Abstract
Bile acid homeostasis is critical in maintaining health and is primarily regulated by the nuclear receptors farnesoid X receptor (FXR) and small heterodimer partner (SHP). Bile acid-activated FXR indirectly inhibits expression of cholesterol 7α hydroxylase (CYP7A1), a key enzyme in conversion of cholesterol to bile acids, by induction of SHP. We recently demonstrated that SHP inhibits CYP7A1 transcription by recruiting chromatin-modifying cofactors, including Brm-Swi/Snf. Swi/Snf complexes contain either Brm or Brg-1 ATPases, and whether these subunits have distinct functions remains unclear. We have examined the role of these subunits in regulation of bile acid metabolism under physiological conditions by FXR and SHP. Brg-1 interacted with FXR and enhanced FXR-mediated transactivation of SHP, whereas Brm interacted with SHP and enhanced SHP-mediated repression of CYP7A1 and, interestingly, auto-repression of SHP. Chromatin immunoprecipitation and remodeling studies revealed that after treatment with FXR agonists, Brg-1 was recruited to the SHP promoter, resulting in transcriptionally active accessible chromatin, whereas Brm was recruited to both CYP7A1 and SHP promoters, resulting in inactive inaccessible chromatin. Our studies demonstrate that Brm and Brg-1 have distinct functions in the regulation of two key genes, CYP7A1 and SHP, within a single physiological pathway, feedback inhibition of bile acid biosynthesis, by differentially targeting SHP and FXR.
Cholesterol is a component of the cell membrane and is an essential precursor for the biosynthesis of steroid hormones, fat-soluble vitamins, and bile acids (33). Bile acids also play an important role in the absorption of dietary lipids and function as signaling molecules which are critically involved in the regulation of lipid and glucose metabolism and energy homeostasis (14, 22, 39, 45). Despite their essential functions, excess levels are associated with hypercholesterolemia and related heart disease, gall stone formation, and liver cholestasis (28, 33, 41). Therefore, cholesterol and bile acid levels must be tightly regulated under physiological conditions. Cholesterol 7α hydroxylase (CYP7A1), the first and rate-limiting enzyme in the conversion of cholesterol into bile acids in the liver, plays a key role in maintaining cholesterol and bile acid homeostasis (4, 33).
Farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily (24) and the primary biosensor for endogenous bile acids (23, 38, 42). It has been demonstrated that feedback regulation of bile acid biosynthesis is primarily achieved by coordinated actions of the nuclear receptors FXR and small heterodimer partner (SHP) (11, 21). In the FXR/SHP pathway, the nuclear bile acid receptor FXR senses elevated hepatic bile acid levels and indirectly suppresses bile acid biosynthesis by inhibiting the expression of CYP7A1 via the induction of the orphan nuclear receptor and transcriptional corepressor SHP (11, 21, 35). FXR-induced SHP, then, interacts with a hepatic activator, liver receptor homolog 1 (LRH-1) that is bound to the CYP7A1 promoter, which results in transcriptional repression of the CYP7A1 gene.
We recently demonstrated in studies of the molecular mechanism of SHP repression that nucleosomes at the CYP7A1 promoter were regularly phased and bile acid treatment did not result in gross structural changes, such as nucleosome sliding or disruption (7, 16). Instead, bile acid treatment resulted in decreased accessibility of DNA in nucleosome cores to endonucleases at the promoter region, indicating a remodeling of the chromatin. Consistent with these results, a chromatin-remodeling Swi/Snf complex, which contains Brm, in addition to the mSin3A/HDACs corepressor complex and G9a lysine methyltransferase, was recruited to the CYP7A1 promoter after bile acid treatment (7, 16).
ATP-dependent Swi/Snf chromatin-remodeling complexes contain a central ATPase, either Brm or Brg-1, and various additional Brm- or Brg1-associated factors. Using the energy from ATP hydrolysis, these complexes alter the nucleosome structure by disrupting DNA and histone interactions and thereby modulate gene expression in the regulation of diverse biological activities (29, 30, 36, 47). While Swi/Snf complexes have been generally implicated in gene activation (5, 12, 29, 30, 36), recent studies, including ours, have shown that these complexes are also associated with corepressor complexes, such as the mSin3A/HDAC1/2 complex, and are involved in gene repression (16, 25, 36, 44, 48). In some cases Brm and Brg-1 have been shown to be functionally redundant (6, 40), but distinct actions have also been reported for each (10, 15, 32). It has been demonstrated that Brg-1 binds to proteins containing the zinc finger DNA binding motifs, but Brm interacts with ankyrin repeat proteins that are critical components of the Notch signaling pathway and, importantly, Brm and Brg-1 are recruited to different promoters during cellular proliferation and differentiation (15). Furthermore, functional specificity of Brm and Brg-1 Swi/Snf complexes during osteoblast differentiation was recently demonstrated (10). Remarkably, mice lacking Brm or Brg exhibit different phenotypes. Brm null mice developed normally but were 10 to 15% heavier than their littermates and showed altered cellular proliferation compared to wild-type mice (32). In contrast, Brg-1 null mice were embryonic lethal, and heterozygous Brg-1 null mice were predisposed to tumor formation (3). These intriguing previous findings indicate that Brm and Brg-1 may have distinct promoter-, tissue-, and development-specific biological functions. However, the functional specificities of these two ATPases on a physiological basis are largely unknown.
The negative feedback regulation of bile acid synthesis involves both induction of the SHP gene and SHP-mediated repression of the CYP7A1 gene in response to elevated hepatic bile acid levels. Since we observed that a Brm-containing Swi/Snf complex was involved in SHP-mediated repression (7, 16), we examined whether Swi/Snf complexes show functional specificity in the feedback regulation of bile acid biosynthesis by differentially regulating CYP7A1 and SHP genes. Our data from molecular, cellular, and in vivo animal studies using gain- or loss-of-function approaches demonstrate that Brm and Brg-1 have distinct roles in the FXR/SHP-mediated regulation of bile acid biosynthesis. While Brg-1 is involved in SHP gene induction by coactivating FXR, Brm, as a component of an inhibitory SHP complex, is critically involved in the SHP-mediated inhibition of the CYP7A1 gene and auto-inhibition of the SHP gene.
MATERIALS AND METHODS
Materials.
Antibodies against FXR, Brm, Brg-1, green fluorescent protein, lamin, tubulin, LRH-1, HNF-4, and SHP were purchased from Santa Cruz Biotechnlogy. M2 antibody and M2 agarose were purchased from Sigma. Protein G-agarose was purchased from GE Healthcare. Restriction enzymes were purchased from New England Biolabs. A synthetic FXR agonist, GW4064, was purchased from Tocis Bioscience.
Construction of plasmid and adenoviral vectors.
For construction of Ad-Flag-Brm, pcDNA3-Flag-Brm (16) was digested with HindIII and EcoRV and subcloned into Ad-Track-CMV (13). For construction of Ad-si-mouse Brm, U6-siBrm (2) was cloned into the Ad-Track vector. Flag-FXR in this report refers to 3Flag-human FXR (8). Ad-Flag-SHP and Ad-Flag-FXR have been previously described (8, 26).
Cell culture.
HepG2 cells (ATCC HB8065) were maintained in Dulbecco's modified Eagle's medium/F-12 medium (1:1). SW13 cells (ATCC CCL 105) and mouse Hepa1c1c7 cells (ATCC CRL 2026) were grown in Dulbecco's modified Eagle's medium. To construct stable HepG2 cell lines that express small interfering RNA (siRNA) for Brm or Brg-1, viral packaging ψcrip cells were transfected with the retroviral vectors pSSSP-siBrm or pSSSP-siBrg (27). Cell supernatants were collected and were used to infect HepG2 cells in the presence of Polybrene. Stable cells were selected with 500 μg/ml of puromycin for 3 to 4 weeks. Drug-resistant colonies were pooled and expanded for further analyses.
Expression of SHP and FXR in mouse liver using adenoviral delivery.
Flag-SHP and Flag-FXR proteins were expressed in mouse liver in vivo using adenoviral delivery as previously described (7, 26, 31). Briefly, recombinant adenoviral vectors expressing Flag-SHP or Flag-FXR or control Ad-empty viral vectors were injected via the tail vein of mice, and 5 days after infection, livers were collected for further analyses. For activation of FXR, BALB/c male mice were fed with chow supplemented with 0.5% cholic acid (CA), a primary bile acid and natural FXR agonist (Harland Teklad TD05271) or were treated with GW4064, a synthetic FXR-specific agonist (30 mg/kg in corn oil) by intraperitoneal injection, and livers were collected for further analyses. All the animal use and adenoviral protocols were approved by the Institutional Animal Care and Use and Institutional Biosafety Committees at the University of Illinois at Urbana-Champaign and were in accordance with National Institutes of Health guidelines.
CoIP and ChIP assays.
Coimmunoprecipitation (CoIP) assays in HepG2 cells and mouse liver were performed as described previously (7, 16, 26, 31). Chromatin immunoprecipitation (ChIP) assays for mouse liver were carried out essentially as described previously (7, 8, 16, 31). Time course ChIP experiments were repeated twice with similar reproducible results.
Quantification of mRNA.
RNA was isolated from liver or cultured cells, and the levels of mRNA were determined by quantitative reverse transcriptase PCR (qRT-PCR) as previously described (7, 26).
Endonuclease accessibility chromatin remodeling assay.
Livers were homogenized by for four to five strokes in a Dounce homogenizer in hypotonic buffer (15 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.2% NP-40, 1 mM EDTA, 5% sucrose, 1 mM dithiothreitol, and protease inhibitors). The homogenate was layered onto a sucrose cushion buffer (300 mM sucrose, 60 mM KCl, 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.15 mM spermine, 0.5 mM spermidine, 1 mM dithiothreitol, and protease inhibitors) and centrifuged at 5,000 rpm for 2 min. For HepG2 cell remodeling studies, cells were resuspended in hypotonic buffer (10 mM Tris-HCl, pH, 7.4, 10 mM NaCl, 5 mM MgCl2, 0.1% NP-40), incubated on ice for 20 min, passed through a 27-gauge syringe, and centrifuged at 2,000 rpm for 5 min. Then, DNA in isolated nuclei from mouse liver or HepG2 cells was further subjected to endonuclease accessibility assays as previously described with some modifications (19, 37). Briefly, DNA in intact nuclei was partially digested with a restriction enzyme (5 to 25 units/100 μl) in 1× buffer (New England Biolabs) at 37°C for 15 to 40 min. Genomic DNA was isolated and further subjected to PCR amplification using primers specific to the SHP or CYP7A1 promoter.
RESULTS
Brm and Brg-1 are differentially associated with CYP7A1 and SHP promoters in mouse liver after cholic acid feeding.
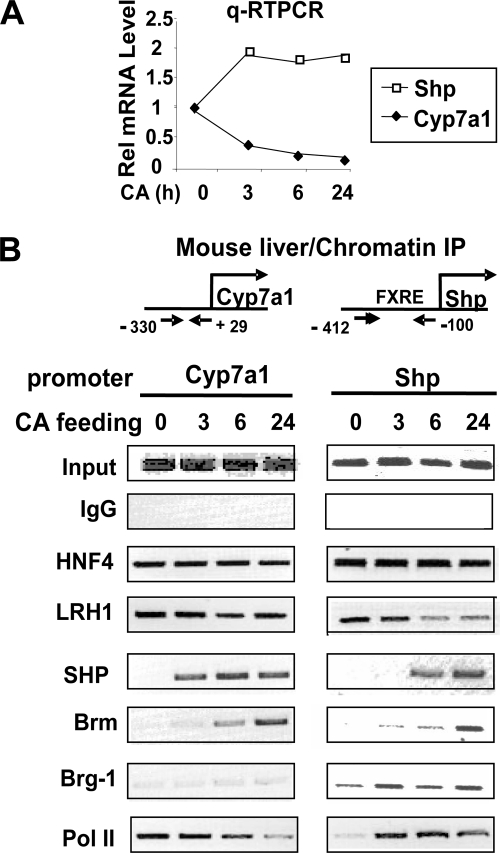
To explore the roles of Brm and Brg-1 in the feedback regulation of bile acid biosynthesis under physiological conditions in vivo, temporal association of these subunits with hepatic CYP7A1 and SHP promoters was examined by ChIP after feeding mice with chow supplemented with CA, a primary bile acid and natural FXR agonist (23, 38, 42). To examine the effectiveness of CA feeding, the mRNA levels of CYP7A1 and SHP were first analyzed by qRT-PCR. CYP7A1 mRNA levels were reduced by 3 h after CA feeding and further reduced at 24 h (Fig. 1A). In contrast, the SHP mRNA levels increased to maximal levels after 3 h of CA feeding and did not increase further at later times (Fig. 1A).
FIG. 1.
Effects of CA feeding on temporal association of Brm and Brg-1 and other regulators with CYP7A1 and SHP promoters in mouse liver Mice were fed normal chow or chow supplemented with 0.5% CA for the indicated times, and livers were collected for qRT-PCR (A) and ChIP (B) analyses. (A) The mRNA levels of SHP and CYP7A1 were determined by qRT-PCR and normalized to those of 36B4. (B) ChIP analysis was performed as described in Materials and Methods to detect association of the indicated proteins with the SHP and CYP7A1 promoters. Reproducible results were observed in two independent analyses.
In ChIP assays, binding of LRH-1 and HNF-4, known hepatic activators of the CYP7A1 genes (11, 21), was examined as a control. Both factors were associated with the CYP7A1 and SHP promoters, independent of CA feeding, although LRH-1 binding to the SHP promoter was slightly decreased after CA feeding (Fig. 1B). SHP was detected at the CYP7A1 promoter as early as 3 h after the start of CA feeding, and Brm was detected as early as 6 h. Importantly, Brg-1 was not markedly associated with the CYP7A1 promoter with or without CA feeding. In contrast, Brg-1 occupancy at the SHP promoter was increased by 3 h of CA feeding. Association of FXR with the SHP promoter was increased after CA feeding (see Fig. S1 in the supplemental material). Interestingly, SHP was also recruited to its own promoter by 6 h of CA feeding, consistent with auto-inhibition of the SHP gene (4, 21). Association of Brm with the SHP promoter was also detected after CA feeding and increased progressively with time, but in a delayed manner compared to Brg-1. Consistent with these results, association of RNA polymerase II was increased at the SHP promoter after 3 h of CA feeding, whereas association with the CYP7A1 promoter progressively decreased, indicating increased transcription of the SHP gene and decreased transcription of the CYP7A1 gene. These results indicate that Brg-1 and Brm are differentially associated with the SHP and CYP7A1 promoters in mouse liver after CA feeding and further suggest that Brm and Brg-1 may have distinct roles in the feedback regulation of bile acid biosynthesis. While dynamic recruitment of Brm to the CYP7A1 and SHP gene promoters correlates with suppression of these genes, the recruitment of Brg-1 to the SHP promoter correlates with induction of the SHP gene by the bile acid-activated FXR.
Brg-1, but not Brm, interacts with FXR in a ligand-regulated manner.
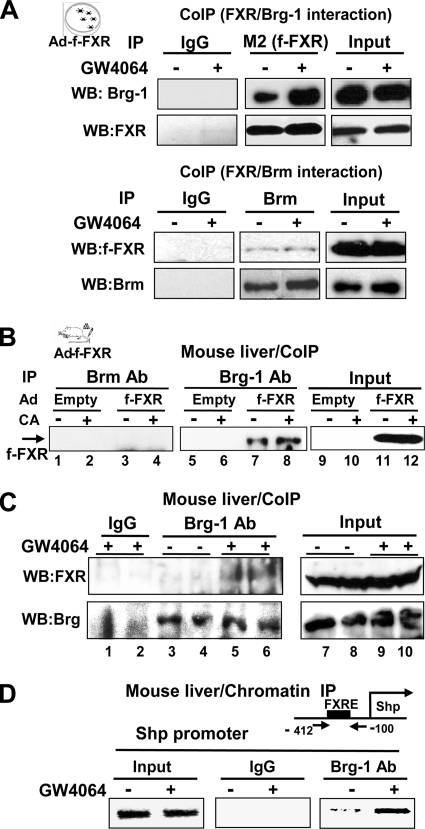
Since Brg-1 was recruited with FXR to the SHP promoter after treatment with CA or GW4064, a synthetic FXR agonist (46) in mouse liver, we examined the the interactions of FXR with these ATPases by CoIP studies in cells. The amount of Brg-1 in anti-FXR immunoprecipitates was substantially increased after treatment with GW4064 (Fig. 2A, upper panel). In parallel experiments, the amount of FXR in Brm immunoprecipitates was barely detectable and was not affected by GW4064 treatment (Fig. 2A, lower panel). These results indicate that Brg-1 interacts with FXR in a ligand-regulated manner, while Brm does not efficiently interact with FXR.
FIG. 2.
Brg-1, but not Brm, interacts with FXR in a ligand-regulated manner. (A) HepG2 cells, cotransfected with expression plasmids for Brg-1 and Brm, were infected with Ad-Flag-FXR and treated with 100 nM GW4064 for 1 h, and cell extracts were prepared. Flag-FXR was immunoprecipitated with M2 antibody, and the presence of Brg-1 in the immunoprecipitates was detected by Western analysis using Brg-1 antibody. Flag-FXR levels in the immunoprecipitates were also detected by Western analysis using M2 antibody. In parallel experiments using the same cell extracts, Brm was immunoprecipitated and the presence of Flag-FXR was detected by Western analysis (lower panel). (B) Mice were infected with adenovirus expressing Flag-FXR or control Ad-empty, and 5 days after infection, mice were fed either normal chow or chow supplemented with 0.5% CA for 3 h and livers were collected for further analyses. Endogenous Brm or Brg-1 was immunoprecipitated from liver extracts, and the presence of Flag-FXR in the immunoprecipitates was determined by Western analysis. Representative results from two independent CoIP assays are shown. (C and D) Mice were injected with GW4064 or vehicle and 1 h later, livers were collected for CoIP (C) and ChIP (D) analyses. (C) Endogenous Brg-1 was immunoprecipitated from liver nuclear extracts, and the presence of endogenous FXR in the immunoprecipitates was detected by Western analysis. Brg-1 levels in the immunoprecipitates were detected by Western analysis (lower panel). Results from two sets of mice are shown. (D) ChIP analysis was performed to detect association of Brg-1 with the SHP promoter region containing the FXR binding site (FXRE). Reproducible results were observed in two independent analyses.
To determine whether these interactions occur in vivo, Flag-FXR was expressed in mouse liver by adenoviral infection, and the mice were fed with normal chow or chow supplemented with CA. Similar infection efficiencies between groups were confirmed by examining green fluorescent protein expression by confocal microscopy (data not shown). Similar levels of expressed Flag-FXR in both groups of mice were detected by Western analysis (see Fig. S2 in the supplemental material). Interaction of FXR with Brm or Brg-1 was detected by immunoprecipitation with antiserum to Brm or Brg-1 followed by detection of Flag-FXR by Western analysis. CA feeding increased the association of Flag-FXR with Brg-1 (Fig. 2B, lanes 5 to 8), but importantly, interaction of Flag-FXR with Brm was not detected (lanes 1 to 4). Moreover, the interaction between endogenous FXR and endogenous Brg-1 in mouse liver was also increased after treatment of mice with GW4064 (Fig. 2C, lanes 3 to 6). In in vitro interaction studies, Brg-1 directly interacted with full-length FXR, but deletion of the N-terminal region containing the DNA binding domain in FXR markedly reduced the interaction (see Fig. S3 in the supplemental material). Consistent with these protein interaction studies, in ChIP analyses, hepatic association of Brg-1 with the promoter of the SHP gene, a well-known FXR target (11, 21), was markedly increased after treatment with GW4064 (Fig. 2D). These results indicate that Brg-1, but not Brm, associates with FXR, and treatment with FXR agonists CA or GW4064 increases their interaction in mouse liver.
Brg-1 enhances FXR transactivation of the SHP promoter.
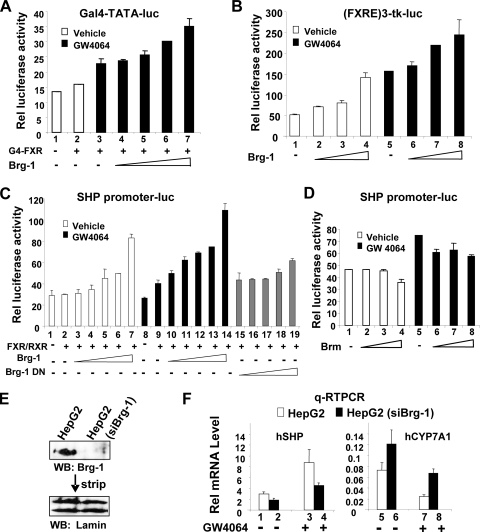
To determine whether the interactions of FXR with Brg-1 are functionally relevant, we examined the effects of overexpression of Brg-1 on FXR transactivation by using a Gal4 DBD-FXR fusion protein. Treatment with GW4064 increased FXR-mediated transactivation of the Gal4 reporter, and increasing amounts of Brg-1 enhanced the transactivation in a dose-dependent manner (Fig. 3A, lanes 3 to 7). Since the SHP gene is a well-known FXR target (11, 21), we also examined the effects of Brg-1 on FXR transactivation by using a SHP promoter-luciferase reporter as well as a synthetic (FXRE)3-tk-luc reporter. With both the (FXRE)3-tk-luc (Fig. 3B) and SHP promoter-luc (Fig. 3C) reporters, FXR-mediated transactivation was stimulated by GW4046 treatment, and Brg-1 enhanced the transactivation in a dose-dependent manner. In contrast, FXR transactivation was not increased when similar levels (data not shown) of a catalytically inactive dominant negative (DN) Brg-1 mutant were cotransfected with the SHP promoter-reporter (Fig. 3C, compare lanes 10 to 14 and 15 to 19). The requirement for the ATPase activity of Brg-1 for the enhanced transactivation suggests that Brg-1 enhances FXR transactivation by chromatin remodeling. In contrast, expression of increasing amounts of Brm did not enhance, and in fact inhibited, FXR transactivation of the SHP promoter (Fig. 3D, lanes 5 to 8). These results strongly suggest that Brg-1 and Brm are functionally distinct and that Brg-1, but not Brm, can act as a coactivator for FXR activity, which is consistent with the interaction of Brg-1, but not Brm, with FXR in the CoIP studies (Fig. 2).
FIG. 3.
Brg-1 coactivates FXR transactivation of the SHP promoter. (A to D) Mouse Hepa1c1c7 cells were cotransfected with 200 ng of the indicated reporter plasmid, 200 ng of CMV-β-galactosidase, 25 ng of G4-FXR, 10, 50, 100, or 200 ng of Brg-1 or Brg-1 DN, 50 ng of pcDNA3FXR, 50 ng of CMX-RXRα, or 10, 50, 100, or 200 ng of Brm expression plasmids, as indicated. Cells were treated with 100 nM GW4064 or vehicle overnight and harvested for reporter assays. The values for firefly luciferase activities were normalized by dividing by β-galactosidase activities. (E and F) Stable HepG2 cell lines that express siRNA for Brg-1 were constructed as described in Materials and Methods. (E) Expression levels of endogenous Brg-1 were detected by Western analysis, and the membrane was stripped and reprobed with lamin antibody. (F) The mRNA levels of SHP and CYP7A1 were determined by qRT-PCR. The standard errors of the means were calculated from three sets of samples.
Downregulation of Brg-1 differently modulates SHP and CYP7A1 gene expression.
To directly determine whether Brg-1 coactivates FXR, we examined the effects of downregulation of Brg-1 on expression of the endogenous SHP and CYP7A1 genes in HepG2 cells stably expressing Brg-1 siRNA. Expression levels of endogenous Brg-1 were substantially decreased in these cells (Fig. 3E). SHP mRNA levels were increased about two- to threefold after GW4064 treatment (Fig. 3F, lanes 1 and 3) in parental HepG2 cells, and this increase was substantially attenuated by downregulation of Brg-1 expression (lanes 2 and 4). Treatment with GW4064 decreased mRNA levels of CYP7A1 as expected (8, 46) (Fig. 3F, lanes 5 and 7), and the repression was partially reversed by downregulation of Brg-1 (lanes 6 and 8). These results demonstrate that Brg-1 increases induction of SHP by FXR, which is consistent with the enhancement of FXR transactivation by Brg-1 in the reporter assays.
Brm, but not Brg-1, interacts with SHP.
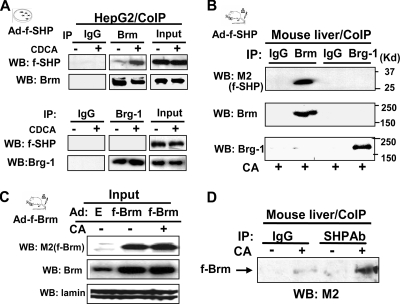
To test whether SHP can interact with Brg-1, Brm, or both, CoIP studies were performed in hepatic cells. We previously showed that SHP directly interacts with Brm through the C-terminal repression domain of SHP (16). Consistent with these findings, Brm was coimmunoprecipitated with SHP (Fig. 4A, upper panel), while Brg-1 was not detectable in the anti-SHP immunoprecipitates (Fig. 4A, lower panel). These results indicate that Brm, but not Brg-1, interacts with SHP in cells.
FIG. 4.
Brm, but not Brg-1, interacts with SHP in mouse liver. (A) HepG2 cells cotransfected with expression plasmids for Brg-1 or Brm were infected with Ad-Flag-SHP and treated with 50 μM CDCA for 1 h, and cell extracts were prepared. Endogenous Brm or Brg-1 was immunoprecipitated from cell extracts, and the presence of total Flag-SHP in the extracts (input) or Flag-SHP in the immunoprecipitates was detected by Western analysis using M2 antibody. (B) Mice were infected with adenovirus expressing Flag-SHP or control Ad-empty, and 5 days after infection, mice were fed CA chow (+) for 3 h. Endogenous Brm or Brg-1 was immunoprecipitated from liver extracts from mice fed CA chow, and the presence of Flag-SHP in the immunoprecipitates was detected by Western analysis. Brm or Brg-1 levels in the immunoprecipitates were detected by Western analysis (lower panels). (C and D) Mice were infected with adenovirus expressing Flag-Brm or control Ad-empty, and 5 days after infection mice were fed either normal chow (-) or CA chow (+) for 3 h and livers were collected for Western (C) and CoIP (D) analyses. Endogenous SHP was immunoprecipitated from liver extracts of mice fed normal chow (-) or CA chow (+), and the presence of Flag-Brm in the immunoprecipitates was detected by Western analysis using M2 antibody.
To test if SHP interacts with Brm or Brg-1 in vivo, Flag-SHP was expressed in mouse liver by adenoviral infection, and mice were fed CA-supplemented chow. Consistent with the hepatic cell studies (Fig. 4A), Flag-SHP was detected in anti-Brm immunoprecipitates in liver extracts from CA-fed mice but not in the anti-Brg-1 immunoprecipitates (Fig. 4B). Further, when similar amounts of Brm were expressed in mice fed normal or CA-supplemented chow (Fig. 4C), the interaction of Brm with SHP was substantially increased in the mice fed the CA chow (Fig. 4D). These results indicate that Brm, but not Brg-1, interacts with SHP and that CA feeding increases the interaction of Brm with SHP in mouse liver, which is consistent with specific roles for Brm and Brg-1 in FXR/SHP-mediated feedback inhibition of bile acid biosynthesis.
Brm enhances inhibition of gene expression by SHP.
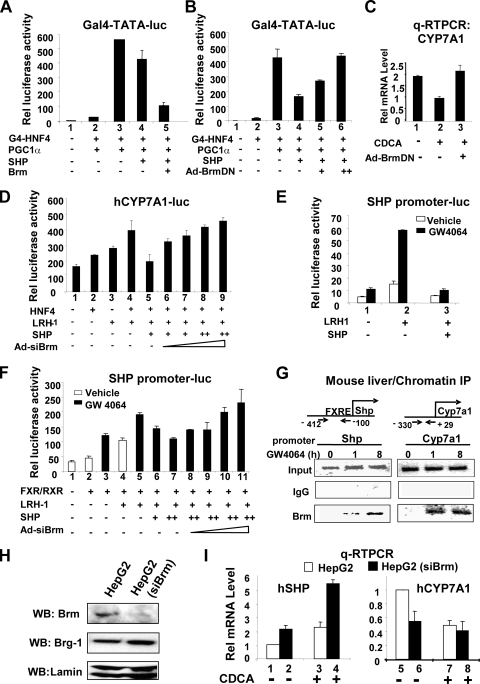
Since Brm, but not Brg-1, interacts with SHP, we further tested whether Brm enhances the inhibition of gene expression by SHP using transfection reporter assays. Transactivation by HNF-4 and its coactivator, peroxisome proliferator-activated receptor (PPAR) cofactor 1γ (PGC-1γ), have been shown to be inhibited by SHP (7, 16, 17). Therefore, we tested whether Brm enhances the SHP-mediated inhibition of the HNF-4/PGC-1γ transactivation activity by using a Gal4 reporter system. Expression of SHP modestly inhibited transactivation mediated by Gal4-HNF-4/PGC-1γ, and overexpression of Brm substantially enhanced the SHP inhibition in Brm/Brg-1-deficient adrenal carcinoma SW13 cells (Fig. 5A).
FIG. 5.
Brm enhances inhibition of gene expression by SHP. (A and B) Brg-1/Brm-deficient SW13 cells were cotransfected with 200 ng of Gal4-TATA-Luc, 300 ng CMV-β-galactosidase, 25 ng of G4-HNF-4, 10 ng of pcDNA3PGC-1α, 25 ng of pcDNA3SHP, or 150 ng of pcDNA3-Flag-Brm. The values for firefly luciferase activities were normalized by dividing by β-galactosidase activities. (C) HepG2 cells were infected with Ad-empty control (lanes 1 and 2) or Ad-Brm DN (lane 3), and 2 days later, cells were treated with 100 nM GW4064 or vehicle for 10 h and mRNA levels of CYP7A1 were determined by qRT-PCR. (D) Cells were infected with Ad-siBrg-1, and 2 days after infection, cells were further transfected with expression plasmids as indicated and treated with GW4064 and harvested for reporter assays. (E) Cells were cotransfected with plasmids as indicated and treated with vehicle or GW4064 overnight. (F) Cells were cotransfected with plasmids as indicated and infected with Ad-si-Brm, and 2 days later, cells were harvested for reporter assays. (G) ChIP assays. Mice were treated with GW4064 for the indicated times, and livers were collected for ChIP assays to detect the association of Brm at the native SHP and CYP7A1 promoters. (H) HepG2 cells that stably express siRNA for Brm were constructed as described in Materials and Methods, and Brm, Brg-1, and tubulin levels in cell extracts were detected by Western analysis. (I) HepG2 cells stably expressing Brm siRNA or normal HepG2 cells were treated with 50 μM CDCA or vehicle for 3 h and harvested for qRT-PCR to measure mRNA levels of SHP and CYP7A1. The standard errors of the means were calculated from three independent sets of samples.
To test whether the ATPase activity of Brm is required for the enhancement of SHP inhibition, we utilized a catalytically inactive Brm with a mutation in the ATPase domain that functions as a DN mutant (6, 36). Increasing amounts of expression plasmids for the Brm DN mutant did not enhance SHP-mediated inhibition of HNF-4/PGC-1γ transactivation and instead reversed the inhibition in a dose-dependent manner (Fig. 5B, lanes 4 to 6). These results demonstrate that the catalytic domain is required for enhancement of SHP repression and further that dominant-negative inhibition of endogenous Brm reverses the SHP repression, demonstrating the importance of catalytically active Brm for SHP repression.
To evaluate the role of Brm in the suppression of the endogenous CYP7A1 gene in HepG2 cells, cells were infected with Ad-empty or Ad-Brm-DN and then treated with chenodeoxy cholic acid (CDCA), a primary bile acid and natural FXR agonist. Since CA, which efficiently activates FXR signaling in vivo, does not activate FXR signaling in cultured cells (20, 23), CDCA instead of CA was utilized in cell culture studies. After CDCA treatment, CYP7A1 mRNA levels were decreased about 50%, and blocking the endogenous Brm function with the Brm DN mutant completely reversed this inhibition (Fig. 5C, lanes 2 and 3).
To further test whether endogenous Brm enhances SHP inhibition of CYP7A1 promoter activity, Brm expression was downregulated with siRNA. Expression of Brm siRNA by adenoviral infection resulted in marked decreases in protein levels of endogenous Brm (see Fig. S4 in the supplemental material). In reporter assays, expression of HNF-4 and LRH-1 additively increased CYP7A1 promoter activity, which was inhibited by expression of SHP (Fig. 5D). Downregulation of Brm by infection with increasing amounts of Ad-siBrm reversed the SHP-mediated inhibition (Fig. 5D, lanes 5 to 9). These results indicate that Brm is involved in SHP-mediated suppression of CYP7A1 expression, most likely by catalyzing chromatin remodeling at the promoter.
Brm is also involved in auto-inhibition of SHP gene expression.
SHP directly interacts with and inhibits numerous nuclear receptors, including LRH-1 and HNF-4 (1, 18), and LRH-1 and HNF-4 sites are present in the SHP promoter (4, 8, 21), which suggests that SHP may inhibit its own transcription by interacting with these nuclear receptors. If so, Brm, a critical component of the inhibitory SHP complex, may also be involved in this negative auto-regulation. Therefore, we tested first whether SHP inhibits its own transcription and then whether Brm enhances the SHP-mediated inhibition. Expression of LRH-1 increased the SHP promoter activity, which was substantially increased further by GW4064 treatment (Fig. 5E, lanes 1 and 2). Overexpression of SHP markedly reduced LRH-1-mediated transactivation, an effect increased by treatment with GW4064 (Fig. 5E, lanes 2 and 3), demonstrating that SHP can inhibit its own transcription.
We directly tested whether endogenous levels of Brm can potentiate the inhibition of the SHP promoter by SHP via downregulation of Brm. Expression of FXR and its heterodimer partner, RXRα, and LRH-1 increased SHP promoter activity, and treatment with GW4064 further increased the activity (Fig. 5F, lanes 4 and 5). Exogenous expression of SHP inhibited the enhanced SHP promoter activity in a dose-dependent manner (Fig. 5F, lanes 5 to 7), and downregulation of Brm by infection with increasing amounts of Ad-siBrm reversed the SHP inhibition (lanes 7 to 11). These results demonstrate the Brm is involved in auto-inhibition of SHP gene expression by enhancing SHP inhibitory activity.
Brm is recruited to the SHP promoter in mouse liver after treatment with GW4064.
If Brm is involved in auto-inhibition of the SHP gene, association of Brm with the SHP promoter as well as the CYP7A1 promoter should be detected. Indeed, in a time course ChIP analysis, Brm was recruited to the SHP as well as CYP7A1 promoters after GW4064 treatment (Fig. 5G). These results further support the conclusion that Brm is involved in auto-regulation of SHP expression.
Effects of downregulation of Brm on expression of CYP7A1 and SHP genes.
In HepG2 cell lines stably expressing Brm siRNA, expression of endogenous Brm was decreased while Brg-1 levels were modestly elevated (Fig. 5H), consistent with previous studies in Brm null mice (32). SHP mRNA levels were increased about twofold after CDCA treatment of wild-type HepG2 cells (Fig. 5I, lanes 1 and 3), and downregulation of Brm significantly increased SHP mRNA levels in both untreated and CDCA-treated HepG2 cells (Fig. 5I, lanes 1 and 2 versus 3 and 4), which is consistent with a role for Brm in repression of the SHP gene. The increased SHP mRNA levels may also be partly due to elevated Brg-1 levels in these cells (Fig. 5H). Consistent with increased SHP expression after downregulation of Brm, expression of CYP7A1 was decreased (Fig. 5I, lanes 5 to 8). Importantly, the inhibition of CYP7A1 expression by CDCA was much greater in the parental HepG2 cells than in the stable cells (compare lanes 5 and 7 with lanes 6 and 8), suggesting that Brm is important for the CYP7A1 suppression after CDCA treatment. These results, taken together with CoIP protein interaction studies, suggest that Brm is a critical component of the functional SHP complex which inhibits gene expression of both SHP and CYP7A1.
Brg-1 and Brm differently alter the chromatin structure of the CYP7A1 and SHP promoters after treatment with FXR agonists.
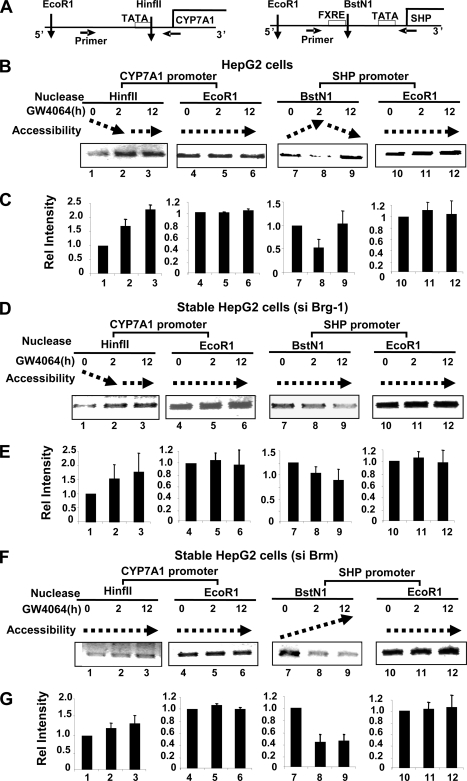
We observed that Brg-1 and Brm were differently recruited to the SHP and CYP7A1 promoters in response to treatment with either GW4064 or bile acids (Fig. 1, 2, and 5), with different functional outcomes (Fig. 3 and 5). Therefore, to determine whether the functional differences were correlated with chromatin remodeling at the SHP and CYP7A1 promoters, endonuclease accessibility chromatin remodeling assays were performed in HepG2 cells that stably express siRNA for either Brg-1 or Brm (Fig. 3 and 5). Chromatin at the SHP and CYP7A1 promoters was digested with endonucleases HinfII and BstNI, respectively, and sensitivity to endonuclease cleavage was determined by PCR amplification (Fig. 6A). Therefore, increased accessibility resulting in increased cleavage of the PCR template should result in detection of decreased amounts of the PCR product.
FIG. 6.
Brg-1 and Brm alter the chromatin structure of the CYP7A1 and SHP promoters differently after treatment with FXR agonists. (A) Endonuclease recognition sites within the human CYP7A1 and SHP promoters are indicated by vertical arrows, and PCR primers are indicated by horizontal arrows. (B to G) Parental HepG2 cells (B and C) or HepG2 cells stably expressing Brg-1 siRNA (D and E) or Brm siRNA (F and G) were treated with GW4064 for the indicated times, and isolated nuclei were partially digested with the indicated endonucleases or EcoRI as a control. Genomic DNA was purified and subjected to PCR analysis. Increased and decreased accessibilities are indicated by upward and downward dotted arrows, respectively. (C, E, and G) Band intensities were measured by densitometry, and the intensities relative to the 0-h time point were plotted with standard deviations indicated (n = 3 to 6).
Accessibility of the CYP7A1 promoter to HinfII was decreased in parental HepG2 cells after GW4064 treatment (Fig. 6B and C, lanes 1 to 3), whereas accessibility of the SHP promoter chromatin to BstNI was increased at 2 h after GW4064 treatment but decreased at 12 h after GW4064 treatment (Fig. 6B and C, lanes 7 to 9). In contrast, accessibility to EcoRI, which does not cleave within the promoter regions, was not changed after GW4046 treatment (Fig. 6B and C, lanes 4 to 6 and 10 to 12).
Interestingly, downregulation of Brg-1 blocked the increased accessibility at the SHP promoter after GW4064 treatment (compare Fig. 6B and C, lanes 7 to 9, and D and E, lanes 7 to 9), while little effect on accessibility of the CYP7A1 promoter chromatin was observed (compare Fig. 6B and C, lanes 1 to 3, and D and E, lanes 1 to 3). In contrast, downregulation of Brm blocked the decrease in accessibility at both the CYP7A1 promoter (compare Fig. 6B and C, lanes 1 to 3, and F and G, lanes 1 to 3) and SHP promoter (compare Fig. 6B, lanes 8 and 9, and F and G, lanes 8 and 9). Importantly, downregulation of Brm did not block the early increase in accessibility at the SHP promoter (compare Fig. 6B and C, lanes 7 and 8, and F and G, lanes 7 and 8). These results demonstrate that Brg-1 is critically involved in SHP gene induction by remodeling the promoter chromatin to a transcriptionally active accessible chromatin structure and that Brm is involved in inhibition of both CYP7A1 and SHP genes by remodeling the promoter chromatin to a transcriptionally suppressed inaccessible chromatin configuration.
Accessibility of the CYP7A1 and SHP promoters to endonucleases is altered after treatment with FXR agonists in mouse liver in vivo.
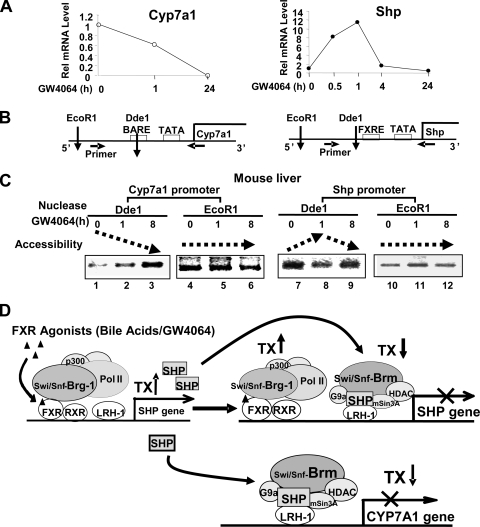
We finally examined whether chromatin remodeling occurs at the CYP7A1 and SHP promoter in mouse liver in vivo after treatment with FXR agonists. To test the effects of GW4064 on chromatin structure at the SHP and CYP7A1 promoters, mice were treated with GW4064 for different times and endonuclease accessibility chromatin remodeling studies were performed. As a control, SHP and CYP7A1 mRNA levels were measured after treatment with GW4064 for different times. The CYP7A1 mRNA levels were reduced by 1 h after GW4064 treatment and further reduced at 24 h (Fig. 7A). In contrast, the SHP mRNA levels were increased to maximal levels after 1 h of GW4064 treatment and decreased to basal levels at later times (Fig. 7A). These results are consistent with auto-inhibition of the SHP gene by SHP and Brm, as observed in functional reporter assays (Fig. 5).
FIG. 7.
(A to C) Accessibility of the CYP7A1 and SHP promoters to endonucleases is altered after treatment with FXR agonists in mouse liver in vivo. Mice were treated with GW4064 for different times, and livers were collected for qRT-PCR (A) and chromatin remodeling studies (B and C). (A) The mRNA levels of SHP and CYP7A1 were determined by qRT-PCR and normalized to those of 36B4. (B) Schematic diagram of endonuclease recognition sites within the mouse CYP7A1 and SHP promoters. (C) Mice were treated with vehicle or GW4064 for the indicated times, nuclei were isolated from livers, and DNA was partially digested by each of the indicated endonucleases. Genomic DNA was purified and subjected to PCR analysis using the primers specific for the SHP and CYP7A1 promoters. Consistent results were obtained from two independent GW4064 experiments and a single CA feeding experiment (see Fig. S5 in the supplemental material). (D) Functional specificities of Brm and Brg-1 in the FXR/SHP-mediated feedback regulation of bile acid biosynthesis The primary nuclear bile acid receptor, FXR, suppresses hepatic bile acid production by inducing SHP and, thereby, indirectly inhibiting transcription of CYP7A1, which encodes the key enzyme in the biosynthesis of bile acids from cholesterol. In response to elevated hepatic bile acid levels or treatment with FXR agonists, the interaction between FXR and Brg-1 is increased. Brg-1 is recruited to the promoter of the SHP gene, resulting in ATP-dependent chromatin remodeling to an open chromatin configuration and subsequent gene activation of SHP. FXR-induced SHP, then, inhibits transcription of the CYP7A1 gene by recruiting chromatin-modifying cofactors, including Swi/Snf-Brm as well as mSin3A/HDACs and G9a methyltransferase, which results in ATP-dependent chromatin remodeling and subsequent gene silencing of CYP7A1. Interestingly, SHP also inhibits transcription of its own gene in a delayed negative auto-regulatory manner. Therefore, accessibility of the SHP promoter chromatin to endonuclease is initially increased after treatment with FXR agonists, but it becomes decreased, which correlates with the delayed recruitment of SHP and Swi/Snf-Brm to the SHP promoter.
In endonuclease accessibility chromatin remodeling assays, chromatin was digested with DdeI, and sensitivity to DdeI cleavage was determined by amplification of fragments containing the DdeI site (Fig. 7B). Accessibility of the DdeI site at the CYP7A1 promoter chromatin was substantially decreased after treatment with GW4064 (Fig. 7C, lanes 1 to 3). Consistent with the studies using GW4064, accessibility of the CYP7A1 promoter to endonuclease was also substantially decreased in mice fed CA chow (see Fig. S5 in the supplemental material). In contrast, accessibility of DdeI sites in the SHP promoter was markedly increased after 1 h of treatment with GW4064 (Fig. 7C, lanes 7 and 8), but the increased sensitivity was reversed and accessibility was decreased after 12 h of treatment (lanes 8 and 9). Accessibility to EcoRI, which does not cleave within the promoter regions, was not substantially changed after GW4046 treatment (Fig. 7C, lanes 4 to 6 and 10 to 12).
These results, taken together, demonstrate that upon treatment with FXR agonists, such as bile acids or GW4064, the chromatin structure at the CYP7A1 promoter is remodeled to a more closed chromatin configuration correlating with transcriptional suppression. In contrast, the SHP promoter chromatin is initially remodeled to transcriptionally active accessible conformation, but at longer times it is remodeled to a more closed, suppressed configuration. These results are consistent with early recruitment of Brg-1 and the delayed recruitment of Brm to the SHP promoter (Fig. 1) and with initial activation of the SHP gene by FXR followed by feedback auto-inhibition by SHP.
DISCUSSION
In this paper we present evidence that the catalytic subunits of Swi/Snf chromatin-remodeling complexes, Brm and Brg-1, have distinct functions in the regulation of two key genes, the CYP7A1 and SHP genes, within a single physiological pathway, the negative feedback regulation of bile acid biosynthesis. Brm is associated with gene repression, and Brg-1 is associated with gene activation. In support of this conclusion, Brg-1, but not Brm, was associated with the nuclear bile acid receptor FXR in mouse liver and in HepG2 cells and enhanced FXR transactivation of the SHP promoter. Downregulation of endogenous Brg-1 in stably transfected HepG2 cells with siRNA resulted in a decrease in SHP expression and a subsequent increase in CYP7A1 expression. These results strongly suggest that Brg-1 substantially enhances SHP induction by bile acid-activated FXR. In contrast, Brm, but not Brg-1, interacted with SHP and potentiated SHP-mediated suppression of CYP7A1. Interestingly, Brm also enhanced SHP-mediated suppression of the SHP gene itself, consistent with existence of auto-inhibition of SHP expression by bile acid-induced SHP (4, 21). Downregulation of endogenous Brm in HepG2 cells by siRNA resulted in increased SHP expression, indicating that Brm is involved in inhibition of the expression of the SHP gene. Despite increases in SHP expression after downregulation of Brm by siRNA, which should have increased bile acid-mediated inhibition of CYP7A1 gene expression, the inhibition was largely blocked, confirming that Brm is an important functional component of the inhibitory SHP complex that is required for bile acid-mediated CYP7A1 inhibition, as shown previously (7, 16). The results indicate that FXR recruits Brg-1-containing Swi/Snf complexes that contribute to gene activation, while SHP recruits Brm-containing complexes that contribute to gene repression.
Swi/Snf complexes are ATP-dependent chromatin-remodeling enzymes that are involved in the activation or repression of eukaryotic gene transcription (12, 15, 29, 30, 36). Each Swi/Snf complex contains Brm or Brg-1 as a catalytic subunit and several Brm- or Brg-1-associated factors (47). It has been shown that Brg-1 can compensate for loss of Brm function in many biological systems, suggesting that the two factors are functionally redundant (6, 40). However, several lines of evidence suggest that Brm and Brg-1 have distinct functions as well. Multiple biochemically distinct Swi/Snf complexes exist (36), suggesting functional specificities of these complexes. For example, the mSin3A/HDAC corepressor complex was found in Brm complexes and in one case in a Brg-1 complex (36). Brm and Brg-1 are recruited to different target promoters during cellular proliferation and differentiation (15). Brg-1, but not Brm, interacts with zinc finger proteins such as ELKF and GATA-1. This zinc finger motif is present in the DNA binding domain of the superfamily of nuclear receptors, including FXR (24), which is consistent with the interaction of Brg-1 with FXR observed in this study while SHP does not contain a DNA binding domain (35), which may explain why Brg-1 does not interact with SHP. While these previous studies suggested that Brm and Brg-1 may have distinct functions, our present studies demonstrated directly that Brm and Brg-1 have distinct functions within a single regulatory pathway. Our results indicate that these distinct inhibitory and activating functions of Brm and Brg-1 are due to the specific interactions of Brg-1 with the activator FXR and of Brm with the repressor SHP.
These results, together with our previous published studies (7, 16), are summarized in the model for the role of Swi/Snf complexes in FXR-mediated induction of the SHP gene and SHP repression of both the CYP7A1 and SHP promoters (Fig. 7D). Bile acid-activated FXR, upon heterodimerization with RXRα, binds to the SHP promoter and recruits transcriptional activation complexes, including Swi/Snf-Brg-1, histone acetyl transferases such as p300 (8), and RNA polymerase II. Brg-1 enhances induction of the SHP gene by bile acid-activated FXR by remodeling the promoter to a transcriptionally active open chromatin structure. The increased expression of SHP results in the repression of CYP7A1, as shown previously (7, 16). SHP was recruited as early as after 3 h of CA feeding, and Brm was detected at the promoter by 6 h of CA feeding. The delayed recruitment of Brm is consistent with previous findings that deacetylation and methylation at H3K9 precede recruitment of the Swi/Snf-Brm complex to the CYP7A1 promoter (7). In addition to recruitment to the CYP7A1 gene, Brm is also recruited to the SHP promoter to which Brg-1 was initially recruited by FXR. Interestingly, both Brg-1 and Brm are present at the promoter simultaneously as detected by ChIP assays, but the increase in accessibility of the chromatin to restriction enzymes at early times is reversed at later times, suggesting that the Brm function is dominant over Brg-1 in the context of the SHP promoter. LRH-1 is present at the SHP promoter, so that SHP may be recruited to the SHP promoter via interaction with LRH-1 as shown for the CYP7A1 promoter (11, 21). These results are consistent with auto-inhibition of the SHP gene. SHP coordinately recruits chromatin-modifying complexes, such as histone deacetyltransferases (HDACs), G9a methyltransferase, and Swi/Snf-Brm, in a sequential manner to the CYP7A1 promoter (7, 16). We have demonstrated that deacetylation of H3K9 and methylation of H3K9 are required for recruitment of the Brm complex (7). These results are consistent with the remodeling of chromatin structure at the CYP7A1 promoter to a transcriptionally silent closed chromatin configuration upon treatment with FXR agonists such as bile acids or GW4064. At the SHP promoter, early recruitment of Brg-1 and delayed recruitment of Brm result initially in remodeling to a transcriptionally active open chromatin structure which is reversed to a closed configuration later.
Potential involvement of Swi/Snf complexes in metabolic regulation has been shown in several systems. Temporal recruitment of Swi/Snf complexes was observed at the PPARγ gene promoter during adipogenic induction (34). Both Brm and Brg-1 were detected at the PPARγ promoter, suggesting that both Brm- and Brg-1-containing Swi/Snf complexes redundantly contribute to adipogenic induction of the PPARγ gene. In studies of the regulation of metabolic genes by insulin, insulin treatment resulted in recruitment of Swi/Snf complexes to its target gene promoters with concomitant changes in chromatin structure and gene expression (19). Interestingly, Swi/Snf interacted with the adipogenic factor, sterol regulatory element-binding protein 1c, to mediate not only insulin-dependent gene regulation but also insulin sensitivity (19).
SHP has been implicated in regulation of diverse biological pathways by virtue of its suppression of the expression of genes for numerous transcriptional factors (1, 17, 18, 35). Abnormal SHP function and activity have been implicated in metabolic disorders such as fatty liver development, obesity, and diabetes (26, 43). Therefore, it will be interesting to test whether SHP induction and SHP activity are modulated by Brm or Brg-1 in regulation of genes within these other metabolic pathways. Since the inhibitory SHP complex also inhibits expression of the SHP gene as well as the CYP7A1 gene, it is likely that chromatin modification and remodeling by SHP-recruited Brm-containing chromatin-modifying complexes may be a common mechanism by which SHP suppresses gene expression in multiple other biological systems. SHP contains a putative ligand binding domain (35), and a recent intriguing study showed that 4-[3-(1-adamantyl)-4-hydroxy pheynl]-3-chlorocinnamic acid, a retinoid-related compound, is a potential ligand for SHP and increased the interaction of SHP with mSin3A (9). We also have found that this compound dramatically increases the interaction of SHP with Brm but not with Brg-1 (J. Miao and K. Kemper, unpublished data), providing further evidence that Brm is selectively a component of functional SHP complexes. Given the important roles of SHP and the bile acid receptor FXR in normal and abnormal metabolism, development of pharmacological agents that target Brg-1 or Brm and, therefore, modulate activity of SHP and FXR may be good therapeutic candidates for metabolic disorders, including fatty liver development, obesity, and diabetes.
Supplementary Material
Acknowledgments
We are grateful to C. Muchardt for providing plasmids for pSIREN-si-mouse Brm, H. Iba for pSSS-si-human Brm and pSSSP-si-human Brg-1, A. Imbalzano and S. Sif for the wild type and DN mutants for Brg-1 and Brm pBS(ks), and R. Sato for CMV-3flag-FXR for this study. We also thank B. W. Kemper for helpful discussion.
This study was supported by grant CA116777 to K.E.K. and grants DK062777 and AHA 0756028Z to J.K.K.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print on 5 October 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bavner, A., S. Sanyal, J. A. Gustafsson, and E. Treuter. 2005. Transcriptional corepression by SHP: molecular mechanisms and physiological consequences. Trends Endocrinol. Metab. 16:478-488. [DOI] [PubMed] [Google Scholar]
- 2.Bourachot, B., M. Yaniv, and C. Muchardt. 2003. Growth inhibition by the mammalian SWI-SNF subunit Brm is regulated by acetylation. EMBO J. 22:6505-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bultman, S., T. Gebuhr, D. Yee, C. La Mantia, J. Nicholson, A. Gilliam, F. Randazzo, D. Metzger, P. Chambon, G. Crabtree, and T. Magnuson. 2000. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell 6:1287-1295. [DOI] [PubMed] [Google Scholar]
- 4.Chiang, J. Y. L. 2002. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr. Rev. 23:443-463. [DOI] [PubMed] [Google Scholar]
- 5.de La Serna, I. L., K. A. Carlson, D. A. Hill, C. J. Guidi, R. O. Stephenson, S. Sif, R. E. Kingston, and A. N. Imbalzano. 2000. Mammalian SWI-SNF complexes contribute to activation of the hsp70 gene. Mol. Cell. Biol. 20:2839-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Serna, I. L., K. A. Carlson, and A. N. Imbalzano. 2001. Mammalian SWI/SNF complexes promote MyoD-mediated muscle differentiation. Nat. Genet. 27:187-190. [DOI] [PubMed] [Google Scholar]
- 7.Fang, S., J. Miao, L. Xiang, B. Ponugoti, E. Treuter, and J. K. Kemper. 2007. Coordinated recruitment of histone methyltransferase G9a and other chromatin modifying enzymes in SHP-mediated regulation of hepatic bile acid metabolism. Mol. Cell. Biol. 27:1407-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang, S., S. Tsang, R. Jones, B. Ponugoti, H. Yoon, S. Y. Wu, C. M. Chiang, T. M. Willson, and J. K. Kemper. 2008. The p300 acetylase is critical for ligand-activated farnesoid X receptor (FXR) induction of SHP. J. Biol. Chem. 283:35086-35095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farhana, L., M. I. Dawson, M. Leid, L. Wang, D. D. Moore, G. Liu, Z. Xia, and J. A. Fontana. 2007. Adamantyl-substituted retinoid-related molecules bind small heterodimer partner and modulate the Sin3A repressor. Cancer Res. 67:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flowers, S., N. G. Nagl, Jr., G. R. Beck, Jr., and E. Moran. 2009. Antagonistic roles for BRM and BRG1 SWI/SNF complexes in differentiation. J. Biol. Chem. 284:10067-10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin, B., S. A. Jones, R. R. Price, M. A. Watson, D. D. McKee, L. B. Moore, C. Galardi, J. G. Wilson, M. C. Lewis, M. E. Roth, P. R. Maloney, T. M. Wilson, and S. A. Kliewer. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6:517-526. [DOI] [PubMed] [Google Scholar]
- 12.Hassan, A. H., K. E. Neely, M. Vignali, J. C. Reese, and J. L. Workman. 2001. Promoter targeting of chromatin-modifying complexes. Front. Biosci. 6:D1054-D1064. [DOI] [PubMed] [Google Scholar]
- 13.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houten, S. M., M. Watanabe, and J. Auwerx. 2006. Endocrine functions of bile acids. EMBO J. 25:1419-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadam, S., and B. M. Emerson. 2003. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell 11:377-389. [DOI] [PubMed] [Google Scholar]
- 16.Kemper, J., H. Kim, J. Miao, S. Bhalla, and Y. Bae. 2004. Role of a mSin3A-Swi/Snf chromatin remodeling complex in the feedback repression of bile acid biosynthesis by SHP. Mol. Cell. Biol. 24:7707-7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, Y., H. Dell, D. H. Dowhan, M. Hadzopoulou-Cladaras, and D. D. Moore. 2000. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol. Cell. Biol. 20:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, Y., and D. D. Moore. 2002. Dual mechanism for repression of the monomeric orphan receptor liver receptor homologous protein-1 (LRH-1) by the orphan small heterodimer partner (SHP). J. Biol. Chem. 277:2463-2467. [DOI] [PubMed] [Google Scholar]
- 19.Lee, Y. S., D. H. Sohn, D. Han, H. W. Lee, R. H. Seong, and J. B. Kim. 2007. Chromatin remodeling complex interacts with ADD1/SREBP1c to mediate insulin-dependent regulation of gene expression. Mol. Cell. Biol. 27:438-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li-Hawkins, J., M. Gafvels, M. Olin, E. G. Lund, U. Andersson, G. Schuster, I. Bjorkhem, D. W. Russell, and G. Eggertsen. 2002. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J. Clin. Investig. 110:1191-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, T. T., M. Makishima, J. J. Repa, K. Schoonjans, T. A. Kerr, J. Auwerx, and D. J. Mangelsdorf. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 6:507-515. [DOI] [PubMed] [Google Scholar]
- 22.Ma, K., P. K. Saha, L. Chan, and D. D. Moore. 2006. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Investig. 116:1102-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makishima, M., A. Y. Okamoto, J. J. Repa, H. Tu, M. Learned, A. Luk, M. V. Hull, K. D. Lustig, D. J. Mangelsdorf, and B. Shan. 1999. Identification of a nuclear receptor for bile acids. Science 284:1362-1365. [DOI] [PubMed] [Google Scholar]
- 24.Mangelsdorf, D. J., and R. M. Evans. 1995. The RXR heterodimers and orphan receptors. Cell 83:841-850. [DOI] [PubMed] [Google Scholar]
- 25.Martens, J. A., and F. Winston. 2002. Evidence that Swi/Snf directly represses transcription in S. cerevisiae. Genes Dev. 16:2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao, J., Z. Xiao, D. Kanamaluru, G. Min, P. M. Yau, T. D. Veenstra, E. Ellis, S. Strom, K. Suino-Powell, H. E. Xu, and J. K. Kemper. 2009. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 23:986-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizutani, T., T. Ito, M. Nishina, N. Yamamichi, A. Watanabe, and H. Iba. 2002. Maintenance of integrated proviral gene expression requires Brm, a catalytic subunit of SWI/SNF complex. J. Biol. Chem. 277:15859-15864. [DOI] [PubMed] [Google Scholar]
- 28.Moschetta, A., A. L. Bookout, and D. J. Mangelsdorf. 2004. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat. Med. 10:1352-1358. [DOI] [PubMed] [Google Scholar]
- 29.Muchardt, C., and M. Yaniv. 1999. The mammalian SWI/SNF complex and the control of cell growth. Semin. Cell Dev. Biol. 10:189-195. [DOI] [PubMed] [Google Scholar]
- 30.Peterson, C. L., and J. L. Workman. 2000. Promoter targeting and chromatin remodeling by the SWI/SNF complex. Curr. Opin. Genet. Dev. 10:187-192. [DOI] [PubMed] [Google Scholar]
- 31.Ponugoti, B., S. Fang, and J. K. Kemper. 2007. Functional interaction of hepatic nuclear factor-4 and peroxisome proliferator-activated receptor-γ coactivator 1α in CYP7A1 regulation is inhibited by a key lipogenic activator, sterol regulatory element-binding protein-1c. Mol. Endocrinol. 21:2698-2712. [DOI] [PubMed] [Google Scholar]
- 32.Reyes, J. C., J. Barra, C. Muchardt, A. Camus, C. Babinet, and M. Yaniv. 1998. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2α). EMBO J. 17:6979-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell, D. W. 1999. Nuclear orphan receptors control cholesterol catabolism. Cell 97:539-542. [DOI] [PubMed] [Google Scholar]
- 34.Salma, N., H. Xiao, E. Mueller, and A. N. Imbalzano. 2004. Temporal recruitment of transcription factors and SWI/SNF chromatin-remodeling enzymes during adipogenic induction of the peroxisome proliferator-activated receptor gamma nuclear hormone receptor. Mol. Cell. Biol. 24:4651-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seol, W., H. Choi, and D. D. Moore. 1996. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272:1336-1339. [DOI] [PubMed] [Google Scholar]
- 36.Sif, S., A. J. Saurin, A. N. Imbalzano, and R. E. Kingston. 2001. Purification and characterization of mSin3A-containing Brg1 and hBrm chromatin remodeling complexes. Genes Dev. 15:603-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simone, C., S. V. Forcales, D. A. Hill, A. N. Imbalzano, L. Latella, and P. L. Puri. 2004. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 36:738-743. [DOI] [PubMed] [Google Scholar]
- 38.Sinal, C., M. Tohkin, M. Miyata, J. Ward, G. Lambert, and F. J. Gonzalez. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731-744. [DOI] [PubMed] [Google Scholar]
- 39.Staels, B., and F. Kuipers. 2007. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs 67:1383-1392. [DOI] [PubMed] [Google Scholar]
- 40.Strobeck, M. W., D. N. Reisman, R. W. Gunawardena, B. L. Betz, S. P. Angus, K. E. Knudsen, T. F. Kowalik, B. E. Weissman, and E. S. Knudsen. 2002. Compensation of BRG-1 function by Brm: insight into the role of the core SWI-SNF subunits in retinoblastoma tumor suppressor signaling. J. Biol. Chem. 277:4782-4789. [DOI] [PubMed] [Google Scholar]
- 41.Trauner, M., and J. L. Boyer. 2003. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83:633-671. [DOI] [PubMed] [Google Scholar]
- 42.Wang, H., J. Chen, K. Hollister, L. Sowers, and B. M. Forman. 1999. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3:543-553. [DOI] [PubMed] [Google Scholar]
- 43.Wang, L., J. Liu, P. Saha, J. Huang, L. Chan, B. Spiegelman, and D. D. Moore. 2005. The orphan nuclear receptor SHP regulates PGC-1α expression and energy production in brown adipocytes. Cell Metab. 2:227-238. [DOI] [PubMed] [Google Scholar]
- 44.Wang, S., B. Zhang, and D. V. Faller. 2002. Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth. EMBO J. 21:3019-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe, M., S. M. Houten, C. Mataki, M. A. Christoffolete, B. W. Kim, H. Sato, N. Messaddeq, J. W. Harney, O. Ezaki, T. Kodama, K. Schoonjans, A. C. Bianco, and J. Auwerx. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484-489. [DOI] [PubMed] [Google Scholar]
- 46.Willson, T. M., S. A. Jones, J. T. Moore, and S. A. Kliewer. 2001. Chemical genomics: functional analysis of orphan nuclear receptors in the regulation of bile acid metabolism. Med. Res. Rev. 21:513-522. [DOI] [PubMed] [Google Scholar]
- 47.Workman, J. L., and R. E. Kingston. 1998. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67:545-579. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, H. S., M. Gavin, A. Dahiya, A. A. Postigo, D. Ma, R. X. Luo, J. W. Harbour, and D. C. Dean. 2000. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 101:79-89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.