Abstract
The oral commensal Streptococcus gordonii must adapt to constantly fluctuating and often hostile environmental conditions to persist in the oral cavity. The arginine deiminase system (ADS) of S. gordonii enables cells to produce, ornithine, ammonia, CO2, and ATP from arginine hydrolysis, augmenting the acid tolerance of the organism. The ADS genes are substrate inducible and sensitive to catabolite repression, mediated through ArcR and CcpA, respectively, but the system also requires low pH and anaerobic conditions for optimal activation. Here, we demonstrate that the CiaRH and ComDE two-component systems (TCS) are required for low-pH-dependent expression of ADS genes in S. gordonii. Further, the VicRK TCS is required for optimal ADS gene expression under anaerobic conditions and enhances the sensitivity of the operon to repression by oxygen. The known anaerobic activator of the ADS, Fnr-like protein (Flp), appeared to act independently of the Vic TCS. Mutants of S. gordonii lacking components of the CiaRH, ComDE, or VicRK grew more slowly in acidified media and were more sensitive to killing at lethal pH values and to agents that induce oxidative stress. This study provides the first evidence that TCS can regulate the ADS of bacteria in response to specific environmental signals and reveals some notable differences in the contribution of CiaRH, ComDE, and VicRK to viability and stress tolerance between the oral commensal S. gordonii and the oral pathogen Streptococcus mutans.
Streptococcus gordonii colonizes the oral cavity very early in life (10, 27, 52), and its presence in oral biofilms is generally associated with oral health (1, 7, 48, 49). Colonization by S. gordonii is believed to be beneficial to the host in large part because of the contribution of this microorganism to pH homeostasis in oral biofilms through the hydrolysis of arginine in saliva and the diet (11). The pH of the oral cavity fluctuates in response to the diet and diurnal rhythms of the host, and adaptation to acidic conditions is important for the survival of S. gordonii and other oral biofilm organisms (58). Residents of oral biofilms also experience other environmental stresses, including modest temperature fluctuations, substantial changes in nutrient source and abundance, and wide variation in oxygen tension and redox potential as oral biofilms mature (26). Despite these challenging conditions, S. gordonii is able to persist as a significant proportion of the biofilm populations of the oral cavity (20, 56) and, like some other viridans streptococci, can cause endocarditis (6, 24, 42). Thus, adaptations by S. gordonii to conditions in multiple intraoral sites or in blood and infected heart valves are essential for persistence.
The arginine deiminase system (ADS), which is present in S. gordonii and a number of other abundant commensal oral streptococci, is a three-enzyme pathway that converts arginine to ornithine, CO2, and ammonia, with the concomitant generation of ATP (13). The ADS augments acid tolerance in S. gordonii by neutralizing the cytoplasm and environment, and the ATP generated can be used for growth, anabolism, and to extrude protons (11, 14, 15, 38). A strong association with the arginolytic potential of human dental biofilms and resistance to caries has been documented (45, 60), and organisms that are ADS positive are believed to have a beneficial impact on oral microbial ecology. Mechanistically, the presence of the ADS in oral biofilms is believed to provide a selective advantage to those organisms that possess it and to moderate biofilm acidification, both of which favor the persistence of a microflora that is compatible with dental health and discourage the emergence of aciduric organisms associated with dental caries.
In S. gordonii, the genes for the three enzymes of the pathway, arginine deiminase (AD; arcA), ornithine carbamyltransferase (arcB) and carbamate kinase (arcC) are cotranscribed in an operon with arcD (arginine:ornithine antiporter) and arcT (arginine aminopeptidase). Induction of the ADS genes (arcABCDT) by arginine is mediated by a transcriptional activator encoded by the divergently transcribed arcR gene, located immediately 3′ to the ADS operon. Immediately 5′ to the ADS operon is a gene for an Fnr-like protein (flp) (14), which activates expression from the arcA promoter (ParcA) under anaerobic conditions. Carbohydrate catabolite repression of the operon by preferred carbohydrate sources, such as glucose, is exerted primarily through CcpA (14, 15). In addition, ADS expression is optimal under acidic conditions, but the basis for pH-dependent control of transcription has not been investigated (38). Other complexities in ADS regulation exist in S. gordonii, including that queosine modification of tRNA may impact translation of the genes (38), ADS expression is higher in stationary phase (38), and other mechanisms for posttranscriptional regulation of expression appear to exist (38). Because of the spectrum of control mechanisms governing ADS production and the wide distribution of this system in abundant commensal organisms and pathogens, analysis of ADS expression has provided many insights into genetic regulation in streptococci. There is also a high degree of relevance of ADS regulation to oral biofilm ecology, oral health, and disease (11). In spite of substantial progress in understanding control of the ADS, critical gaps remain in our knowledge of how this system is regulated.
Transcriptional control of gene expression by two-component signal transduction systems (TCSs) is a common mechanism used by bacteria to modulate cell behaviors in response to environmental conditions (57). A TCS is composed of a histidine kinase that usually detects an environmental signal and a response regulator that can be phosphorylated by the sensor kinase (25). TCSs are involved in the stress responses of many bacteria, including Escherichia coli (16, 50), Pseudomonas aeruginosa (51, 59), Staphylococcus aureus (19, 59), and Streptococcus mutans (4, 9, 29, 54). In S. gordonii, the ComDE TCS was shown to regulate development of genetic competence (23, 41) and was required for efficient in vitro biofilm formation (40). The BfrAB TCS of S. gordonii affects biofilm development and the expression of multiple ABC transporters (28, 64, 65). In addition to ComDE, the CiaRH and VicRK TCSs of oral streptococci and some pathogenic streptococci seem particularly important for regulation of traits associated with colonization, growth in the host, and pathogenesis (4, 35, 37, 54). We investigated here whether the CiaRH, ComDE, and VicRK TCSs are able to influence the expression of the ADS in response to pH and oxidative stress. In addition, we begin to explore whether these TCSs, which play key regulatory roles in a variety of critical cellular functions in low G+C gram-positive bacteria (18, 44, 46), affect traits of S. gordonii that are known to be important for establishment and persistence in the oral cavity.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and reagents.
S. gordonii DL1 and its derivatives were maintained and passaged in brain heart infusion medium (BHI; Difco Laboratories, Detroit, MI) at 37°C in 5% CO2. Recombinant S. gordonii DL1 strains carrying a promoterless chloramphenicol acetyltransferase (CAT) gene (cat) fused to the arcA (ParcA-cat) promoter (38) were selected and maintained on BHI agar supplemented with erythromycin at 5 μg ml−1. Strains carrying insertion/deletion mutations in the ciaRH, comDE, or vicRK genes were selected on BHI agar with kanamycin (Km; 250 μg ml−1). Escherichia coli strains were grown in Luria-Bertani (LB) medium supplemented with tetracycline (12 μg ml−1), if needed. Preparation of competent cells and transformation of S. gordonii were done as previously described (33). Chemical reagents and antibiotics were obtained from Sigma (St. Louis, MO). To monitor AD expression, batch cultures of strains of S. gordonii were grown in a low-carbohydrate tryptone-yeast extract (TY) medium (62) supplemented with 25 mM galactose and 10 mM arginine to an optical density at 600 nm (OD600) of 0.5. For aerobic growth, overnight cultures of S. gordonii strains were diluted 1:50 into a 250-ml conical flask containing 50 ml of BHI, and cultures were grown on a rotary shaker (150 rpm) at 37°C to an OD600 of 0.5. For anaerobic growth, cultures were similarly diluted and incubated, but the medium was overlaid with mineral oil (1).
Construction of mutant strains.
Strains used in the present study are listed in Table 1, and primers used for deletion mutagenesis are listed in Table 2. To make deletions of the genes of interest, 5′ and 3′ flanking regions of each gene were amplified from chromosomal DNA from S. gordonii DL1, ligated together using BamHI sites designed into each primer set, and cloned into the pGEM-T Easy vector (Promega, Madison, WI). These plasmids were digested with BamHI and a nonpolar Km resistance gene (3), which lacks its own promoter, was inserted (Table 1). The desired mutagenic plasmids were selected after PCR amplification using vector-originated M13 primers, isolated, and used to transform S. gordonii/ ParcA-cat. To construct strains lacking the vicRK and flp genes, the mutagenic plasmid containing regions flanking vicRK was transformed into a Flp-deficient mutant of S. gordonii/ ParcA-cat (14). Transformants were selected on BHI agar with Km. In all cases, double-crossover mutants of each gene were confirmed by PCR and DNA sequencing, including sequencing the flanking regions to ensure no unwanted mutations were inadvertently introduced. To construct ciaRH, comDE, and vicRK complemented strains, the DNA fragments of ciaRH, comDE, and vicRK with their respective promoter regions were amplified by using primers described in Table 2 and cloned into the shuttle vector pDL278 (32) to create plasmids pDL-ciaRH, pDL-comDE, and pDL-vicRK, respectively. The ligation mixtures were transformed into E. coli, and transformants were selected on LB plates with spectinomycin (100 μg ml−1). The integrity of the constructs was confirmed by restriction enzyme digestion and DNA sequencing. The plasmids were recovered from E. coli and introduced into ciaRH-, comDE-, and vicRK-deficient mutants of S. gordonii/ ParcA-cat by natural transformation. Transformants were selected on BHI agar with spectinomycin (1,000 μg ml−1) and screened for the correct plasmid content.
TABLE 1.
Strains used in this study
| Strain | Relevant characteristics | Source or reference |
|---|---|---|
| SgWT | S. gordonii DL1/ParcA-cat | 38 |
| SgciaRH | ΔciaRH/S. gordonii /ParcA-cat | This study |
| SgciaR | ΔciaR/S. gordonii/ParcA-cat | This study |
| SgciaH | ΔciaH/S. gordonii/ParcA-cat | This study |
| SgciaRH−/ciaRH | ΔciaRH/pDL-ciaRH/S. gordonii/ParcA-cat | This study |
| SgcomDE | ΔcomDE/S. gordonii/ParcA-cat | This study |
| SgcomD | ΔcomD/S. gordonii/ParcA-cat | This study |
| SgcomE | ΔcomE/S. gordonii/ParcA-cat | This study |
| SgcomDE−/comDE | ΔcomDE/pDL-comDE/S. gordonii/ParcA-cat | This study |
| SgvicK | ΔvicK/S. gordonii/ParcA-cat | This study |
| SgvicR | ΔvicR/S. gordonii/ParcA-cat | This study |
| SgvicRK | ΔvicRK/S. gordonii/ParcA-cat | This study |
| SgvicRK−/vicRK | ΔvicRK/pDL-vicRK/S. gordonii/ParcA-cat | This study |
| Sgflp | Δflp/S. gordonii/ParcA-cat | 14 |
| SgvicRK-flp | ΔvicRK/Δflp/S. gordonii/ParcA-cat | This study |
TABLE 2.
Primers used in this study
| Primer | Sequence (5′-3′)a | Application | Source or reference |
|---|---|---|---|
| ComE-BamHI-3′ | CGGGATCCATTTCTTCGAAGAC | Deletion of comE | This study |
| ComD-5′-2 | GCAAACCGGAATTAACTCAGGTG | Deletion of comE | This study |
| ComE-BamHI-5′ | ACTGTTGACAAAGCGGGATCCACTGG | Deletion of comE | This study |
| ComE-3′ | CCAAGTTCTTTATCTTGTTCTTG | Deletion of comE | This study |
| ComE-3′-3 | GGACTTCCTGTCGTCTC | Deletion of comD | This study |
| ComD-BamHI-5′ | GAAGTAGGATCCTCAACAAAAGG | Deletion of comD | This study |
| ComD-5′ | CCCTACCTCATCAATTAATGCG | Deletion of comD | This study |
| ComD-BamHI-3′ | GAGCGAACGGATCCACCAGTAACTTGC | Deletion of comD | This study |
| Pcom-BamHI-5′ | GGCAGTCCTTATCATAAAGGATCCTTGACGC | Cloning of comDE into pDL278 | This study |
| ComE-SphI-3′ | CCAGTTTATCTCGCATGCTCAACAG | Cloning of comDE into pDL278 | This study |
| CiaR-5′-1 | GTCGGCTTGCTTGGTGGATATACATTCGG | Deletion of ciaR | This study |
| CiaR-BamHI-3′ | CATCTTGGGATCCTCCTGCTATAAGATAC | Deletion of ciaR | This study |
| CiaH-3′-2 | GATTCTGACTGGTTTGCTCC | Deletion of ciaR | This study |
| CiaR-BamHI-5′ | GATCGGATCCGGGGCTTTGACAG | Deletion of ciaR | This study |
| CiaH-BamHI-5′ | TGGTGCTGGTACTATTGGGATCCCATAT | Deletion of ciaH | This study |
| CiaH-3′ | CCCAGATTCTGCTATCGCACCCACC | Deletion of ciaH | This study |
| CiaH-BamHI-3′ | GTG TGAATACACCAGGATCCCG | Deletion of ciaH | This study |
| CiaR-5′-2 | GATGACTTTGCGGATGTCATGCAGGT | Deletion of ciaH | This study |
| Pcia-HindIII-5′ | AGGATAGCTATTCTAGTCAAGCTTATGAAG | Cloning of comDE into pDL278 | This study |
| CiaH-SalI-3′ | GCCAGCCATATGTCGACCCAATAGTACCA | Cloning of comDE into pDL278 | This study |
| VicR-5′-1 | GATGGTCGTGAAGCTCTTGA | Deletion of vicK | This study |
| VicR-BamHI-3′ | GGCGAGGATCCCTTCGATTCTC | Deletion of vicK | This study |
| VicK-BamHI-5′ | GGAGGATCCCTGGGAAAGTGAAG | Deletion of vicK | This study |
| VicK-3′ | CCGATAAAATTGTGGTGCCGCCGC | Deletion of vicK | This study |
| VicR-5′-2 | CAAGGGTGCCTTCCCAACATGGC | Deletion of vicR | This study |
| VicR-BamHI-3′-2 | GCCGGATCCACTTCATAGCCC TC | Deletion of vicR | This study |
| VicR-BamHI-5′ | CTCGTCGTGGATCCGGCTAC | Deletion of vicR | This study |
| VicR-3′ | GAACAGCTACCAAACCAGAG | Deletion of vicR | This study |
| Pvic-BamHI-5′ | GCTATCCTAGCGGATCCCGCCAAC | Cloning of comDE into pDL278 | This study |
| Vic-SphI-3′ | TACCACTGCATGCAGATGC | Cloning of comDE into pDL278 | This study |
| Flp-5′ | CCAGTTTTATATGCCGTA | Deletion of flp | 14 |
| FlP-3′ | GTCCAGTAGACTAACTTTCT | Deletion of flp | 14 |
| Flp-SmaI-S | TCTTTTTTTCTGGAGACCCGGGTGATCGCCTT | Deletion of flp | 14 |
| Flp-SmaI-AS | GAGAAAAAGGCGATCACCCGGGTCTCCAG | Deletion of flp | 14 |
Boldfacing indicates engineered restriction sites.
Growth kinetics.
Growth of all strains in BHI (pH 7.0) or BHI that was acidified to pH 5.0 with HCl (BHI/HCl), under aerobic or anaerobic conditions (1), was monitored by using a Bioscreen C (Growth Curves) Microbiology Reader with multiwell disposable microtiter plates. An aliquot (3 μl) of cell suspension from an overnight culture was inoculated in at least triplicate into each well containing 300 μl of BHI (pH 7.0) or BHI/HCl (pH 5.0) fresh medium. Inocula were adjusted to the same OD600 before dilution. To assess the ability of cells to grow in the presence of oxidative stressors, overnight cultures of cells were transferred to prewarmed BHI and grown at 37°C in a 5% CO2 atmosphere to an OD600 of 0.5. The cells were then diluted into fresh BHI containing 25 mM paraquat (methyl viologen; Sigma), and the impact of the agents on bacterial growth was monitored in a Bioscreen C at 37°C under aerobic or anaerobic conditions.
Biochemical and acid stress tolerance assays.
CAT activity was measured as previously described (38) and expressed as nanomoles of chloramphenicol acetylated (minute × milligram of total protein)−1. AD activity was measured by a method detailed elsewhere (5) and expressed as micrograms of citrulline produced (minute × mg of total protein)−1. The concentration of protein was determined by using a Bradford protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard. The ability of bacterial cells to withstand acid killing was assessed as previously described (61).
RESULTS
ComDE and CiaRH affect low pH induction of the ADS genes.
To examine whether the Cia, Com or Vic TCSs were involved in the regulation of the ADS in S. gordonii, the entire ciaRH, comDE, and vicRK operons were replaced by a nonpolar Km cassette to create strains SgciaRH, SgcomDE, and SgvicRK, respectively. To evaluate the function of individual components of each TCS, ciaR, ciaH, comD, comE, vicR, and vicK were disrupted by nonpolar insertions (Table 1). In all cases, the nonpolar Km insertion was confirmed to allow efficient readthrough to the downstream genes by real-time PCR (data not shown). All mutations were confirmed by PCR analysis and DNA sequencing of the regions flanking the insertion site of the marker to ensure that no mutations had been introduced into flanking genes. Although repeated attempts to generate a vicR mutant in S. mutans strains NG8 and UA159 were unsuccessful (54), both VicR- and VicRK-deficient strains of S. gordonii were isolable.
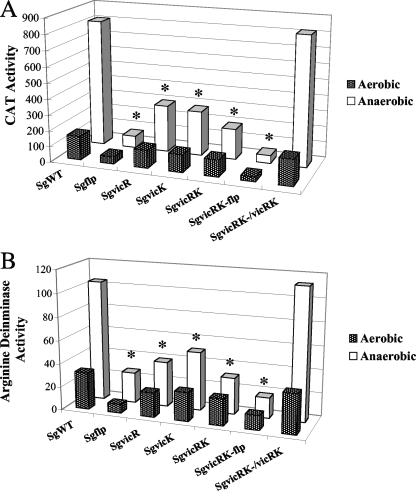
The cia, com, and vic mutants of S. gordonii carrying the arcA promoter (ParcA) fused to a CAT gene (cat) were grown to mid-exponential phase in TY medium that was acidified with HCl to pH 5.5 or buffered at pH 7.0 by using 50 mM potassium phosphate buffer (53). CAT activity and AD activity were measured as outlined in Materials and Methods. In the SgWT background, cells expressed threefold-higher CAT activity from ParcA at pH 5.5 than at pH 7.0. A similar phenotype was observed in the vic mutants (Fig. 1A), whereas only a 1.1- to 2-fold induction of ParcA by low pH could be detected in the cia and com mutants (Fig. 1A). Complementation of the SgciaRH or SgcomDE strains with plasmid-borne ciaRH or comDE genes, respectively, restored induction by low pH to a level comparable to that observed in SgWT (Fig. 1A). Measurements of AD activity (Fig. 1B) supported an involvement of the Com and Cia systems, but not Vic, in low-pH induction of the ADS. Both the histidine kinases (CiaH and ComD) and the response regulators (CiaR and ComE) of these TCSs were required for activation of ADS gene expression by low pH (Fig. 1).
FIG. 1.
CAT (A) and AD (B) activities of S. gordonii carrying ParcA-cat and its derivatives (Table 1) cultured in TY broth containing 25 mM galactose with 20 mM arginine that had been acidified to pH 5.5 with HCl (TY/HCl) or TY buffered at pH 7.0 (TY/KPB) to mid-exponential phase. The values of the columns are the average of a minimum of nine separate cultures for each strain and condition. (A) The standard deviations for the CAT activities of the pH 7.0 group from left to right are 20.46, 40.79, 12.02, 11.86, 23.19, 9.48, 0.95, 16.83, 29.79, 22.95,15.60, and 8.23; those of the pH 5.5 group from left to right are 26.13, 30.18, 1.61, 37.76, 27.10, 40.02, 12.80, 20.16, 37.42, 52.80, 16.90, and 8.44. (B) The standard deviations for the AD activities of pH 7.0 group from left to right are 2.78, 1.40, 3.23, 1.94, 7.07, 2.41, 0.95, 4.94, 3.63, 8.33, 0.84, and 2.22; and those of the pH 5.5 group from left to right are 17.22, 2.31, 1.60, 6.47, 3.22, 2.99, 1.35, 1.25, 1.12, 1.80, 1.67, and 2.38. Activities: ▪, pH 7.0 cultures; □, pH 5.0 cultures. *, statistically significant differences between SgWT and mutants grown under identical culture conditions (P < 0.05 [Student t test]).
Contribution of VicRK to anaerobic induction of the ADS.
The VicK sensor kinase of S. mutans contains a PAS domain (55) and was found to be involved in regulation of oxygen-responsive genes in S. mutans (2, 54). To examine whether the Vic system affected expression of the ADS in response to oxygen in S. gordonii, vic mutants and SgWT were cultured to mid-exponential phase in TY medium containing 25 mM galactose and 10 mM arginine under aerobic or anaerobic conditions, and the CAT and AD activities were measured. In the SgWT background, cells expressed fivefold-higher CAT activity from ParcA under anaerobic conditions compared to aerobic conditions (Fig. 2A). A threefold induction in ParcA expression in anaerobic conditions was detected in both SgvicR and SgvicK strains (Fig. 2A), and only a twofold induction was observed in the SgvicRK strain (Fig. 2A). Both the histidine kinase VicK and the response regulator VicR were shown to contribute to anaerobic induction of the transcription of the arc operon in S. gordonii. CAT activity was consistent with AD activity, demonstrating that the response of the organism to aeration occurred mainly at the transcriptional level (Fig. 2B). Introduction of vicRK on a plasmid into the SgvicRK strain resulted in restoration of the fivefold induction of the ADS under anaerobic conditions that was observed with strain SgWT (Fig. 2). Importantly, no difference in the response of ADS expression to aeration was noted between the SgWT strain and strains lacking one or both components of the CiaRH or the ComDE TCS (data not shown).
FIG. 2.
CAT (A) and AD (B) activities of mutants of S. gordonii carrying ParcA-cat and its derivatives (Table 1) cultured in TY broth containing 25 mM galactose with 20 mM arginine under aerobic or anaerobic conditions to mid-exponential phase. The values of the columns are the average of a minimum of nine separate cultures for each strain and condition. (A) The standard deviations for CAT activities of the aerobic group from left to right are 25.00, 10.13, 18.14, 19.28, 7.04, 3.60, and 7.81; those of the anaerobic group from left to right are 34.12, 3.60, 44.30, 6.36, 38.95, 2.51, and 24.13. (B) The standard deviations for AD activities of the aerobic group from left to right are 5.20, 0.50, 2.40, 2.61, 3.23, 15.23, and 1.78; those of the anaerobic group from left to right are 5.17, 1.11, 1.89, 9.79, 1.29, 1.28, and 7.78. Activities: ▪, aerobic cultures; □, anaerobic cultures. *, statistically significant differences between SgWT and mutants grown under identical culture conditions (P < 0.05 [Student t test]).
The Vic system appears to act independently of Flp.
Flp (for Fnr-like protein) activates arc operon expression in response to low oxygen tension in S. gordonii (14). To examine whether the Flp and the Vic system acted independently in the anaerobic induction of the arc operon, the strain SgvicRK-flp, in which the flp and vicRK genes were deleted, was examined. Inactivation of flp resulted in 10- and 3.3-fold decreases in CAT activity compared to the results seen with SgWT grown under anaerobic and aerobic conditions, respectively (Fig. 2A). Loss of both Flp and VicRK resulted in 15- and 5-fold lower CAT activities than in SgWT cultured under anaerobic or aerobic conditions, respectively (Fig. 2A). Thus, Flp and VicRK may act independently in the anaerobic induction of the ADS in S. gordonii. Measurements of AD activity showed the same trend as the gene fusion results, although the modest differences in fold induction between CAT and ADS in the SgvicRK-flp strain (Fig. 2B) add further support that posttranscriptional events can modulate AD enzyme activity (38).
CiaRH, ComDE, and VicRK contribute to acid tolerance.
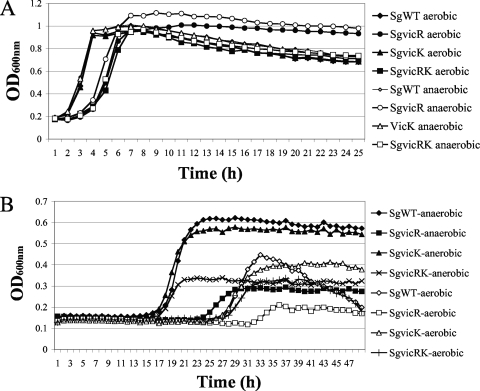
Given the participation of the Vic, Com, and Cia TCS in modulation of the ADS in response to pH and oxidative stress, we explored whether these systems contribute to the tolerance of environmental stresses by S. gordonii. The various strains were cultured in BHI (pH 7.0) or BHI/HCl (pH 5.0) broth, and growth was monitored spectrophotometrically. At pH 7.0, the growth curves of the SgciaR and SgvicK strains were similar to that of the wild-type strain (Fig. 3A and C), whereas longer lag phases were noted in the SgciaH, SgciaRH, SgvicR, and SgvicRK strains (Fig. 3A and C). In addition, all com mutants displayed a decreased final OD after 15 h of incubation compared to that of SgWT (Fig. 3B). When the medium was adjusted to pH 5.0, all cia and com mutants, as well as the SgvicR strain, could not grow (Fig. 3), and a decreased final OD was evident for the SgvicRK strain compared to SgWT (Fig. 3C). However, similar growth curves were observed for the SgvicK and SgWT strains (Fig. 3C).
FIG. 3.
Growth of strains SgWT and cia mutants including SgciaR, SgciaH, and SgciaRH (A); com mutants including SgcomD, SgcomE, and SgcomDE (B); and vic mutants including SgvicR, SgvicK, and SgvicRK (C) in BHI (pH 7.0) or acidified BHI with HCl (pH 5.0). The OD600 was determined every 15 min for 24 h using a Bioscreen C.
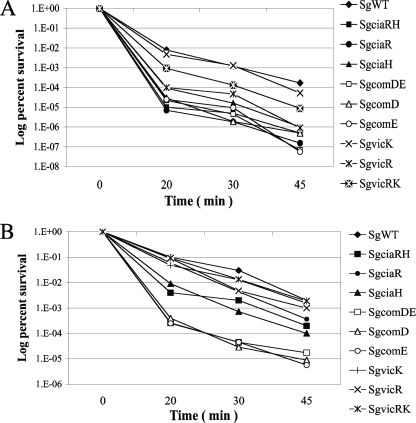
After 45 min of exposure to pH 2.8, the survival rate of the ciaRH, comDE, and vicR mutants was more than 3 logs lower than that of SgWT (Fig. 4A). SgvicRK showed a 1-log lower survival rate than the wild-type strain, whereas no significant differences in the survival rates of the SgvicK and SgWT strains were noted (Fig. 4A). To examine whether S. gordonii was able to mount a classical acid tolerance response (ATR), characterized by an increase in resistance to acid killing after initial exposure to mildly acidic conditions, SgWT was preincubated in BHI medium that was adjusted to pH 5.0 with HCl for 2 h to allow acid adaptation and then incubated in pH 2.8 buffer to monitor the rate of acid killing. After 45 min, a 1-log higher survival rate of cells preincubated at pH 5.0 was observed compared to cells that were not acid adapted (Fig. 4). Interestingly, the ability to resist acid killing of the SgvicR mutants, which showed a substantial deficiency in acid tolerance in unadapted cells, was restored to a level comparable to that of SgWT after acid adaptation (Fig. 4B). Also of note, the com mutants could not mount an effective ATR, as evidenced by the lack of enhanced survival after preexposure to acidic conditions, but the cia mutants could. In all cases, however, the cia and com mutants were less resistant to acid killing than the wild-type strain, provided that the strains were pretreated in the same manner (Fig. 4B).
FIG. 4.
Acid tolerance assay. (A) S. gordonii carrying ParcA-cat and its derivatives were grown in BHI medium adjusted to pH 7.0 to an OD600 of 0.3, washed with 0.1 M glycine buffer (pH 7.0), and subjected to acid killing by incubating the cells in 0.1 M glycine buffer (pH 2.8). (B) The wild type and mutants of S. gordonii were grown in BHI medium adjusted to pH 7.0 to an OD600 of 0.2, and then the cells were harvested and resuspended in fresh BHI medium adjusted to pH 5.0. After two additional hours of incubation, cells with an OD600 of 0.3 were prepared for acid killing as described above. In all cases, the survival rate was determined by plating cells in triplicate on BHI agar plates. The results are expressed as the percent survival rate versus the time at pH 2.8. The data presented are representative of at least nine individual replicates for each strain.
Requirement of VicRK for oxidative stress tolerance in S. gordonii.
To examine whether VicRK in S. gordonii contributed to oxidative stress tolerance, the vic mutants and SgWT were cultured in BHI medium under aerobic and anaerobic conditions, and the growth curves of the cells were monitored. In the SgWT and SgvicK strains, similar growth curves were noted for aerobic and anaerobic cultures (Fig. 5A), whereas the SgvicR and SgvicRK strains grew more slowly and achieved slightly lower final ODs when cells were cultured under aerobic conditions compared to anaerobic conditions (Fig. 5A). When the cells were cultured with 25 mM paraquat, similar growth curves were noted for the SgWT and SgvicK strains under both aerobic and anaerobic conditions (Fig. 5B). However, the SgvicR strain had a greatly extended lag phase and achieved about half the final OD of the SgWT strain when cultured with paraquat under anaerobic conditions (Fig. 5B). Also, the SgvicR strain could barely grow aerobically in the presence of paraquat (Fig. 5B) and the SgvicRK strain achieved about half the final OD of the SgWT strain when cultured with paraquat under aerobic or anaerobic conditions (Fig. 5B).
FIG. 5.
Growth of strains SgWT and vic mutants including SgvicR, SgvicK, and SgvicRK cultured in BHI medium without (A) or with 25 mM paraquat (B) under aerobic or anaerobic conditions. The OD600 was determined every 30 min for 24 h or 48 h by using a Bioscreen C.
DISCUSSION
S. gordonii is a particularly effective colonizer of the oral cavity and is present in significant proportions in healthy supra- and subgingival biofilms. To persist, this organism must adapt to often adverse and fluctuating environmental conditions, particularly variations in oxygen tension, acidification of the surroundings, and transitions between nutrient limitation and excess due to intermittent feeding by the host. A substantial effort has been focused on the adaptation strategies of caries and periodontal disease pathogens, but comparatively little information is available on these traits in the commensal flora associated with dental health. Such information is needed for the design of new strategies to control oral diseases by fostering the persistence of oral biofilms that are compatible with health. To our knowledge, this is the first report demonstrating a role for TCS in low-pH and oxygen-dependent activation of the AD genes in bacteria, yielding insights into the molecular basis for differential expression of one of the two major alkali-generating systems in dental biofilms. Our results also disclose significant differences and similarities in the functions of key TCS components in the modulation of ammonia production, an important protective mechanism against environmental acidification, and in the general stress tolerance properties of S. gordonii and S. mutans, an established oral pathogen.
The CiaRH, ComDE, and VicRK TCSs have been studied in some detail in S. mutans (4, 9, 29, 54) and contribute in various ways to acid tolerance, biofilm formation, and virulence gene expression. Recently, we determined that the Cia and Com TCSs also were involved in activation of the agmatine deiminase system (AgDS) of S. mutans. The AgDS is highly similar to the ADS: generating ammonia, CO2, and ATP from the decarboxylated derivative of arginine, agmatine, with putrescince instead of ornithine as an endproduct. Both systems are substrate inducible, catabolite repressible, and low pH inducible (14, 21, 38, 39), but the expression of the ADS (14), not the AgDS, is sensitive to oxygen (data not shown). The AgDS is believed to augment acid tolerance while concomitantly disposing of exogenous agmatine, which is inhibitory to the growth of S. mutans and other oral streptococci (21, 22). Interestingly, only the histidine kinases CiaH and ComD were required for AgDS induction in S. mutans, whereas the histidine kinases (CiaH and ComD) and the response regulators (CiaR and ComE) were required for ADS induction by low pH in S. gordonii. In S. mutans, the transcription of the comDE genes is influenced by CiaR (4), but there is no evidence yet for regulation of comDE by CiaRH in S. gordonii. Still, it is possible that differences in cross-regulation between CiaRH and ComDE could be one explanation for the disparate influences of components of these TCSs in the regulation of alkali generation in S. mutans and S. gordonii.
The transcription of the ADS operon of S. gordonii was found to be optimally induced under anaerobic conditions (14) and the present study showed contributions of both the Vic system and Flp to optimal expression of the ADS in response to the redox environment. Although our results suggest that Flp and Vic act independently (Fig. 2), further proof is needed to confirm that there is no interaction between Flp and the Vic system.
Another notable finding here is that inactivation of comDE resulted in an inability of S. gordonii to mount an ATR (Fig. 4), whereas mutants lacking cia and vic genes could undergo acid adaptation, as evidenced by enhanced resistance to killing at a lethal pH acquired during preexposure to mildly acidic conditions (Fig. 4). In fact, even though vic mutants were markedly more acid sensitive than the wild-type strain in the absence of acid adaptation, induction of the ATR in the vic mutants restored acid tolerance almost to the levels observed in SgWT cells that had been preadapted to low pH (Fig. 4). Thus, the ComDE system plays a critical role in adaptation to low pH in S. gordonii, whereas the CiaRH and VicRK systems primarily impact constitutive acid tolerance. Similar roles for ComDE, CiaRH, and VicRK vis-à-vis constitutive acid tolerance and the ATR were observed in S. mutans UA159 (4, 37a). Notably, mutants lacking comC, -D, or -E in S. mutans BM71 displayed an attenuated ATR but still acquired enhanced resistance to acid killing after adaptation at a mildly acidic pH (36).
The Vic system of S. mutans is critical for modulation of gene expression in response to aeration and regulates a variety of genes and phenotypes (2, 35), including autolysis. In S. mutans, strains lacking VicK showed modified adherence, biofilm formation, and genetic competence development, and it is believed that VicR can directly regulate the expression of several virulence-associated genes, including gtfBCD, ftf, and gbpB (2, 4, 9, 29, 34, 54). Interestingly, lack of VicR in S. gordonii resulted in slower growth, but also in a higher final yield, perhaps indicating a role for the Vic system in control of autolytic behavior. Consistent with this idea, the vic mutants of S. gordonii formed clumps after incubation in BHI medium, a trait that was not observed in wild-type S. gordonii, but which has been associated with altered autolysis (3). In fact, VicR-deficient strains of S. gordonii do show substantial changes in autolytic behavior (data not shown) and the underlying mechanisms are under investigation.
VicR-deficient S. gordonii were also more sensitive to growth in air or in the presence of the superoxide anion-generating compound paraquat (Fig. 3D), but VicK-deficient strains did not show any obvious defects in tolerance of oxidative stresses (Fig. 3D), perhaps indicating the potential for other sensor kinases to modulate the phosphorylation state of VicR. Consistent with the proposed role in responses to oxygen, the S. gordonii VicK protein contains a PAS domain, which can function in sensing of the redox state (46). Collectively, these results indicate that the Vic system in S. gordonii may function similarly to the Vic system of S. mutans and other WalRK homologs of gram-positive bacteria (17) by sensing redox and monitoring the integrity of the cell envelope. However, it should be reiterated that a vicR mutant of S. gordonii is viable, whereas efforts to generate a deletion mutation of vicR in S. pneumoniae and S. mutans have thus far been unsuccessful (2, 4, 9, 29, 34, 54). In addition, VicRK was required for low-pH induction of the AgDS in S. mutans but not the ADS in S. gordonii. Further analysis of the spectrum of genes and activities under the control of the Vic system in S. gordonii should prove useful for understanding how evolution may have shaped the functions of the Vic TCS in the oral pathogen S. mutans and the oral commensal S. gordonii.
The differences between S. gordonii and S. mutans in terms of the participation of various TCS components in the modulation of alkali-generating capacity and in the phenotypes elicited by inactivation of the TCS components is of particular interest. The evolutionary divergence of these TCSs may be due to the ecological and physiologic differences in the two species, and, in the case of the comDE genes, a result of the genes originating from different ancestral genes (12, 63). In particular, S. mutans is aciduric, and the organism does not generate substantial amounts of H2O2 when grown in aerobic environments (8, 30), whereas S. gordonii is considered only weakly acid tolerant (8), in the absence of arginine, and has an active H2O2-forming NADH-oxidase (47). In addition, evolutionary differences in the competence regulons of streptococci have been described by Martin et al. (43), including that ComDE of S. gordonii are more similar to ComDE of S. pneumoniae, than to those annotated in S. mutans. In fact, the S. mutans ComDE proteins appear to have evolved independently from the bacteriocin regulators BlpRH (43). Thus, the fundamental differences in acid tolerance and oxygen metabolism between these two organisms may be associated, at least in part, with the observations that individual mutations in the TCS studied here impact ADS, AgDS, and stress tolerance in various ways. Moreover, based on the behaviors of mutants lacking individual or double mutations in the TCS, it is also reasonable to predict that the abilities of the sensor kinases of these two organisms to “cross-regulate” (31) response regulators has also diverged.
In summary, the present study reveals additional complexities in the regulation of the ADS, sheds new light on the molecular mechanisms of stress tolerance by S. gordonii, and illustrates important differences of the roles and interactions of key TCSs in S. gordonii and closely related streptococci. S. gordonii has been suggested to play an important role in maintaining pH homeostasis in oral biofilms (11). Recently, a clinical study demonstrated that there were higher levels of salivary ADS activity in caries-free subjects compared to caries-active subjects (45). Therefore, the ADS has significant potential as an avenue to prevent dental caries in humans (11), so understanding how to optimize ADS expression in dental biofilms could be very beneficial. Continued investigation of CiaRH, ComDE, and VicRK TCSs of S. gordonii will be essential to develop a comprehensive understanding of the role of these systems in pathogenic and commensal streptococci.
Acknowledgments
We thank Sang-Joon Ahn and Lin Zeng for helpful suggestions.
This study was supported by Public Health Service grant DE10362 and DE13239 from the National Institute of Dental and Craniofacial Research.
Footnotes
Published ahead of print on 25 September 2009.
REFERENCES
- 1.Aas, J. A., A. L. Griffen, S. R. Dardis, A. M. Lee, I. Olsen, F. E. Dewhirst, E. J. Leys, and B. J. Paster. 2008. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 46:1407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, S. J., and R. A. Burne. 2007. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J. Bacteriol. 189:6293-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, S. J., and R. A. Burne. 2006. The atlA operon of Streptococcus mutans: role in autolysin maturation and cell surface biogenesis. J. Bacteriol. 188:6877-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn, S. J., Z. T. Wen, and R. A. Burne. 2006. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 74:1631-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archibald, R. M. 1944. Determination of citrulline and allantoin and demonstration of citrulline in blood plasma. J. Biol. Chem. 156:121-142. [Google Scholar]
- 6.Bayliss, R., C. Clarke, C. Oakley, W. Somerville, and A. G. Whitfield. 1983. The teeth and infective endocarditis. Br. Heart J. 50:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker, M. R., B. J. Paster, E. J. Leys, M. L. Moeschberger, S. G. Kenyon, J. L. Galvin, S. K. Boches, F. E. Dewhirst, and A. L. Griffen. 2002. Molecular analysis of bacterial species associated with childhood caries. J. Clin. Microbiol. 40:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender, G. R., S. V. Sutton, and R. E. Marquis. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas, I., L. Drake, D. Erkina, and S. Biswas. 2008. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. J. Bacteriol. 190:68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden, G. H. W., D. C. Ellwood, and I. R. Hamilton. 1979. Microbial ecology of the oral cavity. Adv. Microb. Ecol. 3:135-217. [Google Scholar]
- 11.Burne, R. A., and R. E. Marquis. 2000. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 193:1-6. [DOI] [PubMed] [Google Scholar]
- 12.Cock, P. J., and D. E. Whitworth. 2007. Evolution of prokaryotic two-component system signaling pathways: gene fusions and fissions. Mol. Biol. Evol. 24:2355-2357. [DOI] [PubMed] [Google Scholar]
- 13.Cunin, R., N. Glansdorff, A. Pierard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong, Y., Y. Y. Chen, and R. A. Burne. 2004. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J. Bacteriol. 186:2511-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong, Y., Y. Y. Chen, J. A. Snyder, and R. A. Burne. 2002. Isolation and molecular analysis of the gene cluster for the arginine deiminase system from Streptococcus gordonii DL1. Appl. Environ. Microbiol. 68:5549-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorel, C., O. Vidal, C. Prigent-Combaret, I. Vallet, and P. Lejeune. 1999. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol. Lett. 178:169-175. [DOI] [PubMed] [Google Scholar]
- 17.Dubrac, S., P. Bisicchia, K. M. Devine, and T. Msadek. 2008. A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol. Microbiol. 70:1307-1322. [DOI] [PubMed] [Google Scholar]
- 18.Fabret, C., and J. A. Hoch. 1998. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J. Bacteriol. 180:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier, B., and D. C. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 182:3955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frandsen, E. V., V. Pedrazzoli, and M. Kilian. 1991. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol. Immunol. 6:129-133. [DOI] [PubMed] [Google Scholar]
- 21.Griswold, A. R., M. Jameson-Lee, and R. A. Burne. 2006. Regulation and physiologic significance of the agmatine deiminase system of Streptococcus mutans UA159. J. Bacteriol. 188:834-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griswold, A. R., M. M. Nascimento, and R. A. Burne. 2009. Distribution, regulation and role of the agmatine deiminase system in mutans streptococci. Oral Microbiol. Immunol. 24:79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havarstein, L. S., P. Gaustad, I. F. Nes, and D. A. Morrison. 1996. Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol. 21:863-869. [DOI] [PubMed] [Google Scholar]
- 24.Herzberg, M. C., and M. W. Meyer. 1996. Effects of oral flora on platelets: possible consequences in cardiovascular disease. J. Periodontol. 67:1138-1142. [DOI] [PubMed] [Google Scholar]
- 25.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. ASM Press, Washington, D.C.
- 26.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2002. Oxidative stress tolerance is manganese (Mn2+) regulated in Streptococcus gordonii. Microbiology 148:3255-3263. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura, Y., X. G. Hou, F. Sultana, H. Miura, and T. Ezaki. 1995. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int. J. Syst. Bacteriol. 45:406-408. [DOI] [PubMed] [Google Scholar]
- 28.Kilic, A. O., L. Tao, Y. Zhang, Y. Lei, A. Khammanivong, and M. C. Herzberg. 2004. Involvement of Streptococcus gordonii β-glucoside metabolism systems in adhesion, biofilm formation, and in vivo gene expression. J. Bacteriol. 186:4246-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreth, J., D. C. Hung, J. Merritt, J. Perry, L. Zhu, S. D. Goodman, D. G. Cvitkovitch, W. Shi, and F. Qi. 2007. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology 153:1799-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 187:7193-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laub, M. T., and M. Goulian. 2007. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41:121-145. [DOI] [PubMed] [Google Scholar]
- 32.LeBlanc, D. J., Y. Y. Chen, and L. N. Lee. 1993. Identification and characterization of a mobilization gene in the streptococcal plasmid, pVA380-1. Plasmid 30:296-302. [DOI] [PubMed] [Google Scholar]
- 33.LeBlanc, D. J., and F. P. Hassell. 1976. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J. Bacteriol. 128:347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, S. F., G. D. Delaney, and M. Elkhateeb. 2004. A two-component covRS regulatory system regulates expression of fructosyltransferase and a novel extracellular carbohydrate in Streptococcus mutans. Infect. Immun. 72:3968-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levesque, C. M., R. W. Mair, J. A. Perry, P. C. Lau, Y. H. Li, and D. G. Cvitkovitch. 2007. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett. Appl. Microbiol. 45:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, Y. H., M. N. Hanna, G. Svensater, R. P. Ellen, and D. G. Cvitkovitch. 2001. Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. J. Bacteriol. 183:6875-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, Y. H., P. C. Lau, N. Tang, G. Svensater, R. P. Ellen, and D. G. Cvitkovitch. 2002. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 184:6333-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Liu, Y., and R. A. Bourne. 2009. Multiple two-component systems of Streptococcus mutans regulate agmatine deiminase gene expression and stress tolerance. J. Bacteriol. 191:7363-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, Y., Y. Dong, Y. Y. Chen, and R. A. Burne. 2008. Environmental and growth phase regulation of the Streptococcus gordonii arginine deiminase genes. Appl. Environ. Microbiol. 74:5023-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, Y., L. Zeng, and R. A. Burne. 2009. AguR is required for induction of the Streptococcus mutans agmatine deiminase system by low pH and agmatine. Appl. Environ. Microbiol. 75:2629-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 182:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lunsford, R. D. 1998. Streptococcal transformation: essential features and applications of a natural gene exchange system. Plasmid 39:10-20. [DOI] [PubMed] [Google Scholar]
- 42.Manford, M., J. Matharu, and K. Farrington. 1992. Infective endocarditis in a district general hospital. J. R. Soc. Med. 85:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin, B., Y. Quentin, G. Fichant, and J. P. Claverys. 2006. Independent evolution of competence regulatory cascades in streptococci? Trends Microbiol. 14:339-345. [DOI] [PubMed] [Google Scholar]
- 44.Martin, P. K., T. Li, D. Sun, D. P. Biek, and M. B. Schmid. 1999. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J. Bacteriol. 181:3666-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nascimento, M. M., V. V. Gordan, C. W. Garvan, C. M. Browngardt, and R. A. Burne. 2009. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol. Immunol. 24:89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng, W. L., G. T. Robertson, K. M. Kazmierczak, J. Zhao, R. Gilmour, and M. E. Winkler. 2003. Constitutive expression of PcsB suppresses the requirement for the essential VicR (YycF) response regulator in Streptococcus pneumoniae R6. Mol. Microbiol. 50:1647-1663. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen, P. T., J. Abranches, T. N. Phan, and R. E. Marquis. 2002. Repressed respiration of oral streptococci grown in biofilms. Curr. Microbiol. 44:262-266. [DOI] [PubMed] [Google Scholar]
- 48.Nyvad, B., and M. Kilian. 1990. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 24:267-272. [DOI] [PubMed] [Google Scholar]
- 49.Nyvad, B., and M. Kilian. 1990. Microflora associated with experimental root surface caries in humans. Infect. Immun. 58:1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parkins, M. D., H. Ceri, and D. G. Storey. 2001. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 40:1215-1226. [DOI] [PubMed] [Google Scholar]
- 52.Pearce, C., G. H. Bowden, M. Evans, S. P. Fitzsimmons, J. Johnson, M. J. Sheridan, R. Wientzen, and M. F. Cole. 1995. Identification of pioneer viridans streptococci in the oral cavity of human neonates. J. Med. Microbiol. 42:67-72. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook. J., and D. W. Russell. 2001. Preparation of reagents and buffers used in molecular cloning, p. A1.1-A1.30. In J. Argentine, N. Irwin, K. A. Janssen, S. Curtis, and M. Zierler (ed.), Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 54.Senadheera, M. D., B. Guggenheim, G. A. Spatafora, Y. C. Huang, J. Choi, D. C. Hung, J. S. Treglown, S. D. Goodman, R. P. Ellen, and D. G. Cvitkovitch. 2005. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J. Bacteriol. 187:4064-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shemesh, M., A. Tam, M. Feldman, and D. Steinberg. 2006. Differential expression profiles of Streptococcus mutans ftf, gtf, and vicR genes in the presence of dietary carbohydrates at early and late exponential growth phases. Carbohydr. Res. 341:2090-2097. [DOI] [PubMed] [Google Scholar]
- 56.Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent, Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]
- 57.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi, N., and T. Yamada. 1999. Acid-induced acid tolerance and acidogenicity of non-mutans streptococci. Oral Microbiol. Immunol. 14:43-48. [DOI] [PubMed] [Google Scholar]
- 59.Toledo-Arana, A., N. Merino, M. Vergara-Irigaray, M. Debarbouille, J. R. Penades, and I. Lasa. 2005. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J. Bacteriol. 187:5318-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Wuyckhuyse, B. C., H. E. Perinpanayagam, D. Bevacqua, R. F. Raubertas, R. J. Billings, W. H. Bowen, and L. A. Tabak. 1995. Association of free arginine and lysine concentrations in human parotid saliva with caries experience. J. Dent. Res. 74:686-690. [DOI] [PubMed] [Google Scholar]
- 61.Wen, Z. T., and R. A. Burne. 2004. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 186:2682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wexler, D. L., M. C. Hudson, and R. A. Burne. 1993. Streptococcus mutans fructosyltransferase (ftf) and glucosyltransferase (gtfBC) operon fusion strains in continuous culture. Infect. Immun. 61:1259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitworth, D. E., and P. J. Cock. 2009. Evolution of prokaryotic two-component systems: insights from comparative genomics. Amino Acids. 37:459-466. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, Y., Y. Lei, A. Khammanivong, and M. C. Herzberg. 2004. Identification of a novel two-component system in Streptococcus gordonii V288 involved in biofilm formation. Infect. Immun. 72:3489-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, Y., M. Whiteley, J. Kreth, Y. Lei, A. Khammanivong, J. N. Evavold, J. Fan, and M. C. Herzberg. 2009. The two-component system BfrAB regulates expression of ABC transporters in Streptococcus gordonii and Streptococcus sanguinis. Microbiology 155:165-173. [DOI] [PMC free article] [PubMed] [Google Scholar]