Abstract
Rhinovirus infections are the most common cause of viral illness in humans, and there is increasing evidence of their etiological role in severe acute respiratory tract infections (ARTIs). Human rhinoviruses (HRVs) are classified into two species, species A and B, which contain over 100 serotypes, and a recently discovered genetically heterogeneous third species (HRV species C). To investigate their diversity and population turnover, screening for the detection and the genetic characterization of HRV variants in diagnostic respiratory samples was performed by using nested primers for the efficient amplification of the VP4-VP2 region of HRV (and enterovirus) species and serotype identification. HRV species A, B, and C variants were detected in 14%, 1.8%, and 6.8%, respectively, of 456 diagnostic respiratory samples from 345 subjects (6 samples also contained enteroviruses), predominantly among children under age 10 years. HRV species A and B variants were remarkably heterogeneous, with 22 and 6 different serotypes, respectively, detected among 73 positive samples. Similarly, by using a pairwise distance threshold of 0.1, species C variants occurring worldwide were provisionally assigned to 47 different types, of which 15 were present among samples from Edinburgh, United Kingdom. There was a rapid turnover of variants, with only 5 of 43 serotypes detected during both sampling periods. By using divergence thresholds and phylogenetic analysis, several species A and C variants could provisionally be assigned to new types. An initial investigation of the clinical differences between rhinovirus species found HRV species C to be nearly twice as frequently associated with ARTIs than other rhinovirus species, which matches the frequencies of detection of respiratory syncytial virus. The study demonstrates the extraordinary genetic diversity of HRVs, their rapid population turnover, and their extensive involvement in childhood respiratory disease.
Human rhinoviruses (HRVs) have recently been classified in the genus Enterovirus, family Picornaviridae (34). Typically for picornaviruses, they are small, nonenveloped, single-stranded positive-sense viruses with a 7,200-base mRNA genome. HRVs are highly heterogeneous genetically and antigenically. A total of 101 serotypes have been classified, and these fall into two species, HRV species A (HRV-A; 74 serotypes) and HRV-B (25 serotypes); HRV serotype 87 is genetically most similar to members of human enterovirus (HEV) species D (HEV-D). Recently, several reports have described the detection and characterization of a series of new divergent rhinovirus variants that have provisionally been assigned to a new species, HRV-A2 or HRV-C (1, 13, 15, 16, 18, 22, 28).
HRVs are the most common etiological agents of upper respiratory tract infections and regularly cause a mild, self-limiting illness that is often referred to as the common cold. HRV infections are transmitted most commonly by the respiratory-salivary route, both by person-to-person contact and by airborne transmission. In temperate countries, infections occur primarily in two peaks, with the first being in April and May and the second being in September and October (24, 38). HRV infections occur in all age groups during all seasons, with a 90% past infection frequency by the age of 2 years (2). While infections are frequently associated with mild upper respiratory tract symptoms, HRV infections have been implicated in more serious illnesses, including pneumonia, otitis media, exacerbations of asthma, and chronic obstructive pulmonary disease (for a review, see reference 20). Whether HRV-C is more likely to be etiologically associated with the more severe end of the HRV-associated spectrum of respiratory disease is currently under active debate and investigation (23, 40).
In the study described here, we have coupled a sensitive PCR-based method for screening for HRV using primers from the conserved 5′ untranslated region (UTR) with a VP4-VP2 amplification and typing method that can be applied directly to clinical specimens. The method effectively amplifies and allows the genetic characterization of all three species of HRV as well as HEVs. When the method was applied to respiratory specimens collected in Edinburgh, United Kingdom, we were able to document the diversity and rapid turnover of circulating HRV strains, including species C variants, in a predominantly pediatric population.
MATERIALS AND METHODS
Study population and samples.
A total of 456 respiratory samples from 345 patients (189 male patients, 155 female patients, 1 patient whose sex was unknown) referred to the Specialist Virology Centre, Royal Infirmary of Edinburgh, for testing for respiratory virus infection in September 2006 and February 2007 were used in this study. The samples predominantly comprised nasopharyngeal aspirates or swabs (n = 397; 87%) and were routinely screened for the following respiratory viruses by previously described PCR assays (8, 37): adenovirus (AdV), influenza A and B viruses, parainfluenza virus type 1 (PIV-1), PIV-2, PIV-3, and respiratory syncytial virus (RSV). The samples were further tested for human parechovirus (7) and human metapneumovirus (5a). The samples referred were predominantly from hospital inpatients or patients in accident and emergency departments; only seven samples were sent from outpatient attendees, and three were sent from general practitioners.
All samples were anonymously labeled and deposited in the Specialist Virology Centre respiratory sample archive before they were tested. Approval was obtained from the Lothian Regional Ethics Committee (approval number 08/S11/02/2) to retain the collected information for epidemiological purposes but to make the information anonymous to strictly protect patient confidentiality. The stored data included age band, partial postcode, any recorded symptoms or clinical information, referral source, month of sample collection, and the results of other virological tests for each sample.
HRV and HEV detection.
RNA was extracted from 100 μl of the clinical specimens by using a MinElute virus spin kit, according to the manufacturer's instructions (Qiagen, Hemel Hempstead, United Kingdom), and the extracted RNA was stored at −70°C. RNA from pools or individual samples was reverse transcribed into cDNA by using a reverse transcription system (Promega, United Kingdom), according to the manufacturers' instructions, and by using the changes described previously (7). Amplification of the 5′ UTR, the VP4-VP2 region, and the VP1 region of the genome used a previously described nested RT-PCR strategy (7) and the primers listed in Table 1; the three HEV- and HRV-specific 5′ UTR inner sense primers were included in the second amplification step. For a small number of samples that were positive with the 5′ UTR primers but negative for VP4-VP2 amplification, SuperScript III (Invitrogen) was used for reverse transcription before amplification with the VP4-VP2 primers (19). The amplification products were visualized by gel electrophoresis.
TABLE 1.
Primers used for amplification of HRV and HEV 5′ UTR and VP4-VP2 regions and VP1 of HRV-A
| Region | Target | Orientationa | Positionb | Sequencec |
|---|---|---|---|---|
| 5′ UTR | HRV and HEV | OS | 178 | HCAAGYACTTCTGTYWCCCCSG |
| HRV and HEV | OAS | 573 | GAAACACGGACACCCAAAGTAGT | |
| HRV-A and HRV-B | IS | 367 | CYAGCCTGCGTGGCKGCCWRC | |
| HRV-C | IS | 367 | GTAGCCYGCGTGGTGCCCWGC | |
| HEV | IS | 370 | GGCTGCGYTGGCGGCCTRC | |
| HRV and HEV | IAS | 477 | TTAGCCRCATTCAGGGGCCGG | |
| VP4-VP2 | HRV and HEV | OS | 458 | CCGGCCCCTGAATGYGGCTAA |
| HRV and HEV | IS | 547 | ACCRACTACTTTGGGTGTCCGTG | |
| HRV and HEV | IAS | 1087 | TCWGGHARYTTCCAMCACCANCC | |
| HRV and HEV | OAS | 1125 | ACATRTTYTSNCCAAANAYDCCCAT | |
| VP1 | HRV-A | OS | 1995 | MGHTTYAGYTTYATGTTYTGTGG |
| HRV-A | IS | 2430 | TRGAYGCWGCWGARACWGG | |
| HRV-A | IAS | 3333 | GTRTTTGTKCGGTADATGAYTARRTC | |
| HRV-A | OAS | 3525 | CCACARTCWCCWGGYTCACADGG |
Abbreviations: OS, outer sense; OAS, outer antisense; IS, inner sense; IAS, inner antisense.
The 5′ base position numbered according to the HRV-B serotype 14 genome (GenBank accession number NC_001490).
Primer positions that differ between HEV, HRV-A, HRV-B, or HRV-C are indicated in underlined boldface.
Screening of individual samples was accomplished with primers targeting the highly conserved 5′ UTR of the genome. The sensitivity of the assay using the combined inner primers for the detection of HEV was assessed by comparison of the results with those obtained by a previously described procedure (19). The VP4 region and part of the VP2 genome region (VP4-VP2) of samples positive with the 5′ UTR-specific primers were subsequently amplified. VP4-VP2 was sequenced and subjected to phylogenetic analysis, as were published full-length and overlapping partial sequences from VP4-VP2; the previously published full-length sequences predominantly comprised those from HRV reference strains whose serotypes were previously determined by serological methods (14, 25, 36). The VP1 region of reference strains whose sequences were not clearly grouped with previously designated HRV serotypes was amplified and sequenced. The edited sequences generated from this study were aligned with published sequences by using the Simmonics sequence editor (version 1.6) program (33; http://www.virus-evolution.org/). Phylogenetic trees were constructed by neighbor joining from 100 samplings of maximum-composite-likelihood distances by using the MEGA (version 4.0) software package (35) with pairwise deletion for missing data.
Nucleotide sequence accession numbers.
The sequences generated in this study have been assigned GenBank accession numbers GQ476576 to GQ476685.
RESULTS
Sample screening and serotype identification.
Primers for amplification of the 5′ UTR were newly designed to accommodate the sequence variability within the much larger data set of published sequences of all HRV-A and HRV-B serotypes, along with the sequences obtained from the recently described species C variants. Conserved regions between HRV-A, -B, and -C coincided with those of HEV-A to HEV-D, enabling a combined HRV-HEV amplification method, although the incorporation of HEV and HRV-C amplification necessitated the use of separate inner sense primers; for example, HEV 5′ UTR sequences differed from HRV at several positions in the primer-binding target (Table 1; the bases that are different are underlined and in boldface). The sensitivity of the combined HEV-HRV PCR was compared to that of a previously described 5′ UTR-based nested PCR previously used for the screening of clinical specimens (cerebrospinal fluid in suspected viral meningitis/encephalitis cases [19]) and surveillance samples (blood donor screening [39]) (Table 2). On the basis of the proportion of replicates positive at each dilution, the two assays showed identical sensitivities.
TABLE 2.
Sensitivity of HRV-HEV combined PCR assay
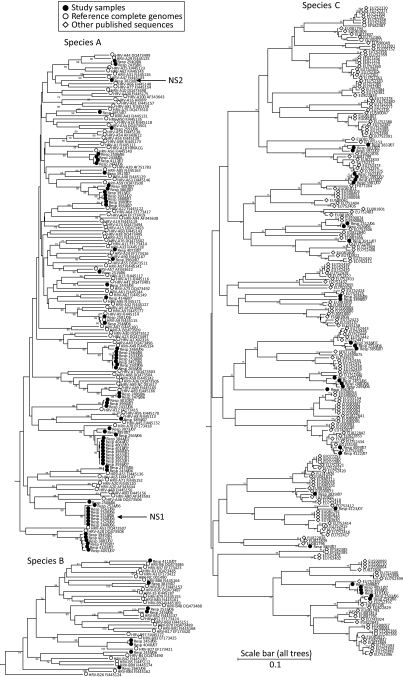
To investigate the frequency and the genetic diversity of HRV and HEV strains among respiratory samples referred for diagnostic testing, a total of 456 samples were collected from 345 study subjects in September 2006 (n = 136) and February 2007 (n = 320). High detection frequencies were observed in both months (51 of 136 [38%] samples in 2006 and 60 of 320 [19%] samples in 2007). To identify the infecting serotypes, the more variable, longer, and more phylogenetically informative VP4-VP2 region of each positive sample was amplified and sequenced. Of the 111 5′ UTR-positive samples, the VP4-VP2 regions of 110 could be amplified and sequenced (Fig. 1). The single exception was a follow-up sample from a subject with a previous VP4-VP2-positive sample.
FIG. 1.
Diversity of HRV variants detected in the study. The phylogeny of the VP4-VP2 sequences amplified from strains from the study subjects and the results of a comparison of those sequences with those from reference strains of species A and B or with other published sequences from species C of currently unassigned types are shown (for clarity, sequences showing less than 0.5% divergence in the analyzed region were excluded from the tree). For phylogeny estimation, neighbor-joining trees were constructed by using maximum-composite-likelihood distances estimated between sequences in the amplified region (positions 629 to 1063 numbered according to the HRV-B14 reference sequence [GenBank accession number NC_001490]). Data were bootstrap resampled 100 times to assess the robustness of the branches; values of 70% or greater are shown. All trees were drawn to the same scale; for species A and C variants, HRV-B (GenBank accession number NC_001490) was used to root the trees; sequence HRVACG (serotype 1B; GenBank accession number D00329) was used to root the species B tree.
For sequences grouping with the HRV-A and the HRV-B clades, serotype assignments were performed by recording the nearest neighbors among the published sequences of serologically defined HRV reference sequences (29) (Table 3). This approach was used because distance thresholds (either nucleotide or amino acid sequences) between serotypes currently remain undetermined, although this issue is investigated below in relation to the putative type assignments for species C variants.
TABLE 3.
HRV serotypes detected among study subjects
| Species and serotype | No. of samples with the indicated serotype in: |
|
|---|---|---|
| September 2006 | February 2007 | |
| Species A | ||
| A9 | 2 | |
| A12 | 1 | 2 |
| A34 | 1 | |
| A41 | 1 | |
| A29/A44 | 2 | |
| A49 | 1 | |
| A55 | 2 | |
| A56 | 3 | 1 |
| A57 | 1 | |
| A89 | 9 | |
| NS1a | 8 | |
| A22 | 2 | |
| A28 | 6 | |
| A33 | 1 | |
| A59 | 5 | |
| A67 | 1 | |
| A75 | 1 | |
| A78 | 11 | |
| A85 | 1 | |
| A90 | 1 | |
| A95 | 1 | |
| NS2a | 1 | |
| Total (n = 22) | 31 | 34 |
| Species B | ||
| B4 | 1 | |
| B52 | 2 | |
| B84 | 1 | |
| B27 | 1 | 1 |
| B6 | 1 | |
| B72 | 1 | |
| Subtotal (n = 6) | 5 | 3 |
| Species Cb | ||
| C1 | 1 | |
| C5 | 2 | |
| C9 | 2 | |
| C10 | 1 | 2 |
| C11 | 3 | 1 |
| C2 | 1 | |
| C3 | 1 | |
| C4 | 1 | |
| C6 | 1 | |
| C7 | 4 | |
| C8 | 2 | |
| C12 | 3 | |
| C13 | 4 | |
| C14 | 1 | |
| C15 | 1 | |
| Subtotal (n = 15) | 9 | 22 |
Provisionally assigned new HRV-A serotypes.
Temporary names based on tree position.
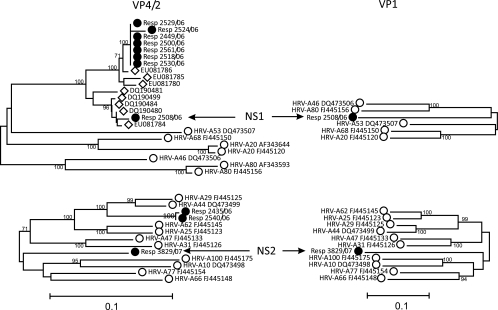
By using the existing nearest-neighbor assignment method, nine subjects were found to be infected with two different HRV-A variants with no single closest neighbor (Fig. 1), and these variants were genetically divergent from all other serotypes. The VP4-VP2 sequence of new serotype 1 (NS1) differed from that of the closest prototype isolate (serotype A46) by 17.6%, while the VP4-VP2 sequence of NS2 differed from that of its closest neighbor (serotype A77) by 14.0%. To investigate whether these variants were similarly divergent elsewhere in the genome, the sequences of the VP1 region were compared by amplification with a new set of nested primers spanning nucleotide positions 2516 to 3285 (Fig. 2). The phylogeny relationships between the most closely grouping sequences in VP4-VP2 were similar to those of the most closely grouping sequences in the VP1 region; the lack of nearest neighbors and the high degree of sequence divergence in the second genomic region (VP1) provide evidence for the existence of two new serotypes of HRV-A. The inclusion of published VP4-VP2 sequences derived from a previous PCR-based screening of clinical specimens revealed that viruses similar to NS1 were present in samples from other surveys (see Discussion). No sequences in the GenBank database were similar to NS2.
FIG. 2.
VP4-VP2 and VP1 phylogenies of putative new serotypes and the most closely related HRV-A serotypes. A comparison of the phylogenies in the VP4-VP2 and VP1 regions of the putative new serotypes, NS1 and NS2, and the most closely related HRV-A serotypes is shown. The symbols correspond to those used in Fig. 1.
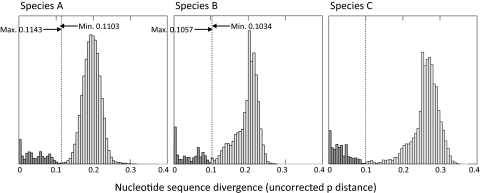
Variants of HRV-C are not currently assigned to serotypes, as methods for their in vitro culture have not been successful to date and their cross-neutralization properties remain unknown. However, variants within the species fell into a number of separate branches or groups that showed pairwise distances equal to or greater than those between the serotypes of other HRV species. To investigate whether assignment on the basis of a distance threshold was feasible, the frequency distributions of the pairwise distances between the 184 VP4-VP2 sequences available in the GenBank database (as of 12 May 2009) of species C variants were compared with those of species A (n = 447) and species B (n = 141) (Fig. 3).
FIG. 3.
Pairwise distances between and within HRV-A and HRV-B serotypes and identification of the divergence threshold in species C. The distributions of the pairwise distances between the available VP4-VP2 sequences (between positions 629 and 958) of HRV-A (n = 447), HRV-B (n = 141), and HRV-C (n = 220) variants are shown (sequences were downloaded from the GenBank database on 12 May 2009). The inter- and intraserotype (shaded light and dark gray, respectively) distance ranges for species A and B were calculated by using reference sequences of assigned serotypes (14, 25, 29, 36). The dotted line shows the upper limit of the intraspecies divergence in HRV-A and HRV-B and a provisionally assigned threshold of 0.1 for the currently unclassified HRV-C variants. For all three species, the y-axis scale for intraserotype comparisons (inferred in the case of HRV-C) was expanded fivefold for clarity.
For the species A and B variants, the distinction between intratype pairwise distances and between serotype pairwise distances was assisted by the inclusion of reference sequences of assigned serotypes, allowing the distributions of the interpairwise distances (Fig. 3, light gray bars) and the intrapairwise distances (Fig. 3, dark gray bars) to be identified. For both species, the pairwise distances fell to a minimum value at sequence divergence values of approximately 11% (HRV-A) and 10% (HRV-B). The only exceptions to these serotype assignment thresholds were distances between eight species A serotype pairs (serotype 25-serotype 62, serotype 29-serotype 44, serotype 8-serotype 95, serotype 1a-serotype 1b, serotype 62-serotype 25, serotype 31-serotype 47, serotype 30-serotype 23, serotype 54-serotype 98) that were less than the maximum recorded intraserotype divergence of 11.4% between two serotype 57 sequences. Similarly, for species B, the divergence for one pairwise comparison between serotypes (serotypes 17 and 91; 10.3%) was below the maximum divergence within a serotype (serotype B52; 10.6%). In many cases, these different serotype assignments for genetically similar viruses have been shown to be incorrect (see Discussion).
The distribution of pairwise distances among the data set of HRV-C sequences was similarly bimodal, with the minimum value between putative intra- and intertype distances being approximately 0.1, which corresponds almost exactly to the thresholds assigned to species A and B (Fig. 3). The pairwise distances between putative HRV-C types were, however, greater than those between species A and B variants (mean value, 27%; mean values for HRV-A and HRV-B, 18% and 20%, respectively). By using the 10% threshold, the pairwise distances between all available sequences of the HRV-C strains were assigned to the same and different type categories, a procedure that produced a total of 47 putative types among the 227 currently available HRV-C sequences in this region. A total of 15 different HRV-C types were found among the 33 positive samples identified in the current study, and 2 of these corresponded to types absent from the larger worldwide collection of HRV-C sequences.
The six remaining samples whose VP4-VP2 regions were sequenced comprised HEV-B (five samples from four individuals) and HEV-C (one sample). The HEV serotypes were identified by comparison of the sequences with those of HEV variants of defined serotype by using the BLAST program. Two of the species B variants were assigned to coxsackievirus B type 4 (CVB4) on the basis of their close sequence similarity (94%) with published sequences of this serotype. For two positive samples obtained from the same individual, one was CVB1 (95% similarity) and one was CVB3 (93% similarity); for the species C variants, the closest matching sequence was that of CVA21 (92% similarity). All samples positive for HEV were detected in the September 2006 sampling period.
As a further check of the sensitivity of the combined HRV-HEV 5′ UTR-based method for screening for HEV, all 456 study samples were screened by using the previously described HEV-specific primers (39). The samples were initially screened in pools of 10, and positive pools were split to identify individual positive samples. This procedure identified the same six samples that were positive by the HEV-HRV screening assay and no other positive samples.
A small proportion of subjects (n = 14) contributed more than one HRV-positive sample (n = 2 to 5) over the study period. In 10 subjects from whom samples were collected during the same month (September 2006 or February 2007), the VP4-VP2 sequences were closely similar or identical. They comprised seven samples with HRV-A infections, two samples with HRV-C infections, and one sample with an HEV infection. A change in the infecting HRV serotype was observed in four subjects (HRV-B4 to HRV-A49, NS1 to HRV-A78, NS1 to HRV-A28, HRV-C to HRV-A39), although in the first three subjects, the change occurred between the two sampling months. In the final case, the switch from HRV-C to a species A serotype occurred between samples collected approximately 5 days apart.
Epidemiological and clinical associations of HRV and HEV infections.
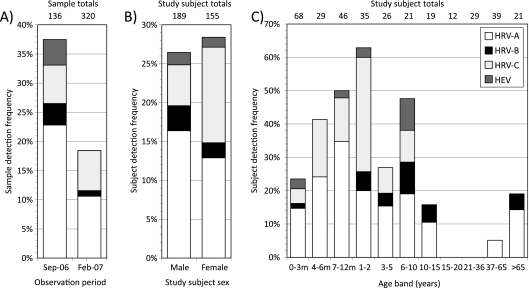
HRV-A and HRV-B were substantially more prevalent in September than in February (Fig. 4A), differences that approached or exceeded statistical significance (for species A, P = 0.0009; for species B, P = 0.055 [Fisher's two-tailed exact test]). By contrast, similar frequencies of species C were found between the two sampling months (9/136 samples and 22/320 samples, respectively; P = 1.0). HRV-A was the most frequently detected and the most genetically diverse species found in the study subjects, accounting for 65 of the 104 HRV-positive samples (63%) whose VP4-VP2 regions could be amplified. HRV-C was the second most common (n = 31; 30%), exceeding the incidence of HRV-B by nearly fourfold (n = 8). HRV (and HEV) showed substantial genetic heterogeneity, with 65 HRV-A-positive samples being classifiable into a total of 22 different serotypes, 8 HRV-B-positive samples being classifiable into 6 different serotypes, and 31 HRV-C-positive samples being classifiable into 15 putatively assigned types (Table 3). Evidence for the rapid turnover of HRV populations was provided by the observation of almost entirely distinct serotype distributions between the two sampling months. In the case of species A, only 2 of the 11 serotypes detected in September were found among the 13 serotypes found in February. For HRV-B and HRV-C, one and two serotypes, respectively, were shared between months.
FIG. 4.
Epidemiological characteristics of HRV- and HEV-infected subjects. Frequencies of detection of HRV and HEV in the two sampling months (proportion of samples) (A), between males and females (proportion of subjects; the sex of one study subject was unknown) (B), and in different age bands (proportion of subjects) (C) are shown. The y axis depicts the proportion of samples or subjects positive from the total in each category. m, months.
All three HRV species (and HEV) showed higher frequencies of infection of children, with all infections being largely confined to those under 10 years of age; and a second peak occurred in those over 65 years of age (Fig. 4C). Extremely high infection frequencies were observed in children in the 1- to 2-year-old age band (60%), whereas none were found in older children and most adult groups (age range, 16 to 36 years). The relative frequency of detection of HRV-A, -B, and -C was broadly similar across the age ranges, with HRV-A being the commonest, followed by species C and then species B in most age groups; the exception to this finding was the rarity of HRV-B variants in children less than 1 year of age and of species C in elderly individuals.
By using the patient, clinical, and other referral information accompanying the samples, the clinical presentations of individuals infected with different HRV species and other respiratory viruses were compared. Most HRV infections were found in children under the age of 10 years as monoinfections, with 81%, 80%, and 77% of infections caused by HRV-A, B-, and -C, respectively, being detected in the absence of coinfection with other respiratory viruses (RSV, AdV, PIV-1 to PIV-3, influenza viruses A and B, human metapneumovirus, human parechovirus). The frequencies of HRV monoinfections were higher than those observed for other respiratory pathogens, such as RSV (69%; P ≈ 0.1) and AdV (39%; P < 0.001). A significantly higher proportion of females than males were infected with HRV-C (infection frequencies, 12% and 5%, respectively; P = 0.03 by Fisher's exact two-tailed test), in contrast to the slight excess of males infected with HRV-A and -B (16% for males and 13% for females for HRV-A and 3.2% for males and 1.9% for females for HRV-B).
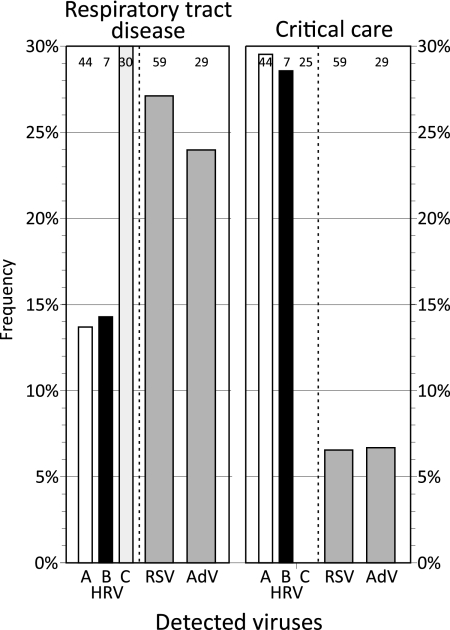
HRV infections were detected the most frequently among those subjects with recorded diagnoses of acute respiratory tract infections and among those in critical care (including intensive therapy, high-dependency, and neonatal units), although the frequencies of occurrence of the different HRV species varied substantially (Fig. 5). Species C infections were found at nearly twice the frequency of HRV-A and -B infections (30%, 13.6%, and 14.3%, respectively; P ≈ 0.1) among those with acute respiratory tract infections, with the frequency of HRV-C infections being similar to the frequencies recorded for RSV and AdV infections (Fig. 5). In contrast, species A and B infections were more frequently found in association with admission to or nursing in a critical care unit (29.5% and 28.6%, respectively), and HRV-C was not detected in that patient group (P = 0.002). This finding is comparable to the low frequencies of RSV and AdV detection (6.8% and 6.9%, respectively).
FIG. 5.
Proportion of subjects under the age of 10 years infected with HRV-A, HRV-B, HRV-C, RSV, and AdV with recorded symptoms or diagnoses of acute respiratory disease or those treated on critical care wards (intensive therapy, high-dependency, and neonatal units). The numbers at the tops of the bars are the total number of subjects in each category; these were used as the denominators to calculate the HRV detection frequencies (recorded on the y axis).
DISCUSSION
This study documents the remarkable diversity and rapid turnover of HRV variants of all three species at two sample collection times 5 months apart. Among the respiratory viruses for which the samples were screened, HRV was by far the most frequently detected in the study group, being particularly prevalent (>50%) among diagnostic specimens collected from children between 1 and 2 years of age. Consistent with the findings of previous studies, variants classifiable as species C were frequently found in respiratory samples (31 of 104; 30%), comparable to the frequencies of detection of this species in diagnostic respiratory specimens collected worldwide (28% to 52%; [3, 11, 23]) and substantially exceeding the frequency of detection of HRV-B serotypes. As mentioned previously (20), it is remarkable how such a prevalent virus infection could have circulated undetected for so many years, although its lack of culturability in vitro and the relatively recent application of PCR-based methods for screening for HRV have undoubtedly contributed historically to this diagnostic gap.
Assays that screen for HRV typically target the highly conserved 5′ UTR for amplification (5, 9, 10, 27, 38). However, in addition to detection, identification of the infecting species or serotype is of key importance diagnostically for the detection of mixed infections or a reinfection with different HRV serotypes, as well as in broader clinical investigations of the relationship between a serotype or species with disease severity and in epidemiological investigations of the circulation and turnover of HRVs (20). Unfortunately, the amplicons generated by most screening assays (including the 5′ UTR-based methods described in this paper) are usually too short and lack sufficient variability to allow serotypes or, often, species to be reliably identified (32). Furthermore, while the recombination events that occur in HRV have not been documented to be as extensive as those that occur in HEV (14, 25, 30, 33), the 5′ UTR-P1 boundary is a recombination hot spot in other picornavirus groups and genera. Consequently, phylogeny relationships in the 5′ UTR may not reflect those in the structural gene-coding region on which the serological properties of HRVs naturally depend. The discordant phylogeny of the 5′ UTR sequences from HRV-C epitomizes this problem, with some variants forming a discrete clade separate from the clades of species A and B (and enteroviruses) and other viruses, including the HRV-C prototype sequence, QPM (22), that falls within species A. This is itself a likely outcome of a recombination event that occurred in the evolutionary history of some species C variants.
For these reasons and despite the recent promotion of 5′ UTR-based HRV (sero)type identification methods (12, 18), we concur with Savolainen-Kopra and colleagues that P1-coding sequences are required for reliable species and serotype assignments (32). We therefore supplemented our screening method with a second PCR for typing of VP4-VP2 using nested primers to ensure reliability and sensitivity for direct use with clinical specimens. Its ability to amplify and provide complete amplicon sequences for 109 of the 110 screen-positive samples (including all 6 of the enterovirus-positive samples) provided a highly effective tool with which to compare the genetic diversity, molecular epidemiology, and disease associations of the three HRV species. A striking finding, mirrored in other recent PCR-based investigations of rhinovirus diversity (1, 3, 6, 11, 16, 23, 28, 31), was the large number of circulating types within the study subject population, including species C. Equally striking was evidence of the rapid turnover of HRV variants, with only 5 of the 43 HRV-A, -B, and -C (sero)types being found in both sampling months (Table 3), findings reminiscent of the detection of multiple HRV serotypes within the same household over even shorter observation periods (26).
While comparison of HRV-A and HRV-B VP4-VP2 sequences with those of the corresponding region of prototype strains provides a guide for serotype assignment (14, 25, 29, 36), HRV-C variants are not, to date, formally classified into types, even though there is equal or greater diversity between variants in this new clade than in variants in species A or B (Fig. 1). The lack of in vitro methods of culturing HRV-C variants would, in any case, preclude the use of the serologically based typing used for other rhinoviruses, therefore necessitating (geno)type assignment on the basis of genetic diversity. Examination of the distribution of pairwise distances in VP4-VP2 justifies the placement of a threshold at an uncorrected pairwise distance of about 10% for such a purpose and is similar to the 10% to 11% boundary that divides intra- and (most) interserotype distances in species A and B in this genomic region (Fig. 3). By using this provisional type assignment method, the 31 sequences obtained in the current study combined with the HRV-C variants collected worldwide to date comprise a total of 47 different types, which is already more than the number in species B and may, possibly, with more extensive temporal and geographical sampling, exceed that in HRV-A.
Reliance on virus isolation techniques may, however, have also led to long-term underestimates of the extent of HRV-A and HRV-B serotype diversity. In the case of HRV-A, assignment by similarity values is complicated by the existence of separately assigned serotypes with similarity values below 11% that divided interserotype from intraserotype similarity values in VP4-VP2. In some cases, genetically similar serotypes (below the 10% threshold in VP4-VP2) have been shown to be serologically cross-reactive (e.g., serotypes A8 and A95 [1% divergent] [17], serotypes A29 and A44 [5.3%] [4], and serotypes 1A and 1B [8.3%] [4]), while others (e.g., serotypes A62 and A25 [7.0%] and serotypes A47 and A31 [8.7%]) are apparently non-cross-neutralizable (4). However, in the current study two HRV-A variants were detected that failed to group phylogenetically with any prototype strain in either VP4-VP2 or VP1 (Fig. 3) and that showed minimum divergence values well above the 11% threshold (17.6% and 14.0% for NS1 and NS2, respectively). Remarkably, comparison of all available VP4-VP2 sequences from HRV-A revealed the frequent detection of NS1 in Australia (1), Germany (28), the United States (13), Spain (3), and China (GenBank accession number EU822866), even though its existence as a likely new serotype was not specifically commented on in most of those studies. With the availability of complete genome sequences from all HRV prototype isolates and the adoption of agreed-upon criteria for the assignment of new HRV types without recourse to virus isolation, it is likely that the number of HRV (sero- or geno)types in all three species will greatly expand in the future.
Although it was not the primary focus of the study, the available clinical and epidemiological information available for patients in whose samples species and serotype were identified provided the basis for an initial examination of differences in the age distributions and clinical presentations of the patients. The age profiles for infection were similar (Fig. 4C), but there were substantial differences between HRV-A and -B and HRV-C in their association with other demographic variables and clinical presentations. In our study group, there was a significant overrepresentation of females among those infected with HRV-C, with the frequency of HRV-C in females being approximately twice that in males (Fig. 4B). This difference has not, however, been found in previous comparisons (e.g., 31 females/77 total [23], 14/25 [28], and 7/12 [16]).
We obtained evidence for an association of HRV-C with more severe primary respiratory infections (Fig. 5) that have been reported in some previous comparisons (11, 21-23) but not all previous comparisons (16, 28) of species-associated differences in the clinical presentations of patients with HRV infections. The frequencies of symptoms or reported respiratory tract disease in patients infected with HRV-C were comparable to those in patients infected with RSV and twice those in patients infected with species A and B serotypes. These observations are consistent with the more frequent detection of this species in children with an underlying severe respiratory disease, particularly asthma (11, 23) and bronchiolitis, as well as other lower respiratory tract diseases (16, 21). However, corroboration of the hypothesis for the greater pathogenicity of species C will require larger numbers of subjects and a comparison of HRV species detection frequencies in control groups of subjects who have mild symptoms or who are nonsymptomatic. The underlying reasons for the high frequency of subjects with species A and B HRV infections (and the absence of HRV-C infections) requiring admission to critical care units remain unclear and need further investigation.
The development of effective methods for HRV detection and species assignment will, in the future, undoubtedly expand our knowledge of the role of HRVs in respiratory disease, a much neglected area of clinical investigation. As discussed previously (20), HRV may indeed rival or exceed RSV and other respiratory pathogens in its association with severe disease requiring hospitalization, as well as in its association with chronic conditions, such as asthma. The ability of molecular methods to identify HRV species and serotypes genetically will additionally allow the heterogeneity in the disease associations of different HRV variants, including species C variants and the recently discovered HRV-A types that are perhaps refractory to conventional virus isolation methods, to be better explored.
Acknowledgments
We are grateful to Kate Templeton, Peter McCullough, Julie White, Mary Notman, Eleanor Leslie, and Carol Thomson for providing samples, data, and other virus testing results from the respiratory sample archive.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Arden, K. E., P. McErlean, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2006. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J. Med. Virol. 78:1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomqvist, S., M. Roivainen, T. Puhakka, M. Kleemola, and T. Hovi. 2002. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J. Med. Virol. 66:263-268. [DOI] [PubMed] [Google Scholar]
- 3.Briese, T., N. Renwick, M. Venter, R. G. Jarman, D. Ghosh, S. Kondgen, S. K. Shrestha, A. M. Hoegh, I. Casas, E. V. Adjogoua, C. Akoua-Koffi, K. S. Myint, D. T. Williams, G. Chidlow, R. van den Berg, C. Calvo, O. Koch, G. Palacios, V. Kapoor, J. Villari, S. R. Dominguez, K. V. Holmes, G. Harnett, D. Smith, J. S. Mackenzie, H. Ellerbrok, B. Schweiger, K. Schonning, M. S. Chadha, F. H. Leendertz, A. C. Mishra, R. V. Gibbons, E. C. Holmes, and W. I. Lipkin. 2008. Global distribution of novel rhinovirus genotype. Emerg. Infect. Dis. 14:944-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooney, M. K., J. P. Fox, and G. E. Kenny. 1982. Antigenic groupings of 90 rhinovirus serotypes. Infect. Immun. 37:642-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gama, R. E., P. J. Hughes, C. B. Bruce, and G. Stanway. 1988. Polymerase chain reaction amplification of rhinovirus nucleic acids from clinical material. Nucleic Acids Res. 16:9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Gaunt, E., E. C. McWilliam-Leitch, K. Templeton, and P. Simmonds. 9 October 2009, posting date. Incidence, molecular epidemiology and clinical presentations of human metapneumovirus; assessment of its importance as a diagnostic screening target. J. Clin. Virol. doi: 10.1016/j.jcv.2009.09.016. [DOI] [PubMed]
- 6.Han, T. H., J. Y. Chung, E. S. Hwang, and J. W. Koo. 2009. Detection of human rhinovirus C in children with acute lower respiratory tract infections in South Korea. Arch. Virol. 154:987-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvala, H., I. Robertson, E. C. McWilliam Leitch, K. Benschop, K. C. Wolthers, K. Templeton, and P. Simmonds. 2008. Epidemiology and clinical associations of human parechovirus respiratory infections. J. Clin. Microbiol. 46:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heim, A., C. Ebnet, G. Harste, and P. Pring-Akerblom. 2003. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 70:228-239. [DOI] [PubMed] [Google Scholar]
- 9.Hyypia, T., P. Auvinen, and M. Maaronen. 1989. Polymerase chain reaction for human picornaviruses. J. Gen. Virol. 70:3261-3268. [DOI] [PubMed] [Google Scholar]
- 10.Kammerer, U., B. Kunkel, and K. Korn. 1994. Nested PCR for specific detection and rapid identification of human picornaviruses. J. Clin. Microbiol. 32:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khetsuriani, N., X. Lu, W. G. Teague, N. Kazerouni, L. J. Anderson, and D. D. Erdman. 2008. Novel human rhinoviruses and exacerbation of asthma in children. Emerg. Infect. Dis. 14:1793-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiang, D., I. Kalra, S. Yagi, J. K. Louie, H. Boushey, J. Boothby, and D. P. Schnurr. 2008. Assay for 5′ noncoding region analysis of all human rhinovirus prototype strains. J. Clin. Microbiol. 46:3736-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kistler, A., P. C. Avila, S. Rouskin, D. Wang, T. Ward, S. Yagi, D. Schnurr, D. Ganem, J. L. DeRisi, and H. A. Boushey. 2007. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J. Infect. Dis. 196:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kistler, A. L., D. R. Webster, S. Rouskin, V. Magrini, J. J. Credle, D. P. Schnurr, H. A. Boushey, E. R. Mardis, H. Li, and J. L. DeRisi. 2007. Genome-wide diversity and selective pressure in the human rhinovirus. Virol. J. 4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamson, D., N. Renwick, V. Kapoor, Z. Liu, G. Palacios, J. Ju, A. Dean, K. St. George, T. Briese, and W. I. Lipkin. 2006. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J. Infect. Dis. 194:1398-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau, S. K., C. C. Yip, H. W. Tsoi, R. A. Lee, L. Y. So, Y. L. Lau, K. H. Chan, P. C. Woo, and K. Y. Yuen. 2007. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J. Clin. Microbiol. 45:3655-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledford, R. M., N. R. Patel, T. M. Demenczuk, A. Watanyar, T. Herbertz, M. S. Collett, and D. C. Pevear. 2004. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 78:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, W. M., C. Kiesner, T. Pappas, I. Lee, K. Grindle, T. Jartti, B. Jakiela, R. F. Lemanske, Jr., P. A. Shult, and J. E. Gern. 2007. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One 2:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leitch, E. C., H. Harvala, I. Robertson, I. Ubillos, K. Templeton, and P. Simmonds. 2009. Direct identification of human enterovirus serotypes in cerebrospinal fluid by amplification and sequencing of the VP1 region. J. Clin. Virol. 44:119-124. [DOI] [PubMed] [Google Scholar]
- 20.Mackay, I. M. 2008. Human rhinoviruses: the cold wars resume. J. Clin. Virol. 42:297-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McErlean, P., L. A. Shackelton, E. Andrews, D. R. Webster, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2008. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C). PLoS One 3:e1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McErlean, P., L. A. Shackelton, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2007. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J. Clin. Virol. 39:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, E. K., K. M. Edwards, G. A. Weinberg, M. K. Iwane, M. R. Griffin, C. B. Hall, Y. Zhu, P. G. Szilagyi, L. L. Morin, L. H. Heil, X. Lu, and J. V. Williams. 2009. A novel group of rhinoviruses is associated with asthma hospitalizations. J. Allergy Clin. Immunol. 123:98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monto, A. S. 2002. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin. Ther. 24:1987-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmenberg, A. C., D. Spiro, R. Kuzmickas, S. Wang, A. Djikeng, J. A. Rathe, C. M. Fraser-Liggett, and S. B. Liggett. 2009. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 324:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peltola, V., M. Waris, R. Osterback, P. Susi, O. Ruuskanen, and T. Hyypia. 2008. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J. Infect. Dis. 197:382-389. [DOI] [PubMed] [Google Scholar]
- 27.Pitkaranta, A., E. Arruda, H. Malmberg, and F. G. Hayden. 1997. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J. Clin. Microbiol. 35:1791-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renwick, N., B. Schweiger, V. Kapoor, Z. Liu, J. Villari, R. Bullmann, R. Miething, T. Briese, and W. I. Lipkin. 2007. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J. Infect. Dis. 196:1754-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83:333-340. [DOI] [PubMed] [Google Scholar]
- 30.Savolainen, C., P. Laine, M. N. Mulders, and T. Hovi. 2004. Sequence analysis of human rhinoviruses in the RNA-dependent RNA polymerase coding region reveals large within-species variation. J. Gen. Virol. 85:2271-2277. [DOI] [PubMed] [Google Scholar]
- 31.Savolainen, C., M. N. Mulders, and T. Hovi. 2002. Phylogenetic analysis of rhinovirus isolates collected during successive epidemic seasons. Virus Res. 85:41-46. [DOI] [PubMed] [Google Scholar]
- 32.Savolainen-Kopra, C., S. Blomqvist, T. Smura, M. Roivainen, T. Hovi, D. Kiang, I. Kalra, S. Yagi, J. K. Louie, H. Boushey, J. Boothby, and D. P. Schnurr. 2009. 5′ noncoding region alone does not unequivocally determine genetic type of human rhinovirus strains. J. Clin. Microbiol. 47:1278-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmonds, P. 2006. Recombination and selection in the evolution of picornaviruses and other mammalian positive-stranded RNA viruses. J. Virol. 80:11124-11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanway, G., F. Brown, P. Christian, T. Hovi, T. Hyypia, A. M. Q. King, N. J. Knowles, S. M. Lemon, P. D. Minor, M. A. Pallansch, A. C. Palmenberg, and T. Skern. 2005. Family Picornaviridae, p. 757-778. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, London, United Kingdom.
- 35.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 36.Tapparel, C., T. Junier, D. Gerlach, S. Cordey, S. Van Belle, L. Perrin, E. M. Zdobnov, and L. Kaiser. 2007. New complete genome sequences of human rhinoviruses shed light on their phylogeny and genomic features. BMC Genomics 8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Templeton, K. E., S. A. Scheltinga, M. F. Beersma, A. C. Kroes, and E. C. Claas. 2004. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J. Clin. Microbiol. 42:1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesa, S., M. Kleemola, S. Blomqvist, A. Takala, T. Kilpi, and T. Hovi. 2001. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr. Infect. Dis. J. 20:574-581. [DOI] [PubMed] [Google Scholar]
- 39.Welch, J. B., K. McGowan, B. Searle, J. Gillon, L. M. Jarvis, and P. Simmonds. 2001. Detection of enterovirus viraemia in blood donors. Vox Sang. 80:211-215. [DOI] [PubMed] [Google Scholar]
- 40.Xiang, Z., R. Gonzalez, Z. Xie, Y. Xiao, L. Chen, Y. Li, C. Liu, Y. Hu, Y. Yao, S. Qian, R. Geng, G. Vernet, G. Paranhos-Baccala, K. Shen, Q. Jin, and J. Wang. 2008. Human rhinovirus group C infection in children with lower respiratory tract infection. Emerg. Infect. Dis. 14:1665-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]