Transportation of sputum samples from remote areas to laboratories for culture of mycobacteria may take several days, which can lead to overgrowth of commensal flora and possible loss of viable mycobacteria (1, 3, 5, 8). To overcome this problem, cetyl pyridinum chloride (CPC) is still the most commonly used preservative (5, 8). The effect of CPC on the mycobacterial culture isolation rate for the Bactec MGIT 960 system in tropical countries where tuberculosis is endemic, in which the rate of environmental contamination of culture media is high, has scarcely been reported (6).
We carried out a prospective study to evaluate the effect of CPC on the recovery of mycobacteria in the Bactec MGIT 960 system (Becton, Dickinson and Company). Sixty patients whose sputa were positive for acid-fast bacilli (AFB) were enrolled in the study. All 60 patients were asked to bring fresh sputum samples the next morning, and each of these freshly collected sputum samples was aliquoted into three equal parts. Two aliquots were added to equal volumes of 1% CPC and stored at 4°C. The third aliquot was processed on the same day of collection by following the standard protocol for smear microscopy and MGIT 960 culture with minor modifications (7). In brief, about 5 to 6 ml of the sputum was mixed with an equal volume of a 0.5% N-acetyl-l-cysteine-4% NaOH mixture in a 50-ml ridge-capped round-bottom processing tube, the mixture was subjected to a vortex, and the tube was incubated at 37°C for 10 min. After incubation, the mixture was neutralized with phosphate buffer (pH 6.8), yielding a total volume of 50 ml, and subjected to centrifugation at 8,000 × g for 10 min. The supernatant was decanted, and the pellet was resuspended in 2 ml of the phosphate buffer. From the decontaminated sample, 0.5 ml of the suspension was inoculated into a Bactec MGIT 960 tube and 0.1 ml was inoculated onto a Lowenstein-Jensen slant under sterile conditions in a biosafety cabinet type 2 (Kartos, India), and from rest of the sample, smears were prepared for Ziehl-Neelsen (Z-N) staining. On the next day of storage, the contents of one aliquot with CPC were washed with phosphate buffer (pH 6.8) and centrifuged at 8,000 × g for 10 min, and the pellet was resuspended in 1 ml of phosphate buffer for smear microscopy and culture. The third aliquot was also taken out from the refrigerator and processed as described above but without being washed with buffer. Both pellets were dissolved in 2 ml of phosphate buffer (pH 6.8) and used for smear analyses and culture as described above. Smear results were interpreted using standard criteria for AFB grading. The number of days taken to give a positive or negative signal in the MGIT 960 system was recorded. Irrespective of a positive or negative signal, the contents of all mycobacterial growth indicator (MGI) tubes were checked for AFB by Z-N staining, either after the Bactec MGIT 960 system gave a positive signal or after 42 days of incubation. To check the viability of mycobacterial growth, 100 μl of culture medium was aspirated out from each MGI tube and 50 μl of yellow MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma, St. Louis, MO)] was added to this aliquot. If the mycobacteria are alive, the MTT is reduced to formazan, changing the color of the culture medium to purple. For comparison, findings for the aliquot without CPC added, which was processed on the same day, were taken as the standard. Growths of mycobacteria were identified by using a standard protocol in our laboratory, described elsewhere (4).
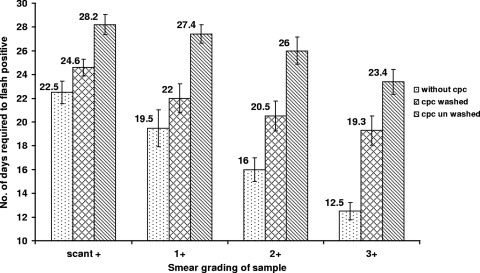
Results of AFB microscopy are provided in Table 1. Sixty samples were collected from 60 treatment-naïve patients known to have pulmonary tuberculosis and were divided into 180 aliquots (3 per sample). Among these, 59 (98.3%) of 60 aliquots without the addition of CPC, 53 (88.8%) of 60 treated with CPC and washed, and 50 (83.3%) of 60 treated with CPC and not washed gave positive signals in the MGIT 960 system within 42 days of incubation (2). The difference in the recovery rate between samples with and without the addition of CPC (irrespective of washing) was statistically significant (Table 1). Surprisingly, upon Z-N staining of the contents of culture tubes with negative signals, 70% of CPC-treated, unwashed samples (7 of 10) and 71.4% of CPC-treated, washed samples (5 of 7) were positive for AFB (Table 1). Upon the addition of MTT to the MGI tubes, the contents of all the above-mentioned 17 tubes developed purple coloring, indicating the presence of viable mycobacteria. Our results show that the addition of CPC significantly delayed mycobacterial detection in the Bactec MGIT 960 system by 6 to 11 days, as shown in Fig. 1. This delay could be reduced significantly by washing the CPC-treated samples with phosphate buffer.
TABLE 1.
Rate of mycobacterial detection by Bactec MGIT 960 system and rate of false-negative signalsa
| Protocol no. | Sample-processing protocol | No. of samples with positive signal | No. of samples with negative signal | No. of negative smears | No. of false-negative results/no. of samples classified as negative by Bactec MGIT 960 system (%) |
|---|---|---|---|---|---|
| 1 | Processed without CPC (n = 60) | 59 | 1 | 1 | 0/1 (0) |
| 2 | Mixed with CPC and washed (n = 60) | 53 | 7 | 2 | 5/7 (71.4) |
| 3 | Mixed with CPC and not washed (n = 60) | 50 | 10 | 3 | 7/10 (70.0) |
P values: for protocols 1 and 2, <0.0001; for protocols 1 and 3, <0.0001; and for protocols 2 and 3, >0.5. Each of the 60 samples was divided into three parts, and one aliquot had no CPC, another had CPC but was washed, and the third aliquot had CPC and was processed without being washed. The Bactec MGIT 960 result was labeled false negative when the tube gave a negative signal but was shown to contain mycobacteria upon smear examination.
FIG. 1.
Relationship between the amount of time required for a Bactec MGIT 960 positive result and the load of AFB in sputum samples. Smear grade: 3+, >10 AFB/field in at least 20 oil fields of smear; 2+, 1 to 9 AFB/field in at least 50 fields; 1+, 10 to 99 AFB in 100 fields; scant+, <10 AFB in 100 fields. For smears classified as negative, no AFB were seen in the entire smear.
Even though CPC remains the first choice to preserve the viability of mycobacteria and simultaneously minimize the overgrowth of bacterial and fungal flora, the results of this study clearly show a negative effect of CPC in the Bactec MGIT 960 system, probably involving mainly the levels of fluorescence and thereby decreasing the mycobacterial detection rate and increasing the time required for mycobacterial growth readings. However, the most important observation of the study was false-negative signals given by the system despite the presence of mycobacterial growth in the medium. Finding AFB in a high proportion (70%) of signal-negative culture tubes is alarming. Recently introduced tuberculosis control programs recommend the use of Bactec MGIT 960 for regional laboratories which cater services to various primary health care laboratories. Our results indicate that in these programs, it should be clearly highlighted that even if the samples need to be transported to regional laboratories, the use of CPC should be discouraged. Nevertheless, our study also shows that, if the use of CPC is unavoidable, the detrimental effect can be minimized by washing the samples with phosphate buffer. We also recommend that all Bactec MGIT 960 tubes with negative signals be rechecked for the presence of AFB before the release of culture-negative reports.
Acknowledgments
We acknowledge Balbir Singh and Mahesh Paliwal for their technical help, and we thank the Department of Biotechnology, Government of India, for funding this work (through grant no. BT/PR7761/MED/14/1105/2006).
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Aparna, S., K. V. Krishna Moorthy, and S. Gokhale. 2005. From microscopy centre to culture laboratory: a viable ride for mycobacteria. Int. J. Tuberc. Lung Dis. 10:447-449. [PubMed] [Google Scholar]
- 2.Becton, Dickinson and Company. 28 June 2009, accession date. Product catalog. Becton, Dickinson and Company, Franklin Lakes, NJ. http://catalog.bd.com/bdCat/viewProduct.doCustomer?productNumber=445870.
- 3.Bobadilla-del-Valle, M., A. Ponce-de-León, M. Kato-Maeda, A. Hernández-Cruz, J. J. Calva-Mercado, B. Chávez-Mazari, B. A. Caballero-Rivera, J. C. Nolasco-García, and J. Sifuentes-Osorniol. 2003. Comparison of sodium carbonate, cetyl-pyridinium chloride, and sodium borate for preservation of sputa for culture of Mycobacterium tuberculosis. J. Clin. Microbiol. 41:4487-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopinath, K., and S. Singh. 2009. Multiplex PCR assay for simultaneous detection and differentiation of Mycobacterium tuberculosis, Mycobacterium avium complexes and other mycobacterial species directly from clinical specimens. J. Appl. Microbiol. 107:425-435. [DOI] [PubMed] [Google Scholar]
- 5.Phillips, B. J., and W. Kaplan. 1976. Effect of cetylpyridinium chloride on pathogenic fungi and Nocardia asteroides in sputum. J. Clin. Microbiol. 3:272-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvakumar, N., P. Vanajakumar, G. Gopi, K. V. Venkataramu, M. Datta, C. N. Paramasivan, and R. Prabhakar. 1995. Isolation of tubercle bacilli from sputum samples of patients in the field studies by the cetylpyridinium chloride-sodium chloride and sodium hydroxide methods. Indian J. Med. Res. 102:149-151. [PubMed] [Google Scholar]
- 7.Singh, S., T. P. Saluja, M. Kaur, and G. C. Khilnani. 2008. Comparative evaluation of FASTPlaque assay with PCR and other conventional in vitro diagnostic methods for the early detection of pulmonary tuberculosis. J. Clin. Lab. Anal. 22:367-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smithwick, R. W., C. B. Stratigos, and H. L. David. 1975. Use of cetylpyridinium chloride and sodium chloride for the decontamination of sputum specimens that are transported to the laboratory for the isolation of Mycobacterium tuberculosis. J. Clin. Microbiol. 1:411-413. [DOI] [PMC free article] [PubMed] [Google Scholar]