Abstract
Endotoxemia is undetectable for up to 60% of cases of bacteremia caused by gram-negative (GN) species, a discordance attributed to the limitations of the Limulus assay for endotoxemia. The lipid A structure of the endotoxin molecule is critical for the sensing of GN bacteria by the host immune system although not so for sensing by the Limulus assay. The lipid A structure of commensal Enterobacteriaceae is hexa-acyl, whereas non-Enterobacteriaceae have a broader range of structures. By using a previously published classification of lipid A structures (R. S. Munford, Infect. Immun. 76:454-465, 2008), the association of endotoxemia with bacteremia caused by GN organisms is reexamined for 580 GN bacteremic patients from 46 studies. Endotoxemia was less commonly detected for cases of bacteremia caused by Salmonella enterica serovar Typhi (four studies; 15 of 55 cases of bacteremia [27%]) than for cases of bacteremia caused by Neisseria meningitidis (five studies; 69 of 84 cases [82%]) and Pseudomonas pseudomallei (one study; 38 of 41 cases [93%]) among studies restricted to those with specified cases of bacteremia caused by GN organisms. Among 23 unrestricted studies, endotoxemia was less commonly detected for cases of bacteremia with a commensal member of the Enterobacteriaceae (104 of 240 cases [43%]) than with non-Enterobacteriaceae (59 of 100 cases [59%]) (summary odds ratio, 0.53 [90% confidence interval, 0.33 to 0.85]). This finding is consistent across all the unrestricted studies, even including studies with seemingly contrary results for endotoxemia diagnosis among cases of bacteremia caused by GN bacteria overall. Surprisingly, with bacteremia caused by commensal Enterobacteriaceae, the diagnosis of endotoxemia appears to be unrelated to the Limulus assay sensitivity. Across these 45 studies, the association of endotoxemia with GN bacteremia is variable but consistent for different types of GN bacteremia.
There are key structural differences between the lipid A components of the endotoxin molecule (lipopolysaccharide) of different gram-negative (GN) bacteria. Members of the Enterobacteriaceae characteristically have a lipid A structure with a hexa-acyl structure, whereas other lipid A structures are present for non-Enterobacteriaceae (45). These differences in lipid A structure are now known to be critically important for the recognition of GN bacteremia by the host immune system (45) by the MD-2-Toll-like receptor 4 receptor but not for sensing by the clotting proteins of the blood cells of the Limulus polyphemus horseshoe crab, from which the Limulus amebocyte lysate assay is derived (56). If the recognition of hexa-acyl lipid A by the host immune system has a role in the pathogenesis of bacteremia, it might be expected that the proportion with detectable endotoxemia among patients with GN bacteremia might depend on the lipid A structure of the isolate.
Attempts to define the concordance between GN bacteremia and endotoxemia in patients with sepsis have been elusive. Indeed, two of those studies (15, 54), with over a hundred patients each, concluded that there is no concordance. The purpose of this review is to attempt to reconcile the disparate findings from studies of clinically detected sepsis by examining the proportion with endotoxemia detected by using the Limulus assay for patients with different types of GN bacteremia for which the lipid A structures are known. Of particular interest is the proportion with endotoxemia among cases of bacteremia caused by commensal Enterobacteriaceae versus non-Enterobacteriaceae. The statistical techniques of meta-analysis are used to derive study-specific and summary estimates of these proportions expressed as an OR and more so to obtain estimates of the consistency in these ORs across the panel of studies (24).
MATERIALS AND METHODS
Data sources.
A comprehensive search of the literature from 1966 to April 2009 was performed by using a search strategy detailed previously (27, 29), and a call for data was issued (30). Clarifications of published data were sought from the original authors. This search was in addition to publications obtained from a library of several hundred publications related to clinical aspects of endotoxemia that I accumulated from repeatedly searching the literature over two decades (29, 33).
Study selection and classification of bacteria.
The following inclusion criteria were used: (i) comparison of the Limulus assay with blood cultures from patients with suspected GN bacteremia, (ii) the sensitivity of the Limulus assay to an internal endotoxin standard stated, and (iii) a minimum of two patients with GN bacteremia.
The studies were classified regarding whether they were restricted to examining one of four specific GN bacteremias (Salmonella enterica serovar Typhi, Neisseria meningitidis, Yersinia pestis, and Burkholderia pseudomallei) for which characteristic clinical features facilitate the diagnosis (restricted studies) versus studies that were unrestricted (unrestricted studies). For the unrestricted studies, the bacteria were classified into two groups according to the lipid A structure reported previously (see Table 1 of reference 45) into those known to have a hexa-acyl lipid A structure and those known to have a non-hexa-acyl lipid A structure. The group of bacterial isolates known to have a hexa-acyl lipid A structure is labeled commensal Enterobacteriaceae for the purposes of this analysis, as in the clinical setting, this group consists predominantly of commensal Enterobacteriaceae (Escherichia coli, Klebsiella pneumoniae, Serratia marcescens, Proteus mirabilis, and Providencia rettgeri). The group of bacterial isolates known to have a non-hexa-acyl lipid A is labeled as non-Enterobacteriaceae for the purposes of this analysis. Among the unrestricted studies, there were small numbers of the four specified GN bacteria, members of the Enterobacter species (which can have either a hexa-acyl [Enterobacter cloacae] or non-hexa-acyl [Enterobacter agglomerans] lipid A structure) and Haemophilus influenzae (hexa-acyl lipid A structure), and these were all analyzed separately.
TABLE 1.
Detection of endotoxemia in association with GN bacteremiaa
| Authors, yr (reference) | Limulus assay sensitivity limit (ng/ml) | No. (%) of patients with GN bacteremia caused by: |
|||
|---|---|---|---|---|---|
| Commensal Enterobacteriaceaeb |
Non-Enterobacteriaceaec |
||||
| Limulus positive | Limulus negative | Limulus positive | Limulus negative | ||
| Assay band Ad | |||||
| van Dissel et al., 1993 (58) | 0.001 | 3 | 0 | ND | ND |
| Goldie et al., 1995 (22)e | 0.002 | 2 | 1 | 3 | 1 |
| Yoshida et al., 1993 (61)e | 0.003 | 3 | 3 | 9 | 3 |
| Ahmed et al., 2004 (2) | 0.004 | 5 | 1 | 3 | 0 |
| Massignon et al., 1996 (42)e | 0.005f | 13 | 2 | 4 | 2 |
| Dofferhoff et al., 1992 (14) | 0.005 | 2 | 2 | 2 | 0 |
| Guidet et al., 1994 (23)e | 0.005 | 13 | 6 | 6 | 0 |
| Ketchum et al., 1997 (37)e | 0.005 | 8 | 32 | 2 | 6 |
| van Deventer et al., 1988 (57) | 0.005 | 14 | 4 | 1 | 0 |
| Subtotal for assay band A | 63 (55)f | 51 (45)f | 30 (71)g | 12 (29)g | |
| Assay band Bd | |||||
| Danner et al., 1991 (11)e | 0.01 | 3 | 4 | 2 | 0 |
| McCartney et al., 1987 (43) | 0.01 | 0 | 1 | 1 | 0 |
| Shenep et al., 1988 (52) | 0.025 | 3 | 1 | 1 | 0 |
| Giamarellos-Bourboulis et al., 1999 (21)e | 0.01 | 9 | 1 | ND | ND |
| Hass et al., 1986 (26) | 0.01 | ND | ND | 2 | 0 |
| Suyasa et al., 1995 (55) | 0.01 | ND | ND | 4 | 0 |
| Hynninen et al., 1995 (34) | 0.013 | 2 | 18 | 0 | 3 |
| Bion et al., 1994 (3) | 0.02 | ND | ND | 1 | 1 |
| Exley et al., 1992 (16) | 0.025 | 2 | 0 | ND | ND |
| Subtotal for assay band B | 19 (43)f | 25 (57)f | 11 (73)g | 4 (27)g | |
| Assay band Cd | |||||
| Prins et al., 1995 (49)e | 0.04 | 4 | 5 | 1 | 0 |
| Fossard and Kakkar, 1974 (19) | 0.1 | 1 | 0 | 1 | 0 |
| Pearson et al., 1985 (47) | 0.1 | 6 | 1 | ND | ND |
| Fink and Grunert, 1984 (18) | 0.1f | 1 | 0 | 2 | 0 |
| Scheifele et al., 1985 (51) | 0.2 | 2 | 1 | ND | ND |
| Stumacher et al., 1973 (54) | 0.5 | 14 | 26 | 5 | 8 |
| Oberle et al., 1974 (46) | 0.5 | 1 | 0 | 2 | 0 |
| van Wieringen et al., 1976 (59) | 0.5 | 3 | 0 | ND | ND |
| Subtotal for assay band C | 32 (49)f | 33 (51)f | 11 (58)g | 8 (42)g | |
| Assay band Dd | |||||
| Cooperstock and Riegle, 1985 (10) | 1 | 3 | 0 | 3 | 1 |
| Feldman and Pearson, 1974 (17) | 1 | 0 | 12 | 2 | 12 |
| Jirillo et al., 1975 (35) | 1 | 1 | 1 | ND | ND |
| Lau et al., 1996 (38) | 1f | 8 | 3 | ND | ND |
| Clumeck et al., 1977 (9) | 3 | 5 | 3 | 2 | 0 |
| Kelsey et al., 1982 (36) | 5 | 0 | 1 | 1 | 0 |
| Levin et al., 1972 (39) | 5 | 10 | 9 | 6 | 1 |
| Martinez et al., 1973 (41) | 5 | 1 | 6 | 0 | 3 |
| Subtotal for assay band D | 28 (44)g | 35 (56)g | 14 (45)h | 17 (55)h | |
| Total excluding studies with ND | 108 (44) | 137 (56) | 59 (60) | 40 (40) | |
| Total all studies | 142 (49) | 144 (51) | 66 (62) | 41 (38) | |
The following bacteria causing bacteremia (with numbers of Limulus amebocyte lysate-positive patients and the total numbers of patients) from 17 studies (2, 9, 10, 11, 17, 22, 23, 34, 37, 39, 41, 52, 54, 57, 58, 59, 61) are not included in this analysis: N. meningitides (six of six), Salmonella species (seven of nine), Enterobacter species (13 of 33), and Haemophilus influenzae (eight of nine). ND, no data.
Commensal Enterobacteriaceae as defined in Materials and Methods.
Non-Enterobacteriaceae include 74 cases of bacteremia with Pseudomonas species, 6 cases of bacteremia with Acinetobacter species, and 20 cases of bacteremia with anaerobic bacteria (Fusobacterium species and Bacteroides species).
The assay bands and assay sensitivity limits of the included studies are as defined in Materials and Methods.
Data were clarified by personal communication from study authors.
The Limulus assay sensitivity limit in these studies was expressed in endotoxin units per ml, and a conversion equivalence of 1 endotoxin unit/ml to 100 pg/ml of endotoxin has been used here.
Test for equality of the proportion of patients with endotoxemia among patients with bacteremia and a commensal Enterobacteriaceae isolate detected among subtotals of bands of studies (chi-squared value, 2.27; P = 0.52; 3 df).
Test for equality of the proportion with endotoxemia among patients with bacteremia and an non-Enterobacteriaceae isolate detected among subtotals of bands of studies (chi-squared value, 5.7; P = 0.13; 3 df) with a test for trend (chi-squared value, 4.9; P = 0.03; 1 df).
Data extraction and analysis: calculation of an OR.
The endotoxemia diagnosis data for all studies were extracted for all those with GN bacteremia on a per-patient basis. For those unrestricted studies with data available for both commensal Enterobacteriaceae and non-Enterobacteriaceae, the data were extracted into a 2-by-2 contingency table format based on the diagnosis of endotoxemia (Limulus positive) versus no diagnosis (Limulus negative) and the type of GN bacteremia (commensal Enterobacteriaceae versus non-Enterobacteriaceae) to enable the calculation of a study-specific OR and 90% confidence interval (CI). The summary OR and 90% CI were derived by using the Metan command (24) in STATA (release 10.0; STATA Corp., College Station, TX).
Assay sensitivity.
The sensitivity of the Limulus assay used in each study is the sensitivity to the internal endotoxin standard stated in each study. The unrestricted studies were stratified into four bands of assay sensitivity as follows: <0.01 ng/ml (assay band A), ≥0.01 to 0.03 ng/ml (assay band B), 0.033 to 0.9 ng/ml (assay band C), and ≥1 ng/ml (assay band D). A nonparametric test for trend was analyzed by using the subtotals derived for each band. The trend in the proportion with detectable endotoxemia across the four bands was then analyzed separately for the commensal Enterobacteriaceae and the non-Enterobacteriaceae.
RESULTS
Characteristics of the studies.
Forty-six studies were identified, reporting the results for a total of 580 patients with GN bacteremia published since 1970 (Tables 1 and 2). The listed sensitivity to the internal endotoxin standard used in the assays was in the range of 0.001 to 5 ng/ml. There were 18 studies that had fewer than six patients with GN bacteremia. There was evidence suggestive of publication bias in that 10 of the 18 of studies with fewer than six patients with GN bacteremia reported that the proportion of cases of GN bacteremia that have endotoxemia was 100% (Tables 1 and 2).
TABLE 2.
Detection of endotoxemia in association with bacteremia among studies of four specified GN infections (restricted studies)
| Organism and authors, yr (reference) | Assay sensitivity limit (ng/ml) | No. (%) of patients |
|
|---|---|---|---|
| Limulus positive | Limulus negative | ||
| Neisseria meningitidis | |||
| Bjorvatn et al., 1984 (4) | 0.005 | 4 | 0 |
| Brandtzaeg et al., 1989 (5) | 0.005 | 24 | 11 |
| Gardlund et al., 1995 (20) | 0.01 | 9 | 1 |
| Prins et al., 1998 (48) | 0.04 | 30 | 1 |
| Harthug et al., 1983 (25) | 0.5 | 2 | 2 |
| Subtotal for N. meningitidis | 69 (82) | 15 (18) | |
| Yersinia pestis | |||
| Butler et al., 1973 (7) | 0.5 | 2 | 0 |
| Butler et al., 1976 (8) | 5 | 3 | 2 |
| Subtotal for Y. pestis | 5 (71) | 2 (29) | |
| Burkholderia pseudomallei | |||
| Simpson et al., 2000 (53) | 0.002 | 38 (93) | 3 (7) |
| Salmonella serovar Typhi | |||
| McGladdery et al., 1993 (44) | 0.04 | 0 | 16 |
| Adinolfi et al., 1987 (1) | 0.3 | 7 | 7 |
| Butler et al., 1978 (6) | 1 | 0 | 13 |
| Magliulo et al., 1976 (40) | 1 | 8 | 4 |
| Subtotal for S. Typhi | 15 (27) | 40 (63) | |
Unrestricted studies.
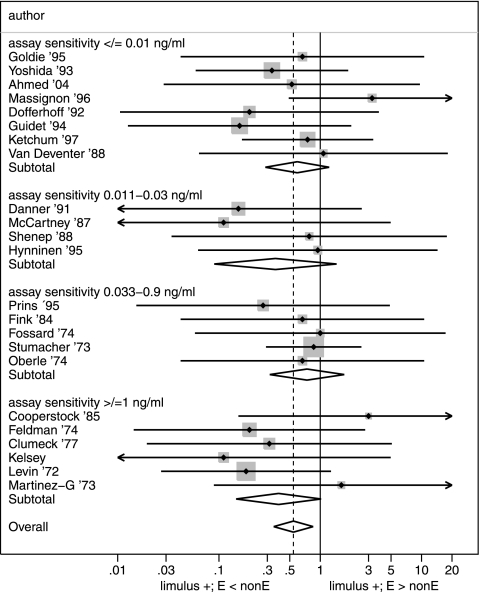
Of the 34 unrestricted studies, there were 23 with data for both commensal Enterobacteriaceae and non-Enterobacteriaceae. Among those 23 studies, the proportion of cases of GN bacteremia in each study that were commensal Enterobacteriaceae varied from 33% to 100% (median, 60%). From these 23 studies, an OR could be calculated for the proportion with detectable endotoxemia among those with GN bacteremia caused by commensal Enterobacteriaceae versus non-Enterobacteriaceae (Fig. 1). The summary OR was 0.53 (90% CI, 0.33 to 0.85), and there was no significant heterogeneity in the study-specific OR (chi-squared value, 8.8; P = 0.98; 22 df). As a sensitivity analysis, the analysis was repeated after excluding studies with fewer than six bacteremic patients, and the summary OR was 0.55 (90% CI, 0.34 to 0.9), also with no significant heterogeneity (chi-squared value, 7.8; P = 0.96; 16 df). Overall, of the 11 studies with data missing for either commensal Enterobacteriaceae or non-Enterobacteriaceae bacteremia, endotoxemia was detected in 41 of 49 (84%) cases of bacteremia (Table 1).
FIG. 1.
Forest plot of ORs for endotoxemia with different types of GN bacteremia. The proportions of positive Limulus assay tests (limulus +) for patients with commensal Enterobacteriaceae versus non-Enterobacteriaceae GN bacteremia are presented as study-specific ORs and summary ORs (and 90% CIs) derived from all 23 unrestricted studies without missing data from Table 1, with studies ranked by order of Limulus assay sensitivity. Summary ORs for each subcategory of assay band are also displayed. Arrowheads indicate 90% CIs that extend out of range; limulus + E < nonE indicates the range in ORs for which positive Limulus tests are less common for bacteremia caused by Enterobacteriaceae than non-Enterobacteriaceae; limulus + E > nonE indicates the range in ORs for which positive Limulus tests are more common for bacteremia caused by Enterobacteriaceae than non-Enterobacteriaceae.
Restricted studies.
Among 12 studies that were restricted to one of the four specified types of GN bacteremia (Table 2), the proportion with detectable endotoxemia was lowest for studies that examined Salmonella serovar Typhi bacteremia (four studies; 15 of 55 cases of bacteremia [27%]) compared to studies of Neisseria meningitidis (five studies; 69 of 84 cases of bacteremia [82%]) and Pseudomonas pseudomallei (one study; 38 of 41 cases of bacteremia [93%]).
Assay sensitivity.
Among all 34 of the unrestricted studies, there was no significant difference in the proportion of cases with detectable endotoxemia over the bands of listed assay sensitivity for the commensal Enterobacteriaceae (chi-squared value, 2.27; P = 0.52; 3 df), although among the non-Enterobacteriaceae, there was a significant trend toward a higher proportion with detectable endotoxemia in the bands with the more sensitive assays (chi-squared value, 4.9; P = 0.03; 1 df). Repetition of the analysis after excluding studies with fewer than six cases of bacteremia reached the same conclusion (data not shown).
DISCUSSION
In this analysis of 46 clinical studies of endotoxemia diagnosis using the Limulus assay for cases of GN bacteremia with different types of lipid A structures, three surprising observations are noted. Among the unrestricted studies, the proportion of cases with detectable endotoxemia is lower when the GN bacterium responsible for bacteremia is a member of the commensal Enterobacteriaceae than when it its not a member of the Enterobacteriaceae. Moreover, this finding is consistent across a broad range of studies, including studies (39, 54) that reached seemingly contrary findings regarding the diagnostic utility of the Limulus assay with regard to endotoxemia diagnosis among cases of bacteremia caused by GN bacteria overall (Fig. 1). A second finding is that the proportion with detectable endotoxemia for cases of bacteremia caused by commensal Enterobacteriaceae appears to be unrelated to the sensitivity limit of the Limulus assay, as used in each study, over a 1,000-fold range in sensitivity to an internal endotoxin standard. Third, the proportion of cases of bacteremia with associated endotoxemia caused by GN species is highest among studies that were restricted to cases of bacteremia with N. meningitidis or B. pseudomallei, and these findings are not representative of the general diagnostic experience among unrestricted studies.
There is possible evidence of publication bias with studies that were smaller or had missing data showing atypical results. However, the conclusions of this analysis are robust regarding the inclusion or exclusion of these studies.
There are a number of limitations of this analysis. The 34 unrestricted studies were heterogeneous with respect to year of publication, patient numbers and demographics, bacteremia diagnosis and isolate identification methods, plasma extraction methods, and types of GN bacteremia. A further limitation is that the number of samples evaluated per patient in each study is not known and cannot be evaluated. Despite this heterogeneity among the studies, there is no statistical heterogeneity in the study-specific ORs among the unrestricted studies. This would not have been so readily appreciated from a either a single study in isolation or a review of the studies in the traditional narrative style without the statistical techniques of meta-analysis. Moreover, this analysis reveals that the range in the proportion of cases of bacteremia caused by commensal Enterobacteriaceae versus non-Enterobacteriaceae among the total number of cases of GN bacteremia varies widely, from 33% to 100%. This could account for the contrary conclusions regarding the diagnostic utility of the Limulus assay made in studies with a low (54) or high (39) proportion of commensal Enterobacteriaceae among the cases of GN bacteremia.
The presumption that in patients with GN sepsis, GN bacteremia and endotoxemia must always be associated with each other, even at low levels, in part contributed to earlier criticisms of the Limulus assay (15, 54) and stimulated the development of more sensitive versions. The findings that in other clinical settings, the concentration of endotoxin detected by the Limulus assay is proportional to the GN bacterial CFU count, such as in bronchoalveolar lavage samples from patients with pneumonia (50) and in urine samples of bacteruric patients (32), reinforced this presumption. However, this analysis of GN bacteremia indicates that the type of bacteremia isolate is critical to the application of the Limulus assay, which helps to explain why the mix of GN bacteremia isolates is influential in the concordance of GN bacteremia with the Limulus test of plasma (29). This is because endotoxin is undetectable for >50% of cases of bacteremia caused by the Enterobacteriaceae, regardless of the level of assay sensitivity.
The Limulus assay, a biological assay, is the most sensitive test available for the diagnosis of endotoxin (13). However, in comparisons using either the Limulus assay or other biological assays, the activity of endotoxin is not a uniform gravimetric property for endotoxins of different bacterial origins (12). Additionally, both the reaction kinetics (33) and the sensing of endotoxin by the Limulus assay (60) are affected by human plasma. It remains to be determined whether these effects of plasma differ for endotoxins from different types of GN bacteria. Moreover, the relationship between the diagnosis of endotoxemia and the clinical manifestations of sepsis has not been examined here (28, 31).
This analysis of 46 previously published clinical studies does not support the presumption that for patients with GN sepsis, GN bacteremia must always be associated with endotoxemia, but it suggests that there is a variable association of endotoxemia with GN bacteremia, which is consistent for different types of GN bacteremia.
Acknowledgments
I thank M. Yoshida, Jichi Medical School, Japan; E. J. Giamarellos-Bourboulis, Laiko General Hospital, Athens, Greece; R. Danner, NIH, Bethesda, MD; B. Guidet, Hopital Saint-Antoine, Paris, France; K. C. H. Fearon, Royal Infirmary, Edinburgh, Scotland; D. Massignon, Center Hospitalier Lyon Sud, France; and D. Bates, Brigham and Women's Hospital, Boston, MA, for the clarification of previously published data.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Adinolfi, L. E., R. Utili, G. B. Gaeta, P. Perna, and G. Ruggiero. 1987. Presence of endotoxemia and its relationship to liver dysfunction in patients with typhoid fever. Infection 15:359-362. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, T., M. A. Azam, N. Armed, K. M. Jamil, F. Hassan, N. Ogura, H. Tamura, and T. Yokochi. 2004. Detection of endotoxin in sera from children hospitalized for treatment of diarrhea in Bangladesh. J. Endotox. Res. 10:223-228. [DOI] [PubMed] [Google Scholar]
- 3.Bion, J. F., I. Badger, H. A. Crosby, P. Hutchings, K. L. Kong, J. Baker, P. Hutton, P. McMaster, J. A. Buckels, and T. S. Elliott. 1994. Selective decontamination of the digestive tract reduces gram-negative pulmonary colonization but not systemic endotoxemia in patients undergoing elective liver transplantation. Crit. Care Med. 22:40-49. [DOI] [PubMed] [Google Scholar]
- 4.Bjorvatn, B., L. Bjertnaes, H. O. Fadnes, T. Flaegstad, T. J. Gutteberg, B. E. Kristiansen, J. Pape, O. P. Rekvig, B. Osterud, and L. Aanderud. 1984. Meningococcal septicaemia treated with combined plasmapheresis and leucapheresis or with blood exchange. BMJ 288:439-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159:195-204. [DOI] [PubMed] [Google Scholar]
- 6.Butler, T., W. R. Bell, J. Levin, N. N. Linh, and K. Arnold. 1978. Typhoid fever. Studies of blood coagulation, bacteremia, and endotoxemia. Arch. Intern. Med. 138:407-410. [DOI] [PubMed] [Google Scholar]
- 7.Butler, T., J. Levin, C. Do Quang, and R. I. Walker. 1973. Bubonic plague: detection of endotoxemia with the limulus test. Ann. Intern. Med. 79:642-646. [DOI] [PubMed] [Google Scholar]
- 8.Butler, T., J. Levin, N. N. Linh, D. M. Chau, M. Adickman, and K. Arnold. 1976. Yersinia pestis infection in Vietnam. II. Quantiative blood cultures and detection of endotoxin in the cerebrospinal fluid of patients with meningitis. J. Infect. Dis. 133:493-499. [DOI] [PubMed] [Google Scholar]
- 9.Clumeck, N., S. Lauwers, A. Kahn, M. Mommens, and J. P. Butzler. 1976. Contribution of the “limulus test” to the diagnosis of endotoxemias and meningitis due to gram negative bacteria. Nouv. Presse Med. 6:1451-1454. (In French.) [PubMed] [Google Scholar]
- 10.Cooperstock, M., and L. Riegle. 1985. Plasma Limulus gelation assay in infants and children: correlation with gram negative bacterial infection and evidence for “intestinal endotoxemia.” Prog. Clin. Biol. Res. 189:329-345. [PubMed] [Google Scholar]
- 11.Danner, R. L., R. J. Elin, J. M. Hosseini, R. A. Wesley, J. M. Reilly, and J. E. Parillo. 1991. Endotoxemia in human septic shock. Chest 99:169-175. [DOI] [PubMed] [Google Scholar]
- 12.Dehus, O., T. Hartung, and C. Hermann. 2006. Endotoxin evaluation of eleven lipopolysaccharides by whole blood assay does not always correlate with Limulus amebocyte lysate assay. J. Endotox. Res. 12:171-180. [DOI] [PubMed] [Google Scholar]
- 13.Devleeschouwer, M. J., M. F. Cornil, and J. Dony. 1985. Studies on the sensitivity and specificity of the Limulus amebocyte lysate test and rabbit pyrogen assays. Appl. Environ. Microbiol. 50:1509-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dofferhoff, A. S., V. J. Bom, H. G. de Vries-Hospers, J. van Ingen, J. van der Meer, B. P. Hazenberg, P. O. Mulder, and J. Weits. 1992. Patterns of cytokines, plasma endotoxin, plasminogen activator inhibitor, and acute-phase proteins during the treatment of severe sepsis in humans. Crit. Care Med. 20:185-192. [DOI] [PubMed] [Google Scholar]
- 15.Elin, R. J., R. A. Robinson, A. S. Levine, and S. M. Wolff. 1975. Lack of clinical usefulness of the limulus test in the diagnosis of endotoxemia. N. Engl. J. Med. 293:521-524. [DOI] [PubMed] [Google Scholar]
- 16.Exley, A. R., T. Leese, M. P. Holliday, R. A. Swann, and J. Cohen. 1992. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut 33:1126-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman, S., and T. A. Pearson. 1974. The Limulus test and gram-negative bacillary sepsis. Am. J. Dis. Child. 128:172-174. [DOI] [PubMed] [Google Scholar]
- 18.Fink, P. C., and J. H. Grunert. 1984. Endotoxemia in intensive care patients: a longitudinal study with the limulus amebocyte lysate test. Klin. Wochenschr. 62:986-991. (In German.) [DOI] [PubMed] [Google Scholar]
- 19.Fossard, D. P., and V. V. Kakkar. 1974. The Limulus test in experimental and clinical endotoxaemia. Br. J. Surg. 61:798-804. [DOI] [PubMed] [Google Scholar]
- 20.Gardlund, B., J. Sjolin, A. Nilsson, M. Roll, C. J. Wickerts, and B. Wretlind. 1995. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J. Infect. Dis. 172:296-301. [DOI] [PubMed] [Google Scholar]
- 21.Giamarellos-Bourboulis, E. J., J. Perdios, M. Lelekis, E. Eoconomou, P. Tsouroulas, and H. Giamarellou. 1999. Impact of cefuroxime administration on endotoxin (LPS) and tumour necrosis factor-a (TNF-a) blood levels in patients suffering from acute pyelonephritis: a preliminary report. Int. J. Antimicrob. Agents 11:115-119. [DOI] [PubMed] [Google Scholar]
- 22.Goldie, A. S., K. C. Fearon, J. A. Ross, G. R. Barclay, R. E. Jackson, I. S. Grant, G. Ramsay, A. S. Blyth, J. C. Howie, et al. 1995. Natural cytokine antagonists and endogenous antiendotoxin core antibodies in sepsis syndrome. JAMA 274:172-177. [PubMed] [Google Scholar]
- 23.Guidet, B., V. Barakett, T. Vassal, J. C. Petit, and G. Offenstadt. 1994. Endotoxemia and bacteremia in patients with sepsis syndrome in the intensive care unit. Chest 106:1194-1201. [DOI] [PubMed] [Google Scholar]
- 24.Harris, R. J., M. J. Bradburn, J. J. Deeks, R. M. Harbord, D. G. Altman, and J. A. C. Sterne. 2008. Metan: fixed- and random-effects meta-analysis. Stata J. 8:3-28. [Google Scholar]
- 25.Harthug, S., B. Bjorvatn, and B. Osterud. 1983. Quantitation of endotoxin in blood from patients with meningococcal disease using a limulus lysate test in combination with chromogenic substrate. Infection 11:192-195. [DOI] [PubMed] [Google Scholar]
- 26.Hass, A., M. I. Rossberg, H. L. Hodes, A. C. Hyatt, and D. S. Hodes. 1986. Endotoxin levels in immunocompromised children with fever. J. Pediatr. 109:265-269. [DOI] [PubMed] [Google Scholar]
- 27.Hurley, J. C. 1994. Concordance of endotoxemia with gram-negative bacteremia in patients with gram-negative sepsis: a meta-analysis. J. Clin. Microbiol. 32:2120-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurley, J. C. 1995. Endotoxemia: methods of detection and clinical correlates. Clin. Microbiol. Rev. 8:268-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurley, J. C. 2000. Concordance of endotoxemia with gram-negative bacteremia. A meta-analysis using receiver operating characteristic curves. Arch. Pathol. Lab. Med. 124:1157-1164. [DOI] [PubMed] [Google Scholar]
- 30.Hurley, J. C. 2001. Endotoxemia and gram-negative bacteremia as predictors of outcome in sepsis: a call for data. J. Endotox. Res. 7:467. [DOI] [PubMed] [Google Scholar]
- 31.Hurley, J. C., and J. Levin. 1999. Relevance of endotoxin detection in sepsis, p. 841-854. In H. Brade, S. Opal, S. Vogel, and D. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker Limited, New York, NY.
- 32.Hurley, J. C., and F. A. Tosolini. 1992. A quantitative micro-assay for endotoxin and correlation with bacterial density in urine. J. Microbiol. Methods 16:91-99. [Google Scholar]
- 33.Hurley, J. C., F. A. Tosolini, and W. J. Louis. 1991. Quantitative Limulus lysate assay for endotoxin and the effect of plasma. J. Clin. Pathol. 44:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hynninen, M., M. Valtonen, M. Vaara, H. Markkanen, P. Kuusela, H. Saxen, and O. Takkunen. 1995. Plasma endotoxin and cytokine levels in neutropenic and non-neutropenic bacteremic patients. Eur. J. Clin. Microbiol. Infect. Dis. 14:1039-1045. [DOI] [PubMed] [Google Scholar]
- 35.Jirillo, E., N. Pasquetto, L. Marcuccio, R. Monno, P. De Rinaldis, and D. Fumarola. 1975. Endotoxemia detected by Limulus assay in severe malnourished children. Plasma effects on leucocyte migration: preliminary investigations. G. Batteriol. Virol. Immunol. 68:174-178. (In Italian.) [PubMed] [Google Scholar]
- 36.Kelsey, M. C., A. P. Lipscomb, and J. M. Mowles. 1982. Limulus amoebocyte lysate endotoxin test: an aid to the diagnosis in the septic neonate? J. Infect. 4:69-72. [DOI] [PubMed] [Google Scholar]
- 37.Ketchum, P. A., J. Parsonnet, L. S. Stotts, T. J. Novitsky, B. Schlain, and D. W. Bates. 1997. Utilization of a chromogenic Limulus amebocyte lysate blood assay in a multi-center study of sepsis. J. Endotox. Res. 4:9-16. [Google Scholar]
- 38.Lau, J. Y., S. C. Chung, J. W. Leung, T. K. Ling, M. Y. Yung, and A. K. Li. 1996. Endoscopic drainage aborts endotoxaemia in acute cholangitis. Br. J. Surg. 83:181-184. [PubMed] [Google Scholar]
- 39.Levin, J., T. E. Poore, N. S. Young, S. Margolis, N. P. Zauber, A. S. Townes, and W. R. Bell. 1972. Gram-negative sepsis: detection of endotoxemia with the limulus test. With studies of associated changes in blood coagulation, serum lipids, and complement. Ann. Intern. Med. 76:1-7. [DOI] [PubMed] [Google Scholar]
- 40.Magliulo, E., D. Scevola, D. Fumarola, R. Vaccaro, A. Bertotto, and S. Burberi. 1976. Clinical experience in detecting endotoxemia with the limulus test in typhoid fever and other Salmonella infections. Infection 4:21-24. [DOI] [PubMed] [Google Scholar]
- 41.Martinez, L. A., R. Quintiliani, and R. C. Tilton. 1973. Clinical experience on the detection of endotoxemia with the limulus test. J. Infect. Dis. 127:102-105. [DOI] [PubMed] [Google Scholar]
- 42.Massignon, D., A. Lepape, G. Debize, M. F. Remillieux, V. DePasquale, V. Banssillon, P. Coeur, et al. 1996. Detection of gram-negative bacteraemia in early sepsis by a quantitative chromogenic and kinetic endotoxin assay. Eur. J. Clin. Investig. 26:596-601. [DOI] [PubMed] [Google Scholar]
- 43.McCartney, A. C., M. R. Robertson, B. I. Piotrowicz, and N. P. Lucie. 1987. Endotoxaemia, fever and clinical status in immunosuppressed patients: a preliminary study. J. Infect. 15:201-206. [DOI] [PubMed] [Google Scholar]
- 44.McGladdery, S., R. Larasati, N. Silitonga, N. Punjabi, M. Lesmana, S. Pulungsih, and P. O'Hanley. 1993. Acute inflammatory cytokine response in typhoid fever, abstr. 284. Abstracts of the 1993 IDSA Annual Meeting. Clin. Infect. Dis. 17:578. [Google Scholar]
- 45.Munford, R. S. 2008. Sensing gram-negative bacterial lipopolysaccharides: a human disease determinant? Infect. Immun. 76:454-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oberle, M. W., G. G. Graham, and J. Levin. 1974. Detection of endotoxemia with the Limulus test: preliminary studies in severely malnourished children. J. Pediatr. 85:570-573. [DOI] [PubMed] [Google Scholar]
- 47.Pearson, F. C., J. Dubczak, M. Weary, G. Bruszer, and G. Donohue. 1985. Detection of endotoxin in the plasma of patients with gram-negative bacterial sepsis by the Limulus amoebocyte lysate assay. J. Clin. Microbiol. 21:865-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prins, J. M., F. N. Lauw, B. H. Derkx, P. Speelman, E. J. Kuijper, J. Dankert, and S. J. van Deventer. 1998. Endotoxin release and cytokine production in acute and chronic meningococcaemia. Clin. Exp. Immunol. 114:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prins, J. M., M. A. van Agtmael, E. J. Kuijper, S. J. van Deventer, and P. Speelman. 1995. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J. Infect. Dis. 172:886-891. [DOI] [PubMed] [Google Scholar]
- 50.Pugin, J., R. Auckenthaler, O. Delaspre, E. van Gessel, and P. M. Suter. 1992. Rapid diagnosis of gram negative pneumonia by assay of endotoxin in bronchoalveolar lavage fluid. Thorax 47:547-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheifele, D. W., E. M. Olsen, and M. R. Pendray. 1985. Endotoxinemia and thrombocytopenia during neonatal necrotizing enterocolitis. Am. J. Clin. Pathol. 83:227-229. [DOI] [PubMed] [Google Scholar]
- 52.Shenep, J. L., P. M. Flynn, F. F. Barrett, G. L. Stidham, and D. F. Westenkirchner. 1988. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J. Infect. Dis. 157:565-568. [DOI] [PubMed] [Google Scholar]
- 53.Simpson, A. J., S. M. Opal, B. J. Angus, J. M. Prins, J. E. Palardy, N. A. Parejo, W. Chaowagul, and N. J. White. 2000. Differential antibiotic-induced endotoxin release in severe melioidosis. J. Infect. Dis. 181:1014-1019. [DOI] [PubMed] [Google Scholar]
- 54.Stumacher, R. J., M. J. Kovnat, and W. R. McCabe. 1973. Limitations of the usefulness of the Limulus assay for endotoxin. N. Engl. J. Med. 288:1261-1264. [DOI] [PubMed] [Google Scholar]
- 55.Suyasa, I. G., I. G. Reka, K. Inada, H. Suda, M. Kojima, K. Mushiaki, S. Okamoto, and M. Yoshida. 1995. Plasma endotoxin in typhoid fever. Kobe J. Med. Sci. 41:175-186. [PubMed] [Google Scholar]
- 56.Takada, H., S. Kotani, S. Tanaka, T. Ogawa, I. Takahashi, M. Tsujimoto, T. Komuro, T. Shiba, S. Kusumoto, N. Kusunose, A. Hasegawa, and M. Kiso. 1988. Structural requirements of lipid A species in activation of clotting enzymes from the horseshoe crab, and the human complement cascade. Eur. J. Biochem. 175:573-580. [DOI] [PubMed] [Google Scholar]
- 57.van Deventer, S. J., H. R. Buller, J. W. ten Cate, A. Sturk, and W. Pauw. 1988. Endotoxaemia: an early predictor of septicaemia in febrile patients. Lancet i:605-609. [DOI] [PubMed] [Google Scholar]
- 58.van Dissel, J. T., R. van Furth, B. A. Compier, H. D. Feuth, and M. Frolich. 1993. Survival in selected patients with gram-negative sepsis after adjunctive therapy with HA-1A. Lancet 341:959-960. [DOI] [PubMed] [Google Scholar]
- 59.van Wieringen, P. M., L. A. Monnens, and J. A. Bakkeren. 1976. Hemolytic-uremic syndrome: absence of circulating endotoxin. Pediatrics 58:561-563. [PubMed] [Google Scholar]
- 60.Warren, H. S., T. J. Novitsky, P. A. Ketchum, P. F. Roslansky, S. Kania, and G. R. Siber. 1985. Neutralization of bacterial lipopolysaccharides by human plasma. J. Clin. Microbiol. 22:590-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida, M., T. Obayashi, H. Tamura, S. Tanaka, T. Kawai, S. Sakamoto, and Y. Miura. 1994. Diagnostic and prognostic significance of plasma endotoxin determination in febrile patients with haematological malignancies. Eur. J. Cancer 30A:145-147. [DOI] [PubMed] [Google Scholar]