Abstract
Species limits within the clinically important Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes (FIESC and FCSC, respectively) were investigated using multilocus DNA sequence data. Maximum-parsimony and maximum-likelihood analyses of aligned DNA sequences from four loci resolved 28 species within the FIESC, within which the species were evenly divided among two clades designated Incarnatum and Equiseti, and four species within the FCSC. Sequence data from a fifth locus, β-tubulin, was excluded from the study due to the presence of highly divergent paralogs or xenologs. The multilocus haplotype nomenclature adopted in a previous study (K. O'Donnell, D. A. Sutton, A. Fothergill, D. McCarthy, M. G. Rinaldi, M. E. Brandt, N. Zhang, and D. M. Geiser, J. Clin. Microbiol. 46:2477-2490, 2008) was expanded to all of the species within the FIESC and FCSC to provide the first DNA sequence-based typing schemes for these fusaria, thereby facilitating future epidemiological investigations. Multilocus DNA typing identified sixty-two sequence types (STs) among 88 FIESC isolates and 20 STs among 26 FCSC isolates. This result corresponds to indices of discrimination of 0.985 and 0.966, respectively, for the FIESC and FCSC four-locus typing scheme using Simpson's index of discrimination. Lastly, four human and two veterinary isolates, received as members of the FIESC or FCSC, were resolved as five phylogenetically distinct species nested outside these species complexes. To our knowledge, these five species heretofore have not been reported to cause mycotic infections (i.e., F. armeniacum, F. brachygibbosum, F. flocciferum, and two unnamed Fusarium species within the F. tricinctum species complex).
Fusarium species are hyaline filamentous molds (Hypocreales, Ascomycota) that can cause superficial infections, such as onychomycoses and keratitis in immunocompetent individuals, or deeply invasive and hematogenously disseminated infections with high mortality in persistently and severely neutropenic patients (11). Despite a poor response, liposomal amphotericin B remains the antifungal of choice for the treatment of fusarioses (41). Unfortunately, most fusaria exhibit broad resistance to the spectrum of antifungals currently available, including amphotericin B, azoles, echinocandins, and terbinafine, which typically show high MICs in vitro (1, 2, 35, 41, 43).
Recent multilocus molecular phylogenetic studies have revealed that the most commonly reported fusaria causing infections in humans and other animals, such as Fusarium solani, F. oxysporum, and F. moniliforme (F. verticillioides pro parte), harbor multiple species, several of which are morphologically cryptic (30, 31, 36). To date, detailed molecular evolutionary studies have been published on clinically important members of the F. solani species complex (FSSC) (2, 30, 35, 51), F. oxysporum species complex (FOSC) (32, 36), Gibberella (Fusarium) fujikuroi species complex (GFSC) (31, 33), and F. dimerum species complex (FDSC) (44). Species within these four complexes account for approximately 85% of all fusarioses within the United States. Members of these complexes are estimated to cause infections at the following frequencies: FSSC 60%; FOSC, 10%; GFSC, 10%; and FDSC, 5%. Results of the present study indicate that the remaining approximately 15% of clinically relevant fusaria from the United States are mostly nested within two closely related lineages, the F. chlamydosporum species complex (FCSC) and F. incarnatum-F. equiseti species complex (FIESC). Although several members of the FIESC included in the Centers for Disease Control and Prevention (CDC) Fusarium keratitis investigation in 2005 to 2006 were analyzed phylogenetically (8), species limits within this complex and the FCSC have never been critically examined employing genealogical concordance phylogenetic species recognition (GCPSR) (49) using multilocus DNA sequence data.
Chang et al. (8) first introduced a multilocus haplotype nomenclature for members of the FSSC and FOSC involved in CDC's Fusarium keratitis investigation, which elucidated their epidemiology and population structure and facilitated accurate communication of their genetic diversity within the public health community. The multilocus species/haplotype nomenclature developed for these fusaria is important for accurately reporting on pathogen identity and their genetic diversity, primarily because several molecular phylogenetic studies have revealed that most fusaria pathogenic to humans and other animals lack Latin binomials (5, 34, 35, 44, 51). In the present study, species limits and evolutionary relationships within the FCSC and FIESC were investigated via GCPSR for the first time using DNA sequence data from portions of four loci. In addition, we report on five Fusarium species that to our knowledge have not been reported previously to cause infections of humans or other animals.
MATERIALS AND METHODS
Fungal isolates.
Eighty-eight of the 120 isolates included in this study (Tables 1 and 2) were cultured from human or veterinary sources. The remaining 32 isolates were chosen to represent the phylogenetic breadth of the FIESC and FCSC represented within the culture collections of the Centraalbureau voor Schimmelcultures (CBS) Biodiversity Center (Utrecht, The Netherlands) and the Fusarium Research Center (FRC, Pennsylvania State University, State College, PA). With the exception of six clinical or veterinary isolates and the outgroup sequences of Fusarium concolor NRRL 13459 (received as the ex-type strain of F. polyphialidicum, which is a later synonym of F. concolor), the remaining isolates were members of the FCSC or FIESC. The 26 FCSC and 88 FIESC isolates were identified as members of these two species complexes via morphological analysis at the respective culture collections (Tables 1 and 2) and subsequent molecular phylogenetic analyses of aligned partial sequences of the RNA polymerase second largest subunit (RPB2) (34). All isolates are stored cryogenically in liquid nitrogen vapor (−175°C) in the Agricultural Research Service (NRRL) Culture Collection, National Center for Agricultural Utilization Research, Peoria, IL, where they are available upon request.
TABLE 1.
FIESC isolates subjected to DNA MLST
| NRRL no. | FIESC MLST (species)a | Equivalent no(s).b | Isolate source | Origin |
|---|---|---|---|---|
| 3020 | 10-a | FRC R-6053, 7.12 MRC | Unknown | Unknown |
| 3214 | 10-a | FRC R-6054, 7.13 MRC | Unknown | Unknown |
| 5537 | 8-a | ATCC 28805 | Fescue hay | Missouri |
| 6548 | 12-a | IMI 112503 | Wheat | Germany |
| 13335 | 21-a | FRC R-2138 | Alfalfa | Australia |
| 13379 | 23-b | FRC R-5198, BBA 62200 | Oryza sativa | India |
| 13402 | 9-b (F. scirpi) | FRC R-6363 | Pine soil | Australia |
| 13459 | (F. concolor)c | CBS 691.87 | Plant debris | South Africa |
| 20423 | 4-a (F. lacertarum) | IMI 300797 | Lizard skin | India |
| 20697 | 14-b (F. equiseti) | CBS 245.61 | Beet | Chile |
| 20722 | 27-a | IMI 190455 | Chrysanthemum sp. | Kenya |
| 22244 | 25-a | H.-K. Chen F64 | Rice | China |
| 25795 | 5-c | CBS 394.93, BBA 64265 | Disphymacrassifolium seed | Germany |
| 26417 | 26-a | CBS 544.96 | Leaf litter | Cuba |
| 26419 | 14-a (F. equiseti) | CBS 307.94, BBA 68556 (neotype) | Soil | Germany |
| 26921 | 12-a | CBS 731.87 | Wheat | Germany |
| 26922 | 9-c (F. scirpi) | CBS 610.95 | Soil | France |
| 28029 | 3-b | CDC B-3335 | Human eye | California |
| 28577 | 28-a | CBS 430.81 | Grave stone | Romania |
| 28714 | 26-b | ATCC 74289 | Acacia sp. branch | Costa Rica |
| 29134 | 9-a (F. scirpi) | CBS 448.84 | Pasture soil | Australia |
| 31011 | 12-a | BBA 69079 | Thuja sp. | Germany |
| 31160 | 15-c | MDA 3 | Human lung | Texas |
| 31167 | 18-a | MDA 10 | Human sputum | Texas |
| 32175 | 15-a | MDA F10 | Human sputum | Texas |
| 32181 | 15-c | MDA F20 | Human blood | Oklahoma |
| 32182 | 15-b | MDA F22 | Human blood | Texas |
| 32522 | 18-b | Loyola W-14182 | Human diabetic cellulitis | Illinois |
| 32864 | 17-a | FRC R-7245 | Human | Texas |
| 32865 | 21-b | FRC R-8480 | Human endocarditis | Brazil |
| 32866 | 23-a | FRC R-8822 | Human cancer patient | Texas |
| 32867 | 23-a | FRC R-8837 | Human | Texas |
| 32868 | 25-c | FRC R-8880 | Human blood | Texas |
| 32869 | 15-c | FRC R-9445 | Human cancer patient | Texas |
| 32871 | 5-a | FRC R-9561 | Human abscess | Texas |
| 32993 | 25-b | UTHSC 00-755 | Human nasal tissue | Texas |
| 32994 | 15-c | UTHSC 00-494 | Human ethmoid sinus | Texas |
| 32995 | 15-c | UTHSC 99-1964 | Human sinus | Texas |
| 32996 | 15-c | UTHSC 99-1741 | Human leg wound | Texas |
| 32997 | 7-a | UTHSC 99-423 | Human toenail | Colorado |
| 34001 | 15-e | UTHSC 95-1945 | Human foot wound | Texas |
| 34002 | 22-a | UTHSC 95-1545 | Human ethmoid sinus | Texas |
| 34003 | 20-a | UTHSC 95-28 | Human sputum | Texas |
| 34004 | 16-a | UTHSC 94-2581 | Human BAL | Texas |
| 34005 | 24-a | UTHSC 94-2471 | Human intravitreal fluid | Minnesota |
| 34006 | 15-a | UTHSC 93-2692 | Human eye | Texas |
| 34007 | 15-a | UTHSC 93-933 | Human sputum | Texas |
| 34008 | 15-d | UTHSC 92-1955 | Human lung | Texas |
| 34010 | 15-c | UTHSC 02-1698 | Human maxillary sinus | Texas |
| 34011 | 15-a | UTHSC 02-2060 | Human sputum | Texas |
| 34032 | 5-a | UTHSC 98-2172 | Human abscess | Texas |
| 34034 | 1-c | UTHSC 94-1167 | Human leg | Arizona |
| 34035 | 5-d | UTHSC 91-569 | Human sinus | Colorado |
| 34037 | 5-b | UTHSC 02-966 | Human abscess | Colorado |
| 34039 | 1-b | UTHSC 96-1394 | Human | Connecticut |
| 34056 | 16-b | Loyola M54234 | Human bronchial wash | Illinois |
| 34059 | 16-c | Loyola S8158 | Human blood | Illinois |
| 34070 | 17-c | Loyola W37591 | Tortoise | Illinois |
| 36123 | 4-b | CBS 102300, BBA 70843 | Unknown | Unknown |
| 36136 | 14-a (F. equiseti) | CBS 107.07, IMI 091982 | Unknown | Unknown |
| 36269 | 12-b | CBS 162.57 | Pinusnigra seedling | Croatia |
| 36318 | 3-a | CBS 185.31 | Unknown | Unknown |
| 36321 | 14-a (F. equiseti) | CBS 185.34 | Soil | Netherlands |
| 36323 | 3-a | CBS 186.31 | Cotton yarn | England |
| 36372 | 11-a | CBS 235.79 | Air | Netherlands Antilles |
| 36392 | 12-c | CBS 259.54 | Seedling | Germany |
| 36401 | 2-a | CBS 264.50 | Cotton | Mozambique |
| 36448 | 2-b | CBS 384.92 | Phaseolus vulgaris seed | Sudan |
| 36466 | 14-a (F. equiseti) | CBS 414.86 | Potato peel | Denmark |
| 36478 | 9-a (F. scirpi) | CBS 447.84 | Pasture soil | Australia |
| 36548 | 17-b | CBS 190.60 | Banana | Congo |
| 36575 | 20-b | CBS 976.97 | Juniperuschinensis leaf | Hawaii |
| 43297 | 24-b | W. Elmer 22 | Spartina rhizomes | Connecticut |
| 43498 | 8-b | CDC 2006743466 | Human eye | Pennsylvania |
| 43619 | 15-a | UTHSC 05-2847 | Human finger | Texas |
| 43622 | 15-c | UTHSC 03-2501 | Human lung | Texas |
| 43623 | 5-e | UTHSC 03-59 | Human maxillary sinus | Colorado |
| 43635 | 13-a | UTHSC 06-638 | Horse | Nebraska |
| 43636 | 14-c (F. equiseti) | UTHSC 06-170 | Dog | Texas |
| 43637 | 1-a | UTHSC 05-1729 | Dog | Pennsylvania |
| 43638 | 6-a | UTHSC R-3500 | Manatee | Florida |
| 43639 | 19-a | UTHSC 04-135 | Manatee | Florida |
| 43640 | 1-a | UTHSC 04-123 | Dog nose | Texas |
| 43694 | 6-a | CDC 2006743607 | Human eye | Texas |
| 43730 | 16-c | CDC 2006743605 | Contact lens | Mississippi |
| 45995 | 5-b | UTHSC 02-966 | Human abscess | Colorado |
| 45996 | 1-a | UTHSC 03-3101 | Human sinus | New York |
| 45997 | 5-f | UTHSC 04-1902 | Human sinus | Colorado |
| 45998 | 6-b | UTHSC 06-2315 | Human toe | Texas |
Arabic numerals identify species, and lowercase roman letters identify unique haplotypes within each species.
Sequences of F.concolor NRRL 13459 were used to root the phylogeny (Fig. 1).
ATCC, American Type Culture Collection, Manassas, VA; BBA, Biologische Bundesanstalt für Land-und Forstwirtschaft, Institute für Mikrobiologie, Berlin, Germany; CBS, CBS-KNAW Fungal Biodiversity Center, Utrecht, The Netherlands; CDC, Centers for Disease Control and Prevention, Atlanta, GA; Loyola, Loyola University, Maywood, IL; IMI, CABI Biosciences, Egham, Surrey, England; MDA, M. D. Anderson Cancer Center, Houston, TX; WE, Wade Elmer, Connecticut Agricultural Experiment Station, New Haven, CT.
TABLE 2.
FCSC and novel pathogenic isolates subjected to DNA MLST
| NRRL no. | MLST and/or speciesa | Equivalent no.c | Isolate source | Origin |
|---|---|---|---|---|
| 13338 | FCSC 4-a (F. nelsonii) | FRC R-2181 | Soil | Australia |
| 13444 | FCSC 2-a | FRC T-550 | Corn soil | Australia |
| 13459 | F. concolorb | CBS 691.87 | Plant debris | South Africa |
| 28505 | FCSC 4-b (F. nelsonii) | FRC R-8670 | Soil debris | South Africa |
| 28578 | FCSC 1-a | CBS 615.87 | Colocasiaesculenta leaf | Cuba |
| 32521 | FCSC 1-e | FRC T-0852 | Human cancer patient | Texas |
| 34012 | FCSC 1-g | UTHSC 01-451 | Human toe | Texas |
| 34013 | FCSC 2-a | UTHSC 01-2416 | Human toenail | Pennsylvania |
| 34014 | FCSC 1-l | UTHSC 01-2668 | Human maxillary sinus | Illinois |
| 34015 | FCSC 2-b | UTHSC 02-1276 | Horse eye | Alabama |
| 34016 | FCSC 2-a | UTHSC 98-2537 | Human leg | Texas |
| 34017 | FCSC 1-h | UTHSC 97-59 | Human maxillary sinus | Texas |
| 34018 | FCSC 1-m | UTHSC 97-17 | Human arm | Florida |
| 34019 | FCSC 1-c | UTHSC 96-2036 | Human eye | Texas |
| 34021 | FCSC 2-a | UTHSC 95-1488 | Human upper lobe wash | Texas |
| 34022 | FCSC 1-j | UTHSC 93-2120 | Human paranasal sinus | Georgia |
| 34023 | FCSC 2-c | UTHSC 93-1353 | Human finger | Texas |
| 34033 | F. brachygibbosum | UTHSC 97-99 | Human foot cellulites | Texas |
| 34036 | Fusarium sp. strain 1, FTSC | UTHSC 01-1965 | Human ethmoid sinus | Colorado |
| 36147 | Fusarium sp. strain 2, FTSC | CBS 109232 | Human bronchial secretion | Unknown |
| 36495 | FCSC 2-b | CBS 491.77 | Soil | Kuwait |
| 36539 | FCSC 1-k | CBS 677.77 | Cultivated soil | Solomon Islands |
| 43627 | FCSC 2-d | UTHSC 05-3559 | Human bronchial lavage | Texas |
| 43628 | FCSC 1-i | UTHSC 05-3396 | Human finger | Florida |
| 43629 | FCSC 1-b | UTHSC 05-3200 | Human blood | Utah |
| 43630 | FCSC 2-a | UTHSC 05-2743 | Human sputum | Texas |
| 43631 | FCSC 3-a | UTHSC 05-2441 | Human leg | Texas |
| 43632 | FCSC 1-d | UTHSC 05-1260 | Human eye | Florida |
| 43633 | FCSC 1-f | UTHSC 03-3472 | Human maxillary sinus | Tennessee |
| 43641 | F. armeniacum | UTHSC 06-1377 | Horse eye | Missouri |
| 45992 | FCSC 1-j | UTHSC 06-3823 | Human leg | South Carolina |
| 45994 | Fusarium sp. strain 2, FTSC | UTHSC 06-2616 | Cloaca | Texas |
| 45999 | F. flocciferum | UTHSC 06-3449 | Human scalp | California |
MLST within FCSC. Arabic numerals designate species, and lowercase roman letters identify unique haplotypes within each species.
Sequences of F.concolor NRRL 13459 were used to root the phylogeny (Fig. 2).
CBS, CBS-KNAW Fungal Biodiversity Center, Utrecht, The Netherlands; CDC, Centers for Disease Control and Prevention, Atlanta, GA.
DNA manipulations for multilocus DNA sequencing.
Mycelium was grown in yeast extract-malt broth (20 g of dextrose, 5 g of peptone, 3 g of yeast extract, and 3 g of malt extract per liter; Difco, Detroit, MI) on a rotary shaker at 100 rpm for 2 to 3 days and freeze dried, and then total genomic DNA was extracted using a hexadecyltrimethyl-ammonium bromide (Sigma, St. Louis, MO) protocol as previously described (31). Portions of five nuclear gene fragments were selected for multilocus sequence typing (MLST) based on previous analyses (33-35): translation elongation factor (EF-1α), RPB2, the internal transcribed spacer (ITS) region, domains D1 and D2 of the nuclear large-subunit (LSU) rRNA, calmodulin (CAM), and β-tubulin. Data obtained from this last locus, however, was excluded from the study due to the presence of highly divergent paralogs (homologs evolved by gene duplication) or xenologs (homologs evolved by lateral gene transfer among different species) that complicated phylogenetic reconstruction. PCR and sequencing primers for the MLST scheme have been published previously (34, 35). All PCRs employed Platinum Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA) and identical cycling parameters in an Applied Biosystems 9700 Thermocycler (Emeryville, CA), as previously reported (31). Applied Biosystems BigDye, version 3.1, Terminator reaction mixture was used in all DNA sequencing reactions (31).
Chromatograms were edited and aligned with Sequencher, version 4.1.2 (Gene Codes, Ann Arbor, MI), prior to manual improvement of the alignments to establish positional homology.
Phylogenetic analysis.
Maximum-parsimony (MP) analyses implemented in PAUP*, version 4.0b10 (47), and maximum likelihood (ML) employing GARLI (52) were conducted as previously described (35), except that nonparametric ML bootstrapping was conducted with a 2.6-Ghz MacBook Pro. The Akaike information criterion in MrModeltest, version 2.2 (29), was used to identify the best-fit model of nucleotide substitution for the ML analyses. Multilocus haplotypes or sequence types (STs) were identified using COLLAPSE, version 1.1 (http://inbio.byu.edu/Faculty/kac/crandall_lab/Computer.html).
Nucleotide sequence accession numbers.
The DNA sequences determined in this study have been deposited in the GenBank under accession numbers GQ505373 to GQ505852.
RESULTS
Phylogenetic diversity of FIESC clinical isolates.
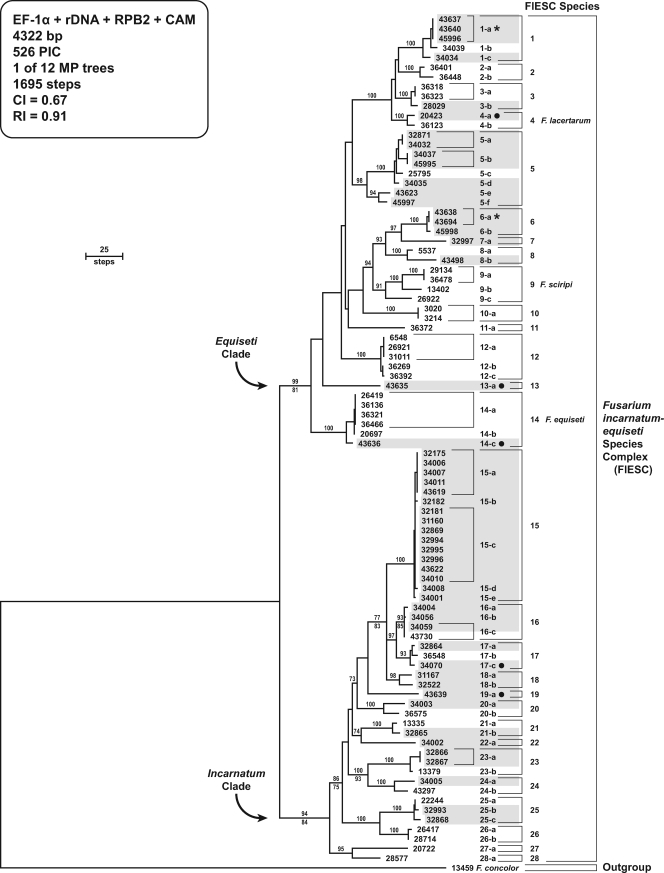
Evolutionary relationships and species limits of 52 human or veterinary isolates, together with 36 nonclinical isolates within the FIESC, were inferred using multilocus DNA sequence data from four loci. All of the clinically relevant isolates were from the United States except for one from an endocarditic patient from Brazil and the ex-type strain of F. lacertarum, which was isolated from lizard skin in India (45) (Table 1). Tree statistics and summary sequence for the individual and combined data sets are provided in Table 3. The combined data set comprised portions of the EF-1α gene (717 bp), the ITS plus LSU (ITS+LSU) 28S rRNA gene (1,135 bp), RPB2 (1,766 bp), and CAM (704 bp), totaling 4,322 bp of aligned nucleotide sequence data from each isolate. Analyses of the individual partitions revealed that the EF-1α and ITS+LSU 28S rRNA genes were the most and least phylogenetically informative loci, respectively, based on parsimony-informative characters per bp and number of species resolved as monophyletic by MP bootstrapping of the four individual data sets (Table 3). Results of these analyses resolved the 21 FIESC species represented by two or more isolates as reciprocally monophyletic in the majority of the bootstrapped individual genealogies, thereby fulfilling a stringent interpretation of species recognition under GCPSR. The remaining seven putatively phylogenetically distinct FIESC species were each represented by a single highly divergent isolate, and therefore additional sampling is required to fully assess their species limits.
TABLE 3.
FIESC tree statistics for the individual and combined partitions
| Locus | Size (bp) | No. of PICs | No. of PICs/bp | AUTa | MPT length (no. of steps)b | CIc | RId | No. of haplotypese | No. of FIESC species supported as monophyleticf |
|---|---|---|---|---|---|---|---|---|---|
| EF-1-α | 717 | 162 | 0.23 | 95 | 517 | 0.61 | 0.92 | 51 | 20 |
| LSU+ITS rRNA genes | 1,135 | 11 | 0.01 | 35 | 58 | 0.57 | 0.92 | 21 | 1 |
| RPB2 | 1,766 | 220 | 0.12 | 184 | 629 | 0.58 | 0.92 | 46 | 19 |
| CAM | 704 | 133 | 0.19 | 109 | 385 | 0.64 | 0.94 | 45 | 14 |
| EF-1-α plus rRNA genes | 1,852 | 173 | 0.09 | 130 | 602 | 0.57 | 0.91 | 59 | — |
| EF-1-α plus RPB2 | 2,483 | 382 | 0.15 | 279 | 1,181 | 0.57 | 0.91 | 57 | — |
| EF-1-α plus CAM | 1,421 | 295 | 0.21 | 204 | 934 | 0.59 | 0.92 | 54 | — |
| rRNA genes plus RPB2 | 2,901 | 231 | 0.08 | 219 | 714 | 0.55 | 0.91 | 54 | — |
| rRNA genes plus CAM | 1,839 | 144 | 0.08 | 144 | 469 | 0.58 | 0.93 | 56 | — |
| RPB2 plus CAM | 2,470 | 353 | 0.14 | 293 | 1,062 | 0.69 | 0.92 | 53 | — |
| Combined | 4,322 | 526 | 0.12 | 423 | 1,695 | 0.67 | 0.91 | 62 | 21 |
AUT, autapomorphy or a derived character.
MPT, most parsimonious tree.
CI, consistency index.
RI, retention index.
Number of multilocus haplotypes or STs determined using COLLAPSE, version 1.1.
Based on maximum parsimony bootstrap support. —, comparison not made.
To determine whether DNA sequence data from the various gene partitions could be concatenated into a single data set, an MP bootstrap value of ≥70% was used as a threshold for identifying topological incongruence. Results of these analyses indicated that the individual data sets could be combined and analyzed phylogenetically using MP in PAUP* (47) and ML in GARLI (52). DNA sequence data from a fifth locus, β-tubulin, was excluded from the study due to the widespread presence of highly divergent paralogs or xenologs. MP and ML phylogenetic analyses of the combined data set recovered trees that were highly concordant topologically (Fig. 1; only the MP tree is shown) and in which there was a deep basal split between two early diverging lineages, here informally designated the Equiseti and Incarnatum clades. The 12 most-parsimonious trees were 4,322 steps in length; the ML tree with the best negative log-likelihood score was −15,737.46164 based on 10 independent heuristic analyses, using the general time-reversible (GTR) model of nucleotide substitution with a proportion of invariant (I) sites and gamma-distributed (G) rate heterogeneity (i.e., GTR+I+G) in GARLI (52). Only relatively minor differences were observed between the MP and ML topologies. These differences were restricted to five internodes along the backbone of the phylogeny within the Incarnatum clade; however, the MP and ML bootstrap values differed by only 6 to 11% (Fig. 1). Analyses of the individual and combined partitions support the recognition of 14 phylogenetically distinct species within each clade. Of the 28 species within the FIESC, 9 species within the Equiseti clade and 11 within the Incarnatum clade were recovered from mycotic infections, and these spanned the phylogenetic breadth of each clade (Fig. 1). Latin binomials, however, can be applied with confidence to only three of the species within the Equiseti clade, namely, F. lacertarum (FIESC 4) (45), F. scirpi (FIESC 9) (7), and F. equiseti (FIESC 14), and none of the 14 species within the Incarnatum clade. F. scirpi is broadly circumscribed here to include two STs from Australia (FIESC 2-a and 2-b) and the highly divergent NRRL 26922 (FIESC 9-c) from France, which suggests that additional sampling may reveal that FIESC 9-c represents a phylogenetically distinct species.
FIG. 1.
One of 12 most-parsimonious trees inferred from MP analysis of the combined four-locus data set for 88 isolates within the FIESC. The phylogram is rooted by the outgroup method using sequences of F. concolor NRRL 13459. Arabic numbers and lowercase Roman letters identify species and their multilocus STs, respectively. Human and veterinary isolates are distinguished from nonclinical isolates by shading. Veterinary isolates are distinguished from those from humans by a solid dot to the right of the NRRL number/ST. In addition, a star to the right of ST 6-a indicates that one isolate was from a veterinary source and the other from a human. Note that Latin binomials can be applied confidently to only 3 of 14 species within the Equiseti clade and to none of the species within the Incarnatum clade. MP bootstrap values based on 1,000 pseudoreplicates of the data are indicated above internodes. ML bootstrap values are indicated below internodes only when they differed by ≥5% of the MP value.
In the absence of our ability to confidently apply Latin binomials to 25 of the 28 species within the FIESC, the species and haplotype nomenclature previously adopted within the medically important clade 3 of the FSSC (35, 51) has been extended herein to all of the species within the species-rich FIESC. Results of the typing scheme revealed that, with the exception of FIESC 10 whose two isolates shared the same ST, the 20 other species represented by two or more isolates possessed between two and six STs with unique combinations of alleles. FIESC 15-a (n = 5) and FIESC 15-c (n = 8), which were restricted to Texas and Oklahoma, represented the most commonly sampled clinically relevant STs in the present study. Although six species exhibited intercontinental distributions (i.e., FIESC 3, 4, 9, 14, 23, and 25), FIESC 1-a represented the only ST out of the 62 unique haplotypes typed in the present study isolated on separate continents. In addition, FIESC 1-a and FIESC 6-a were the only two STs within this complex recovered from humans and other animals (Table 1). Employing Simpson's index of diversity, the four-locus FIESC typing scheme achieved a 0.985 index of discrimination (18).
Phylogenetic diversity of FCSC clinical isolates.
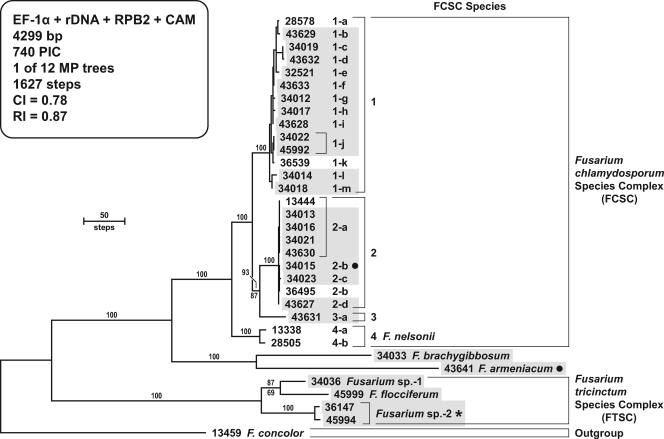
Twenty of the 26 isolates analyzed phylogenetically within the FCSC were isolated within the United States from mycotic infections, and they included 19 from humans and a single isolate from a horse eye. Summary sequence and tree statistics of the four loci sampled are presented in Table 4. Based on parsimony informative characters (PIC) per bp (Table 4), CAM and the ITS+LSU 28S rRNA genes were the most and least phylogenetically informative loci, respectively, with 0.26 and 0.06 PIC/bp, respectively. An ambiguously aligned 37-bp indel-containing region within CAM was excluded from all phylogenetic analyses. The same conditional combination approach employed above for the FIESC indicated that trees inferred from the four FCSC loci sampled represented the same underlying phylogeny, and therefore the data were analyzed as a combined data set using MP and ML. As noted above, the homoplastic distribution of highly divergent β-tubulin paralogs or xenologs precluded the use of this locus for phylogeny reconstruction. The 12 most-parsimonious trees were 4,299 steps in length; the ML tree with the best negative log-likelihood score, using the GTR+I+G model of nucleotide substitution, was −14,131.69216 based on 10 independent analyses in GARLI (52). MP and ML phylogenies resolved F. nelsonii as the earliest diverging lineage within the FCSC, forming a basal sister to the remaining members of this complex (Fig. 2; only the MP tree is shown). Analyses of the individual and combined data sets support the recognition of four genealogically exclusive, phylogenetically distinct species within this complex (Fig. 2). Three of the four FCSC species were associated with mycotic infections. However, because it is unclear which of these species, if any, represents F. chlamydosporum, herein these three species are designated FCSC 1, 2, and 3. High allelic diversity was observed, with 12 STs among 13 isolates within FCSC 1 and four STs among 9 isolates within FCSC 2. The latter species contained the most common ST sampled, FCSC 2-a, with human isolates from Texas and Pennsylvania and a soil isolate from Australia (Table 2). Lastly, the DNA typing results revealed that three of the four FCSC species exhibited transoceanic distributions (FCSC 1, 2, and 4 [equivalent, F. nelsonii]). Overall the FCSC four-locus typing scheme achieved an index of discrimination of 0.966 employing Simpson's index of diversity (18).
TABLE 4.
FCSC tree statistics for the individual and combined partitions
| Locus | Size (bp) | No. of PICs | No. of PICs/bp | AUTa | MPT length (no. of steps)b | CIc | RId | No. of haplotypese | No. of FCSC species supported as monophyleticf |
|---|---|---|---|---|---|---|---|---|---|
| EF-1-α | 710 | 159 | 0.22 | 74 | 379 | 0.81 | 0.89 | 17 (7) | 3 |
| LSU+ITS rRNA genes | 1,153 | 69 | 0.06 | 45 | 156 | 0.85 | 0.9 | 14 (6) | 2 |
| RPB2 | 1,766 | 335 | 0.19 | 111 | 697 | 0.71 | 0.88 | 17 (7) | 3 |
| CAM | 670 | 177 | 0.26 | 59 | 364 | 0.82 | 0.88 | 12 (5) | 2 |
| EF-1-α plus rRNA genes | 1,863 | 228 | 0.12 | 119 | 551 | 0.73 | 0.87 | 24 (7) | — |
| EF-1-α plus RPB2 | 2,476 | 494 | 0.2 | 185 | 1,084 | 0.72 | 0.88 | 23 (7) | — |
| EF-1-α plus CAM | 1,380 | 336 | 0.24 | 133 | 745 | 0.77 | 0.88 | 21 (7) | — |
| rRNA genes plus RPB2 | 2,919 | 404 | 0.14 | 156 | 872 | 0.71 | 0.87 | 21 (7) | — |
| rRNA genes plus CAM | 1,823 | 246 | 0.13 | 104 | 540 | 0.8 | 0.86 | 20 (6) | — |
| RPB2 plus CAM | 2,436 | 512 | 0.21 | 170 | 1,065 | 0.78 | 0.88 | 21 (7) | — |
| Combined | 4,299 | 740 | 0.17 | 289 | 1,627 | 0.78 | 0.87 | 27 (7) | 3 |
AUT, autapomorphy or a derived character.
MPT, most parsimonious tree.
CI, consistency index.
RI, retention index.
Number of multilocus haplotypes or STs determined using COLLAPSE, version 1.1. Note that the number in parentheses refers to the number of STs for the non-FCSC isolates within the ingroup.
Based on maximum parsimony bootstrap support. —, comparison not made.
FIG. 2.
One of 12 most-parsimonious MP phylograms inferred from the combined data from four loci for the FCSC and related clinically relevant fusaria rooted with sequences of F. concolor NRRL 13459. Arabic numbers and lowercase Roman letters are used to identify four species and their multilocus STs within the FCSC. Dark shading identifies isolates from human clinical and veterinary sources, the latter of which are further distinguished by a solid dot to the right of the NRRL number/ST. A star to the right of Fusarium sp. strain 2 indicates that it was represented by one human and one veterinary isolate. It is unclear which of the three unnamed species within the FCSC represents F. chlamydosporum, if any. Numbers above nodes represent the frequency (percent) with which they were recovered from 1,000 MP bootstrap pseudoreplicates of the data. ML bootstrap values are indicated only below those internodes that differed by ≥5% of the MP value. The present study represents the first report of the five species basal to the FCSC causing mycotic infections in humans and other animals. Fusarium brachygibbosum and F. armeniacum are members of the trichothecene toxin-producing clade, whereas the other non-FCSC ingroup species are members of the FTSC.
Five novel Fusarium species causing mycotic infections.
Six isolates tentatively identified morphologically as members of the FIESC or FCSC were resolved as five phylogenetically distinct species nested outside these complexes, based on comparisons of partial EF-1α sequences with the FUSARIUM-ID database (12) and partial RPB2 gene sequences from a more inclusive data set (K. O'Donnell, unpublished data). Results of these analyses identified three isolates as described species: NRRL 34033 (from human foot cellulites; Texas) was identified as F. brachygibbosum by comparison with the ex-type strain NRRL 20954 (= BBA 64691); NRRL 43641 (from horse eye; Missouri) as F. armeniacum by comparison with the ex-type strain NRRL 26908 (CBS 485.94 and FRC R-9372); and NRRL 45999 (from human scalp; California) formed a genealogically exclusive group with F. flocciferum isolates NRRL 25471 (CBS 792.70 and BBA 11141) and NRRL 25473 (BBA 64346). The first two species are members of the trichothecene toxin-producing clade of fusaria (21). NRRL 45999 F. flocciferum, together with the remaining two clinical species, represented by NRRL 34036 Fusarium sp. strain 1 (from human ethmoid sinus; Colorado) and NRRL 36147 Fusarium sp. strain 2 (from human bronchial secretion; geographic origin unknown), and NRRL 45994 Fusarium sp. strain 2 (from cloaca; Texas), are members of a clade designated herein as the F. tricinctum species complex (FTSC) (21). NRRL 34036 Fusarium sp. strain 1 (from human ethmoid sinus; Colorado) and Fusarium sp. strain 2, represented by NRRL 36147 strain 1 (from human bronchial secretion; geographic origin unknown) and NRRL 45994 strain 2 (from cloaca; Texas), appear to represent two undescribed phylogenetically distinct species. To our knowledge, the current study represents the first report implicating these five species in causing mycotic infections of humans and other animals.
DISCUSSION
Species limits and evolutionary relationships within two closely related fusaria lineages, the FIESC and FCSC, together with five clinically novel Fusarium species that are important in medical and veterinary contexts, were investigated for the first time employing multilocus GCPSR (49). The major finding of the present study is that the FCSC and FIESC appear to comprise, respectively, 4 and 28 phylogenetically distinct species; that over 70% of the species within these two complexes are represented by isolates recovered from infections of humans or other animals; and that they comprise approximately 15% of all fusarial infections within the United States. The 3 species within the FCSC and all 21 species within the FIESC represented by two or more isolates fulfilled the highly conservative requirements of GCPSR as applied here in that they were resolved as genealogically exclusive in the majority of the four bootstrapped single-locus genealogies (37, 40). In addition, bootstrapping revealed that none of the individual genealogies contradicted the monophyly of these species (i.e., genealogical nondiscordance sensu Dettman et al.) (10). Additional sampling of more isolates is needed to assess the monophyly of one putative species within the FCSC and seven within the FIESC, given that they were each represented by a single genetically divergent isolate in the present study.
Phylogenetic diversity of FIESC clinical isolates.
This study describes the first MLST typing scheme for species and haplotypes based on nucleotide polymorphism within portions of four nuclear genes among members of the FIESC that are important in both clinical and veterinary contexts. Previous molecular phylogenetic studies of the FIESC have not focused on identifying GCPSR-based species limits among clinically relevant or mycotoxigenic isolates, given that their genetic diversity was assessed only via partial DNA sequence data from single nuclear genes such as EF-1α (21, 42), RPB2 (34), β-tubulin (3), 28S rDNA (15), or restriction fragment polymorphisms from the nuclear ribosomal intergenic spacer region (rDNA) (20). Based on the results of the present study, EF-1α was the most phylogenetically informative gene and the ITS+LSU 28S rDNA was the least informative. Even though the latter locus possessed relatively little phylogenetic signal, the typing schemes benefited from its inclusion by an increase of six STs within the FIESC and three STs within the FCSC.
One of the most surprising results to emerge from the present study is that the species-rich FIESC comprises at least 20 mycoses-associated species among the 28 reciprocally monophyletic lineages resolved by the multilocus molecular phylogenetics. What makes this finding all the more remarkable is that only one of the 52 clinical isolates was recovered from outside the United States, revealing that phylogenetically diverse human-opportunistic members of this complex are well represented in North America. Moreover, phenotypically based taxonomic treatments of the genus have underestimated species diversity within the FIESC by close to 1 order of magnitude (13, 22, 28). Similar GCPSR-based studies within other clinically important clades within Fusarium, such as the FSSC (30, 35, 51), GFSC (31, 33), FDSC (44), and FOSC (36) have revealed similar levels of cryptic speciation.
The result is that Latin binomials can be applied with confidence to only 3 of the 28 species within the FIESC (Fig. 1). This is due primarily to the discovery of the large number of phylogenetically distinct but morphologically cryptic species reported herein and also to unresolved taxonomic and nomenclatural problems associated with applying validly published names such as F. incarnatum and F. pallidoroseum and their varieties (19, 46), which might represent phylogenetically distinct species, to members of the FIESC. Although the name F. semitectum has been used in the literature more than any other species within the Incarnatum clade, study of the type collection surprisingly revealed that this binomial has been misapplied because it is a later synonym of Colletotrichum musae (6). These systematic problems are exacerbated by the dearth of and homoplasious morphological characters within the FIESC and because type specimens, where known, are too old for DNA typing using the present four-locus MLST scheme.
In the absence of binomials for most of the species within the FIESC, one of the primary objectives of the present study was to extend the standardized multilocus species/haplotype nomenclature, first proposed in the CDC's keratitis outbreak investigation (8), to each member of the FIESC to facilitate communication of epidemiologically relevant data within the public health, phytopathological, and mycotoxin research communities. In this connection, it is worth mentioning that 59% of the FIESC isolates typed represented unique STs, with FIESC 15's 16 isolates and five STs from Texas or Oklahoma being the most common species sampled. This finding, however, undoubtedly represents a sampling bias given that close to two-thirds of the clinically relevant isolates we typed were obtained from the University of Texas Health Science Center's (UTHSC) Fungus Testing Laboratory in San Antonio, TX. Clearly, future studies are needed to elucidate how clinically relevant STs are distributed throughout North America and on other continents with the aim of identifying their environmental reservoirs. Surveys of the FIESC in nature indicate that they are common on phylogenetically diverse plants and plant debris and in soil in both hemispheres (13). Moreover, members of the Incarnatum clade are especially prevalent in the tropics and subtropics (13). Because members of the FIESC have been reported to produce type A and B trichothecene mycotoxins (14, 17, 25), which can alter immune function (39) and inhibit eukaryotic protein synthesis (50), as well as cytotoxic enniatins (16, 24) and estrogenic mycotoxins (14, 17, 20, 25), studies are needed to evaluate whether any of these toxins function as virulence factors in animal pathogenesis. In addition, ongoing studies are directed at investigating their mycotoxin potential in vitro using the phylogenetic framework developed in the present study.
Phylogenetic diversity of FCSC clinical isolates.
Herein, we report on the first MLST scheme for members of the FCSC. This scheme was used to type 20 relevant isolates from clinical or veterinary sources from the United States, employing portions of the same four loci used for the FIESC. The discovery of highly divergent β-tubulin paralogs or xenologs, as in the FIESC and FSSC (30), precluded the use of this locus for phylogeny reconstruction in the present study. Even so, Azor et al. (3) recently reported that phylogenetic analysis of a 378-bp portion of the β-tubulin gene from eight isolates identified as F. chlamydosporum formed a weakly supported clade (61% bootstrap), which suggests that orthologous alleles were sampled. The only other published phylogenetic analysis of the FCSC, which strongly supported its monophyly (100% bootstrap), was conducted using a 1.8-kb portion of RPB2 (34), but only four isolates were studied. Results of the present study represent the first GCPSR-based assessment of species limits within the FCSC. Species were recognized only if they were reciprocally monophyletic in at least half of the individual partitions and no genealogical discordance was observed (37, 40). Using these ranking criteria, three phylogenetically distinct, clinically relevant species were resolved within the morphotaxon F. chlamydosporum.
Given that the type specimen of this species was isolated from banana in Honduras (13) and that isolates from this host and/or geographic location were unavailable for study, it is unclear which of the three FCSC species, if any, corresponds to F. chlamydosporum. Because the type specimen of this species was collected in 1925 and an ex-type strain does not exist, study of isolates collected from banana at the type locality may help resolve this taxonomic problem. The species/haplotype nomenclature originally developed by Chang et al. (8) for the fusaria keratitis outbreak adopted in the present study, using Arabic numbers for species and lowercase roman letters for each unique ST, obviates these taxonomic issues and promotes precise communication of the MLST data within the scientific community. Results of the present study, which show that the two most common STs, FCSC 2-a and 2-b, exhibited intercontinental distributions and were represented by soil isolates, is consistent with reports that the broadly defined morphospecies F. chlamydosporum is common in soils and the rhizosphere of numerous vascular plants worldwide (13). Isolates of the fourth species within the FCSC, F. nelsonii, have not been reported to cause mycotic infections, possibly because they have been recovered only from remote or sparsely populated regions in South Africa (26) and Australia (this study) or possibly because they may have been misidentified as F. chlamydosporum or F. incarnatum. Given that the FCSC isolates from humans and other animals included in the present study were all from the United States, future studies are needed to elucidate the global distribution of clinically important species/STs, which should help identify their environmental reservoirs and identify widespread clones or clonal lineages (36). Further, because members of the FCSC are able to elaborate several mycotoxins, including trichothecenes and moniliformin (25), the phylogenetic framework developed in the present study will be used to evaluate species/ST mycotoxin potential to better understand the risk these strains pose to food safety and human health (38).
Five novel Fusarium species causing mycotic infections.
The extremely homoplasious morphological characters within the FIESC and FCSC contributed significantly to the initial phenotypic misidentifications of the five novel fusaria causing infections of humans and other animals as F. equiseti, F. incarnatum, or F. chlamydosporum. Fortunately, accurate molecular identifications were easily obtained by simply comparing partial EF-1α sequences with those in the FUSARIUM-ID database (12) and/or molecular phylogenetic analysis of a comprehensive data set of partial RPB2 sequences for human pathogenic and phytopathogenic fusaria (O'Donnell, unpublished). Significant advantages of the molecular approach, based on results of the present study, include that it can provide accurate identifications of rare and novel mycotic agents that are named (i.e., F. brachygibbosum, F. flocciferum, and F. armeniacum) as well as those that apparently lack Latin binomials (i.e., Fusarium sp. strain 1 and Fusarium sp. strain 2). It is worth mentioning that a third isolate of Fusarium sp. strain 2, NRRL 28032, was received from the CDC as B-4271 in 1998, isolated from a toenail infection from a patient in Colorado.
Conclusions and future directions.
It is important that the MLST schemes developed for the FIESC and FCSC in the present study, in contrast to those available via the Internet for some of the most important human pathogenic species (4, 23), focused primarily on identifying species limits within these closely related species complexes. Nevertheless, the four-locus typing schemes for the FIESC and FCSC achieved indices of discrimination of 0.985 and 0.966, respectively, using Simpson's index of diversity (18). Should the necessity arise, identification of additional phylogenetically informative loci for the MLST schemes will be greatly facilitated by four phylogenetically diverse fusarial genomes that are available online, one representing the FSSC from the Joint Genome Institute (http://www.jgi.doe.gov) and three from the Broad Institute of Massachusetts Institute of Technology and Harvard representing the FOSC, GFSC, and the trichothecene toxin-producing fusaria (9; http://www.broad.mit.edu/annotation/fungi/fgi/). With the development of GCPSR-based MLST schemes for the six most important human-pathogenic species complexes within Fusarium (i.e., FSSC, FOSC, GFSC, FDSC, FIESC, and FCSC) (see Fig. 1 in reference 34), which collectively comprise close to 100% of all medically important isolates, a uniform finding that has emerged from these studies is the dramatic discrepancy between species identifications using morphology alone versus molecular phylogenetics. Results of the present study and those published previously (34, 35, 51) have revealed that only 30% of clinically relevant fusaria (i.e., 20 of 65) have Latin binomials that can be applied with confidence. This is largely due to high levels of cryptic speciation and the concomitant extreme morphological homoplasy, especially within the FIESC and FSSC (30, 35, 51), as reflected by the fact that only 3 of the 21 FIESC and 3 of the 20 FSSC mycoses-associated species have known scientific names. In the absence for morphological apomorphies, the MLST schemes provide the only means by which isolates can be identified to species/haplotype with confidence and be accurately reported on in the scientific literature. Because species limits were delimited within the FIESC and FCSC for the first time in the present study, it is possible for us to recommend using a partial EF-1α gene sequence for identifying species within these two complexes. With the present set of isolates, sequence data from this locus was used to identify all 28 species within the FIESC and all 4 species within the FCSC. However, as putatively novel species are detected, GCPSR-based studies will be required to fully assess their genealogical exclusivity. It is worth mentioning that matrix-assisted laser desorption ionization-time of flight analysis appears to provide a potential avenue for rapidly identifying clinical fusaria to the level of species complex (27) or in some cases to species level, assuming their boundaries have been defined previously by GCPSR. In this preliminary study, 35 of the 62 isolates analyzed were identified to one of three species complexes, with only isolates of F. verticillioides and F. proliferatum being identified to species. Even though these results are encouraging, it remains to be determined whether matrix-assisted laser desorption ionization-time of flight analysis can be used to identify most or all of the approximately 65 clinically relevant fusaria to the species level.
To further promote identification of pathogenic fusaria, Internet-accessible standardized MLST databases of clinically relevant fusaria will be made available at the CBS and the FUSARIUM-ID website (http://fcgp.fusariumdb.org/) at Pennsylvania State University. These databases will be updated regularly as new species/STs are discovered, contingent on the deposit of associated chromatograms, which are essential to ensure that sequences are error free, and of cultures in an international, publically accessible culture collection to promote further study by the scientific community. The MLST databases should be viewed as a work in progress (48), providing a novel baseline for understanding Fusarium population biology and potential changes in the spectrum of clinically relevant fusaria within a robust phylogenetic framework.
Acknowledgments
Special thanks are due Allison Strom, Stacy Sink, and Jean Juba for excellent technical assistance; Nathane Orwig for running all of the DNA sequences in the National Center for Agricultural Utilization Research DNA core facility; Don Fraser for preparation of the tree figures; and the culture collections and individuals who supplied isolates used in this study.
The mention of trade products or firm names does not imply that they are recommended by the U.S. Department of Agriculture over similar products or other firms not mentioned.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Alastruey-Izquierdo, A., M. Cuenca-Estrella, A. Monzón, E. Mellado, and J. L. Rodríguez-Tudela. 2008. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J. Antimicrob. Chemother. 61:805-809. [DOI] [PubMed] [Google Scholar]
- 2.Azor, M., J. Gené, J. Cano, and J. Guarro. 2007. Universal in vitro antifungal resistance of genetic clades of the Fusarium solani species complex. Antimicrob. Agents Chemother. 51:1500-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azor, M., J. Gené, J. Cano, P. Manikandan, N. Venkatapathy, and J. Guarro. 2009. Less-frequent Fusarium species of clinical interest: Correlation between morphological and molecular identification and antifungal susceptibility. J. Clin. Microbiol. 47:1463-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain, J. M., A. Tavanti, A. D. Davidson, M. D. Jacobsen, D. Shaw, N. A. R. Gow, and F. C. Odds. 2007. Multilocus sequence typing of the pathogenic fungus Aspergillus fumigatus. J. Clin. Microbiol. 45:1469-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balajee, S. A., A. M. Borman, M. E. Brandt, J. Cano, M. Cuenca-Estrella, E. Dannaoui, J. Guarro, G. Haase, C. C Kibbler, W. Meyer, K. O'Donnell, C. A. Petti, J. L. Rodriguez-Tudela, D. Sutton, A. Velegraki, and B. L. Wickes. 2009. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J. Clin. Microbiol. 47:877-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth, C., and B. C. Sutton. 1984. Fusarium pallidoroseum, the correct name for F. semitectum Auct. Trans. Br. Mycol. Soc. 83:702-704. [Google Scholar]
- 7.Burgess, L. W., P. E. Nelson, T. A. Toussoun, and W. F. O. Marasas. 1985. Fusarium scirpi: emended description and notes on geographic distribution. Mycologia 77:212-218. [Google Scholar]
- 8.Chang, D. C., G. B. Grant, K. O'Donnell, K. A. Wannemuehler, J. Noble-Wang, C. Y. Rao, L. M. Jacobson, C. S. Crowell, R. S. Sneed, F. M. T. Lewis, J. K. Schaffzin, M. A. Kainer, C. A. Genese, E. C. Alfonso, D. B. Jones, A. Srinivasan, S. K. Fridkin, and B. J. Park. 2006. A multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 296:953-963. [DOI] [PubMed] [Google Scholar]
- 9.Cuomo, C. A., U. Güldener, J.-R. Xu, F. Trail, B. G. Turgeon, A. Di Pietro, J. D. Walton, L.-J. Ma, S. E. Baker, M. Rep., G. Adam, J. Antoniw, T. Baldwin, S. Calvo, Y.-L. Chang, D. DeCaprio, L. R. Gale, S. Gnerre, R. S. Goswami, K. Hammond-Kosack, L. J. Harris, K. Hilburn, J. C. Kennell, S. Kroken, J. K. Magnuson, G. Mannhaupt, E. Mauceli, H.-W. Mewes, R. Mitterbauer, G. Muehlbauer, M. Münsterkőtter, D. Nelson, K. O'Donnell, T. Ouellet, W. Qi, H. Quesneville, M. I. G. Roncero, K.-Y. Seong, I. V. Tetko, M. Urban, C. Waalwijk, T. J. Ward, J. Yao, B. W. Birren, and H. C. Kistler. 2007. The Fusarium graminearum genome reveals localized polymorphism and pathogen specialization. Science 317:1400-1402. [DOI] [PubMed] [Google Scholar]
- 10.Dettman, J. R., D. J. Jacobson, and J. W. Taylor. 2003. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evol. 57:2703-2720. [DOI] [PubMed] [Google Scholar]
- 11.Dignani, M. C., and E. J. Anaissie. 2004. Human fusariosis. Clin. Microbiol. Infect. 10(Suppl. 1):67-75. [DOI] [PubMed] [Google Scholar]
- 12.Geiser, D. M., M. del M. Jiménez-Gasco, S. Kang, I. Makalowska, N. Veeraraghavan, T. J. Ward, N. Zhang, G. A. Kuldau, and K. O'Donnell. 2004. FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 110:473-479. [Google Scholar]
- 13.Gerlach, W., and H. Nirenberg. 1982. The genus Fusarium--a pictorial atlas. Mitt. Biol. Bundesanst. Land-Forstwirtsch. 209:1-406. [Google Scholar]
- 14.Goswami, R. S., Y. Dong, and Z. K. Punja. 2008. Host range and mycotoxin production by Fusarium equiseti isolates originating from ginseng fields. Can. J. Plant Pathol. 30:155-160. [Google Scholar]
- 15.Hennequin, C., E. Abachin, F. Symoens, V. Lavarde, G. Reboux, N. Nolard, and P. Berche. 1999. Identification of Fusarium species involved in human infections by 28S rRNA gene sequencing. J. Clin. Microbiol. 37:3586-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann, M., R. Zocher, and A. Haese. 1996. Enniatin production by Fusarium strains and its effect on potato tuber tissue. Appl. Environ. Microbiol. 62:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hestbjerg, H., K. F. Nielsen, U. Thrane, and S. Elmholt. 2002. Production of trichothecenes and other secondary metabolites by Fusarium culmorum and Fusarium equiseti on common laboratory media and a soil organic matter agar: an ecological interpretation. J. Agric. Food Chem. 50:7593-7599. [DOI] [PubMed] [Google Scholar]
- 18.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of tying systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoa, L. V., K. Hatai, and T. Aoki. 2004. Fusarium incarnatum isolated from black tiger shrimp, Penaeus monodon Fabricius, with black gill disease cultured in Vietnam. J. Fish Dis. 27:507-515. [DOI] [PubMed] [Google Scholar]
- 20.Kosiak, E. B., A. Holst-Jensen, T. Rundberget, M. T. Gonzalez-Jaen, and M. Torp. 2005. Morphological, chemical and molecular differentiation of Fusarium equiseti isolated from Norwegian cereals. Int. J. Food Microbiol. 99:195-206. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen, R., M. Torp, B. Kosiak, and A. Holst-Jensen. 2005. Phylogeny and toxigenic potential is correlated in Fusarium species as revealed by partial translation elongation factor 1 alpha gene sequences. Mycol. Res. 109:173-186. [DOI] [PubMed] [Google Scholar]
- 22.Leslie, J. F., and B. A. Summerell. 2006. The Fusarium laboratory manual. Blackwell Publishing, Ames, Iowa.
- 23.Litvintseva, A. P., R. Thakur, R. Vilgalys, and T. G. Mitchell. 2006. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics 172:2223-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logrieco, A., A. Moretti, G. Castella, M. Kostecki, P. Golinski, A. Ritieni, and J. Chelkowski. 1998. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 64:3084-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marasas, W. F. O., P. E. Nelson, and T. A. Tousson. 1984. Toxigenic Fusarium species: Identity and mycotoxicology. Pennsylvania State University Press, University Park, PA.
- 26.Marasas, W. F. O., J. P. Rheeder, A. Logrieco, P. S. Van Wyk, and J. H. Juba. 1998. Fusarium nelsonii and F. musarum: two new species in section Arthrosporiella related to F. camptoceras. Mycologia 90:505-513. [Google Scholar]
- 27.Marinach-Patrice, C., A. Lethuillier, A. Marly, J.-Y. Brossas, J. Gene, F. Symoens, A. Datry, J. Guarro, D. Mazier, and C. Hennequin. 2009. Use of mass spectrometry to identify clinical Fusarium isolates. Clin. Microbiol. Infect. 15:634-642. [DOI] [PubMed] [Google Scholar]
- 28.Nelson, P. E., T. A. Toussoun, and W. F. O. Marasas. 1983. Fusarium species: An illustrated manual for identification. Pennsylvania State University Press, University Park, PA.
- 29.Nylander, J. A. A. 2004. MrModeltest version 2.2. Evolutionary Biology Centre, Uppsala, Sweden.
- 30.O'Donnell, K. 2000. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 92:919-938. [Google Scholar]
- 31.O'Donnell, K., E. Cigelnik, and H. Nirenberg. 1998. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465-493. [Google Scholar]
- 32.O'Donnell, K., C. Gueidan, S. Sink, P. R. Johnston, P. W. Crous, A. Glenn, R. Riley, N. C. Zitomer, P. Colyer, C. Waalwijk, T. van der Lee, A. Moretti, S. Kang, H.-S. Kim, D. M. Geiser, J. H. Juba, R. P. Baayen, M. G. Cromey, S. Bithell, D. A. Sutton, K. Skovgaard, R. Ploetz, H. C. Kistler, M. Elliott, M. Davis, and B. A. J. Sarver. 26 August 2009, posting date. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. doi: 10.1016/j.fgb.2009.08.006. [DOI] [PubMed]
- 33.O'Donnell, K., H. I. Nirenberg, T. Aoki, and E. Cigelnik. 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 41:61-78. [Google Scholar]
- 34.O'Donnell, K., B. A. J. Sarver, M. Brandt, D. C. Chang, J. Noble-Wang, B. J. Park, D. A. Sutton, L. Benjamin, M. Lindsley, A. Padhye, D. M. Geiser, and T. J. Ward. 2007. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 45:2235-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Donnell, K., D. A. Sutton, A. Fothergill, D. McCarthy, M. G. Rinaldi, M. E. Brandt, N. Zhang, and D. M. Geiser. 2008. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 46:2477-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell, K., D. A. Sutton, M. G. Rinaldi, K. C. Magnon, P. A. Cox, S. G. Revankar, S. Sanche, D. M. Geiser, J. H. Juba, J.-A. H. van Burik, A. Padhye, E. J. Anaissie, A. Francesconi, T. J. Walsh, and J. S. Robinson. 2004. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. J. Clin. Microbiol. 42:5109-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Donnell, K., T. J. Ward, D. M. Geiser, H. C. Kistler, and T. Aoki. 2004. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum species complex. Fungal Genet. Biol. 41:600-623. [DOI] [PubMed] [Google Scholar]
- 38.Peraica, M., B. Radic, A. Lucic, and M. Pavolovic. 1999. Toxic effects of mycotoxins in humans. Bull. W. H. O. 77:754-766. [PMC free article] [PubMed] [Google Scholar]
- 39.Peska, M., and A. T. Smolinski. 2005. Deoxynivalenol: toxicology and potential effects on human health. J. Toxicol. Environ. Health B 8:39-69. [DOI] [PubMed] [Google Scholar]
- 40.Pringle, A., D. M. Baker, J. L. Platt, J. P. Wares, J. P. Latgé, and J. W. Taylor. 2005. Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evol. 59:1886-1899. [PubMed] [Google Scholar]
- 41.Pujol, I., J. Guarro, J. Gené, and J. Sala. 1997. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J. Antimicrob. Chemother. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 42.Punja, Z. K., A. Wan, M. Rahman, R. S. Goswami, T. Barasubiye, K. A. Seifert, and C. A. Lévesque. 2008. Growth, population dynamics, and diversity of Fusarium equiseti in ginseng fields. Eur. J. Plant Pathol. 121:173-184. [Google Scholar]
- 43.Reuben, A., E. Anaissie, P. E. Nelson, R. Hashem, C. Legrand, D. H. Ho, and G. P. Bodey. 1989. Antifungal susceptibility of 44 clinical isolates of Fusarium species determined by using a broth microdilution method. Antimicrob. Agents Chemother. 33:1647-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroers, H.-J., K. O'Donnell, S. C. Lamprecht, P. L. Kammeyer, S. Johnson, D. A. Sutton, M. G. Rinaldi, D. M. Geiser, and R. C. Summerbell. 2009. Taxonomy and phylogeny of the Fusarium dimerum species group. Mycologia 101:44-70. [DOI] [PubMed] [Google Scholar]
- 45.Subrahmanyam, A. 1983. Fusarium laceratum. Mykosen 26:478-480. [PubMed] [Google Scholar]
- 46.Summerbell, R. C. 2003. Aspergillus, Fusarium, Sporothrix, Piedraia, and their relatives, p. 237-498. In D. H. Howard (ed.), Pathogenic fungi in humans and animals. Marcel Dekker, Inc., New York, NY.
- 47.Swofford, D. L. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA.
- 48.Taylor, J. W., and M. C. Fisher. 2003. Fungal multilocus sequence typing—it's not just for bacteria. Curr. Opinion Microbiol. 6:351-356. [DOI] [PubMed] [Google Scholar]
- 49.Taylor, J. W., D. J. Jacobson, S. Kroken, T. Kasuga, D. M. Geiser, D. S. Hibbett, and M. C. Fisher. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31:21-32. [DOI] [PubMed] [Google Scholar]
- 50.Ueno, Y., M. Nakajima, K. Sakai, K. Ishii, N. Sato, and N. Shimada. 1973. Comparative toxicology of trichothecene mycotoxins: inhibition of protein synthesis in animal cells. J. Biochem. (Tokyo) 74:285-296. [PubMed] [Google Scholar]
- 51.Zhang, N., K. O'Donnell, D. A. Sutton, F. A Nalim, R. C. Summerbell, A. A. Padhye, and D. M. Geiser. 2006. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 44:2186-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zwickl, D. J. 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. dissertation. The University of Texas, Austin.