Abstract
Anthrax is a zoonotic disease caused by the gram-positive spore-forming bacterium Bacillus anthracis. Human infection occurs after the ingestion, inhalation, or cutaneous inoculation of B. anthracis spores. The subsequent progression of the disease is largely mediated by two native virulence plasmids, pXO1 and pXO2, and is characterized by septicemia, toxemia, and meningitis. In order to produce meningitis, blood-borne bacteria must interact with and breach the blood-brain barrier (BBB) that is composed of a specialized layer of brain microvascular endothelial cells (BMEC). We have recently shown that B. anthracis Sterne is capable of penetrating the BBB in vitro and in vivo, establishing the classic signs of meningitis; however, the molecular mechanisms underlying the central nervous system (CNS) tropism are not known. Here, we show that attachment to and invasion of human BMEC by B. anthracis Sterne is mediated by the pXO1 plasmid and an encoded envelope factor, BslA. The results of studies using complementation analysis, recombinant BslA protein, and heterologous expression demonstrate that BslA is both necessary and sufficient to promote adherence to brain endothelium. Furthermore, mice injected with the BslA-deficient strain exhibited a significant decrease in the frequency of brain infection compared to mice injected with the parental strain. In addition, BslA contributed to BBB breakdown by disrupting tight junction protein ZO-1. Our results identify the pXO1-encoded BslA adhesin as a critical mediator of CNS entry and offer new insights into the pathogenesis of anthrax meningitis.
Bacillus anthracis, the etiologic agent of anthrax, is a gram-positive spore-forming bacterium that is commonly found in soil (29). The bacterium can infect animals and humans by ingestion, inhalation, or cutaneous inoculation of B. anthracis spores (8). Spores are taken up by resident macrophages that migrate to the lymph nodes (15). Here, the spores germinate into vegetative bacteria, multiply, and then disseminate throughout the host, causing septicemia and toxemia (8). Systemic disease can be complicated by the onset of a fulminant and rapidly fatal hemorrhagic meningitis and meningoencephalitis (27). Anthrax meningitis is associated with a high mortality rate despite intensive antibiotic therapy (24). Biopsy studies after an outbreak of inhalational anthrax and experimental studies of inhalational infection in rhesus monkeys demonstrated the presence of bacilli in the central nervous system (CNS) and pathologies consistent with suppurative and hemorrhagic meningitis in the majority of cases (1, 12). The intentional release of B. anthracis spores (19) during the 2001 bioterrorism event resulted in a case of meningitis (19), necessitating a need for a better understanding of the pathogenesis of anthrax meningitis and CNS infection.
To cause meningitis, blood-borne bacteria must interact with and breach the blood-brain barrier (BBB). The majority of the BBB is anatomically represented by the cerebral microvascular endothelium; brain microvascular endothelial cells (BMEC) are joined by tight junctions and display a paucity of pinocytosis, thereby effectively limiting the passage of substances and maintaining the CNS microenvironment (4, 5). Despite its highly restrictive nature, certain bacterial pathogens are still able to penetrate the BBB and gain entry into the CNS. The presence of bacilli in the brains of patients (1, 24) and in experimental models of anthrax infection (42, 44) suggests that vegetative B. anthracis cells are able to cross the BBB to initiate meningeal inflammation and the classic pathology associated with meningitis.
B. anthracis harbors two large virulence plasmids, pXO1 and pXO2 (8), which are required for full virulence, as strains lacking these plasmids are attenuated in animal models of infection (29). B. anthracis Sterne (pXO1+ pXO2−) has been utilized as a vaccine strain (41) but is still widely used in both in vitro and in vivo studies of anthrax infection since it causes lethal disease in mouse models of infection (46). Despite the crucial roles of pXO1 and pXO2 in anthrax disease pathogenesis, very few plasmid-encoded factors have been characterized. The best described are the antiphagocytic polyglutamyl capsule, encoded by biosynthetic enzymes on pXO2, and the anthrax toxin complex comprised of protective antigen, lethal factor (LF), and edema factor (EF), encoded by pXO1 (8, 29). Sequence analysis of the pXO1 plasmid revealed that the majority of plasmid-encoded factors, ∼70%, were of unknown function (31). More recently, in silico analysis identified novel pXO1-encoded proteins with immunogenic potential and relevance for pathogenesis. These included factors with putative adherent and invasive properties (2). Interestingly, two of the immunoreactive proteins were predicted surface layer (S-layer) proteins (2), one of which, B. anthracis S-layer protein A (BslA, pXO1-90), has recently been described and shown to mediate adherence of the vegetative form to host cells (20).
Using in vitro and in vivo model systems, we have recently shown that B. anthracis Sterne adheres to and invades brain endothelium (44). This interaction was partially dependent on the pXO1-encoded anthrax toxins; however, the molecular mechanisms that contribute to B. anthracis penetration of the BBB are currently unknown. In this study, we investigate the role of pXO1 in B. anthracis Sterne's interaction with brain endothelium and identify the encoded BslA adhesin as a critical mediator for BBB attachment and penetration during the pathogenesis of anthrax meningitis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Parental Bacillus anthracis Sterne (pXO1+ pXO2−) strains 34F2 (38) and 7702 (6), their mutant derivatives, and Bacillus thuringiensis HD1 (obtained from the Bacillus Genetic Stock Center, OH) (Table 1) were grown in brain heart infusion broth (Sigma) as shaken cultures under aerobic conditions at 37°C. The B. anthracis Sterne 34F2 pXO1− strain (provided by Mojgan Sabet and Donald Guiney, University of California, San Diego, CA) was cured of pXO1 by passage at 43°C using a method described previously (28). Plasmid loss was confirmed by PCR and Southern blot analysis. Log-phase cultures were grown in brain heart infusion broth to an optical density at 600 nm of 0.4 (1 × 107 CFU/ml for B. anthracis Sterne and its derivatives and 1 × 108 CFU/ml for B. thuringiensis). The toxin-deficient (ΔLF/EF) Sterne strain 7702, described previously (18), was generously provided by Scott Stibitz (Center for Biologics Evaluation and Research, Bethesda, MD). The growth kinetics of all strains were similar under the experimental conditions used in our assays (data not shown).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 | Invitrogen |
| TOP10 | araΔ139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 nupG | |
| Bacillus anthracis strains | ||
| Sterne 7702 | pXO1+ pXO2−; toxigenic, unencapsulated | 6 |
| Sterne 34F2 | pXO1+ pXO2−; toxigenic, unencapsulated bovine isolate | 38 |
| ΔLF/EF strain | 7702 with markerless deletion of lef and cya | 18 |
| ΔpXO1 strain | Sterne 34F2 pXO1− pXO2− | 44 |
| bslA::ΩSpr strain | 34F2 with in-frame allelic replacement of bslA by spectinomycin | 20 |
| bslA::ΩSpr pbslA strain | bslA::ΩSpr strain expressing bslA in pLM5 (pbslA) | 20 |
| bslA::ΩSpr pLM5 strain | bslA::ΩSpr strain expressing pLM5 | 20 |
| B. thuringiensis strains | ||
| HD1 | Bacillus thuringiensis subsp. kurstaki, wild-type isolate | Bacillus Stock Center |
| pUTE-bslA strain | HD1 expressing bslA in pUTE29 | This study |
| pUTE29 strain | HD1 expressing pUTE29 | This study |
| Plasmids | ||
| pLM5 | E. coli/B. anthracis shuttle vector, Kanr in B. anthracis | 20 |
| pblsA | pLM5 containing bslA | 20 |
| pUTE29 | E. coli/B. anthracis shuttle vector, Apr in E. coli and Tcr in B. anthracis | 22 |
| pUTE-bsla | pUTE29 containing bslA | This study |
| pbslA(260-652) | bslA codons 260 to 652 in pGEX-2TK | 20 |
Complementation and heterologous expression of BslA.
For complementation analysis, the full-length bslA gene was amplified from the chromosome and cloned into pLM5 as described previously (20). The resultant construct, pbslA, which carries bslA under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Pspac promoter, was used to transform the BslA-deficient bslA::ΩSpr strain. To induce BslA expression, the complemented strain was grown in the presence of 1 mM of IPTG at 30°C. For the heterologous expression of BslA in B. thuringiensis, bslA plus 150 base pairs of the upstream region was PCR amplified from chromosomal DNA using primers BslA-Fwd (5′-CCACCATTTAACCCACATTC-3′) and BslA-Rev (5′-AATGTTATAGATCAGGAGATTGGC-3′) and cloned into plasmid pUTE29 (22) to yield pUTE-bslA. The pUTE-bslA plasmid was introduced into B. thuringiensis by electroporation using an established protocol described for B. anthracis (22).
Endothelial cell culture.
The human BMEC (hBMEC) cell line, obtained from Kwang Sik Kim (Johns Hopkins University, Baltimore, MD), has been described previously (39, 40). hBMEC were cultured using RPMI 1640 (Gibco) supplemented with 10% fetal calf serum (Gibco), 10% Nuserum (BD Biosciences, San Jose, CA), and 1% modified Eagle's medium with nonessential amino acids (Gibco) without the addition of antibiotics. Cultures were incubated at 37°C with 5% CO2. Tissue culture flasks and 24-well plates were precoated with 1% rat tail collagen to support hBMEC monolayers.
hBMEC adherence and invasion assays.
B. anthracis Sterne, B. thuringiensis, and mutant/derivative strains were analyzed as described previously for their capacities to adhere to and invade hBMEC (9, 44). Briefly, hBMEC were seeded in collagen-coated 24-well tissue culture plates until they reached 90 to 100% confluence. Early-log-phase bacteria were pelleted, washed with 1× phosphate-buffered saline (PBS), and diluted in RPMI 1640-10% FBS. An inoculum of 1 × 105 CFU/well (multiplicity of infection [MOI] of 1 to 3) was added to hBMEC monolayers in a final volume of 500 μl. Plates were centrifuged at 800 × g for 5 min to synchronize the infection and subsequently incubated at 37°C with 5% CO2. After 45 min, hBMEC monolayers were washed five times with PBS to remove nonadherent bacilli. Monolayers were disrupted by the addition of 0.025% trypsin-EDTA-Triton X-100 solution, and the total number of surface-adherent (total cell associated) bacteria were quantified by plating serial dilutions on Todd Hewitt broth agar plates. To quantify the number of intracellular bacteria, hBMEC monolayers were incubated with bacteria for 2 h, followed by the addition of gentamicin (50 μg/ml) for 15 min to kill extracellular bacteria. The monolayers were washed three times with PBS and treated as described above to enumerate intracellular organisms. Data are expressed as the total cell-associated or intracellular CFU recovered compared to the input inoculum (MOI of 1, ∼1 × 105 CFU). All assays were performed at least in triplicate and repeated at least three times. The recombinant BslA protein fused to glutathione S-transferase (GST-BslA260-652), used for BslA protein competition assays, has been described previously (20). Confluent hBMEC monolayers were preincubated with 5 μM of purified GST or GST-BslA260-652 for 1 h, after which B. anthracis Sterne was added to the monolayers (MOI of 1 to 3). After 45 min, hBMEC monolayers were washed five times with PBS to remove nonadherent bacilli and disrupted by the addition of 0.025% trypsin-EDTA-Triton X-100 solution, and the total number of surface-adherent (total cell associated) bacteria was quantified as described above.
Gram stain and microscopy.
To visualize bacterial attachment, hBMEC monolayers were grown to 90 to 100% confluence on collagen-coated glass coverslips and infected with B. anthracis Sterne and isogenic mutants or B. thuringiensis (1 × 106 CFU) for 45 min at 37°C. Cells were washed five times with PBS and heat fixed. Gram staining was performed using standard methods. Slides were mounted in Cytoseal-60 (Thermo Scientific), and images were digitally collected on a Zeiss Axio inverted microscope equipped with a charge-coupled-device camera.
BslA protein purification.
BslA protein lacking amino acids 1 to 260 was expressed as a C-terminal fusion to GST in Escherichia coli TOP10 (Invitrogen) and purified using glutathione-Sepharose affinity chromatography as described previously (20), with minor modifications. Briefly, E. coli TOP10 strains expressing GST or GST-BslA260-652 were induced with 10 mM IPTG for 2.5 h. Following sonication and centrifugation of bacterial extracts, proteins in the supernatant were loaded onto glutathione-Sepharose 4B affinity columns and purified under native conditions. GST and GST-BslA260-652 were eluted with 20 mM glutathione and buffer exchanged in 1× PBS prior to use in cell assays.
Mouse model of hematogenous meningitis.
All animal experiments were approved by the Committee on the Use and Care of Animals and performed using accepted veterinary standards. Eight-week-old out-bred female CD-1 mice (Charles River Laboratories, Wilmington, MA) were injected intravenously with 0.1 ml of B. anthracis Sterne or the bslA::ΩSpr strain (4 × 104 CFU). Mice were monitored for signs of infection at least twice a day and euthanized at 48 h postinfection. Blood, brains, and kidneys were collected and plated to determine bacterial counts in each tissue. Half of the brain was stored in 4% paraformaldehyde for further histological analysis performed at the University of California San Diego Histopathology Core Facility (N. Varki, director).
Immunostaining.
To visualize tight junctions, hBMECs were grown to 100% confluence on collagen-coated glass coverslips and infected with B. anthracis Sterne and its isogenic mutants (1 × 105 CFU) for 4 h at 37°C. Cells were washed three times with PBS, fixed in 4% paraformaldehyde, and permeabilized in 0.25% Triton X-100. Cells were incubated in 2% goat serum for 1 h, followed by incubation with 5 μg/ml anti-ZO-1 antibody (Invitrogen) overnight at 4°C. Cells were washed three times in PBS and then incubated with 5 μg/ml of secondary antibody (Alexa Fluor 594-conjugated anti-rabbit) for 1 h at room temperature. Slides were mounted in Vectashield (Vector Laboratories), and images were digitally collected as stacks at a ×63 magnification on a Zeiss Axio Observer Z1 inverted microscope optimized for fluorescence and equipped with a monochrome AxioCam MRm for imaging. Quantification of ZO-1 fluorescence was determined using a directed pattern correlation method to correlate pixels using an algorithm that we wrote. Images were decomposed by defining cell boundaries with a symlet wavelet decomposition algorithm (42), and noise was reduced using our block filtering method.
Statistical analyses.
Graphpad Prism version 4.03 was used for statistical analyses. Differences in adherence, invasion, and bacterial counts in tissues and ZO-1 staining were evaluated using Student's t test or chi-square analysis. Statistical significance was accepted at a P value of <0.05.
RESULTS
The pXO1-encoded protein, BslA, promotes adherence to and invasion of brain endothelium.
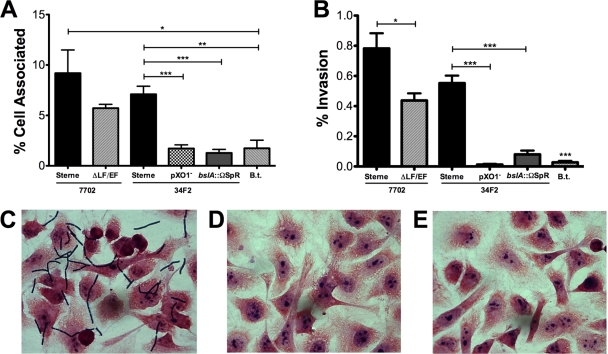
The ability of meningeal pathogens to interact with and penetrate the BBB represents an important first step in the development of bacterial meningitis. We have previously shown that B. anthracis Sterne is able to adhere to and invade brain endothelium (44). However, as the toxin-deficient mutant (ΔLF/EF) exhibited only a partial decrease of BBB interaction, we hypothesized that other genes on the virulence pXO1 plasmid may contribute to this process. To test this hypothesis, we used our previously established quantitative hBMEC adherence and invasion assays (9, 44) in an in vitro BBB model. hBMEC were grown to confluence and infected with B. anthracis Sterne and its isogenic strains lacking pXO1 (pXO1−) or both lethal and edema toxin (ΔLF/EF). Data are expressed as the percentage of total cell-associated or intracellular CFU recovered compared to the inoculum added to hBMEC monolayers. Consistent with previous findings, B. anthracis Sterne was able to adhere to and invade hBMEC, while the ΔLF/EF strain showed a partial reduction in adherence and invasion compared to the results for B. anthracis Sterne (Fig. 1A and B). Strikingly, however, the Sterne pXO1− strain exhibited little to no hBMEC adherence and invasion compared to the results for the parental strain (P = 0.005) (Fig. 1A and B). These results suggest that additional factor(s) on pXO1 mediate the interaction of B. anthracis with brain endothelium.
FIG. 1.
The pXO1-encoded BslA protein plays a prominent role in promoting adherence to and invasion of brain endothelium. (A and B) Adherence to (A) and invasion of (B) hBMEC by B. anthracis Sterne 7702 and its isogenic ΔLF/EF mutant, B. anthracis Sterne 34F2 and its isogenic pXO1− and bslA::ΩSpr mutants, and the closely related B. thuringiensis (B.t.). Data are expressed as the total cell-associated or intracellular CFU recovered compared to the input inoculum (MOI of 1, ∼1 × 105 CFU). All experiments were repeated at least three times in triplicate; data from a representative experiment are shown. Error bars indicate 95% confidence intervals of mean values from three wells. *, P < 0.05; **, P < 0.005; ***, P < 0.001. (C to E) Micrographs of Gram-stained hBMEC monolayers infected with vegetative B. anthracis Sterne 34F2 (C) or the pXO1− (D) or bslA::ΩSpr (E) strain.
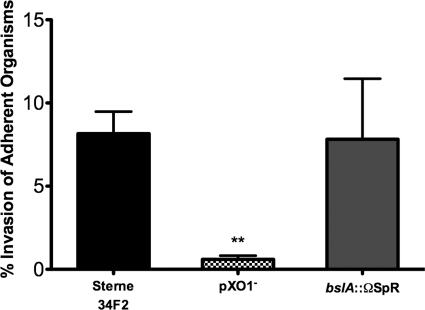
The pXO1 plasmid harbors two predicted S-layer-associated proteins (pXO1-54 and pXO1-90) (31). One of these, BslA (pXO1-90), has recently been demonstrated to be an S-layer protein and mediate adherence to fibroblasts and colonic and lung epithelial cells (20). We therefore examined the role of BslA in B. anthracis Sterne's interaction with hBMEC using an isogenic B. anthracis Sterne strain lacking the bslA gene (bslA::ΩSpr strain). As shown in Fig. 1, the bslA::ΩSpr mutant strain exhibited marked decreases in hBMEC adherence and invasion, with levels similar to the levels observed with Sterne pXO1−. These very low levels of adherence/invasion were also comparable to those observed during infection of hBMEC with the nonhuman pathogen B. thuringiensis, which served as a negative control (Fig. 1A and B). Adherent bacilli were visualized by microscopy following stringent washing and Gram staining. Micrographs revealed cell-associated organisms for B. anthracis Sterne (Fig. 1C) but no hBMEC association of the Sterne pXO1− or the bslA::ΩSpr strain (Fig. 1D and E, respectively). Consistent with our previous results, approximately 7.5% of the total number of hBMEC-associated B. anthracis Sterne strain bacteria had invaded the intracellular compartment (Fig. 2). Interestingly, although there was a very low percentage of adherent BslA-deficient bacteria compared to the input inoculum used (Fig. 1A), still, ∼7% of total hBMEC-associated bslA::ΩSpr bacteria were able to invade hBMEC (Fig. 2). This level is similar to that of the B. anthracis Sterne parent strain, whereas with the Sterne pXO1− mutant, little to no invasion by adherent organisms (0.7%) was observed (Fig. 2). These data suggest that BslA acts primarily as an adhesin, while additional factors on the pXO1 plasmid may contribute to invasive ability.
FIG. 2.
BslA acts primarily as an hBMEC adhesin. Data are expressed as the percentage of intracellular organisms (CFU) recovered compared to the total cell-associated (adherent) bacteria recovered for each strain. Strains were B. anthracis Sterne 34F2 and its isogenic pXO1− and bslA::ΩSpr mutants. Data from a representative experiment are shown; error bars indicate 95% confidence intervals of mean values from three wells. **, P < 0.005.
BslA is necessary and sufficient for hBMEC adherence.
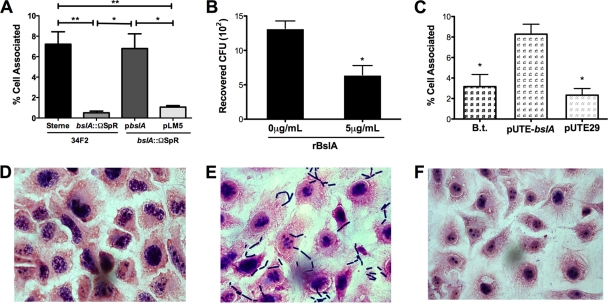
To establish unambiguously that the B. anthracis BslA protein specifically contributes to the hBMEC adherence phenotype, we performed single gene complementation analysis. The BslA-deficient strain complemented with the bslA gene carried by pbslA or with the pLM5 vector-only control was tested in the hBMEC adherence assay. Complementation of the bslA::ΩSpr mutant strain with pbslA restored the adherence level to that of B. anthracis Sterne, while the bslA::ΩSpr mutant transformed with pLM5 alone retained the hypoadherent phenotype (Fig. 3A). It has been shown previously that the bslA::ΩSpr pbslA strain expresses the BslA protein at a level similar to that of the parental Sterne strain (20).
FIG. 3.
BslA is necessary and sufficient for hBMEC adherence. (A) Adherence to hBMEC by B. anthracis Sterne 34F2, the bslA::ΩSpr strain, and the bslA::ΩSpr strain complemented with pbslA or pLM5 vector only. (B) Inhibition of B. anthracis Sterne 34F2 adherence to hBMEC by recombinant BslA protein (rBslA). (C) Adherence to hBMEC by B. thuringiensis (B.t.) or B. thuringiensis harboring pUTE-bslA or vector only (pUTE29). Data are expressed as the total cell-associated organisms recovered compared to the input inoculum. All experiments were repeated at least three times; data from a representative experiment are shown. The error bars indicate 95% confidence intervals of mean values from three wells. *, P < 0.05; **, P < 0.005. (D to F) Micrographs of hBMEC monolayers infected with B. thuringiensis (D) or B. thuringiensis transformed with pUTE-pbslA (E) or pUTE29 vector only (F) were taken at ×63 magnification following Gram staining.
Our results suggest that BslA acts as an adhesin to promote bacterial interactions with the hBMEC surface. To further probe this possibility, an exogenous recombinant BslA protein (GST-BslA260-652) was added to the adherence assays to determine whether its presence blocked B. anthracis Sterne-hBMEC interactions. We found that pretreatment of hBMEC monolayers with recombinant BslA inhibited B. anthracis Sterne's hBMEC adherence by 49% (Fig. 3B). The addition of purified GST alone had no effect on B. anthracis Sterne's adherence (data not shown). This is consistent with competitive inhibition of a cellular receptor(s), suggesting that B. anthracis BslA can function directly as an adhesin for brain endothelium.
To determine if BslA is sufficient for hBMEC adherence, we expressed the bslA gene (pUTE-bslA) in the nonadherent and noninvasive bacterium B. thuringiensis (37). The empty pUTE29 vector in B. thuringiensis served as a control. Heterologous expression of bslA resulted in a significant increase in the adherence of B. thuringiensis bacteria to hBMEC compared to that of B. thuringiensis bacteria expressing the empty vector (Fig. 3C). Micrographs of infected monolayers revealed adherent bacilli only in the case of B. thuringiensis expressing BslA (Fig. 3D, E, and F). Taken together, these results demonstrate that BslA is both necessary and sufficient for adherence to hBMEC.
BslA contributes to BBB penetration in a mouse model of anthrax meningitis.
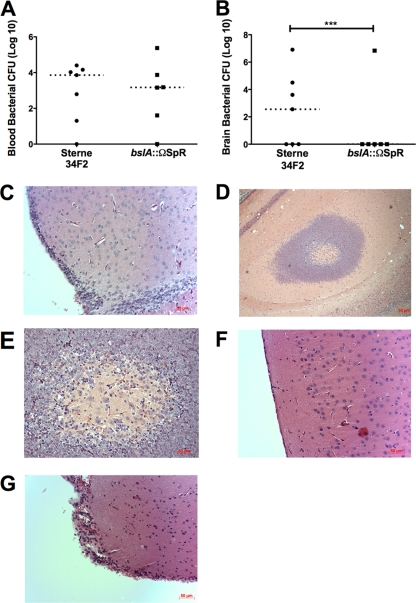
Our results thus far suggest a primary role for BslA in B. anthracis Sterne's interaction with brain endothelium. We next sought to corroborate whether this in vitro phenotype translated into a diminished ability to penetrate the BBB and produce meningitis in vivo. Using our established mouse model of hematogenous anthrax meningitis (44), we infected mice intravenously with B. anthracis Sterne or the bslA::ΩSpr mutant. Mice were euthanized at 48 h postinfection, after which the blood and brain were harvested from each mouse for quantitative bacterial culture. Despite similar levels of bacteremia at the experimental endpoint (Fig. 4A), only one out of six mice infected with the BslA-deficient mutant exhibited CNS infection (Fig. 4B). Representative histopathologic images of brains from experimentally infected mice are shown. Microscopic examination of brains from mice infected with B. anthracis Sterne revealed a thickening of the meninges and leukocytic infiltration (Fig. 4C). Additionally, areas of microabscess formation and hemorrhagic damage in the brain parenchyma were observed (Fig. 4C, D, and E). In contrast, a section from a mouse infected with the bslA::ΩSpr mutant with no brain bacterial counts displayed normal brain architecture and meningeal lining (Fig. 4F). However, in the case where a mouse did exhibit CNS invasion with the bslA::ΩSpr mutant, brain sections showed evidence of meningeal inflammation that was qualitatively similar to that observed with B. anthracis Sterne infection (Fig. 4G). In a subsequent experiment, we analyzed brain bacterial loads in infected mice at a later time point (when mice were moribund) and similarly found that only two out of seven mice infected with the BslA-deficient mutant had bacteria in the brain (data not shown).
FIG. 4.
BslA contributes to BBB penetration in vivo. (A and B) Bacterial counts from the blood (A) and brains (B) of CD-1 mice 48 h after intravenous infection with 4 × 104 CFU of B. anthracis Sterne 34F2 (black circles) or the bslA::ΩSpr mutant (black squares). Dotted lines represent median bacterial CFU. ***, P < 0.001. (C to E) Histopathology of hematoxylin-and-eosin-stained brain tissues of representative individual mice infected with B. anthracis Sterne 34F2, showing meningeal thickening and cellular infiltration (C and D), and abscess formation and damage (D and E). (F) Brain of a mouse infected with the bslA::ΩSpr strain that had no CFU recovered in the brain, displaying normal brain pathology. (G) Meningeal inflammation in the brain of a mouse infected with the bslA::ΩSpr mutant that had high levels of CFU recovered in the brain.
Contribution of BslA to hBMEC tight junction disruption.
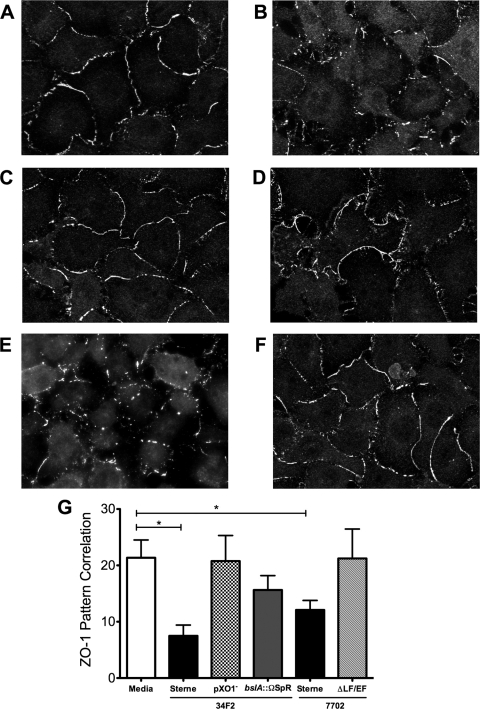
While our findings demonstrate that BslA contributes significantly to CNS entry in vivo, in some cases, we observed that BslA-deficient bacilli were still able to penetrate the BBB and cause meningitis. We hypothesized that B. anthracis Sterne may also be able to disrupt the BBB by modulating tight junction formation and permeability. It has been shown previously that anthrax toxins induce endothelial barrier dysfunction by disrupting the adherens junction protein vascular endothelial cadherin in lung endothelial cells (45). We examined the expression in hBMEC of tight junction protein ZO-1, a primary regulatory protein of tight junction formation in the BBB (3, 7), upon infection with B. anthracis Sterne and the bslA::ΩSpr mutant strain. Immunofluorescence staining showed intense ZO-1 staining at the intercellular junctions in the noninfected control (Fig. 5A). Infection of hBMEC monolayers with either of the B. anthracis Sterne strains 34F2 or 7702 resulted in an overall reduction and disruption in ZO-1 immunofluorescence compared to that in the control (Fig. 5B and E). In contrast, ZO-1 distribution following infection with Sterne lacking the pXO1 plasmid (pXO1−) or the anthrax toxins (ΔLF/EF) was similar to that in the uninfected control (Fig. 5C and F). Interestingly, infection with the bslA::ΩSpr mutant strain resulted in only partial disruption of tight junction formation and ZO-1 staining compared to the results for uninfected hBMEC (Fig. 5D and G).
FIG. 5.
Tight junction ZO-1 staining in hBMEC. (A to F) Immunofluorescence of ZO-1 staining in hBMEC monolayers incubated with cell culture medium (A), B. anthracis Sterne 34F2 (B) and its isogenic pXO1− (C) and bslA::ΩSpr (D) mutants, and B. anthracis Sterne 7702 (E) and its isogenic ΔLF/EF mutant (F). (G) ZO-1 fluorescence was quantified by pixel pattern correlation as described in Materials and Methods, based on six to eight different sections for each condition. Error bars indicate 95% confidence intervals of mean values from three wells. *, P < 0.05.
DISCUSSION
Anthrax meningitis/meningoencephalitis is the main neurological complication of anthrax infection and is associated with extremely high mortality despite antimicrobial therapy and supportive care (24, 27). We have previously shown that B. anthracis Sterne is capable of invading brain endothelium in a process involving host actin cytoskeleton rearrangement to facilitate CNS entry (44); however, the basic pathogenic mechanisms by which B. anthracis penetrates the BBB have not been described. Our studies using mutational analysis and heterologous expression revealed a requirement for the newly described S-layer protein, BslA, in BBB attachment by B. anthracis Sterne. Decreased adherence to hBMEC by the BslA-deficient mutant in vitro was correlated with a reduced risk for development of CNS infection in vivo. Thus, BslA represents the first known factor to directly promote B. anthracis Sterne attachment to brain endothelium and BBB penetration.
While the early stages of anthrax infection, including macrophage interaction and spore germination, have been well studied (16, 23, 29, 34), less is known about the molecular interactions of the vegetative form with host cells during disease pathogenesis. At later stages of infection, vegetative bacilli replicate in the bloodstream, reaching 108 organisms per ml of blood (8, 11, 34). Vegetative forms can also disseminate in vivo by directly infecting nonphagocytic cells (35). We initially observed that the interaction of B. anthracis Sterne with brain endothelium was pXO1 dependent, as an isogenic strain cured of pXO1 (Sterne pXO1−) exhibited a hypoadherent and hypoinvasive phenotype. Sequence analysis of pXO1 has revealed the presence of secreted and/or surface-associated factors and products that are predicted to be involved in microbial pathogenesis (2). Yet, there are ∼50 pXO1-carried genes whose functions remain unknown and which, in most cases, are unique to B. anthracis (2). Interestingly, virulence genes of pXO1 are located on pathogenicity islands in a type of arrangement similar to that found on bacterial chromosomes (31). One such pathogenicity island contains open reading frames (pXO1 base pairs 96 to 127) for known virulence genes, including cya (EF), lef (LF), and pagA (protective antigen), that encode the tripartite anthrax toxin complex; atxA and pagR, which encode trans-acting regulatory factors; and additional genes whose function affects germination. While we found that B. anthracis Sterne strains cured of the pXO1 plasmid (pXO1−) did not adhere to or invade hBMEC, this phenotype could be only partially explained by the presence of lethal and edema toxins, as a mutant lacking both lef and cya exhibited only a 50% reduction in hBMEC interaction compared to that of the parental B. anthracis Sterne strain (Fig. 1).
The surface of B. anthracis contains an elaborate independent S-layer underneath the poly-γ-d-glutamic acid capsule (25). The S-layer, which is thought to contribute to virulence, displays a highly ordered ultrastructure array comprised primarily of two abundant surface proteins, EA1 and Sap (10, 26). An additional 22 genes are predicted to encode S-layer proteins (32); the pXO1-encoded BslA protein has recently been identified as an S-layer component and adhesin (20). We found that BslA promoted attachment to human brain endothelium (Fig. 1) and keratinocytes (data not shown). When hBMEC monolayers were infected with the bslA::ΩSpr mutant, little to no adherence was observed. Additionally, heterologous expression of bslA in B. thuringiensis conferred adherence capabilities and purified recombinant BslA protein reduced the ability of B. anthracis Sterne to bind to hBMEC. These results demonstrate that BslA is both necessary and sufficient to promote host cell interaction.
Finally, we demonstrate that BslA contributes significantly to the ability of B. anthracis Sterne to penetrate the BBB and cause meningitis in vivo. Mice injected intravenously with the bslA::ΩSpr mutant exhibited a significant decrease in the CNS bacterial load (Fig. 4). This was not due to a generalized reduction in the virulence of the BslA-deficient mutant, as bacterial counts in the blood were similar at the experimental endpoint. Microscopic examination of brain sections from mice injected with B. anthracis Sterne confirmed that the development of meningitis correlated with the presence of bacteria in the brain. These results are the first to demonstrate an in vivo role for BslA in the pathogenesis of anthrax meningitis. Although our studies were performed in the Sterne (pXO1+ pXO2−) background, a previous study aimed at vaccine development demonstrated that BslA was highly and equally immunoreactive with serum isolated from rabbits and guinea pigs after infection with B. anthracis Sterne (pXO1+ pXO2−) or B. anthracis Vollum (pXO1+ pXO2+) (13). These results suggest that BslA is accessible on the bacterial surface even in the encapsulated organism.
Our results further suggest that BslA-mediated attachment may also contribute to the ability of B. anthracis to alter and disrupt the tight junction formation of the BBB. In vascular endothelium, tight junctions play a central role in maintaining barrier function by promoting intercellular contacts and monolayer integrity (7). Tight junction proteins ZO-1 and occludin have been shown to be primary regulatory proteins that modulate BBB permeability and contribute to the restrictive nature of the BBB (3). ZO-1 has been shown previously to be expressed in this hBMEC cell line (36). We observed that B. anthracis Sterne infection of hBMEC monolayers resulted in marked alterations in ZO-1 staining. This was due to the expression of the anthrax toxins, as infection with the toxin-deficient strain did not disrupt ZO-1 staining. These results are consistent with those of previous studies where purified LT altered the permeability of human lung microvascular endothelial cells by disrupting the junctional protein vascular endothelial cadherin (45). In addition, LT induced endothelial barrier dysfunction, resulting in major vascular leakage following intraperitoneal injection (14). Our results showed that infection of hBMEC with the bslA::ΩSpr mutant resulted in only partial disruption of ZO-1 tight junction staining (Fig. 5). These results suggest that host cell attachment also contributes to tight junction disruption and/or that BslA-mediated adherence is required for optimal toxin activity.
The hBMEC cell line maintains the morphological and functional characteristics of primary brain endothelium and has proven valuable in the analysis of a wide variety of human CNS pathogens, including group B Streptococcus (30), Escherichia coli K1 (21), Neisseria Streptococcus (43), Streptococcus pneumoniae (33), and recently, B. anthracis (44). We now identify for the first time a B. anthracis surface-associated adhesin that promotes hBMEC interaction and BBB penetration in vivo, contributing to the pathogenesis of anthrax meningitis. The results of our and previous studies suggest that BslA acts as a global adherence factor important for anthrax disease pathogenesis. Interestingly, characterization of Bacillus cereus isolates associated with fatal pneumonias showed that they harbor the bslA (pXO1-90) gene (17). In summary, BslA may represent an attractive new target for pharmacological intervention or vaccine purposes to prevent disease progression during systemic anthrax infection.
Acknowledgments
We are grateful to Monique Stins and Kwang Sik Kim for providing hBMEC, Mojgan Sabet and Donald Guiney for the pXO1-cured strain, Scott Stibitz for the ΔLF/EF B. anthracis Sterne strain, and Theresa Koehler for plasmid pUTE29. The histopathologic analysis was performed at the University of California San Diego Histopathology Core Facility, Nissi Varki, director.
This work was supported by grant no. R01 NS051247 from the NINDS/NIH and an SDSU University Grant Program (UGP) award to K.S.D.
The authors declare that they have no conflict of interest.
Footnotes
Published ahead of print on 9 October 2009.
REFERENCES
- 1.Abramova, F. A., L. M. Grinberg, O. V. Yampolskaya, and D. H. Walker. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. USA 90:2291-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariel, N., A. Zvi, H. Grosfeld, O. Gat, Y. Inbar, B. Velan, S. Cohen, and A. Shafferman. 2002. Search for potential vaccine candidate open reading frames in the Bacillus anthracis virulence plasmid pXO1: in silico and in vitro screening. Infect. Immun. 70:6817-6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballabh, P., A. Braun, and M. Nedergaard. 2004. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 16:1-13. [DOI] [PubMed] [Google Scholar]
- 4.Betz, A. L. 1992. An overview of the multiple functions of the blood-brain barrier. NIDA Res. Monogr. 120:54-72. [PubMed] [Google Scholar]
- 5.Betz, A. L. 1985. Epithelial properties of brain capillary endothelium. Fed. Proc. 44:2614-2615. [PubMed] [Google Scholar]
- 6.Cataldi, A., E. Labruyere, and M. Mock. 1990. Construction and characterization of a protective antigen-deficient Bacillus anthracis strain. Mol. Microbiol. 4:1111-1117. [DOI] [PubMed] [Google Scholar]
- 7.Dejana, E. 2004. Endothelial cell-cell junctions: happy together. Nat. Rev. Mol. Cell Biol. 5:261-270. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 9.Doran, K. S., E. J. Engelson, A. Khosravi, H. C. Maisey, I. Fedtke, O. Equils, K. S. Michelsen, M. Arditi, A. Peschel, and V. Nizet. 2005. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Investig. 115:2499-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etienne-Toumelin, I., J. C. Sirard, E. Duflot, M. Mock, and A. Fouet. 1995. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J. Bacteriol. 177:614-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedlander, A. M. 1999. Clinical aspects, diagnosis and treatment of anthrax. J. Appl. Microbiol. 87:303. [DOI] [PubMed] [Google Scholar]
- 12.Fritz, D. L., N. K. Jaax, W. B. Lawrence, K. J. Davis, M. L. Pitt, J. W. Ezzell, and A. M. Friedlander. 1995. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab. Invest. 73:691-702. [PubMed] [Google Scholar]
- 13.Gat, O., H. Grosfeld, N. Ariel, I. Inbar, G. Zaide, Y. Broder, A. Zvi, T. Chitlaru, Z. Altboum, D. Stein, S. Cohen, and A. Shafferman. 2006. Search for Bacillus anthracis potential vaccine candidates by a functional genomic-serologic screen. Infect. Immun. 74:3987-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gozes, Y., M. Moayeri, J. F. Wiggins, and S. H. Leppla. 2006. Anthrax lethal toxin induces ketotifen-sensitive intradermal vascular leakage in certain inbred mice. Infect. Immun. 74:1266-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidi-Rontani, C. 2002. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 10:405-409. [DOI] [PubMed] [Google Scholar]
- 16.Guidi-Rontani, C., M. Weber-Levy, E. Labruyere, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9-17. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmaster, A. R., K. K. Hill, J. E. Gee, C. K. Marston, B. K. De, T. Popovic, D. Sue, P. P. Wilkins, S. B. Avashia, R. Drumgoole, C. H. Helma, L. O. Ticknor, R. T. Okinaka, and P. J. Jackson. 2006. Characterization of Bacillus cereus isolates associated with fatal pneumonias: strains are closely related to Bacillus anthracis and harbor B. anthracis virulence genes. J. Clin. Microbiol. 44:3352-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janes, B. K., and S. Stibitz. 2006. Routine markerless gene replacement in Bacillus anthracis. Infect. Immun. 74:1949-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jernigan, J. A., D. S. Stephens, D. A. Ashford, C. Omenaca, M. S. Topiel, M. Galbraith, M. Tapper, T. L. Fisk, S. Zaki, T. Popovic, R. F. Meyer, C. P. Quinn, S. A. Harper, S. K. Fridkin, J. J. Sejvar, C. W. Shepard, M. McConnell, J. Guarner, W. J. Shieh, J. M. Malecki, J. L. Gerberding, J. M. Hughes, and B. A. Perkins. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kern, J. W., and O. Schneewind. 2008. BslA, a pXO1-encoded adhesin of Bacillus anthracis. Mol. Microbiol. 68:504-515. [DOI] [PubMed] [Google Scholar]
- 21.Kim, K. S. 2001. Escherichia coli translocation at the blood-brain barrier. Infect. Immun. 69:5217-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koehler, T. M., Z. Dai, and M. Kaufman-Yarbray. 1994. Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J. Bacteriol. 176:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaForce, F. M. 1994. Anthrax. Clin. Infect. Dis. 19:1009-1013. [DOI] [PubMed] [Google Scholar]
- 24.Lanska, D. J. 2002. Anthrax meningoencephalitis. Neurology 59:327-334. [DOI] [PubMed] [Google Scholar]
- 25.Mesnage, S., E. Tosi-Couture, P. Gounon, M. Mock, and A. Fouet. 1998. The capsule and S-layer: two independent and yet compatible macromolecular structures in Bacillus anthracis. J. Bacteriol. 180:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesnage, S., E. Tosi-Couture, M. Mock, P. Gounon, and A. Fouet. 1997. Molecular characterization of the Bacillus anthracis main S-layer component: evidence that it is the major cell-associated antigen. Mol. Microbiol. 23:1147-1155. [DOI] [PubMed] [Google Scholar]
- 27.Meyer, M. A. 2003. Neurologic complications of anthrax: a review of the literature. Arch. Neurol. 60:483-488. [DOI] [PubMed] [Google Scholar]
- 28.Mikesell, P., B. E. Ivins, J. D. Ristroph, and T. M. Dreier. 1983. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect. Immun. 39:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 30.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okinaka, R. T., K. Cloud, O. Hampton, A. R. Hoffmaster, K. K. Hill, P. Keim, T. M. Koehler, G. Lamke, S. Kumano, J. Mahillon, D. Manter, Y. Martinez, D. Ricke, R. Svensson, and P. J. Jackson. 1999. Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J. Bacteriol. 181:6509-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Read, T. D., S. N. Peterson, N. Tourasse, L. W. Baillie, I. T. Paulsen, K. E. Nelson, H. Tettelin, D. E. Fouts, J. A. Eisen, S. R. Gill, E. K. Holtzapple, O. A. Okstad, E. Helgason, J. Rilstone, M. Wu, J. F. Kolonay, M. J. Beanan, R. J. Dodson, L. M. Brinkac, M. Gwinn, R. T. DeBoy, R. Madpu, S. C. Daugherty, A. S. Durkin, D. H. Haft, W. C. Nelson, J. D. Peterson, M. Pop, H. M. Khouri, D. Radune, J. L. Benton, Y. Mahamoud, L. Jiang, I. R. Hance, J. F. Weidman, K. J. Berry, R. D. Plaut, A. M. Wolf, K. L. Watkins, W. C. Nierman, A. Hazen, R. Cline, C. Redmond, J. E. Thwaite, O. White, S. L. Salzberg, B. Thomason, A. M. Friedlander, T. M. Koehler, P. C. Hanna, A. B. Kolsto, and C. M. Fraser. 2003. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 423:81-86. [DOI] [PubMed] [Google Scholar]
- 33.Ring, A., J. N. Weiser, and E. I. Tuomanen. 1998. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Investig. 102:347-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross, J. 1957. Pathogenesis of anthrax following administration of spores by the respiratory route. J. Pathol. Bacteriol. 73:485-494. [Google Scholar]
- 35.Russell, B. H., R. Vasan, D. R. Keene, and Y. Xu. 2007. Bacillus anthracis internalization by human fibroblasts and epithelial cells. Cell. Microbiol. 9:1262-1274. [DOI] [PubMed] [Google Scholar]
- 36.Siddharthan, V., Y. V. Kim, S. Liu, and K. S. Kim. 2007. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 1147:39-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel, J. P. 2001. The mammalian safety of Bacillus thuringiensis-based insecticides. J. Invertebr. Pathol. 77:13-21. [DOI] [PubMed] [Google Scholar]
- 38.Sterne, M. 1937. Variation in Bacillus anthracis. Onderstepoort J. Vet. Sci. Anim. Ind. 8:271-349. [Google Scholar]
- 39.Stins, M. F., F. Gilles, and K. S. Kim. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76:81-90. [DOI] [PubMed] [Google Scholar]
- 40.Stins, M. F., N. V. Prasadarao, J. Zhou, M. Arditi, and K. S. Kim. 1997. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell. Dev. Biol. Anim. 33:243-247. [DOI] [PubMed] [Google Scholar]
- 41.Turnbull, P. C. 1991. Anthrax vaccines: past, present and future. Vaccine 9:533-539. [DOI] [PubMed] [Google Scholar]
- 42.Twenhafel, N. A., E. Leffel, and M. L. Pitt. 2007. Pathology of inhalational anthrax infection in the African green monkey. Vet. Pathol. 44:716-721. [DOI] [PubMed] [Google Scholar]
- 43.Unkmeir, A., K. Latsch, G. Dietrich, E. Wintermeyer, B. Schinke, S. Schwender, K. S. Kim, M. Eigenthaler, and M. Frosch. 2002. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol. Microbiol. 46:933-946. [DOI] [PubMed] [Google Scholar]
- 44.van Sorge, N. M., C. M. Ebrahimi, S. M. McGillivray, D. Quach, M. Sabet, D. G. Guiney, and K. S. Doran. 2008. Anthrax toxins inhibit neutrophil signaling pathways in brain endothelium and contribute to the pathogenesis of meningitis. PLoS One 3:e2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warfel, J. M., A. D. Steele, and F. D'Agnillo. 2005. Anthrax lethal toxin induces endothelial barrier dysfunction. Am. J. Pathol. 166:1871-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welkos, S. L., and A. M. Friedlander. 1988. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb. Pathog. 4:53-69. [DOI] [PubMed] [Google Scholar]