Abstract
An intervention study was conducted to determine whether discontinuing the feeding of milk replacer medicated with oxytetracycline and neomycin to preweaned calves reduced antimicrobial resistance in Salmonella, Campylobacter, and Escherichia coli bacteria. Results demonstrated that the intervention did reduce multidrug resistance in these bacteria but that other factors also influenced multidrug resistance.
Antimicrobial agents are commonly added to preweaned calf milk replacer diets to improve calf growth and decrease morbidity and mortality (1, 13, 14). However, this practice has been implicated in the development of drug resistance in bacteria (6, 11, 12) and subsequent risk of human infection with resistant zoonotic bacteria (4, 7). To address this issue, a study was conducted to determine whether discontinuing feeding of milk replacer medicated with oxytetracycline and neomycin to preweaned calves resulted in increased antimicrobial susceptibility in Salmonella, Campylobacter, and Escherichia coli bacteria isolated from calves and dairy farm environments (8).
Detailed descriptions of sample and data collection, sample processing, and laboratory methods for this study have been given elsewhere (8). Four dairy herds from Michigan and four from New York were enrolled from an earlier project (3, 5). Two large and two small herds from each state were selected, and each pair was divided into the intervention and control groups. All farms in the 3-month preintervention phase fed calves milk replacer containing oxytetracycline and neomycin without ionophores. Intervention herds then began receiving the same brand of milk replacer without antimicrobials (during the postintervention phase). Samples from all herds were obtained monthly: 3 times preintervention and 12 times postintervention.
Preweaned female dairy calves were randomly selected on the day of each farm visit, and individual 10-g fecal samples were obtained by rectal retrieval. Separate composite samples from calf and maternity pens were collected by using individual sterile gauze swabs (soaked in sterile double-strength skim milk) for four calf hutches and the corresponding maternity pen.
Commercially prepared broth microdilution antimicrobial panels (Trek Diagnostics, Inc.) were used for susceptibility testing for E. coli (CMV7CNCD), Salmonella (CMV7CNCD), and Campylobacter (CAMPY) isolates, and E. coli ATCC 25922 was used for quality control. The CMV7CNCD panel contained tetracycline, amikacin, Amoxicillin (amoxicilline)-clavulanic acid, ampicillin, ceftiofur, ceftriaxone, cephalothin (cefalotin), chloramphenicol, ciprofloxacin, cefoxitin, gentamicin, kanamycin, nalidixic acid, streptomycin, sulfamethoxazole, and trimethoprim-sulfamethoxazole, and the CAMPY panel contained azithromycin, ciprofloxacin, clindamycin, erythromycin, florfenicol, gentamicin, nalidixic acid, telithromycin, and tetracycline. Bacterial suspensions for broth microdilution were prepared and processed according to the instructions of the panel manufacturer (Trek Diagnostics, Inc.). MICs from each panel for E. coli and Salmonella were read with an autoreader, and Campylobacter panel results were read manually. The breakpoints used were those recommended by the Clinical and Laboratory Standards Institute (CLSI) and the National Antimicrobial Resistance Monitoring System 2000 annual report (16) for E. coli and Salmonella and by the National Antimicrobial Resistance Monitoring System for Campylobacter in retail meat (15). Isolates classified as intermediate or resistant were considered to be resistant for the purposes of analysis.
Differences in antimicrobial susceptibility between intervention and control herds, measured as percentages of isolates demonstrating susceptibility and numbers of isolates corresponding to specific dilutions, were calculated for E. coli, Salmonella, and Campylobacter and assessed for significance using Fisher's exact test for categorical outcomes and the Kruskal-Wallis (KW) χ2 rank-sum test for continuous outcomes. Multidrug resistance (MDR) was measured by determining the average number of agents to which fecal isolates demonstrated resistance (NAIR) at each sampling visit and the percentages of isolates demonstrating one of the following three commonly reported resistance patterns at each visit: kanamycin-streptomycin-sulfamethoxazole-tetracycline (KSSuT) resistance, ampicillin-kanamycin-streptomycin-sulfamethoxazole-tetracycline (AKSSuT) resistance, and ampicillin-chloramphenicol-streptomycin-sulfamethoxazole-tetracycline (ACSSuT) resistance. Outcomes used in analyses were the differences between each of these measures at a given visit and the corresponding measures for isolates collected during the preintervention period.
Repeated-measures generalized linear mixed models for the E. coli MDR outcomes were developed (using PROC GLIMMIX in SAS software version 9.3.1) by a hierarchical backwards model-building approach. Models were developed for both herd and isolate levels and included random-effects terms for the herd, the state and the herd size, and the month relative to the start of intervention to account for time in the models (analyses were not conducted for Salmonella and Campylobacter due to the low numbers of isolates). The main risk factor included in the models was the intervention status (association with the intervention or control group), and potential covariates included the average age of animals tested, the rates of diarrhea in preweaned calves and adult cattle, and the mortality rates in preweaned calves and adult cattle at the time of sampling; the use of oxytetracycline (yes or no) for calves and cows; and MDR of E. coli isolates from calf pens and maternity pens at the time of sampling.
Results.
Characteristics of the herds enrolled in the study have been reported elsewhere (8). Briefly, there were 126 preweaned calves, 1,120 weaned calves, and 1,517 cows at the beginning of the study. The recovery rates for E. coli, Salmonella, and Campylobacter isolates were 97, 9, and 4%, respectively, from calf fecal samples and 67, 10, and 5%, respectively, from environmental samples.
A total of 1,439 E. coli, 161 Salmonella, and 82 Campylobacter isolates were available for antimicrobial susceptibility testing. Only 9.9% of E. coli isolates, 15.5% of Salmonella isolates, and 12.2% of Campylobacter isolates were pansusceptible. Fecal samples had the highest proportions of resistant isolates (95.5, 95.6, and 90.1% of E. coli, Salmonella, and Campylobacter isolates, respectively), followed by samples from calf pens for E. coli and Salmonella isolates (81 and 40%, respectively) and those from maternity pens for E. coli and Salmonella isolates (22.2 and 9.1%, respectively). Maternity pen Campylobacter isolates had higher rates of resistance than calf pen Campylobacter isolates (75 and 60%, respectively).
MDR was common in both E. coli and Salmonella but was uncommon in Campylobacter. Overall, 88.5% of E. coli isolates, 84.5% of Salmonella isolates, and 11% of Campylobacter isolates demonstrated MDR. Differences in levels of pentaresistance (resistance to five or more agents) between groups of E. coli isolates were found, with 67.4% of isolates from control herds and 50.5% of isolates from intervention herds having pentaresistance (P, <0.01 by Fisher's exact two-tailed test; odds ratio [OR] and 95% confidence interval [95% CI] for intervention, 0.5 and 0.4 to 0.6). For Salmonella, intervention herds had statistically significantly (P < 0.0001) lower levels of pentaresistant isolates (45.4%; OR, 0.05; 95% CI, 0.03 to 0.1) than control herds (94%). The highest levels of MDR were seen in calf fecal samples (94.2% of E. coli isolates and 95.6% of Salmonella isolates were MDR). Commonly reported patterns of antimicrobial resistance (KSSuT, AKSSuT, and ACSSuT resistance) were present among E. coli and Salmonella isolates (Table 1). The most common pattern of MDR seen in Campylobacter was combined resistance to tetracycline and nalidixic acid. Isolates from diarrheal calves corresponded to higher NAIRs than nondiarrhea isolates (5.6% versus 4.9%; P = 0.04 by KW χ2 test).
TABLE 1.
Common patterns of MDR in E. coli and Salmonellaa
| Organism | Resistance pattern(s) | Intervention group |
Control group |
P value (Fisher's exact test) | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| No. of isolates | % of isolates | No. of isolates | % of isolates | |||||
| E. coli | All patterns | 628 | 811 | |||||
| KSSuT resistance | 346 | 55.1 | 560 | 69.1 | <0.01 | 0.54 | 0.46-0.65 | |
| AKSSuT resistance | 189 | 30.1 | 323 | 39.8 | <0.01 | 0.64 | 0.54-0.78 | |
| ACSSuT resistance | 94 | 15.0 | 125 | 15.4 | 0.81 | 0.85 | 0.67-1.08 | |
| Salmonella | All patterns | 35 | 126 | |||||
| KSSuT resistance | 34 | 97.1 | 126 | 100.0 | 0.21 | 0.09 | 0.003-2.13 | |
| AKSSuT resistance | 34 | 97.1 | 126 | 100.0 | 0.21 | 0.09 | 0.003-2.13 | |
| ACSSuT resistance | 12 | 34.3 | 124 | 98.4 | <0.01 | 0.01 | 0.001-0.03 | |
Data from all isolates are included.
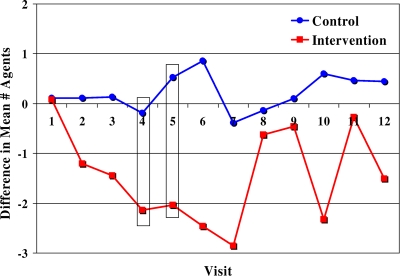
After the intervention was started, isolates from intervention herds had statistically significantly greater decreases in MDR than those from control herds (P < 0.05; KW χ2 test), as indicated by the NAIRs and percentages of isolates with KSSuT and ACSSuT resistance. Time from the beginning of the intervention was associated with reducing resistance in E. coli isolates from intervention herds in the first 2 to 5 months postintervention, but this effect decreased afterwards (Fig. 1). MDR levels declined after the initiation of the intervention but later returned to preintervention levels. This pattern has been reported in other studies (10), and investigators found that it takes 8 years for significant shifts in the genetic composition of E. coli after starting organic practices (20), which suggests that more time is necessary to see long-term changes in cattle gut flora on the intervention farms.
FIG. 1.
Differences in mean numbers of agents to which E. coli isolates demonstrated resistance at the preintervention time points and at each postintervention visit, by group. Boxed results are significantly different (P ≤ 0.05).
Results of herd- and isolate-level (Table 2) multivariable analyses found that the intervention, oxytetracycline use, the presence of MDR in farm environments, calf age, and herd-level measures of disease and mortality were significantly associated with the NAIR and proportions of isolates with KSSuT, AKSSuT, and ACSSuT resistance. Calf age was associated with changes in MDR in fecal E. coli isolates, which agrees with results from studies indicating that resistance declines as calves age (5, 9, 20). Positive associations between MDR and morbidity have been reported in other studies (2, 17, 18, 19) and were confirmed by the positive associations found in this study between resistance and herd-level rates of diarrhea in preweaned calves and cows in the month prior to sample collection.
TABLE 2.
Results from multivariable repeated-measures generalized linear regression modelsa
| Risk factor | OR (95% CI) for NAIR at: |
OR (95% CI) for KSSuT resistance at: |
OR (95% CI) for AKSSuT resistance at: |
OR (95% CI) for ACSSuT resistance at: |
||||
|---|---|---|---|---|---|---|---|---|
| Isolate level | Herd level | Isolate level | Herd level | Isolate level | Herd level | Isolate level | Herd level | |
| Intervention | 0.18 (0.07-0.48) | 0.13 (0.04-0.46) | 0.83 (0.67-1.03) | 0.82 (0.69-0.97) | 1.09 (0.96-1.23) | 0.88 (0.79-0.98) | 0.97 (0.93-1.01) | 0.87 (0.76-1.00) |
| NAIR for calf pen isolates | 1.13 (1.01-1.26) | 1.04 (1.01-1.07) | 1.19 (1.06-1.34) | |||||
| Proportion of calf pen isolates with KSSuT resistance | 1.55 (1.00-2.41) | |||||||
| Proportion of calf pen isolates with ACSSuT resistance | 0.85 (0.76-0.95) | |||||||
| NAIR for maternity pen isolates | 1.02 (1.00-1.04) | 0.99 (0.98-1.00) | ||||||
| Preweaned calf diarrhea rate | 1.97 (1.11-3.29) | 1.43 (1.01-2.03) | ||||||
| Preweaned calf mortality rate | 0.01 (0.00-0.39) | 0.36 (0.18-0.72) | ||||||
| Cow diarrhea rate | 2.47 (1.36-4.51) | |||||||
| Cow mortality rate | 0.0 (0.00-0.12) | |||||||
| Oxytetracycline use in cows | 3.37 (1.81-6.29) | 3.89 (1.31-11.57) | 1.16 (1.04-1.29) | 1.12 (1.02-1.24) | 1.16 (1.02-1.32) | |||
| Age in days (per 10 days) | 0.87 (0.80-0.96) | NA | NA | 0.99 (0.97-1.00) | NA | 0.98 (0.97-0.99) | NA | |
| Treatment of calves before sampling | 2.14 (1.19-3.85) | NA | NA | NA | NA | |||
| Model−2 log likelihood | 5,771.14 | 272.28 | 1,612.15 | 78.05 | 1,641.90 | 35.06 | 961.25 | 28.35 |
Results from multivariable repeated-measures generalized herd-level (n = 84 isolates) and isolate-level (n = 1,179 isolates) linear regression models for change in the proportion of isolates demonstrating different patterns of resistance from the pre- to postintervention periods, with matching for state and herd size and controlling for time (visit number). NA, not applicable.
Conclusions.
We demonstrated that stopping the feeding of medicated milk replacer can reduce MDR in E. coli and Salmonella isolates from calves, maternity pens, and calf pens on the farm, but additional work is needed to conclusively confirm these findings. Increasing the numbers of herds in the study and monitoring these herds for longer periods of time would be useful in determining if there are longer-term effects of discontinuing medicated milk replacers.
Acknowledgments
This work was supported by grant USDA-IREEGCP 2002-5110-01980 from the U.S. Department of Agriculture Research, Education, and Extension Competitive Grants Program.
Footnotes
Published ahead of print on 21 October 2009.
REFERENCES
- 1.Berge, A. C. B., P. Lindeque, D. A. Moore, and W. M. Sischo. 2005. A clinical trial evaluating prophylactic and therapeutic antibiotic use on health and performance of preweaned calves. J. Dairy Sci. 88:2166-2177. [DOI] [PubMed] [Google Scholar]
- 2.Bettelheim, K. A., M. A. Hornitzky, S. P. Djordevic, and A. Kuzevski. 2003. Antibiotic resistance among verocytotoxigenic Escherichia coli (VTEC) and non-VTEC isolated from domestic animals and humans. J. Med. Microbiol. 52:155-162. [DOI] [PubMed] [Google Scholar]
- 3.Fossler, C. P., S. J. Wells, J. B. Kaneene, P. L. Ruegg, L. D. Warnick, J. B. Bender, S. M. Godden, L. W. Halbert, A. M. Campbell, and A. M. Zwald. 2004. Prevalence of Salmonella spp. on conventional and organic dairy farms. J. Am. Vet. Med. Assoc. 225:567-573. [DOI] [PubMed] [Google Scholar]
- 4.Halbert, L. W., J. B. Kaneene, P. L. Ruegg, L. D. Warnick, S. J. Wells, L. S. Mansfield, C. P. Fossler, A. M. Campbell, and A. M. Geiger-Zwald. 2006. Evaluation of antimicrobial susceptibility patterns in Campylobacter spp isolated from dairy cattle and farms managed organically and conventionally in the midwestern and northeastern United States. J. Am. Vet. Med. Assoc. 228:1074-1081. [DOI] [PubMed] [Google Scholar]
- 5.Hoyle, D. V., H. I. Knight, D. J. Shaw, K. Hillman, M. C. Pearce, J. C. Low, G. J. Gunn, and M. E. J. Woolhouse. 2004. Acquisition and epidemiology of antibiotic-resistant Escherichia coli in a cohort of newborn calves. J. Antimicrob. Chemother. 53:867-871. [DOI] [PubMed] [Google Scholar]
- 6.Inglis, G. D., T. A. McAllister, H. W. Busz, L. J. Yanke, D. W. Morck, M. E. Olson, and R. R. Read. 2005. Effects of subtherapeutic administration of antimicrobial agents to beef cattle on the prevalence of antimicrobial resistance in Campylobacter jejuni and Campylobacter hyointestinalis. Appl. Environ. Microbiol. 71:3872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juhasz-Kaszanyitzky, E., S. Janosi, P. Somogyi, A. Dan, L. van der Graaf-van Bloois, E. van Duijkeren, and J. A. Wagenaar. 2007. MRSA transmission between cows and humans. Emerg. Infect. Dis. 13:630-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneene, J. B., L. D. Warnick, C. A. Bolin, R. J. Erskine, K. May, and R. Miller. 2008. Changes in tetracycline susceptibility of enteric bacteria following switching to nonmedicated milk replacer for dairy calves. J. Clin. Microbiol. 46:1968-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khachatryan, A. R., D. D. Hancock, T. E. Besser, and D. R. Call. 2004. Role of calf-adapted Escherichia coli in maintenance of antimicrobial drug resistance in dairy calves. Appl. Environ. Microbiol. 70:752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langlois, B. E., K. A. Dawson, T. S. Stahly, and G. L. Cromwell. 1984. Antibiotic resistance of fecal coliforms from swine fed subtherapeutic and therapeutic levels of chlortetracycline. J. Anim. Sci. 58:666-674. [DOI] [PubMed] [Google Scholar]
- 11.Luangtongkum, T., T. Y. Morishita, A. J. Ison, S. Huang, P. F. McDermott, and Q. Zhang. 2006. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl. Environ. Microbiol. 72:3600-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez, J. L. 2009. The role of natural environments in the evolution of resistance traits in pathogenic bacteria. Proc. R. Soc. B 276:2521-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrill, J. L., A. D. Dayton, and R. Mickelson. 1977. Cultured milk and antibiotics for young calves. J. Dairy Sci. 60:1105-1109. [Google Scholar]
- 14.Morrill, J. L., J. M. Morrill, A. M. Feyerham, and J. F. Laster. 1995. Plasma protein and probiotics as ingredients in milk replacer. J. Dairy Sci. 78:902-907. [DOI] [PubMed] [Google Scholar]
- 15.National Antimicrobial Resistance Monitoring System—Enteric Bacteria. 2004. Retail meat annual report, 2004. Food and Drug Administration, Washington, DC. http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm062597.htm.
- 16.National Antimicrobial Resistance Monitoring System—Enteric Bacteria (Veterinary Isolates). 12 January 2005, revision date. Salmonella—final report, 2000. USDA Agricultural Research Service, Washington, DC. http://www.ars.usda.gov/Business/docs.htm?docid=6760.
- 17.Oloya, J., M. Theis, D. Doetkott, N. Dyer, P. Gibbs, and M. L. Khaitsa. 2007. Evaluation of Salmonella occurrence in domestic animals and humans in North Dakota (2000-2005). Foodborne Pathog. Dis. 4:551-563. [DOI] [PubMed] [Google Scholar]
- 18.Ray, K. A., L. D. Warnick, R. M. Mitchell, J. B. Kaneene, P. L. Ruegg, S. J. Wells, C. P. Fossler, L. W. Halbert, and K. May. 2006. Antimicrobial susceptibility of Salmonella from organic and conventional dairy farms. J. Dairy Sci. 89:2038-2050. [DOI] [PubMed] [Google Scholar]
- 19.Ray, K. A., L. D. Warnick, R. M. Mitchell, J. B. Kaneene, P. L. Ruegg, S. J. Wells, C. P. Fossler, L. W. Halbert, and K. May. 2007. Prevalence of antimicrobial resistance among Salmonella on midwest and northeast USA dairy farms. Prev. Vet. Med. 79:204-223. [DOI] [PubMed] [Google Scholar]
- 20.Walk, S. T., J. M. Mladonicky, J. A. Middleton, A. J. Heidt, J. R. Cunningham, P. Bartlett, K. Sato, and T. S. Whittam. 2007. Influence of antibiotic selection on genetic composition of Escherichia coli populations from conventional and organic dairy farms. Appl. Environ. Microbiol. 73:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]