of the effects of acid, i.e., [H+], from those due to undissociated organic acid. The same limitation applied to the investigation of the contribution of nitrite. The data gaps identified here indicate specific parameters that require systematic studies in order to evaluate their influence on E. coli inactivation in fermented meats.
The results of the present investigation have revealed the dominance of nonlethal temperature as an explanatory variable for the inactivation of E. coli in fermented meats and illustrate that the inactivation of E. coli in these products will be enhanced best by holding products at higher temperatures for increased times. While we recognize that growth-preventative factors, such as low pH and aw, are crucial for causing the inactivation of cells, once these parameters (alone or in combination) have reached inimical levels, it is increases in the time and temperature of manufacture that will most dramatically improve product safety by enhancing the inactivation of E. coli. Arrhenius models fitted to the combined data set (Fig. 1) and to individual studies (Fig. 2) where inactivation rates were determined at three or more temperatures in the range of 0 to 47°C suggest a strong and consistent effect of temperature. Therefore, we propose that the Arrhenius model developed in this study (equation 2) can be used to make a conservative estimate of the amount of inactivation of E. coli that will occur during fermented meat processing based on temperatures in the range of 0 to 47°C. Although its use to make absolute predictions of inactivation is limited by the wide confidence intervals of the fitted equations, it is a valuable tool for assessing the relative changes in product safety associated with changes in temperature and time of fermentation and maturation. An earlier version of this temperature-only model is now used for this purpose within the Australian and New Zealand food industries by both regulators and manufacturers. The model and the report of Ross and Shadbolt (51) can be downloaded from http://www.foodsafetycentre.com.au/fermenter.php.
Studies pertaining to E. coli survival during fermented meat manufacture have typically focused on the effects of various lethal factors, such as low pH and aw and the presence of lactic acid and nitrites. While maintenance of moderate temperatures has long been recognized as a critical processing parameter in achieving desirable sensory qualities in fermented meats (i.e., to prevent defects due to the melting, and separating out, of fat particles), this study is the first to quantify the effect of nonlethal temperature on E. coli under otherwise inimical conditions relevant to fermented meats and to recognize its significant effect relative to further reductions in lethal pH and aw. The nonbactericidal temperatures of fermented meat manufacture may influence the survival of E. coli directly, by altering processes within the cell, and indirectly, by affecting the activity of lactic acid bacteria and hydrogen ion production during fermentation or the rate and amount of moisture loss during maturation. Whatever the mechanism, this research highlights the usefulness of considering factors that are in themselves nonlethal to bacterial pathogens in assessing the safety of food processing regimens.
Acknowledgments
This study was funded by Meat and Livestock Australia. Their support, in particular that of Ian Jenson, Manager of the Food Safety and Strategic Science program, is gratefully acknowledged.
We thank David Ratkowsky for his valuable comments on model development and his critical review of the manuscript.
Footnotes
Published ahead of print on 18 September 2009.
REFERENCES
- 1.Aertsen, A., and C. W. Michiels. 2004. Stress and how bacteria cope with death and survival. Crit. Rev. Microbiol. 30:263-273. [DOI] [PubMed] [Google Scholar]
- 2.Al-Nabulsi, A. A., and R. A. Holley. 2007. Effects on Escherichia coli O157:H7 and meat starter cultures of bovine lactoferrin in broth and microencapsulated lactoferrin in dry sausage batters. Int. J. Food Microbiol. 113:84-91. [DOI] [PubMed] [Google Scholar]
- 3.Brown, J. L. 2002. Kinetics and mechanisms of the low pH-induced inactivation of Escherichia coli. Ph.D. thesis. University of Tasmania, Hobart, Australia.
- 4.Calicioglu, M., N. G. Faith, D. R. Buege, and J. B. Luchansky. 1997. Viability of Escherichia coli O157:H7 in fermented semidry low-temperature-cooked beef summer sausage. J. Food Prot. 60:1158-1162. [DOI] [PubMed] [Google Scholar]
- 5.Calicioglu, M., N. G. Faith, D. R. Buege, and J. B. Luchansky. 2001. Validation of a manufacturing process for fermented, semidry Turkish soudjouk to control Escherichia coli O157:H7. J. Food Prot. 64:1156-1161. [DOI] [PubMed] [Google Scholar]
- 6.Calicioglu, M., N. G. Faith, D. R. Buege, and J. B. Luchansky. 2002. Viability of Escherichia coli O157:H7 during manufacturing and storage of a fermented, semidry soudjouk-style sausage. J. Food Prot. 65:1541-1544. [DOI] [PubMed] [Google Scholar]
- 7.Casey, P., and S. Condon. 2000. Synergistic lethal combination of nitrite and acid pH on a verotoxin-negative strain of Escherichia coli O157. Int. J. Food Microbiol. 55:255-258. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1995. Community outbreak of hemolytic uremic syndrome attributable to Escherichia coli O111:NM—South Australia, 1995. MMWR Morb. Mortal. Wkly. Rep. 44:550-551. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1995. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami—Washington and California, 1994. MMWR Morb. Mortal. Wkly. Rep. 44:157-160. [PubMed] [Google Scholar]
- 10.Chacon, P. A., P. Muthukumarasamy, and R. A. Holley. 2006. Elimination of Escherichia coli O157:H7 from fermented dry sausages at an organoleptically acceptable level of microencapsulated allyl isothiocyanate. Appl. Environ. Microbiol. 72:3096-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chikthimmah, N., R. C. Anantheswaran, R. F. Roberts, E. W. Mills, and S. J. Knabel. 2001. Influence of sodium chloride on growth of lactic acid bacteria and subsequent destruction of Escherichia coli O157:H7 during processing of Lebanon bologna. J. Food Prot. 64:1145-1150. [DOI] [PubMed] [Google Scholar]
- 12.Chikthimmah, N., and S. J. Knabel. 2001. Survival of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in and on vacuum packaged Lebanon bologna stored at 3.6 and 13.0°C. J. Food Prot. 64:958-963. [DOI] [PubMed] [Google Scholar]
- 13.Chirife, J., and S. L. Resnik. 1984. Unsaturated solutions of sodium chloride as reference sources of water activity at various temperatures. J. Food Sci. 49:1486-1488. [Google Scholar]
- 14.Clavero, M. R. S., and L. R. Beuchat. 1996. Survival of Escherichia coli O157:H7 in broth and processed salami as influenced by pH, water activity, and temperature and suitability of media for its recovery. Appl. Environ. Microbiol. 62:2735-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavero, M. R. S., L. R. Beuchat, and M. P. Doyle. 1998. Thermal inactivation of Escherichia coli O157:H7 isolated from ground beef and bovine feces, and suitability of media for enumeration. J. Food Prot. 61:285-289. [DOI] [PubMed] [Google Scholar]
- 16.Cosansu, S., and K. Ayhan. 2000. Survival of enterohaemorrhagic Escherichia coli O157:H7 strain in Turkish soudjouck during fermentation, drying and storage periods. Meat Sci. 54:407-411. [DOI] [PubMed] [Google Scholar]
- 17.Duffy, G., D. C. R. Riordan, and J. J. Sheridan. 1999. Development of a critical control step for Escherichia coli O157:H7 in pepperoni. The Irish Agriculture and Food Development Authority, Dublin, Ireland.
- 18.Duffy, G., D. C. R. Riordan, J. J. Sheridan, B. S. Eblen, R. C. Whiting, I. S. Blair, and D. A. McDowell. 1999. Differences in thermotolerance of various Escherichia coli O157:H7 strains in a salami matrix. Food Microbiol. 16:83-91. [Google Scholar]
- 19.Duffy, L. L., F. H. Grau, and P. B. Vanderlinde. 2000. Acid resistance of enterohaemorrhagic and generic Escherichia coli associated with foodborne disease and meat. Int. J. Food Microbiol. 60:83-89. [DOI] [PubMed] [Google Scholar]
- 20.Duffy, L. L., and P. B. Vanderlinde. 2000. Escherichia coli and salami manufacture—meeting the challenge of the ANZFA requirements. Food Aust. 52:269-270. [Google Scholar]
- 21.Ellajosyula, K. R., S. Doores, E. W. Mills, R. A. Wilson, R. C. Anantheswaran, and S. J. Knabel. 1998. Destruction of Escherichia coli O157:H7 and Salmonella Typhimurium in Lebanon bologna by interaction of fermentation pH, heating temperature, and time. J. Food Prot. 61:152-157. [DOI] [PubMed] [Google Scholar]
- 22.Erkkila, S., M. Venalainen, S. Hielm, E. Petaja, E. Puolanne, and T. Mattila-Sandholm. 2000. Survival of Escherichia coli O157:H7 in dry sausage fermented by probiotic lactic acid bacteria. J. Sci. Food Agric. 80:2101-2104. [Google Scholar]
- 23.Faith, N. G., N. Parniere, T. Larson, T. D. Lorang, C. W. Kaspar, and J. B. Luchansky. 1998. Viability of Escherichia coli O157:H7 in salami following conditioning of batter, fermentation and drying of sticks, and storage of slices. J. Food Prot. 61:377-382. [DOI] [PubMed] [Google Scholar]
- 24.Faith, N. G., N. Parniere, T. Larson, T. D. Lorang, and J. B. Luchansky. 1997. Viability of Escherichia coli O157:H7 in pepperoni during the manufacture of sticks and the subsequent storage of slices at 21, 4 and −20°C under air, vacuum and CO2. Int. J. Food Microbiol. 37:47-54. [DOI] [PubMed] [Google Scholar]
- 25.Faith, N. G., R. K. Wierzba, A. M. Ihnot, A. M. Roering, T. D. Lorang, C. W. Kaspar, and J. B. Luchansky. 1998. Survival of Escherichia coli O157:H7 in full and reduced-fat pepperoni after manufacture of sticks, storage of slices at 4°C or 21°C under air and vacuum, and baking of slices on frozen pizza at 135, 191 and 246°C. J. Food Prot. 61:383-389. [DOI] [PubMed] [Google Scholar]
- 26.Finkel, S. E. 2006. Long-term survival during stationary phase: evolution and the GASP phenotype. Nat. Rev. Microbiol. 4:113-120. [DOI] [PubMed] [Google Scholar]
- 27.Getty, K. J. K., R. K. Phebus, J. L. Marsden, J. R. Schwenke, and C. L. Kastner. 1999. Control of Escherichia coli O157:H7 in large (115 mm) and intermediate (90 mm) diameter Lebanon-style bologna. J. Food Sci. 64:1100-1107. [Google Scholar]
- 28.Glass, K. A., J. M. Loeffelholz, J. P. Ford, and M. P. Doyle. 1992. Fate of Escherichia coli O157:H7 as affected by pH or sodium chloride and in fermented, dry sausage. Appl. Environ. Microbiol. 58:2513-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grau, F. H. 1996. Survival of enterohaemorrhagic Escherichia coli during the production of fermented meats. Meat Research Corporation and Pig Research and Development Corporation, Sydney, Australia.
- 30.Hinkens, J. C., N. G. Faith, T. D. Lorang, P. Bailey, D. Buege, C. W. Kaspar, and J. B. Luchansky. 1996. Validation of pepperoni processes for control of Escherichia coli O157:H7. J. Food Prot. 59:1260-1266. [DOI] [PubMed] [Google Scholar]
- 31.Huang, L. H., and V. K. Juneja. 2003. Thermal inactivation of Escherichia coli O157:H7 in ground beef supplemented with sodium lactate. J. Food Prot. 66:664-667. [DOI] [PubMed] [Google Scholar]
- 32.Ingraham, J. L., and A. G. Marr. 1996. Effect of temperature, pressure, pH and osmotic stress on growth, p. 1570-1578. In F. G. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC.
- 33.International Commission for the Microbiological Specifications for Foods. 1996. Microorganisms in foods 5—specifications of food pathogens. Blackie Academic & Professional, London, United Kingdom.
- 34.Juneja, V. K., and J. S. Novak. 2003. Heat resistance of Escherichia coli O157:H7 in cook-in-bag ground beef as affected by pH and acidulant. Int. J. Food Sci. Technol. 38:297-304. [Google Scholar]
- 35.Kang, D. H., and D. Y. C. Fung. 1999. Effect of diacetyl on controlling Escherichia coli O157:H7 and Salmonella Typhimurium in the presence of starter culture in a laboratory medium and during meat fermentation. J. Food Prot. 62:975-979. [DOI] [PubMed] [Google Scholar]
- 36.Kang, D. H., and D. Y. C. Fung. 1999. Reduction of Escherichia coli O157:H7 by stimulated Pediococcus acidilactici. Lett. Appl. Microbiol. 29:206-210. [DOI] [PubMed] [Google Scholar]
- 37.Kang, D. H., and D. Y. C. Fung. 2000. Stimulation of starter culture for further reduction of foodborne pathogens during salami fermentation. J. Food Prot. 63:1492-1495. [DOI] [PubMed] [Google Scholar]
- 38.Lahti, E., T. Johansson, T. Honkanen-Buzalski, P. Hill, and E. Nurmi. 2001. Survival and detection of Escherichia coli O157:H7 and Listeria monocytogenes during the manufacture of dry sausage using two different starter cultures. Food Microbiol. 18:75-85. [Google Scholar]
- 39.Leyer, G. J., L. L. Wang, and E. A. Johnson. 1995. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl. Environ. Microbiol. 61:3752-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindqvist, R., and M. Lindblad. 2009. Inactivation of Escherichia coli, Listeria monocytogenes and Yersinia enterocolitica in fermented sausages during maturation/storage. Int. J. Food Microbiol. 129:59-67. [DOI] [PubMed] [Google Scholar]
- 41.McKellar, R. C., and K. P. Knight. 1999. Growth and survival of various strains of enterohemorrhagic Escherichia coli in hydrochloric and acetic acid. J. Food Prot. 62:1466-1469. [DOI] [PubMed] [Google Scholar]
- 42.McQuestin, O. J., T. A. McMeekin, and T. Ross. 2006. Effect of suspension media on nonthermal inactivation of Escherichia coli. Lett. Appl. Microbiol. 43:523-527. [DOI] [PubMed] [Google Scholar]
- 43.Murphy, R. Y., E. M. Martin, L. K. Duncan, B. L. Beard, and J. A. Marcy. 2004. Thermal process validation for Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in ground turkey and beef products. J. Food Prot. 67:1394-1402. [DOI] [PubMed] [Google Scholar]
- 44.Muthukumarasamy, P., and R. A. Holley. 2007. Survival of Escherichia coli O157:H7 in dry fermented sausages containing micro-encapsulated probiotic lactic acid bacteria. Food Microbiol. 24:82-88. [DOI] [PubMed] [Google Scholar]
- 45.Nissen, H., and A. Holck. 1998. Survival of Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Kentucky in Norwegian fermented, dry sausage. Food Microbiol. 15:273-279. [Google Scholar]
- 46.Normanno, G., A. Dambrosio, A. Parisi, N. C. Quaglia, L. Laporta, and G. Celano. 2002. Survival of Escherichia coli O157:H7 in a short ripened fermented sausage. Ital. J. Food Sci. 14:181-185. [Google Scholar]
- 47.Pidcock, K., G. M. Heard, and A. Henriksson. 2002. Application of nontraditional meat starter cultures in production of Hungarian salami. Int. J. Food Microbiol. 76:75-81. [DOI] [PubMed] [Google Scholar]
- 48.Presser, K. A., D. A. Ratkowsky, and T. Ross. 1997. Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl. Environ. Microbiol. 63:2355-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riordan, D. C. R., G. Duffy, J. J. Sheridan, B. S. Eblen, R. C. Whiting, I. S. Blair, and D. A. McDowell. 1998. Survival of Escherichia coli O157:H7 during the manufacture of pepperoni. J. Food Prot. 61:146-151. [DOI] [PubMed] [Google Scholar]
- 50.Ross, T. 1996. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 81:501-508. [DOI] [PubMed] [Google Scholar]
- 51.Ross, T., and C. T. Shadbolt. 2001. Predicting Escherichia coli inactivation in uncooked comminuted fermented meat products. Meat and Livestock Australia, Sydney. http://www.foodsafetycentre.com.au/fermenter.php.
- 52.Salter, M., T. Ross, and T. A. McMeekin. 1998. Applicability of models for non-pathogenic Escherichia coli for predicting the growth of pathogenic Escherichia coli. J. Appl. Microbiol. 85:357-384. [DOI] [PubMed] [Google Scholar]
- 53.Samelis, J., A. Kakouri, I. N. Savvaidis, K. Riganakos, and M. G. Kontominas. 2005. Use of ionizing radiation doses of 2 and 4 kGy to control Listeria spp. and Escherichia coli O157:H7 on frozen meat trimmings used for dry fermented sausage production. Meat Sci. 70:189-195. [DOI] [PubMed] [Google Scholar]
- 54.Schimmer, B., K. Nygard, H. M. Eriksen, J. Lassen, B.-A. Lindstedt, L. T. Brandal, G. Kapperud, and P. Aavitsland. 2008. Outbreak of haemolytic uraemic syndrome in Norway caused by stx2-positive Escherichia coli O103:H25 traced to cured mutton sausages. BMC Infect. Dis. 8:41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semanchek, J. J., and D. A. Golden. 1998. Influence of growth temperature on inactivation and injury of Escherichia coli O157:H7 by heat, acid, and freezing. J. Food Prot. 61:395-401. [DOI] [PubMed] [Google Scholar]
- 56.Shadbolt, C. T. 2004. Non-thermal inactivation of Escherichia coli under conditions relevant to the production of uncooked comminuted fermented meats. Ph.D. thesis. University of Tasmania, Hobart, Australia.
- 57.Shadbolt, C. T., T. Ross, and T. A. McMeekin. 1999. Nonthermal death of Escherichia coli. Int. J. Food Microbiol. 49:129-138. [DOI] [PubMed] [Google Scholar]
- 58.Shadbolt, C. T., T. Ross, and T. A. McMeekin. 2001. Differentiation of the effects of lethal pH and water activity: food safety implications. Lett. Appl. Microbiol. 32:99-102. [DOI] [PubMed] [Google Scholar]
- 59.Storz, G., and R. Hengge-Aronis (ed.). 2000. Bacterial stress responses. ASM Press, Washington, DC.
- 60.Tomicka, A., J. R. Chen, S. Barbut, and M. W. Griffiths. 1997. Survival of bioluminescent Escherichia coli O157:H7 in a model system representing fermented sausage production. J. Food Prot. 60:1487-1492. [DOI] [PubMed] [Google Scholar]
- 61.Uyttendaele, M., I. Taverniers, and J. Debevere. 2001. Effect of stress induced by suboptimal growth factors on survival of Escherichia coli O157:H7. Int. J. Food Microbiol. 66:31-37. [DOI] [PubMed] [Google Scholar]
- 62.Uyttendaele, M., S. Vankeirsbilck, and J. Debevere. 2001. Recovery of heat-stressed Escherichia coli O157:H7 from ground beef and survival of Escherichia coli O157:H7 in refrigerated and frozen ground beef and in fermented sausage kept at 7°C and 22°C. Food Microbiol. 18:511-519. [Google Scholar]
- 63.Vanderlinde, P. B. 1999. Escherichia coli in uncooked fermented meat products. Meat and Livestock Australia, Canberra.
- 64.Williams, R. C., S. Isaacs, M. L. Decou, E. A. Richardson, M. C. Buffett, R. W. Slinger, M. H. Brodsky, B. W. Ciebin, A. Ellis, and J. Hockin. 2000. Illness outbreak associated with Escherichia coli O157:H7 in Genoa salami. Can. Med. Assoc. J. 162:1409-1413. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao, T., M. P. Doyle, M. C. Kemp, R. S. Howell, and P. Zhao. 2004. Influence of freezing and freezing plus acidic calcium sulfate and lactic acid addition on thermal inactivation of Escherichia coli O157:H7 in ground beef. J. Food Prot. 67:1760-1764. [DOI] [PubMed] [Google Scholar]