Abstract
The semaphorin 4D (Sema4D) receptor plexin-B1 constitutively interacts with particular Rho guanine nucleotide exchange factors (RhoGEFs) and thereby mediates Sema4D-induced RhoA activation, a process which involves the tyrosine phosphorylation of plexin-B1 by ErbB-2. It is, however, unknown how plexin-B1 phosphorylation regulates RhoGEF activity. We show here that activation of plexin-B1 by Sema4D and its subsequent tyrosine phosphorylation creates docking sites for the SH2 domains of phospholipase Cγ (PLCγ). PLCγ is thereby recruited into the plexin-B1 receptor complex and via its SH3 domain activates the Rho guanine nucleotide exchange factor PDZ-RhoGEF. PLCγ-dependent RhoGEF activation is independent of its lipase activity. The recruitment of PLCγ has no effect on the R-Ras GTPase-activating protein activity of plexin-B1 but is required for Sema4D-induced axonal growth cone collapse as well as for the promigratory effects of Sema4D on cancer cells. These data demonstrate a novel nonenzymatic function of PLCγ as an important mechanism of plexin-mediated signaling which links tyrosine phosphorylation of plexin-B1 to the regulation of a RhoGEF protein and downstream cellular processes.
Mammalian semaphorins were originally identified as axon guidance factors but are now recognized also as important regulators of morphogenesis and homeostasis in various organ systems, including the immune, cardiovascular, and renal systems (3-5, 7, 19, 23, 30, 35, 40, 56, 64, 76). Most effects of semaphorins are mediated by a group of large transmembrane proteins called plexins, of which four families exist in the mammalian system: plexin-A1 to -4, plexin-B1 to -3, plexin-C1, and plexin-D1 (60, 61). The four members of the plexin-A family in most cases require neuropilins as ligand binding partners to respond to semaphorins, whereas the three members of the plexin-B family are directly activated by semaphorins. While plexin-B1 binds Sema4D, plexin-B2 can be activated by Sema4C and Sema4D, and plexin-B3 has been shown to respond to Sema5A (31, 35).
The activation of plexins by semaphorins initiates a variety of signaling processes, which involve several small GTPases of the Ras and Rho families (31, 34, 43). All plexin family members possess an R-Ras GTPase-activating protein (GAP) domain (36). Activated plexin-B1 and -A1 have been shown to also interact with other small GTPases, including GTP-bound Rac1 and RhoD as well as Rnd1, Rnd2, and Rnd3 (14, 37, 48, 63, 67, 68, 74). Different from other plexin families, the C terminus of B-family plexins contains a PDZ domain-binding motif which mediates a stable interaction with the guanine nucleotide exchange factors PDZ-RhoGEF and LARG (1, 15, 26, 39, 57). Activation of the plexin-B1/PDZ-RhoGEF complex by semaphorin 4D (Sema4D) results in RhoA activation downstream of plexin-B1 (15, 39, 57). Members of the plexin-B family also interact with and are phosphorylated by the receptor tyrosine kinases ErbB-2 and c-Met (12, 22, 58). ErbB-2-mediated phosphorylation of plexin-B1 is required for plexin-mediated RhoA activation and downstream cellular effects, including the promigratory effects of Sema4D on cancer cells and the induction of axonal growth cone collapse by Sema4D (58, 59). However, the molecular mechanisms linking ErbB-2-mediated phosphorylation of plexin-B1 to the regulation of RhoA activity and subsequent cellular effects are unknown.
Here we report that upon activation by Sema4D, plexin-B1 becomes phosphorylated by ErbB-2 at particular tyrosine residues on its intracellular portion. These phosphorylated tyrosine residues serve as docking sites for the SH2 domains of PLCγ. PLCγ is thereby recruited into the plexin-B1 receptor complex and through its SH3 domain mediates RhoA activation and downstream cellular effects.
MATERIALS AND METHODS
Antibodies and reagents.
The following antibodies were used: mouse monoclonal anti-Myc (9E10), rabbit polyclonal anti-R-Ras, rabbit polyclonal anti-PLCγ2, and goat polyclonal anti-plexin-B2 (Santa Cruz Biotechnology); mouse monoclonal anti-G protein of vesicular stomatitis virus (VSV), mouse monoclonal anti-α-tubulin, mouse monoclonal and rabbit polyclonal anti-FLAG, and rabbit polyclonal anti-glutathione S-transferase (anti-GST; all from Sigma-Aldrich); and rabbit polyclonal anti-ErbB-2[pY1248], anti-PLCγ1, anti-Grb2, and anti-RhoA (Cell Signaling Technology); mouse monoclonal antiphosphotyrosine (4G10; Upstate Biotechnology); mouse monoclonal anti-Rac (23A8) and anti-ErbB-2 (Biosource Invitrogen); goat polyclonal anti-plexin-B1 (R&D Systems); and rabbit polyclonal anti-PDZ-RhoGEF (Imgenex). Human recombinant Sema3A was purchased from R&D Systems.
Plasmids.
Eukaryotic expression plasmids carrying the cDNAs of PDZ-RhoGEF, Sema4D, Myc-RhoA, GST-RhoA, c-Met, and Rnd1, were described previously (29, 57, 58). VSV-plexin-B1 was kindly provided by L. Tamagnone (University of Torino, Turin, Italy). R-Ras was provided by Monique Dail (The Burnham Institute, La Jolla, CA). GST-RacL61 was provided by Dominique T. Brandt. Myc-PLCγ1 was a kind gift from Peter Gierschik (Institute of Pharmacology and Toxicology, University of Ulm, Ulm, Germany). Specific mutations introduced in VSV-plexin-B1, Myc-PLCγ1, and FLAG-PDZ-RhoGEF were carried out using the QuikChange site-directed mutagenesis procedure (Stratagene, La Jolla, CA) employing Pfu Turbo as a proofreading DNA polymerase. Correct mutagenesis was confirmed by DNA sequencing. Myc-PLCγ1 lacking amino acids 1 to 142 (ΔPH1), 152 to 187 (ΔEF), 320 to 464 (ΔXbox), 550 to 657 (ΔSH2.N), 668 to 756 (ΔSH2.C), 550 to 756 (ΔSH2), 791 to 851 (ΔSH3), 489 to 523 and 895 to 931 (ΔPH2), 953 to 1070 (ΔYbox), or 1075 to 1177 (ΔC2) was generated using standard molecular biology methods. Bacterial expression constructs containing FLAG- and hemagglutinin-tagged C-terminal portions of PDZ-RhoGEF (amino acids 1080 to 1522), FLAG-tagged C-terminal portion of LARG (amino acids 1132 to 1544), and the SH3 domains of PLCγ1 and Grb2 (amino acids 788 to 854 and 153 to 217, respectively) were generated using standard methods.
Cell culture, immunoprecipitation studies, and transfection.
HEK 293, MCF-7, and MDA-MB-468 cells were cultured, and immunoprecipitations were performed as described previously (59). Primary hippocampal neurons were prepared and cultured as described previously (57). HEK 293 cells were transfected using the calcium phosphate method, primary cells were transfected using FuGENE HD (Roche), and cells were transfected with small interfering RNAs (siRNAs) using HiPerFect reagent according to the manufacturer's instructions (Qiagen). Cell migration was assayed using polystyrene Transwell inserts (Greiner) with pore sizes of 0.8 μm (59).
Determination of activated RhoA and R-Ras.
The amounts of activated cellular Rho and R-Ras were determined by precipitation with a fusion protein consisting of GST and the Rho-binding domain of Rhotekin (GST-RBD) or the Ras-binding domain of Raf1 (GST-Raf1) as described previously (45, 65). In vitro determination of RhoA activation was performed as described before (29).
siRNAs.
The target sequences for siRNAs specific to ErbB-2 and c-Met used in this study were published before (16, 33). The sequence of the siRNA used to suppress plexin-B1 expression was GAAUCCGGGUGACCGUGG, and siRNAs for PLCγ1 (AAGAAGUCGCAGCGACCCGAG), PLCγ2 (CTCCGTGTTCATCCTAGGAAA), and Grb2 (TACAAATGTGTTACACATAAA) were purchased from Qiagen. A control siRNA, CGCGUAGAAGAUGAAGUUG, was purchased from IBA.
Production and purification of recombinant Sema4D.
Generation of Lec3.2.8.1 CHO cells expressing the extracellular part of human Sema4D (residues 1 to 657 followed by a lysine residue and a carboxy-terminal histidine tag for purification) was described previously (32, 59).
Retroviral infections.
In order to obtain siRNA-insensitive plexin-B1, silent mutations were introduced at positions 3855 (C→T) and 3858 (G→A) of the coding region of the cDNAs encoding wild-type and mutated (Y1708F/Y1732F) plexin-B1. In order to obtain siRNA-insensitive PLCγ1, silent mutations were introduced at positions 123 (G→T) and 126 (G→T) of wild-type PLCγ1 and its ΔXbox and ΔSH3 mutants. The resulting sequences were subcloned into the retroviral vector pLNCX2 (Clontech). Selection and retroviral transfection were carried out as described before (59).
Peptide synthesis and isolation of binding partners.
Peptides C-DSVGEPLYMLFRGIK and C-SVTGKAKYTLNDNRL corresponding to residues 1701 to 1715 and 1725 to 1739 of plexin-B1, respectively, were synthesized in the Analytical Laboratory of the University Hospital in Düsseldorf (Germany). For phosphorylated peptides, a phosphotyrosine was inserted in positions corresponding to amino acid residues 1708 and 1732 of plexin-B1. Peptides were coupled to the SulfoLink coupling resin (Thermo Scientific), nonbound peptide was removed by washing with radioimmunoprecipitation assay buffer, and the resin was incubated with 50 mM cysteine to reduce unspecific binding. HEK 293 cells were lysed in radioimmunoprecipitation assay buffer as described above, and cleared lysates were incubated with the peptide-coupled SulfoLink for 2 h at 4°C. The resin was then extensively washed and boiled in Laemmli buffer, and proteins bound to peptides were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were visualized using Coomassie blue staining.
Mass spectrometry.
Mass spectrometry analysis was performed in the Core Facility for Mass Spectrometry and Proteomics, Centrum of Molecular Biology, University of Heidelberg, Heidelberg, Germany. Briefly, individual spots were excised from gels and digested with trypsin. Matrix-assisted laser desorption ionization of quadruple time-of-flight mass spectrometry was performed using an Ultraflex time-of-flight apparatus (Bruker Daltonik). For protein identification, the peptide mass fingerprint was run against the NCBI-nr database using Mascot (Matrix Science).
Reporter gene assays.
For the SRE reporter gene assays, cells were grown in six-well plates and transfected with 100 ng 3D.ALuc, a reporter plasmid expressing firefly luciferase under the control of the mutant SRE (SRE.L) lacking a ternary complex factor-binding site (24), and with 100 ng of pRLTK (plasmid expressing Renilla luciferase under the control of the thymidine kinase promoter) together with the experiment-specific plasmids (as described in the figure legends). At 36 h after transfection, cells were starved for 12 h, and the activities of firefly and Renilla luciferases were measured using the Stop and Glow kit (Promega) according to the manufacturer's instructions. Relative luciferase activity was determined by normalizing firefly luciferase activity against Renilla luciferase activity.
Protein purification.
Proteins were produced and purified in Escherichia coli strain DE3 or in HEK cells [(FLAG)-PDZ-RhoGEF]. GST fusion proteins were eluted from glutathione disulfide beads (Amersham), and FLAG-tagged proteins were purified using anti-FLAG antibody-coated Sepharose and eluted with the FLAG peptide (Sigma), followed by gel filtration chromatography, and were then concentrated by using Amicon Ultra centrifugal filter devices (Millipore).
Statistical analysis.
Statistical significance was evaluated by Student's t test. Levels of significance were 0.05, 0.01, and 0.001.
RESULTS
Phosphorylation of specific tyrosine residues of plexin-B1 is required for Sema4D-induced RhoA activation.
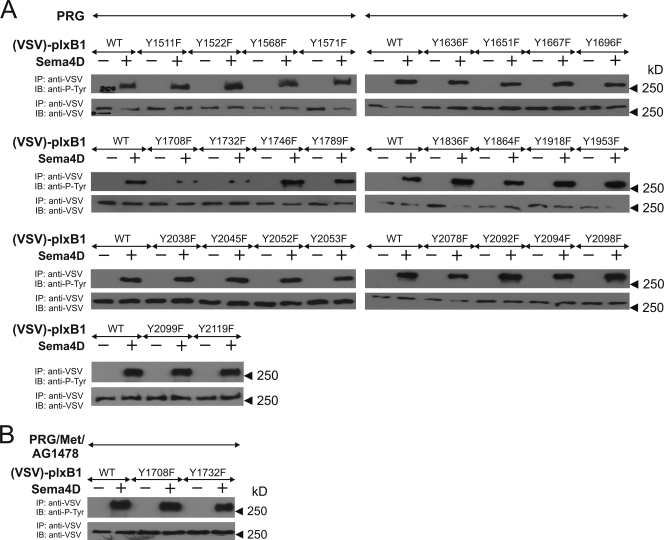
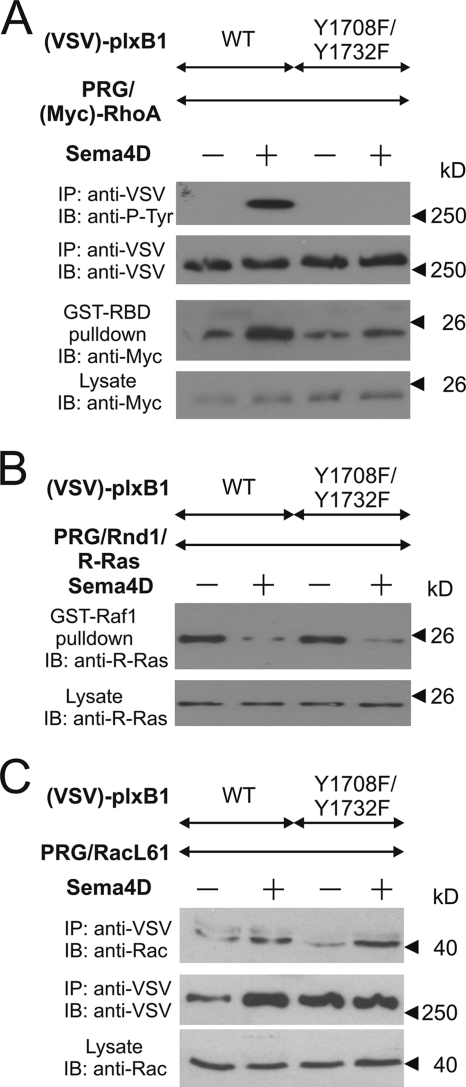
We have previously shown that tyrosine phosphorylation of plexin-B1 by ErbB-2 is required for plexin-B1-mediated RhoA activation (58). In order to identify the tyrosine residues of plexin-B1 phosphorylated by ErbB-2, we generated tyrosine-to-phenylalanine (Y/F) mutants of all 26 tyrosine residues of the cytoplasmic portion of plexin-B1 and tested whether they were still phosphorylated by ErbB-2 in response to Sema4D (Fig. 1A). The analysis of three independently performed experiments revealed that only the mutation of tyrosine residue 1708 or 1732 led to a significant decrease in Sema4D-induced phosphorylation of plexin-B1. The mutation of both tyrosine residues [plexin-B1(Y1708F/Y1732F)] completely abrogated Sema4D-induced phosphorylation of plexin-B1 by ErbB-2 (Fig. 2A), whereas c-Met-mediated phosphorylation remained unaffected (Fig. 1B). Consistent with the established role of plexin-B1 tyrosine phosphorylation in Sema4D-induced RhoA activation, plexin-B1(Y1708F/Y1732F) was not able to mediate Sema4D-induced activation of RhoA (Fig. 2A). In contrast, we found no difference in Sema4D-regulated R-Ras GAP activity or Rac1 association in cells expressing any of the tyrosine mutants of plexin-B1 (data not shown) or the Y1708F/Y1732F double mutant of plexin-B1 (Fig. 2B and C, respectively). This indicates that the Y1708F/Y1732F double mutant of plexin-B1 had not lost its ability to become activated by Sema4D and that the tyrosine phosphorylation of both residues is required for RhoA activation but not for the regulation of other plexin-B1 signaling pathways.
FIG. 1.
Characterization of ErbB-2-mediated phosphorylation of plexin-B1. (A) HEK 293 cells were transfected with wild-type plexin-B1 or the indicated tyrosine to phenylalanine (Y/F) mutants and PDZ-RhoGEF (PRG). Cells were then incubated without (−) or with (+) 150 nM Sema4D and lysed, and plexin-B1 was immunoprecipitated (IP). Immunoprecipitates were subjected to SDS-PAGE and were then immunoblotted (IB) using anti-VSV and antiphosphotyrosine antibodies (anti-P-Tyr). (B) HEK 293 cells were transfected with wild-type plexin-B1 or the indicated Y/F mutants together with PDZ-RhoGEF (PRG) and c-Met (Met). Cells were preincubated with 1 μM of the ErbB-2 inhibitor AG1478 and were then incubated without or with 150 nM Sema4D. Samples were processed as described above. Shown are representative autoluminograms.
FIG. 2.
Effects of the plexin-B1(Y1708F/Y1732F) mutant on plexin-B1 signaling. HEK 293 cells were transfected with cDNAs encoding wild-type (WT) VSV-tagged plexin-B1 [(VSV)-plxB1] or its mutant (Y1708/1732F) together with either Myc-tagged RhoA [(Myc)-RhoA] and wild-type PDZ-RhoGEF (PRG) (A), R-Ras, Rnd1, and PRG (B), or the constitutively active GST-tagged Rac1 mutant [Rac(Q61L)] and wild-type PRG (C). After 48 h cells were kept in 0.5% FBS (A) or 10% FBS (B and C) for 12 h followed by incubation without (−) or with (+) 150 nM Sema4D for 20 min. Thereafter, cells were lysed, and plexin-B1 was immunoprecipitated (IP) using anti-VSV-Sepharose (A and C). (A) Precipitated proteins were analyzed by immunoblotting using antiphosphotyrosine antibodies. In a parallel experiment active RhoA was precipitated from cell lysates as described in Materials and Methods. (B) Activated R-Ras was precipitated from the cell lysates with a GST-fused Ras-binding domain of Raf1. Shown are autoluminograms of immunoblots (IB) of lysed (lysate) or precipitated samples (IP/GST-RBD or GST-Raf1 pulldown) and the indicated antibodies.
Sema4D-induced phosphorylation of plexin-B1 generates docking sites for PLCγ.
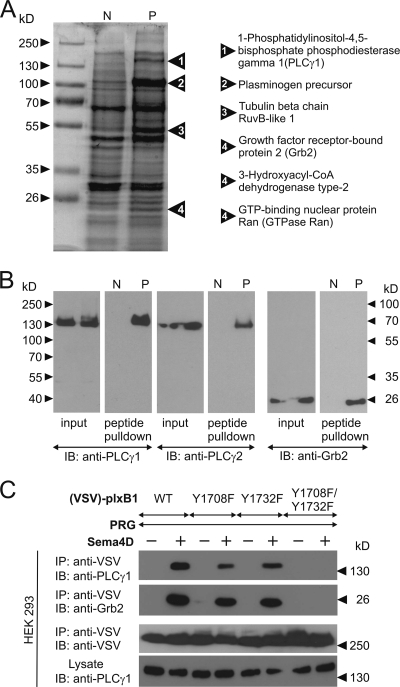
Phosphorylated tyrosine residues can provide docking sites for SH2 or PTB domain-containing proteins (49). In order to test whether the phosphorylated tyrosine residues 1708 and 1732 of plexin-B1 serve as docking sites for other proteins, we synthesized peptides corresponding to 15-amino-acid-long sequences of the plexin-B1 cytoplasmic portion containing tyrosine residues 1708 or 1732 in a nonphosphorylated or a phosphorylated version. Peptides were then immobilized on agarose beads and incubated with lysates of HEK 293 cells. After washing of beads, bound proteins were eluted and separated using SDS-PAGE. Whereas no additional bands were obtained with the peptide containing phosphorylated Y1732 compared to the nonphosphorylated version (data not shown), several proteins specifically interacted with the peptide containing phosphorylated tyrosine residue 1708 (Fig. 3A). Bands detected by Coomassie blue staining were excised and analyzed by matrix-assisted laser desorption ionization mass spectrometry. In two independent experiments, 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase γ1 (PLCγ1) and growth factor receptor-bound protein 2 (Grb2) were identified as SH2 domain-containing proteins interacting with phosphorylated tyrosine 1708 (Fig. 3A, right panel). To test whether PLCγ1 and Grb2 indeed bound only to the peptide containing phosphorylated tyrosine 1708, the material isolated with the different peptides was subjected to an immunoblot analysis using antibodies against PLCγ1, its homologue PLCγ2, and Grb2. All three proteins were identified in the material isolated with the phosphorylated peptide 1708 but not with the unphosphorylated peptide (Fig. 3B).
FIG. 3.
Detection of proteins interacting with pY1708. (A) Peptides representing amino acids 1701 to 1715 of plexin-B1 containing a nonphosphorylated (N) or phosphorylated (P) tyrosine residue at 1708 were bound to agarose beads and incubated with lysates from HEK 293 cells for 2 h. Bound proteins were separated by SDS-PAGE and visualized by Coomassie blue staining. Bands appearing only in samples eluted from phosphorylated peptides were cut out and analyzed by mass spectrometry. Proteins identified in the corresponding band are shown on the right. (B) HEK 293 cell lysates (input) were incubated with immobilized peptide containing nonphosphorylated (N) or phosphorylated (P) tyrosine residue 1708 (peptide pull down). After washing and elution, proteins bound to the peptide were separated by SDS-PAGE, and immunoblotting (IB) was performed with the indicated antibodies. (C) HEK 293 cells were transfected with wild-type (WT) plexin-B1 or the indicated mutants. Cells were starved and incubated without (−) or with (+) Sema4D. Thereafter, WT or mutated plexin-B1 was immunoprecipitated from cell lysates using anti-VSV antibody. Immunoprecipitates were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies.
To test whether PLCγ and Grb2 can interact with plexin-B1 in a cellular context, we expressed VSV-tagged plexin-B1 in HEK 293 cells. Endogenous PLCγ1 and Grb2 coimmunoprecipitated with wild-type plexin-B1 in a Sema4D-dependent manner (Fig. 3C). Mutation of the tyrosine residue 1708 or 1732 resulted in a decreased interaction, and the Y1708F/Y1732F plexin-B1 double mutant completely lost the ability to bind PLCγ1 and Grb2 in response to Sema4D. Thus, Sema4D-dependent phosphorylation of tyrosine residues 1708 and 1732 induced interaction of plexin-B1 with PLCγ1 and Grb2.
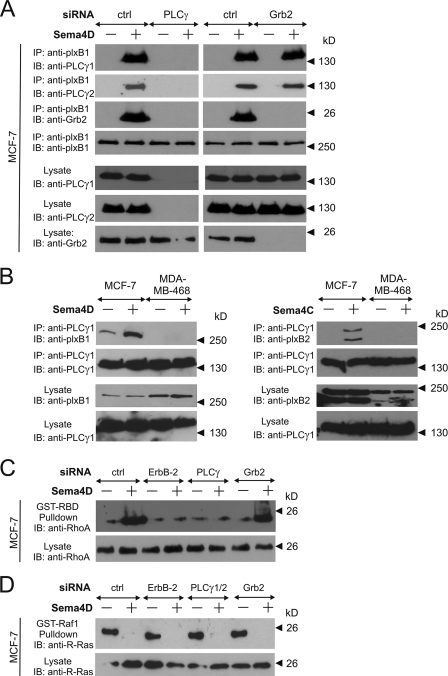
Endogenous plexin-B1 and plexin-B2 interact with PLCγ upon activation by semaphorins.
In order to further evaluate the Sema4D-dependent interaction of plexin-B1 with PLCγ1 and Grb2 we studied the human breast cancer cell line MCF-7. MCF-7 cells express both plexin-B1 and ErbB-2, and stimulation with Sema4D results in an ErbB-2-dependent phosphorylation of plexin-B1 (59). Upon stimulation of cells with Sema4D, endogenous plexin-B1 could be coimmunoprecipitated with endogenous PLCγ1, PLCγ2, and Grb2 (Fig. 4A). Interestingly, the siRNA-mediated knockdown of PLCγ1 and PLCγ2 abrogated not only the interactions between plexin-B1 and both PLCγ proteins but also blocked the interaction of plexin-B1 and Grb2, whereas knockdown of Grb2 had no effect on the interaction between plexin-B1 and PLCγ (Fig. 4A). This strongly suggests that the interaction between plexin-B1 and Grb2 is indirect and dependent on the presence of PLCγ.
FIG. 4.
PLCγ interacts with B-family plexins. MCF-7 cells or MDA-MB-468 cells were not transfected (B) or transfected (A, C, and D) with the indicated siRNAs (PLCγ represents PLCγ1 plus PLCγ2). At 48 h after transfection cells were starved (0.5% FBS) and incubated in the absence (−) or presence (+) of 150 nM Sema4D. Cells were then lysed, and the proteins interacting with plexin-B1 (A) or PLCγ1 (B) were coimmunoprecipitated (IP) using anti-plexin-B1 or anti-PLCγ1 antibodies. Lysates and immunoprecipitates were analyzed using the indicated antibodies (IB). (C and D) MCF-7 cells were transfected with siRNAs as indicated, and 48 h later cells were transferred to 0.5% FBS (C) or were kept in 10% FBS (D) and incubated in the absence or presence of 150 nM Sema4D for 20 min. Active RhoA (C) or R-Ras (D) was precipitated from cell lysates as described in Materials and Methods. Shown are autoluminograms of immunoblots (IB) analyzed with the indicated antibodies.
In contrast to MCF-7 cells, MDA-MB-468 cells do not contain ErbB-2 but express plexin-B1 together with the receptor tyrosine kinase c-Met (59). Although PLCγ1 is expressed in both cell lines, a Sema4D-dependent interaction with plexin-B1 can only be seen in the ErbB-2-expressing MCF-7 cell line (Fig. 4B). This supports the notion that ErbB-2-dependent phosphorylation of plexin-B1 is necessary for this interaction. MCF-7 cells also express plexin-B2, and stimulation of cells with the plexin-B2 ligand Sema4C (13) induced interaction of PLCγ1 with plexin-B2 (Fig. 4B right panel). We did not find any Sema4C-dependent interaction of PLCγ1 and plexin-B2 in MDA-MB-468 cells.
Plexin-B1-induced RhoA activation but not R-Ras inactivation requires PLCγ.
Knockdown of PLCγ1 or PLCγ2 in MCF-7 cells decreases plexin-B1/PDZ-RhoGEF-mediated RhoA activation (data not shown), whereas the depletion of both isoforms blocked Sema4D-induced RhoA activation (Fig. 4C). Consistent with previous data (59), we observed also that siRNA-mediated knockdown of ErbB-2 abolished RhoA activation downstream of plexin-B1, whereas Grb2 knockdown had no effect on RhoA activation (Fig. 4C). In contrast to the Sema4D-induced RhoA activation, the regulation of R-Ras GAP activity of plexin-B1 did not depend on PLCγ or ErbB-2, as indicated by the lack of any effect of PLCγ1/2 or ErbB-2 knockdown on Sema4D-induced inhibition of R-Ras (Fig. 4D). Hence, the recruitment of PLCγ into the plexin-B1 receptor complex is necessary for RhoA activation but not for R-Ras function regulated by plexin-B1.
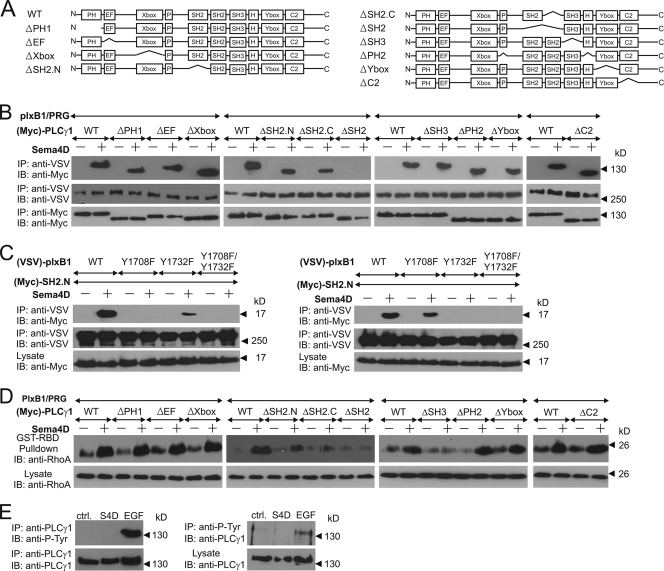
Analysis of the interaction between plexin-B1 and PLCγ1.
To obtain insight into the mechanism underlying the PLCγ-dependent RhoA activation through plexin-B1, PLCγ1 mutants lacking functional domains (Fig. 5A) were expressed in HEK 293 cells. The removal of the N- or C-terminal SH2 domain decreased the interaction between Sema4D-activated plexin-B1 and PLCγ1, whereas deletion of both SH2 domains blocked interaction of PLCγ1 with plexin-B1 (Fig. 5B).
FIG. 5.
Analysis of PLCγ domains required for plexin-B1-mediated signaling. (A) Graphical representation of the analyzed PLCγ1 mutants. (B and D) HEK 293 cells were transfected with plexin-B1, PDZ-RhoGEF (PRG), wild-type PLCγ1 (WT), or its mutants. Cells were incubated without (−) or with (+) 150 nM Sema4D and lysed, and the interaction between plexin-B1 and PLCγ1 and its mutants was analyzed by immunoprecipitation (B), or the amount of activated RhoA was analyzed as described in Materials and Methods (D). (C) Wild-type or Y/F mutants of plexin were cotransfected with the Myc-tagged N- or C-terminal SH2 domain of PLCγ1 (SH2.N or SH2.C, respectively). Protein-protein interactions were analyzed by coimmunoprecipitation. (E) MCF-7 cells were treated with phosphate-buffered saline (ctrl.), 150 nM Sema4D, or 10 ng/ml EGF. Cells were lysed, and immunoprecipitation (IP) was performed using anti-PLCγ1 or antiphosphotyrosine antibodies (left and right panels, respectively). Samples were then analyzed by immunoblotting (IB) using the indicated antibodies.
Consistent with these results we observed that the point mutation (R586K) (62) in one of the SH2 domains decreased the amount of PLCγ1 that coimmunoprecipitates with plexin-B1 (data not shown). Thus, both SH2 domains of PLCγ1 mediated the interaction with tyrosine-phosphorylated plexin-B1, while none of the other domains appeared to be critically involved. Interestingly, a remarkable specificity in the interaction of phosphorylated Y1708 and Y1732 and each of the two SH2 domains of PLCγ1 was observed. While the more N-terminally localized SH2 domain (SH2.N) did not interact with plexin-B1(Y1708F) but it did with plexin-B1(Y1732F) (Fig. 5C, left panel), the more C-terminal SH2 domain (SH2.C) was unable to interact with the Y1732F mutant, while it did interact with plexin-B1(Y1708F) (Fig. 5C, right panel).
In order to study the role of different PLCγ domains in mediating downstream effects of plexin-B1 signaling, we determined RhoA activity in transfected HEK 293 cells (Fig. 5D). Removal of one of the SH2 domains led to an impaired RhoA activation in response to Sema4D, while removal of both SH2 domains completely blocked plexin-B1-mediated RhoA activation. Interestingly, PLCγ1 mutants lacking the X or Y box, which are required for the enzymatic activity of the phospholipase (47), were still able to mediate Sema4D-induced RhoA activation, thereby indicating that PLCγ functions in plexin-B-mediated signaling independently of its lipase activity. Consistent with this, stimulation of cells with epidermal growth factor (EGF) but not with Sema4D resulted in tyrosine phosphorylation of PLCγ, a well-described prerequisite for the activation of its phospholipase activity (47) (Fig. 5E). Among other PLCγ domains, only the removal of the SH3 domain affected plexin-B1-mediated RhoA activation (Fig. 4D). While the ΔSH3 mutant was recruited into the plexin-B1 complex upon Sema4D stimulation (Fig. 5B), it was unable to promote Sema4D-induced RhoA activation. Consistent with the results obtained with the ΔSH3 mutant of PLCγ1 we found that the point mutation (W828K) (62) in the SH2 domain of PLCγ1 also blocks Sema4D-mediated RhoA activation, whereas the interaction between plexin-B1 and PLCγ1 remains unaffected (data not shown). This indicates that the SH3 domain of PLCγ but not its enzymatic activity is required for Sema4D-induced RhoA activation.
The SH3 domain of PLCγ1 mediates activation of PDZ-RhoGEF.
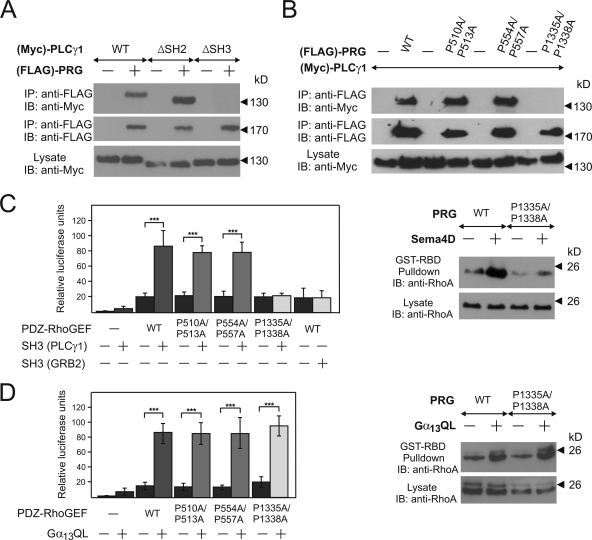
Since plexin-B1 stably interacts with particular RhoGEF proteins to mediate RhoA activation and since the SH3 domain of PLCγ appeared to be crucial for plexin-B1-mediated RhoA activation, we tested the possibility that the SH3 domain of PLCγ1 interacts with PDZ-RhoGEF. In cotransfected HEK 293 cells, we found that PDZ-RhoGEF coimmunoprecipitated with PLCγ1 or its SH2 domain-lacking mutant, but not with a PLCγ1 mutant lacking the SH3 domain (Fig. 6A), suggesting that PLCγ1 interacts with PDZ-RhoGEF via its SH3 domain.
FIG. 6.
PLCγ1 interaction with PDZ-RhoGEF. (A) HEK 293 cells were transfected without or with FLAG-tagged PDZ-RhoGEF [(FLAG)-PRG] and Myc-tagged wild-type (WT) PLCγ1 or its indicated mutants. From cell lysates, PDZ-RhoGEF was immunoprecipitated (IP) and precipitates were then separated and immunoblotted (IB) with the indicated antibodies. (B) HEK 293 cells transfected with Myc-tagged PLCγ1 and FLAG-tagged wild-type PDZ-RhoGEF or its indicated mutants were lysed, and PLCγ1 was immunoprecipitated. Precipitates were analyzed by immunoblotting using the indicted antibodies. (C and D, left panels) HEK 293 cells were cotransfected with SRE.L firefly luciferase reporter plasmid, Renilla luciferase expression plasmid without (−) or with various PDZ-RhoGEF versions, the SH3 domains of Grb2 and PLCγ, or constitutively active Gα13 (Gα13QL) as indicated, and relative luciferase activity was determined as described in Materials and Methods. Shown values are the means of three independent experiments ± standard deviations. (Right panels) HEK 293 cells were transfected with wild-type PDZ-RhoGEF (WT) or its mutant (P1335A/P1338A) and were incubated without or with 150 nM Sema4D (C) or were cotransfected with control or with a constitutively active version of Gα13 (Gα13QL) (D). Thereafter, RhoA activity was determined as described in Materials and Methods section. ***, P ≤ 0.001.
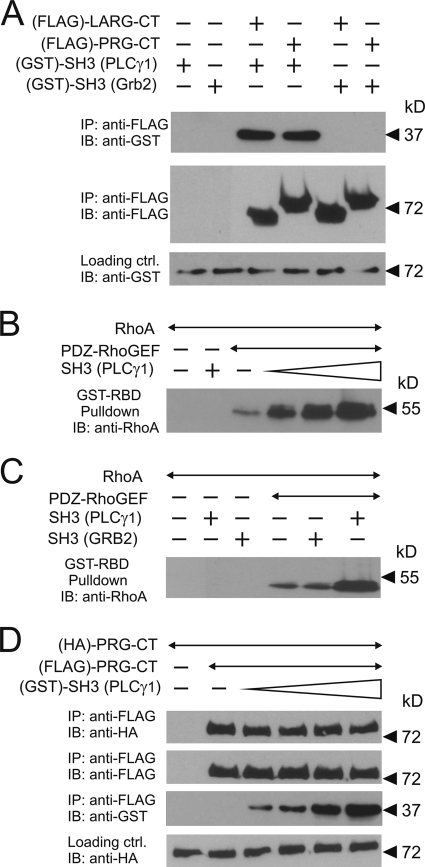
Among six proline-rich regions of the RhoGEF protein, the software SH3 Hunter (17) identified one region at positions 1332 to 1338 as a potential binding partner for the SH3 domain of PLCγ1. Immunoprecipitation experiments with PDZ-RhoGEF carrying proline-to-alanine mutations in this region (P1335A/P1338A) and two other regions (P510A/P513A and P554A/P557A) indicated that the proline-rich region at positions 1332 to 1338 of PDZ-RhoGEF was necessary for the interaction with the SH3 domain of PLCγ1 (Fig. 6B). In order to test whether the SH3 domain of PLCγ1 and the C terminus of PDZ-RhoGEF interact directly, we purified both proteins after expression in bacteria and tested their interaction in vitro. We found that the C terminus of PDZ-RhoGEF indeed interacted with the SH3 domain of PLCγ1 but not with the SH3 domain of Grb2 (Fig. 7A).
FIG. 7.
In vitro characterization of the role of the SH3 domain of PLCγ in PDZ-RhoGEF signaling. (A) Equal amounts of purified GST-tagged SH3 domains of PLCγ1 or Grb2 were incubated without (−) or with (+) purified FLAG-tagged C-terminal portions of LARG or PDZ-RhoGEF (LARG-CT and PRG-CT, respectively). The RhoGEF proteins were then immunoprecipitated (IP) using anti-FLAG antibody. Shown are immunoblots (IB) of the precipitates using the indicated antibodies. (B) One nanomole of FLAG-PDZ-RhoGEF was incubated without or with a 1-, 10-, or 100-fold molar excess of GST-SH3 of PLCγ1. The reaction was started by addition of 100 nanomole of GDP-loaded RhoA. After 10 min of incubation, activated RhoA was precipitated as described in Materials and Methods. Shown are immunoblots using the anti-RhoA antibody. (C) FLAG-PDZ-RhoGEF was incubated with the SH3 domains of PLCγ1 or Grb2. The experiment was performed as described in B. (D) Equal amounts of hemagglutinin (HA)- and FLAG-tagged C-terminal portions of PDZ-RhoGEF were incubated alone or together with increasing concentrations of the SH3 domain of PLCγ1. FLAG-tagged PDZ-RhoGEF was immunoprecipitated, and precipitates were analyzed by immunoblotting with the indicated antibodies.
Expression of PDZ-RhoGEF together with the SH3 domain of PLCγ1 resulted in a synergistic activation of a mutated serum response element (SRE.L) which lacks the binding site for the ternary complex factor and is strongly activated by RhoA (25) (Fig. 6C, left panel). In contrast, the SH3 domain of Grb2 had no effect. Consistent with the results of the interaction studies, the mutation of the proline-rich regions 507 to 513 and 551 to 557 of PDZ-RhoGEF had no effect on the PLCγ-SH3 domain-dependent RhoA activation, whereas mutation of the proline-rich region 1332 to 1338 of PDZ-RhoGEF abolished the effect of the SH3 domain of PLCγ (Fig. 6C, left panel). This effect was specific for the SH3 domain of PLCγ, since wild-type PDZ-RhoGEF and all P/A mutants were equally activated by a constitutively active Gα13 mutant (Gα13Q226L) (Fig. 6D, left panel). These data were confirmed by direct determination of RhoA activity using RhoA pull-down assays (Fig. 6C and D, right panels). In order to test whether the SH3 domain of PLCγ can directly stimulate the catalytic activity of PDZ-RhoGEF, we measured the effect of the purified SH3 domain of PLCγ on RhoA activation in vitro. We found that the PLCγ1 SH3 domain increases the guanine nucleotide exchange activity of PDZ-RhoGEF toward RhoA (Fig. 7B). This effect was specific for the SH3 domain of PLCγ1, since addition of the purified Grb2 SH3 domain to purified PDZ-RhoGEF had no effect (Fig. 7C).
Oligomerization of PDZ-RhoGEF has been suggested to be involved in the regulation of its catalytic activity. Since our data showed that the SH3 domain of PLCγ1 directly influences the catalytic activity of PDZ-RhoGEF, we tested the possibility that binding of the SH3 domain may interfere with the oligomerization of PDZ-RhoGEF. In coimmunoprecipitation experiments with PDZ-RhoGEF and increasing amounts of the SH3 domain of PLCγ1, we were unable to detect any influence of even a 100-fold molar excess of the SH3 domain on homooligomerization of PDZ-RhoGEF (Fig. 7D).
PLCγ regulates plexin-B1-mediated migration in MCF-7 cells.
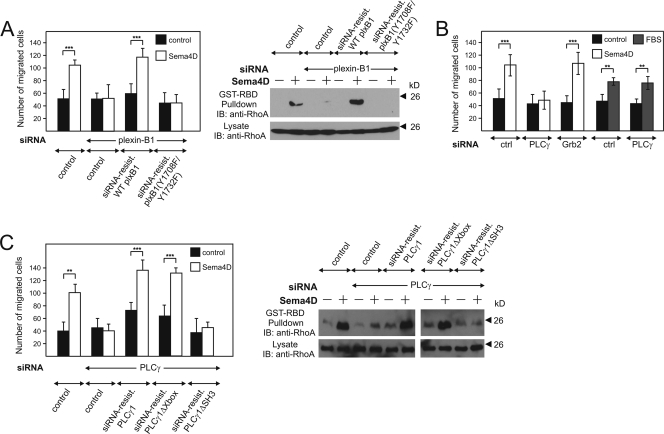
Activation of plexin-B1 by Sema4D has been shown to exert promigratory effects in various cells, including MCF-7 cells (2, 3, 21, 22, 59), a process requiring ErbB-2-dependent activation of RhoA (59). MCF-7 cells were stably transfected with cDNAs encoding wild-type or mutant (Y1708F/Y1732F) plexin-B1 RNAs carrying mutations which made them insensitive to particular siRNAs (see Materials and Methods) (data not shown). While an siRNA-induced knockdown of endogenous plexin-B1 resulted in the inability of MCF-7 cells to respond to Sema4D with migration and RhoA activation, the stable expression of siRNA-insensitive wild-type plexin-B1 fully restored the effect of Sema4D (Fig. 8A). However, cells expressing the siRNA-insensitive Y1708F/Y1732F mutant of plexin-B1 were unresponsive (Fig. 8A), indicating that the phosphorylation of the tyrosine residues 1708 and 1732 is crucial for Sema4D-induced cell migration and RhoA activation.
FIG. 8.
PLCγ mediates plexin-B1-dependent cellular migration. Normal MCF-7 cells or MCF-7 cells stably expressing siRNA-insensitive mutants of wild-type plexin-B1 and PLCγ1 or their indicated mutants (A and C) were transfected with siRNA directed against the indicated targets (PLCγ indicates PLCγ1 plus PLCγ2) and kept at 0.5% FBS. Cells were then incubated in the absence (control) or presence of 150 nM Sema4D or 10% FBS. Sema4D-induced cellular migration (B and left panels of A and C) and RhoA activation (right panels of A and C) were analyzed as described in Materials and Methods. Control cells were stably transfected with the viral vector pLNCX2 alone. Data presented are representative of at least three independently performed experiments. Data of the analysis of migration are shown as means of triplicates (±standard errors of the means). **, P ≤ 0.01; ***, P ≤ 0.001.
Given that these phosphorylated tyrosine residues serve as docking sites for PLCγ, we analyzed the relevance of PLCγ for plexin-B1-mediated regulation of cell migration. Depletion of PLCγ1 and PLCγ2, which are both expressed in MCF-7 cells, abolished Sema4D-stimulated cell migration, whereas the siRNA-mediated knockdown of Grb2 had no effect (Fig. 8B). The ability of cells to migrate was not generally affected by the lack of PLCγ, as indicated by the insensitivity of fetal bovine serum (FBS)-stimulated cell migration to the knockdown of PLCγ (Fig. 8B).
We stably transfected MCF-7 cells with cDNAs encoding wild-type and mutant forms of PLCγ RNAs additionally carrying mutations which made them insensitive to particular siRNAs (see Materials and Methods). The expression of the recombinant proteins and the knockdown efficiency were proven by Western blotting (data not shown). In cells treated with anti-PLCγ siRNA, the expression of siRNA-resistant cDNAs of wild-type and ΔXbox mutant PLCγ1 restored the Sema4D-induced promigratory effect and RhoA activation (Fig. 8C). However, expression of the siRNA-insensitive ΔSH3 mutant of PLCγ1 did not rescue these Sema4D effects (Fig. 8C).
PLCγ is required for plexin-B1-mediated growth cone collapse.
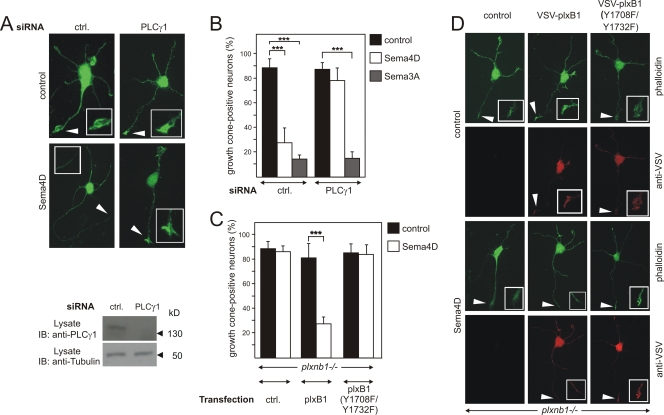
Sema4D has been shown to induce axonal growth cone collapse in hippocampal neurons via plexin-B1 (36, 57). In control siRNA-transfected hippocampal neurons, Sema4D decreased the number of growth cone-positive cells from 85% to 30%; however, siRNA-mediated knockdown of PLCγ1 abolished this effect (Fig. 9A and B). In contrast, knockdown of PLCγ1 had no effects on the ability of Sema3A to promote growth cone collapse of hippocampal neurons (Fig. 9B), indicating that depletion of PLCγ1 did not affect the ability of neurons to respond with growth cone collapse in general.
FIG. 9.
Role of PLCγ1 in plexin-B1-mediated growth cone collapse. Primary E17.5 hippocampal neurons from wild-type (A and B) or plexin-B1-deficient embryos (C and D) were transfected with control (ctrl.) siRNA or siRNA against PLCγ1 (A and B) or with vector alone (ctrl.) or vectors carrying wild-type VSV-plexin-B1 or its mutant, [VSV-plxB1(Y1708F/Y1732F)]. At 48 h later, cells were incubated without (control) or with 150 nM Sema4D or 2 nM Sema3A. Knockdown of PLCγ1 was verified by immunoblotting of cell lysates with an anti-PLCγ1 antibody. Shown are representative hippocampal neurons incubated without or with Sema4D and stained with phalloidin-FITC (A and D) and counterstained with an anti-VSV antibody (D) to identify transfected cells. Insets show a magnified view of axonal ends or growth cones, which are indicated by arrows. (B) Hippocampal neurons were scored for growth cone collapse after treatment with control medium, 150 nM Sema4D, or 2 nM Sema3A. (C) Transfected neurons, identified by immunocytochemistry using anti-VSV antibodies, were scored for growth cone collapse. Shown are mean values of three independent experiments (n = 300; ± standard errors of the means). ***, P ≤ 0.001.
To further analyze the potential role of plexin-B1-phosphorylation of tyrosine residues 1708 and 1732 in Sema4D-induced growth cone collapse, we studied hippocampal neurons isolated from plexin-B1-deficient mice (plxnb1−/−) (13) and transfected them with either VSV-tagged wild-type plexin-B1 or the plexin-B1(Y1708F/Y1732F) mutant. The expression of wild-type or mutated plexin-B1 was visualized by staining with anti-VSV antibodies (Fig. 9D). While plexin-B1-deficient neurons had lost the ability to respond to Sema4D, expression of wild-type plexin-B1 in neurons isolated from plxnb1−/− mice restored Sema4D-induced growth cone collapse (Fig. 9C and D). However, the expression of the Y1708F/Y1732F mutant of plexin-B1 was not able to rescue Sema4D-induced growth cone collapse in plexin-B1-deficient cells (Fig. 9C and D).
DISCUSSION
Plexins of the B-family serve as receptors for particular semaphorins and mediate their cellular effects. During recent years some of their downstream signaling mechanisms have been described. Besides the regulation of the small GTPase R-Ras through an inherent GAP function (36), plexin-B family members mediate the activation of the small GTPase RhoA by direct interaction with the RhoA guanine nucleotide exchange factors PDZ-RhoGEF and LARG (1, 39, 57). Activation of RhoA requires the tyrosine phosphorylation of plexin by ErbB-2 (58). However, the mechanism linking plexin-B1 tyrosine phosphorylation to the activation of RhoA is completely unclear. Here we have shown that upon activation by Sema4D, the intracellular tyrosine residues 1708 and 1732 of plexin-B1 become phosphorylated and serve as docking sites for SH2 domains of PLCγ1 and -2. The recruitment of PLCγ into the plexin-B1 receptor complex is a requirement for plexin-B-mediated RhoA activation, as well as for downstream cellular effects of Sema4D, like plexin-B1-mediated promigratory effects and the Sema4D-induced growth cone collapse of hippocampal neurons.
The tyrosine residue 1708 of plexin-B1 is conserved among all three members of the plexin-B family, and tyrosine 1732 of plexin-B1 is also found in plexin-B2 but not in plexin-B3 (18, 61). Our data show that also plexin-B2 interacts with PLCγ upon activation by its ligand semaphorin 4C. Thus, the observed tyrosine phosphorylation-dependent recruitment of PLCγ is likely to be a common mechanism of plexin-B family members. The phosphorylation of plexin-B1 by c-Met was not affected by the mutations of Y1708 and Y1732, indicating that Erb-2 and c-Met regulate the function of plexin-B1 by phosphorylation of different tyrosine residues of plexin-B1. This is consistent with the observation that the phosphorylation of plexin-B1 by ErbB-2 and c-Met induces different and sometimes opposing downstream signaling and cellular effects (21, 22, 58, 59).
Phosphorylation of tyrosine residues results in the formation of docking sites for SH2 and PTB domain-containing proteins (49). Using a peptide-based as well as several functional approaches, we identified the SH2 domain-containing protein PLCγ as an interaction partner of ErbB-2-phosphorylated plexin-B1. The analysis of the amino acid sequences surrounding tyrosine residues 1708 and 1732 of plexin-B1, GEPLYMLFR and GKAKYTLND, respectively, shows that especially the region surrounding tyrosine 1708 fits the consensus sequence recognized by the SH2 domain of PLCγ1 (pY-hydrophobic-X-hydrophobic) (52, 53). While in most receptor tyrosine kinases able to interact with PLCγ a single phosphorylated tyrosine residue has been shown to mediate interaction with the enzyme (47), at least two autophosphorylated tyrosine residues of the EGF receptor appear to interact with PLCγ1 (9, 51, 66).
The individual role of the two SH2 domains of PLCγ in the interaction of the enzyme with tyrosine phosphorylated proteins has been analyzed for the EGF and platelet-derived growth factor receptors (9, 28, 41). In both cases, a predominant role of the more N-terminally localized SH2 domain was observed. The C-terminally localized SH2 domain has recently been shown to be involved in the activation mechanism of lipase activity by intramolecular interaction with the phosphorylated Y783 residue of PLCγ1 (42). Our data show that efficient binding of PLCγ to activated plexin-B1 requires phosphorylation of both Y1708 and 1732 as well as the presence of both SH2 domains of PLCγ. Since PLCγ does not become tyrosine phosphorylated after recruitment into the plexin receptor complex, the more C-terminal SH2 domain is free to interact with a pY residue of plexin-B1. Interestingly, mutagenesis of individual tyrosine phosphorylation sites of plexin-B1 and of each of the SH2 domains revealed a remarkable specificity in that the N-terminal SH2 domain of PLCγ interacted with pY1708, while the more C-terminally localized SH2 domain preferentially interacted with pY1732. This specificity suggests that the exact orientation of plexin-B1-bound PLCγ is critical for its correct function in plexin-B1-mediated signaling.
Our immunoprecipitation data indicate that phosphorylated plexin-B1 can interact with Grb2, which was also isolated by a peptide containing phosphorylated Y1708. The fact that the siRNA-mediated knockdown of PLCγ1 and PLCγ2 abolished the interaction between plexin-B1 and Grb2 indicates, however, that Grb2 is brought to the plexin-B receptor complex through its interaction with PLCγ rather than through the direct interaction with the phosphorylated receptor. Grb2 could interact with PLCγ directly through an interaction of its SH3 domain with a proline-rich domain of PLCγ, as recently described (6). Alternatively, it is conceivable that Grb2 and PLCγ interact indirectly through a protein able to interact with both Grb2 and PLCγ like LAT (8). Our data showed that knockdown of Grb2 had no effect on Sema4D-induced RhoA activation or promigratory effects. Thus, the potential role of Grb2 in plexin-B-mediated signaling remains unclear.
Two types of mammalian PLCγ are encoded in mammalian genomes, PLCγ1, which is ubiquitously expressed, and PLCγ2, whose expression pattern is more restricted (47). Despite the fact that both PLCγ isoforms are homologous and share the domain organization, studies in cells expressing both isoforms have shown that PLCγ1 and PLCγ2 can have distinct functions (44, 71). Nevertheless, our data show that both enzymes can interact with phosphorylated plexin-B1. According to their expression patterns PLCγ1 may mediate plexin-B1-dependent RhoA activation in the nervous system, whereas PLCγ2 may preferentially be involved in functions of plexin-B1 in the immune system (40).
The classic paradigm of PLCγ activation involves binding of the SH2 domain to activated receptor tyrosine kinases, resulting in the tyrosine phosphorylation of PLCγ and activation of its lipase function (55). Interestingly we did not observe phosphorylation of PLCγ upon treatment with Sema4D, and a mutational analysis revealed that the phospholipase catalytic domain was dispensable for mediation of plexin-B1 signaling. However, Sema4D-induced, plexin-B1-mediated effects required the presence of the SH3 domain of the PLCγ enzyme. There is evidence for PLCγ functions independent of its lipase activity (38, 50), and the SH3 domain of PLCγ1 in particular has been shown to be necessary for downstream cellular effects of PLCγ1, like the mitogenic response (27). The SH3 domain of PLCγ binds to multiple proteins, including SLP-76, tau, and Akt (46, 69, 72), and it can act as a guanine nucleotide exchange factor for the GTPases phosphatidylinositol-3-OH kinase enhancer and dynamin-1 (11, 73). Since PLCγ and PDZ-RhoGEF interacted through the PLCγ SH3 domain and the C-terminal proline-rich region of the RhoGEF protein and since the SH3 domain of PLCγ alone was able to activate PDZ-RhoGEF but not a PDZ-RhoGEF version with a mutated proline-rich region 1332 to 1338, we think that binding of the PLCγ SH3 domain to the proline-rich region of PDZ-RhoGEF mediates RhoA activation once PLCγ has been recruited to the plexin-B1/RhoGEF receptor complex. Thus, our data identify a novel lipase-independent function of PLCγ involving its SH3 domain.
It has been shown that PDZ-RhoGEF and its homologue, LARG, oligomerize via a mechanism involving C-terminal parts of the RhoGEF proteins (10). Removal of a large C-terminal region of PDZ-RhoGEF results in constitutive RhoGEF activation, suggesting that the C terminus contains sequences which are involved in the inhibition of basal GEF activity. (10) Activation of PDZ-RhoGEF has been reported to also require the release of an autoinhibitory, intramolecular interaction involving the RGS domain and another region upstream of the DH domain of PDZ-RhoGEF and the catalytic surface of the DH domain (70, 75). However, the exact mechanism of PDZ-RhoGEF activation remains unclear (20, 54). Our data further support the regulatory role of the C-terminal region of PDZ-RhoGEF. Although we were able to rule out the influence of the SH3 domain of PLCγ1 on homooligomerization of PDZ-RhoGEF, it is still possible that binding of the PLCγ SH3 domain to the C-terminal proline-rich region of PDZ-RhoGEF releases an inhibitory mechanism involving intra- or intermolecular interactions within the PDZ-RhoGEF oligomer, which then results in RhoGEF activation. One of the well-established functions of plexin-B1 is its role in regulating growth cone morphology in neurons. Both RhoA activation downstream of plexin-B1 and GAP activity toward R-Ras have been reported to be involved in plexin-B1-mediated axonal growth cone collapse (36, 57). Our data show that knockdown of PLCγ1 abolishes the ability of Sema4D to induce growth cone collapse and that the expression of an ErbB-2 phosphorylation-resistant plexin-B1 mutant that retains its full R-Ras GAP activity does not rescue the Sema4D-induced growth cone collapse of hippocampal neurons isolated from plexin-B1-deficient mice. This further supports the concept that plexin-B1-mediated neuronal growth cone collapse requires signaling through R-Ras as well as RhoA and that the activation of RhoA depends on the recruitment of PLCγ1 to ErbB-2-tyrosine-phosphorylated plexin-B1.
In summary, we have shown here that the activation of the plexin-B1/ErbB-2 receptor complex results in the phosphorylation of specific tyrosine residues of plexin-B1, thereby providing docking sites for the SH2-domains of PLCγ. After recruitment of PLCγ into the plexin-B1 receptor complex, PLCγ via its SH3 domain mediates the activation of PDZ-RhoGEF, which interacts constitutively with the C terminus of plexin-B1. This PLCγ-dependent signaling mechanism is required for Sema4D-induced, plexin-B1-mediated regulation of cell migration and neuronal growth cone morphology. Interestingly, the lipase activity of PLCγ is dispensable for its function in plexin-B-mediated signaling. These data identify an important new component of plexin-B-mediated signaling and reveal a novel function of PLCγ, a versatile protein acting here as a regulator of RhoGEF proteins in plexin-B-mediated signaling.
Acknowledgments
We thank Evelyne Jones (London) for kindly providing Lec3.2.8.1 CHO cells expressing part of human Sema4D and Peter Gierschik (Ulm) for kindly providing (Myc)-PLCγ1 cDNA. We thank Martina Kurejova for support, H.-P. Gensheimer for technical assistance, and R. LeFaucheur for help with the preparation of the manuscript.
J. Swiercz and T. Worzfeld were recipients of a postdoctoral scholarship of the Medical Faculty Heidelberg.
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Aurandt, J., H. G. Vikis, J. S. Gutkind, N. Ahn, and K. L. Guan. 2002. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc. Natl. Acad. Sci. USA 99:12085-12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile, J. R., T. Afkhami, and J. S. Gutkind. 2005. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol. Cell. Biol. 25:6889-6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basile, J. R., A. Barac, T. Zhu, K. L. Guan, and J. S. Gutkind. 2004. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 64:5212-5224. [DOI] [PubMed] [Google Scholar]
- 4.Basile, J. R., R. M. Castilho, V. P. Williams, and J. S. Gutkind. 2006. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc. Natl. Acad. Sci. USA 103:9017-9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielenberg, D. R., and M. Klagsbrun. 2007. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 26:421-431. [DOI] [PubMed] [Google Scholar]
- 6.Browaeys-Poly, E., I. Broutin, A. F. Antoine, M. Marin, A. Lescuyer, J. P. Vilain, A. Ducruix, and K. Cailliau. 2007. A non-canonical Grb2-PLC-gamma1-Sos cascade triggered by lipovitellin 1, an apolipoprotein B homologue. Cell Signal. 19:2540-2548. [DOI] [PubMed] [Google Scholar]
- 7.Bussolino, F., D. Valdembri, F. Caccavari, and G. Serini. 2006. Semaphoring vascular morphogenesis. Endothelium 13:81-91. [DOI] [PubMed] [Google Scholar]
- 8.Cantrell, D. 1998. The real LAT steps forward. Trends Cell Biol. 8:180-182. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay, A., M. Vecchi, Q. Ji, R. Mernaugh, and G. Carpenter. 1999. The role of individual SH2 domains in mediating association of phospholipase C-γ1 with the activated EGF receptor. J. Biol. Chem. 274:26091-26097. [DOI] [PubMed] [Google Scholar]
- 10.Chikumi, H., A. Barac, B. Behbahani, Y. Gao, H. Teramoto, Y. Zheng, and J. S. Gutkind. 2004. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene 23:233-240. [DOI] [PubMed] [Google Scholar]
- 11.Choi, J. H., J. B. Park, S. S. Bae, S. Yun, H. S. Kim, W. P. Hong, I. S. Kim, J. H. Kim, M. Y. Han, S. H. Ryu, R. L. Patterson, S. H. Snyder, and P. G. Suh. 2004. Phospholipase C-γ1 is a guanine nucleotide exchange factor for dynamin-1 and enhances dynamin-1-dependent epidermal growth factor receptor endocytosis. J. Cell Sci. 117:3785-3795. [DOI] [PubMed] [Google Scholar]
- 12.Conrotto, P., S. Corso, S. Gamberini, P. M. Comoglio, and S. Giordano. 2004. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene 23:5131-5137. [DOI] [PubMed] [Google Scholar]
- 13.Deng, S., A. Hirschberg, T. Worzfeld, J. Y. Penachioni, A. Korostylev, J. M. Swiercz, P. Vodrazka, O. Mauti, E. T. Stoeckli, L. Tamagnone, S. Offermanns, and R. Kuner. 2007. Plexin-B2, but not plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo. J. Neurosci. 27:6333-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driessens, M. H., H. Hu, C. D. Nobes, A. Self, I. Jordens, C. S. Goodman, and A. Hall. 2001. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr. Biol. 11:339-344. [DOI] [PubMed] [Google Scholar]
- 15.Driessens, M. H., C. Olivo, K. Nagata, M. Inagaki, and J. G. Collard. 2002. B plexins activate Rho through PDZ-RhoGEF. FEBS Lett. 529:168-172. [DOI] [PubMed] [Google Scholar]
- 16.Faltus, T., J. Yuan, B. Zimmer, A. Kramer, S. Loibl, M. Kaufmann, and K. Strebhardt. 2004. Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia 6:786-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraro, E., D. Peluso, A. Via, G. Ausiello, and M. Helmer-Citterich. 2007. SH3-Hunter: discovery of SH3 domain interaction sites in proteins. Nucleic Acids Res. 35:W451-W454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco, M., and L. Tamagnone. 2008. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 9:865-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujisawa, H. 2004. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J. Neurobiol. 59:24-33. [DOI] [PubMed] [Google Scholar]
- 20.Fukuhara, S., H. Chikumi, and J. S. Gutkind. 2001. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene 20:1661-1668. [DOI] [PubMed] [Google Scholar]
- 21.Giacobini, P., A. Messina, F. Morello, N. Ferraris, S. Corso, J. Penachioni, S. Giordano, L. Tamagnone, and A. Fasolo. 2008. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. J. Cell Biol. 183:555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giordano, S., S. Corso, P. Conrotto, S. Artigiani, G. Gilestro, D. Barberis, L. Tamagnone, and P. M. Comoglio. 2002. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat. Cell Biol. 4:720-724. [DOI] [PubMed] [Google Scholar]
- 23.Gitler, A. D., M. M. Lu, and J. A. Epstein. 2004. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev. Cell 7:107-116. [DOI] [PubMed] [Google Scholar]
- 24.Hill, C. S., J. Wynne, and R. Treisman. 1995. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 81:1159-1170. [DOI] [PubMed] [Google Scholar]
- 25.Hill, C. S., J. Wynne, and R. Treisman. 1994. Serum-regulated transcription by serum response factor (SRF): a novel role for the DNA binding domain. EMBO J. 13:5421-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirotani, M., Y. Ohoka, T. Yamamoto, H. Nirasawa, T. Furuyama, M. Kogo, T. Matsuya, and S. Inagaki. 2002. Interaction of plexin-B1 with PDZ domain-containing Rho guanine nucleotide exchange factors. Biochem. Biophys. Res. Commun. 297:32-37. [DOI] [PubMed] [Google Scholar]
- 27.Huang, P. S., L. Davis, H. Huber, P. J. Goodhart, R. E. Wegrzyn, A. Oliff, and D. C. Heimbrook. 1995. An SH3 domain is required for the mitogenic activity of microinjected phospholipase C-gamma 1. FEBS Lett. 358:287-292. [DOI] [PubMed] [Google Scholar]
- 28.Ji, Q. S., A. Chattopadhyay, M. Vecchi, and G. Carpenter. 1999. Physiological requirement for both SH2 domains for phospholipase C-γ1 function and interaction with platelet-derived growth factor receptors. Mol. Cell. Biol. 19:4961-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitzing, T. M., A. S. Sahadevan, D. T. Brandt, H. Knieling, S. Hannemann, O. T. Fackler, J. Grosshans, and R. Grosse. 2007. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 21:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korostylev, A., T. Worzfeld, S. Deng, R. H. Friedel, J. M. Swiercz, P. Vodrazka, V. Maier, A. Hirschberg, Y. Ohoka, S. Inagaki, S. Offermanns, and R. Kuner. 2008. A functional role for semaphorin 4D/plexin B1 interactions in epithelial branching morphogenesis during organogenesis. Development 135:3333-3343. [DOI] [PubMed] [Google Scholar]
- 31.Kruger, R. P., J. Aurandt, and K. L. Guan. 2005. Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 6:789-800. [DOI] [PubMed] [Google Scholar]
- 32.Love, C. A., K. Harlos, N. Mavaddat, S. J. Davis, D. I. Stuart, E. Y. Jones, and R. M. Esnouf. 2003. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat. Struct. Biol. 10:843-848. [DOI] [PubMed] [Google Scholar]
- 33.Maeda, A., K. Nakashiro, S. Hara, T. Sasaki, Y. Miwa, N. Tanji, M. Yokoyama, H. Hamakawa, and R. Oyasu. 2006. Inactivation of AR activates HGF/c-Met system in human prostatic carcinoma cells. Biochem. Biophys. Res. Commun. 347:1158-1165. [DOI] [PubMed] [Google Scholar]
- 34.Negishi, M., I. Oinuma, and H. Katoh. 2005. R-ras as a key player for signaling pathway of plexins. Mol. Neurobiol. 32:217-222. [DOI] [PubMed] [Google Scholar]
- 35.Neufeld, G., and O. Kessler. 2008. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat. Rev. Cancer 8:632-645. [DOI] [PubMed] [Google Scholar]
- 36.Oinuma, I., Y. Ishikawa, H. Katoh, and M. Negishi. 2004. The semaphorin 4D receptor plexin-B1 is a GTPase activating protein for R-Ras. Science 305:862-865. [DOI] [PubMed] [Google Scholar]
- 37.Oinuma, I., H. Katoh, A. Harada, and M. Negishi. 2003. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by plexin-B1 and induces cell contraction in COS-7 cells. J. Biol. Chem. 278:25671-25677. [DOI] [PubMed] [Google Scholar]
- 38.Patterson, R. L., D. B. van Rossum, N. Nikolaidis, D. L. Gill, and S. H. Snyder. 2005. Phospholipase C-gamma: diverse roles in receptor-mediated calcium signaling. Trends Biochem. Sci. 30:688-697. [DOI] [PubMed] [Google Scholar]
- 39.Perrot, V., J. Vazquez-Prado, and J. S. Gutkind. 2002. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J. Biol. Chem. 277:43115-43120. [DOI] [PubMed] [Google Scholar]
- 40.Potiron, V., P. Nasarre, J. Roche, C. Healy, and L. Boumsell. 2007. Semaphorin signaling in the immune system. Adv. Exp. Med. Biol. 600:132-144. [DOI] [PubMed] [Google Scholar]
- 41.Poulin, B., F. Sekiya, and S. G. Rhee. 2000. Differential roles of the Src homology 2 domains of phospholipase C-γ1 (PLC-γ1) in platelet-derived growth factor-induced activation of PLC-γ1 in intact cells. J. Biol. Chem. 275:6411-6416. [DOI] [PubMed] [Google Scholar]
- 42.Poulin, B., F. Sekiya, and S. G. Rhee. 2005. Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-γ1. Proc. Natl. Acad. Sci. USA 102:4276-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Püschel, A. W. 2007. GTPases in semaphorin signaling. Adv. Exp. Med. Biol. 600:12-23. [DOI] [PubMed] [Google Scholar]
- 44.Regunathan, J., Y. Chen, S. Kutlesa, X. Dai, L. Bai, R. Wen, D. Wang, and S. Malarkannan. 2006. Differential and nonredundant roles of phospholipase Cγ2 and phospholipase Cγ1 in the terminal maturation of NK cells. J. Immunol. 177:5365-5376. [DOI] [PubMed] [Google Scholar]
- 45.Ren, X. D., and M. A. Schwartz. 2000. Determination of GTP loading on Rho. Methods Enzymol. 325:264-272. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds, C. H., C. J. Garwood, S. Wray, C. Price, S. Kellie, T. Perera, M. Zvelebil, A. Yang, P. W. Sheppard, I. M. Varndell, D. P. Hanger, and B. H. Anderton. 2008. Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cγ1, Grb2, and Src family kinases. J. Biol. Chem. 283:18177-18186. [DOI] [PubMed] [Google Scholar]
- 47.Rhee, S. G. 2001. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70:281-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohm, B., B. Rahim, B. Kleiber, I. Hovatta, and A. W. Puschel. 2000. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett. 486:68-72. [DOI] [PubMed] [Google Scholar]
- 49.Schlessinger, J., and M. A. Lemmon. 2003. SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 2003:RE12. [DOI] [PubMed] [Google Scholar]
- 50.Smith, M. R., Y. L. Liu, N. T. Matthews, S. G. Rhee, W. K. Sung, and H. F. Kung. 1994. Phospholipase C-gamma 1 can induce DNA synthesis by a mechanism independent of its lipase activity. Proc. Natl. Acad. Sci. USA 91:6554-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soler, C., C. V. Alvarez, L. Beguinot, and G. Carpenter. 1994. Potent SHC tyrosine phosphorylation by epidermal growth factor at low receptor density or in the absence of receptor autophosphorylation sites. Oncogene 9:2207-2215. [PubMed] [Google Scholar]
- 52.Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson, W. G. Haser, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, et al. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72:767-778. [DOI] [PubMed] [Google Scholar]
- 53.Songyang, Z., S. E. Shoelson, J. McGlade, P. Olivier, T. Pawson, X. R. Bustelo, M. Barbacid, H. Sabe, H. Hanafusa, T. Yi, et al. 1994. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol. Cell. Biol. 14:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sternweis, P. C., A. M. Carter, Z. Chen, S. M. Danesh, Y. F. Hsiung, and W. D. Singer. 2007. Regulation of Rho guanine nucleotide exchange factors by G proteins. Adv. Protein Chem. 74:189-228. [DOI] [PubMed] [Google Scholar]
- 55.Suh, P. G., J. I. Park, L. Manzoli, L. Cocco, J. C. Peak, M. Katan, K. Fukami, T. Kataoka, S. Yun, and S. H. Ryu. 2008. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 41:415-434. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, K., A. Kumanogoh, and H. Kikutani. 2008. Semaphorins and their receptors in immune cell interactions. Nat. Immunol. 9:17-23. [DOI] [PubMed] [Google Scholar]
- 57.Swiercz, J. M., R. Kuner, J. Behrens, and S. Offermanns. 2002. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 35:51-63. [DOI] [PubMed] [Google Scholar]
- 58.Swiercz, J. M., R. Kuner, and S. Offermanns. 2004. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J. Cell Biol. 165:869-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swiercz, J. M., T. Worzfeld, and S. Offermanns. 2008. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J. Biol. Chem. 283:1893-1901. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi, T., A. Fournier, F. Nakamura, L. H. Wang, Y. Murakami, R. G. Kalb, H. Fujisawa, and S. M. Strittmatter. 1999. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99:59-69. [DOI] [PubMed] [Google Scholar]
- 61.Tamagnone, L., S. Artigiani, H. Chen, Z. He, G. I. Ming, H. Song, A. Chedotal, M. L. Winberg, C. S. Goodman, M. Poo, M. Tessier-Lavigne, and P. M. Comoglio. 1999. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99:71-80. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka, M., R. Gupta, and B. J. Mayer. 1995. Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol. Cell. Biol. 15:6829-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong, Y., P. Chugha, P. K. Hota, R. S. Alviani, M. Li, W. Tempel, L. Shen, H. W. Park, and M. Buck. 2007. Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. J. Biol. Chem. 282:37215-37224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tran, T. S., A. L. Kolodkin, and R. Bharadwaj. 2007. Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 23:263-292. [DOI] [PubMed] [Google Scholar]
- 65.van Triest, M., and J. L. Bos. 2004. Pull-down assays for guanoside 5′-triphosphate-bound Ras-like guanosine 5′-triphosphatases. Methods Mol. Biol. 250:97-102. [DOI] [PubMed] [Google Scholar]
- 66.Vega, Q. C., C. Cochet, O. Filhol, C. P. Chang, S. G. Rhee, and G. N. Gill. 1992. A site of tyrosine phosphorylation in the C terminus of the epidermal growth factor receptor is required to activate phospholipase C. Mol. Cell. Biol. 12:128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vikis, H. G., W. Li, and K. L. Guan. 2002. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 16:836-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vikis, H. G., W. Li, Z. He, and K. L. Guan. 2000. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc. Natl. Acad. Sci. USA 97:12457-12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang, Y., J. Wu, and Z. Wang. 2006. Akt binds to and phosphorylates phospholipase C-gγ1 in response to epidermal growth factor. Mol. Biol. Cell 17:2267-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wells, C. D., M. Y. Liu, M. Jackson, S. Gutowski, P. M. Sternweis, J. D. Rothstein, T. Kozasa, and P. C. Sternweis. 2002. Mechanisms for reversible regulation between G13 and Rho exchange factors. J. Biol. Chem. 277:1174-1181. [DOI] [PubMed] [Google Scholar]
- 71.Wilde, J. I., and S. P. Watson. 2001. Regulation of phospholipase C gamma isoforms in haematopoietic cells: why one, not the other? Cell Signal. 13:691-701. [DOI] [PubMed] [Google Scholar]
- 72.Yablonski, D., T. Kadlecek, and A. Weiss. 2001. Identification of a phospholipase C-γ1 (PLC-γ1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-γ1 and NFAT. Mol. Cell. Biol. 21:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye, K., B. Aghdasi, H. R. Luo, J. L. Moriarity, F. Y. Wu, J. J. Hong, K. J. Hurt, S. S. Bae, P. G. Suh, and S. H. Snyder. 2002. Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415:541-544. [DOI] [PubMed] [Google Scholar]
- 74.Zanata, S. M., I. Hovatta, B. Rohm, and A. W. Puschel. 2002. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J. Neurosci. 22:471-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng, M., T. Cierpicki, K. Momotani, M. V. Artamonov, U. Derewenda, J. H. Bushweller, A. V. Somlyo, and Z. S. Derewenda. 2009. On the mechanism of autoinhibition of the RhoA-specific nucleotide exchange factor PDZRhoGEF. BMC Struct. Biol. 9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou, Y., R. A. Gunput, and R. J. Pasterkamp. 2008. Semaphorin signaling: progress made and promises ahead. Trends Biochem. Sci. 33:161-170. [DOI] [PubMed] [Google Scholar]