Abstract
Rhizomucor variabilis and Hormographiella aspergillata rarely cause human infections. This report details a fatal case of a 14-year-old female with leukemia posthematopoietic cell transplant and relapse with refractory pancytopenia. The patient first developed an R. variabilis var. regularior palate infection and later developed a cutaneous H. aspergillata infection while on posaconazole and caspofungin therapy.
CASE REPORT
The patient was a 14-year-old female with a history of acute myelogenous leukemia diagnosed in July 2007. The patient underwent an allogeneic HLA-matched bone marrow transplant in December 2007 and experienced relapse in April 2008, with a bone marrow aspirate showing a preponderance of blasts, and later failed reinduction chemotherapy. In September 2008, a computed tomography (CT) scan of the chest 2 weeks prior to admission revealed patchy ground-glass opacities with tiny peripheral nodular densities in both lung fields (sparing the left upper lobe) and a 1.2-cm nodule in the right upper lobe. The patient's condition was deemed too fragile to tolerate a diagnostic lung biopsy. She was empirically treated with broad antibacterial and antifungal coverage that included voriconazole. Of interest, the patient also started drinking an herbal tea remedy of an unknown variety. Shortly afterwards, the patient presented with a 2-week history of odynophagia and persistent febrile neutropenia. An examination showed that the patient had white plaques involving the soft palate and pharynx. A smear stained with calcofluor white from a throat culture showed hyphal elements and conidiophores. A biopsy of the palate lesion showed submucosa and mucosa infiltrated with hyphal forms with sparse septation, rare branching, and chlamydoconidia. The patient was started empirically on 800-mg oral (p.o.) doses of posaconazole twice a day (BID). A CT scan of the head and sinuses was negative, while a CT scan of the lungs confirmed pulmonary nodules that had been previously visualized. Serial galactomannan assay results were negative. The fungal isolate obtained in culture from palate biopsy was tentatively identified as a Rhizomucor sp. by the Mount Sinai clinical microbiology laboratory. The isolate was sent to the Mycology Laboratory of the New York State Department of Health for further characterization. The option of surgical debridement was declined by the family due to potentially severe morbidity. At week 2 of therapy, caspofungin was added as an adjunct therapy. By week 2 of therapy, the lesion had decreased in size, with improved symptoms. At week 3 of therapy, she began receiving a new regimen of chemotherapy, along with granulocyte infusions. By week 4, the patient's symptoms had resolved. By week 5 of therapy, the palate lesion was no longer visualized. Throughout, the patient had persistent severe refractory pancytopenia. One and one-half months after initiation of therapy, she developed an altered mental status and had a generalized seizure. A CT scan of the brain showed multiple hypodense lesions of the cerebral hemispheres and cerebellum. Serum and cerebrospinal fluid Toxoplasma and cryptococcal studies were negative. A repeat CT scan of the lungs showed cavitating lesions in the right upper lobe/right middle lobe. At that time, the patient was switched to liposomal amphotericin (Ambisome) for better central nervous system penetration, caspofungin was maintained, and posaconazole discontinued. At this time, she developed high fevers, and a new small erythematous skin papule developed on the right knee. The skin was biopsied. Ten days later, a new skin lesion appeared on the left arm. The patient's respiratory status progressively deteriorated, and she died from respiratory failure 2 weeks after the appearance of the initial skin lesion. Cultures obtained from the skin biopsy yielded a white mold, which was identified postmortem as Hormographiella aspergillata and sent to the Mycology Laboratory of the NYS Department of Health for confirmation and susceptibility testing. No autopsy was performed.
The throat biopsy specimen was cultured on Sabouraud dextrose agar (SDA), Mycosel, and inhibitory mold agar. All cultures were incubated at 30°C in ambient air. Growth of a mold from this culture was evident on the SDA plate after 3 days. The isolate was transferred to a potato dextrose agar plate and overlaid with sterile coverslips and incubated as described above. The mold was examined by placing the coverslip onto a glass slide with a lactophenol cotton blue stain. The slide culture revealed an organism with branched round sporangia arising from hyphae which possessed rhizoids between the stolons. The identification of Rhizomucor sp. was made based on morphology and was confirmed as R. variabilis by genetic analysis (Fig. 1). Internal transcribed spacer 2 (ITS2) PCR, nucleotide sequencing, and BLAST searches using two databases—GenBank (www.ncbi.nlm.nih.gov/) and Centraalbureau voor Schimmelcultures (www.cbs.knaw.nl/yeast/BioloMICSSequences.aspx)-revealed the Rhizomucor isolate to be 100% identical to Rhizomucor variabilis var. regularior (CBS 384.95). The ITS2 gene sequence of this isolate was deposited in GenBank (accession number GQ338322). Growth from the skin biopsy specimen was evident first on the blood agar plate from the bacteriology culture. The mold was subcultured onto a potato dextrose agar plate with sterile coverslips and examined as described above. Macroscopically, there was rapid growth of a white cottony mold with a brownish reverse. Microscopically, the mold exhibited septate hyphae with cylindrical arthroconidia that formed whorls at the apex. The mold was identified as Hormographiella sp. based on its morphological characteristics (Fig. 2) and as Coprinus cinereus (of which H. aspergillata is the anamorph) by genetic analysis. This isolate was 100% identical to Coprinus cinereus (GenBank accession number AB097562; anamorph, Hormographiella aspergillata). Its ITS2 gene sequence was also deposited in GenBank (accession number GQ338323). Molecular identifications in these two instances were reconciled with descriptions of morphological characteristics (2). Antifungal susceptibility tests (with single drugs and two-drug combinations) were performed on both isolates according to CLSI M-38A2 protocol and checkerboard titrations (1). Rhizomucor variabilis var. regularior was susceptible to amphotericin B (1.0 μg/ml), posaconazole (1.0 μg/ml), and voriconazole (1.0 μg/ml) and nonsusceptible to echinocandins (>4.0 μg/ml). The combination of echinocandins with amphotericin B was synergistic (ΣFICi = 0.265, where ΣFICi is the summation fractional inhibitory concentration index). Hormographiella aspergillata was susceptible to posaconazole (0.5 μg/ml) and voriconazole (0.25 μg/ml) and resistant to amphotericin B (4.0 μg/ml) and echinocandins (>4.0 μg/ml). The combination of echinocandins with amphotericin B was synergistic (ΣFICi = 0.075).
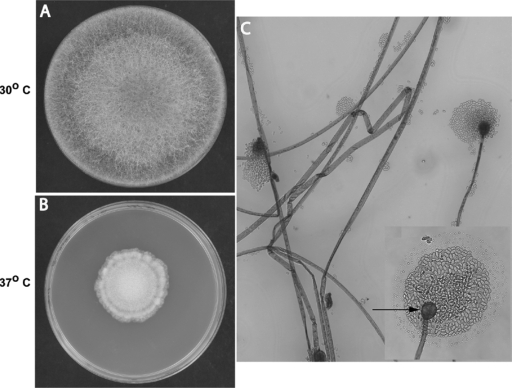
FIG. 1.
Rhizomucor variabilis var. regularior. (A and B) Macroscopic morphology on potato dextrose agar. Colonies were filling the entire plate in 5 days at 30°C (A) and showed restricted growth at 37°C (B). Colonies varied in color from brown to tan and were hairy, with reverse buff-to-brown color. (C) Microscopic morphology in lactophenol cotton blue stain after 3 days (magnification, ×200): hyaline, unbranched, ribbon-like hyphae; long, simple sporangiophores arising from hyphae and ending in sporangium; spherical sporangia with globose columella and no apophysis; hyaline, ellipsoidal, smooth-walled sporangiospores. The inset shows details of the spherical columella (arrow) with sporangiospores (magnification, ×400).
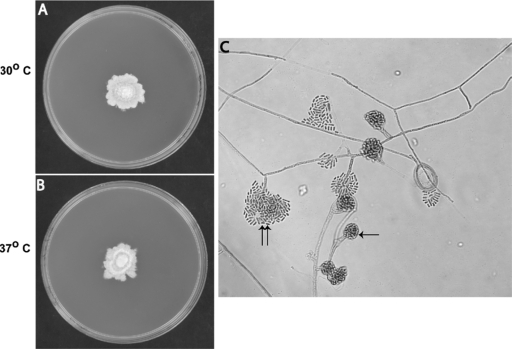
FIG. 2.
Hormographiella aspergillata. (A and B) Macroscopic morphology on potato dextrose agar. Colonies grew 3 cm in 10 days at 30°C (A) and at 37°C (B). Colonies varied in color from white to cream and were velvety with cottony tufts, with irregular margin with pale reverse color. (C) Microscopic morphology in lactophenol cotton blue stain after 10 days: hyaline septate hyphae; simple, well-differentiated conidiophores; catenate, hyaline, smooth-walled, single-cell arthroconidia produced at the end of conidiophores either in clusters (single arrow) or in irregular groups (double arrow).
Rhizomucor species are an infrequent cause of human disease, while Rhizomucor variabilis is rarely reported as an etiologic agent, with few case reports published recently (8, 14). In one retrospective review of zygomycosis in children, among 77 culture-confirmed cases, only one was found to be due to Rhizomucor species (13), while another review of 929 cases documented only 19 secondary infections due to Rhizomucor species (9). Our patient's localized palate infection responded to the combination of posaconazole and caspofungin with granulocyte infusions. Although it is not possible to establish the relative contribution of each of these therapies, it is important to note that the granulocyte infusions were initiated 1 week after the antifungal agents and that a decrease in the size of the lesion along with alleviation of the patient's symptoms was seen prior to their initiation. The patient's isolate showed an MIC of 1 μg/ml for both posaconazole and amphotericin and was resistant to caspofungin. Several papers demonstrate the efficacy of posaconazole as salvage therapy for zygomycosis in patients refractory to standard therapy (4, 6, 11). Our patient's R. variabilis isolate had a ΣFIC of 0.265 for the combinations of amphotericin-caspofungin and amphotericin-posaconazole and 0.18 for the combination of caspofungin-posaconazole, which underscores the fact that the combination used in this case was synergistic at least in vitro.
The patient's second isolate, H. aspergillata, isolated from a cutaneous lesion, is also a rare cause of human pathology. It is probably underdiagnosed, as it is hard to identify by routine methods. Published reports on this pathogen include a case of prosthetic mitral valve endocarditis that was successfully treated with amphotericin B (5). Cases of pulmonary disease include a post-stem-cell-transplant acute myelogenous leukemia patient who died while on caspofungin therapy and a non-Hodgkin's lymphoma patient who had recovered from a neutropenic episode and was successfully treated with amphotericin B (7, 10, 12). In vitro studies have shown that H. aspergillata shows variable susceptibility to amphotericin B, resistance to flucytosine, and uniform susceptibility to the azoles with the exception of fluconazole (3). Our patient's isolate was in fact resistant to amphotericin with an MIC of 4 μg/ml, resistant to caspofungin with an MIC of >2 μg/ml, resistant to micafungin with an MIC of >4 μg/ml, susceptible to voriconazole with an MIC of 0.25 μg/ml, and susceptible to posaconazole with an MIC of 0.5 μg/ml.
Due to the lack of an autopsy, it is impossible to know definitively whether this patient's disseminated disease with brain and lung involvement was secondary to the R. variabilis or H. aspergillata infection. However, given the resolution of her initial palate lesion while on therapy, the timing of the development of the brain lesions, which coincided with the development of her cutaneous lesions, and her clinical deterioration after the appearance of the skin lesions, one can hypothesize that the disseminated disease was likely secondary to H. aspergillata infection. The possibility of two different simultaneous fungal infections in the immunocompromised host is underscored in this report. This possibility along with data showing synergy among different agents illustrates the importance of considering combination therapy in cases of invasive fungal infections. Posaconazole, as illustrated by our patient's isolate and other in vitro susceptibility studies, seems to have one of the lower MICs for zygomycosis, although the clinical significance of such MICs has yet to be established (13). Although better, larger in vivo studies and clinical trials are needed, posaconazole with its broad spectrum shows promise in combination with other agents as a potential treatment option for invasive fungal infections.
Acknowledgments
We thank Tom Walsh at The National Institutes of Health, Bethesda, MD, for his valuable input.
Footnotes
Published ahead of print on 21 October 2009.
REFERENCES
- 1.Chaturvedi, V., R. Ramani, M. A. Ghannoum, S. B. Killian, N. Holliday, C. Knapp, L. Ostrosky-Zeichner, S. A. Messer, M. A. Pfaller, N. J. Iqbal, B. A. Arthington-Skaggs, J. A. Vazquez, T. Sein, J. H. Rex, and T. J. Walsh. 2008. Multilaboratory testing of antifungal combinations against a quality control isolate of Candida krusei. Antimicrob. Agents Chemother. 52:1500-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 3.Gené, J., J. M. Guillamon, J. Guarro, I. Pujol, and K. Ulfig. 1996. Molecular characterization, relatedness and antifungal susceptibility of the basidiomycetous Hormographiella species and Coprinus cinereus from clinical and environmental sources. Antonie van Leeuwenhoek 70:49-57. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg, R. N., K. Mullane, J.-A. H. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greer, E. L., T. J. Kowalski, M. L. Cole, D. V. Miller, and L. M. Baddour. 2008. Truffle's revenge: a pig-eating fungus. Cardiovasc. Pathol. 17:342-343. [DOI] [PubMed] [Google Scholar]
- 6.Guembe, M., J. Guinaca, T. Pelaez, M. Torres-Narbona, and E. Bouza. 2007. Synergistic effect of posaconazole and caspofungin against clinical zygomycetes. Antimicrob. Agents Chemother. 51:3457-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagrou, K., C. Massonet, K. Theunissen, W. Meersseman, M. Lontie, E. Verbeken, J. Van Eldere, and J. Maertens. 2005. Fatal pulmonary infection in a leukaemic patient caused by Hormographiella aspergillata. J. Med. Microbiol. 54:685-688. [DOI] [PubMed] [Google Scholar]
- 8.Lu, X. L., Z. H. Liu, Y. N. Shen, X. D. She, G. X. Lu, P. Zhan, M. H. Fu, X. L. Zhang, Y. P. Ge, and W. D. Liu. 2009. Primary cutaneous zygomycosis caused by Rhizomucor variabilis: a new endemic zygomycosis? A case report and review of 6 cases reported from China. Clin. Infect. Dis. 49:e39-e43. [DOI] [PubMed] [Google Scholar]
- 9.Roden, M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaufele, M. Sein, T. Sein, J. H. Chu, D. P. Kontoyiannis, and T. J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 10.Surmont, I., F. Van Aelst, J. Verbanck, and G. S. De Hoog. 2002. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med. Mycol. 40:217-219. [DOI] [PubMed] [Google Scholar]
- 11.van Burik, J., R. Hare, H. F. Solomon, M. L. Corrado, and D. P. Kontoyiannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:e61-e65. [DOI] [PubMed] [Google Scholar]
- 12.Verweij, P. E., M. van Kasteren, J. van de Nex, G. S. de Hoog, B. E. de Paux, and J. F. Meis. 1997. Fatal pulmonary infection caused by basidiomycete Hormographiella aspergillata. J. Clin. Microbiol. 35:2675-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaoutis, T., E. Roilides, C. C. Chiou, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaefele, M. Sein, T. Sein, P. A. Prasad, J. H. Chu, and T. J. Walsh. 2007. Zygomycosis in children: a systematic review and analysis of reported cases. Pediatr. Infect. Dis. J. 26:723-727. [DOI] [PubMed] [Google Scholar]
- 14.Zhao, Y., Q. Zhang, L. Li, J. Zhu, K. Kang, and L. Chen. 2009. Primary cutaneous mucormycosis caused by Rhizomucor variabilis in an immunocompetent patient. Mycopathologia DOI: 10.1007/s11046-009-9219-3. [DOI] [PubMed]