Abstract
The host innate immune response provides a critical first line of defense against invading pathogens, inducing an antiviral state to impede the spread of infection. While numerous studies have documented antiviral responses within actively infected tissues, few have described the earliest innate response induced systemically by infection. Here, utilizing Venezuelan equine encephalitis virus (VEE) replicon particles (VRP) to limit infection to the initially infected cells in vivo, a rapid activation of the antiviral response was demonstrated not only within the murine draining lymph node, where replication was confined, but also within distal tissues. In the liver and brain, expression of interferon-stimulated genes was detected by 1 to 3 h following VRP footpad inoculation, reaching peak expression of >100-fold over that in mock-infected animals. Moreover, mice receiving a VRP footpad inoculation 6, 12, or 24 h prior to an otherwise lethal VEE footpad challenge were completely protected from death, including a drastic reduction in challenge virus titers. VRP pretreatment also provided protection from intranasal VEE challenge and extended the average survival time following intracranial challenge. Signaling through the interferon receptor was necessary for antiviral gene induction and protection from VEE challenge. However, VRP pretreatment failed to protect mice from a heterologous, lethal challenge with vesicular stomatitis virus, yet conferred protection following challenge with influenza virus. Collectively, these results document a rapid modulation of the host innate response within hours of infection, capable of rapidly alerting the entire animal to pathogen invasion and leading to protection from viral disease.
Venezuelan equine encephalitis virus (VEE) is an arthropod-borne, single-stranded, message-sense RNA virus belonging to the Alphavirus genus and Togaviridae family. Associated with periodic epidemics and equine epizootics in the Western Hemisphere, VEE also serves as a leading model for the study of alphavirus pathogenesis in vivo. In the murine model, which closely mimics infection of horses in nature, VEE causes a two-phase disease: an initial, acute lymphotropic phase characterized by a high serum viremia, followed by invasion of the central nervous system during a neurotropic phase that leads to fatal encephalitis (22, 27). Using the infectious molecular clone of VEE and an extensive panel of mutants blocked at various stages of infection, the course of infection and disease in the mouse model has been well characterized (3, 14, 15, 17, 27).
Studies examining the molecular aspects of VEE pathogenesis have underscored the critical role of virus genetics and the subsequent host response in dictating the course and outcome of infection (6, 12, 23, 27, 35, 60, 64, 73). However, many details of the earliest host-pathogen interactions during VEE infection remain largely unknown. A tool paramount to studying early events in infection are VEE replicon particles (VRP). VRP are propagation-defective particles that undergo only one round of infection, as the structural genes which normally drive the assembly of progeny virions are deleted from the replicon genome (51). Infection of cells by VRP results in amplification of replicon viral RNA, but there is no packaging of new progeny and thus no further spread to other cells. As such, VRP infection is limited to the first round of targeted cells, allowing examination of the earliest interactions between virus and host.
VRP infection of mice facilitated the identification of the draining lymph node (DLN) as the initial site of VEE viral amplification in vivo (44). Following footpad inoculation of mice with VRP, resident dendritic cells in the skin serve as the cellular target for infection. These infected dendritic cells then rapidly migrate from the site of inoculation in the skin to the local DLN (44). In the case of VRP infection, while no new viral progeny are packaged or released, the replicon genome continues to be replicated within these initially infected skin dendritic cells that have migrated and reside in the DLN. However, during infection with VEE virus, new viral progeny are eventually released into the DLN environment and infection spreads to adjacent cells.
Based on these observations, we hypothesize that the earliest host-pathogen interactions within the DLN set the stage for the specific course of events that define VEE-induced pathogenesis. The innate immune response, including interferon (IFN) signaling, has been extensively documented as a critical component of controlling viral infection and spread (45, 47, 62, 66). In fact, utilizing a VRP-based mRNP-tagging system in vivo, we recently reported the robust activation of the host innate antiviral response directly within the infected cells of the DLN, as well as in surrounding uninfected bystander cells, at early times postinoculation (39). A consequence of this early, robust innate immune response at the initial site of replication is likely a contemporaneous induction of an antiviral state in tissues distal to the primary infection.
We postulated that if early viral replication in the DLN induces the production of soluble immune mediators, such as IFN-α/β, then the induction of innate immune responses may be rapidly transmitted downstream from this primary site to distal tissues. Utilizing VRP to limit viral spread, we examined the host antiviral response within the DLN and tissues remote from the site of replication at early times following infection. In the liver and brain, the robust expression of a panel of IFN-stimulated genes, a hallmark of the antiviral state, was detected by 1 to 3 h following VRP footpad inoculation and peaked at expression levels >100-fold over mock animals. These results suggest that the early innate response to VRP infection is capable of rapidly inducing a systemically active antiviral state within the entire infected animal. Moreover, we found that mice pretreated by footpad inoculation with VRP for 6, 12, or 24 h were protected from an otherwise lethal VEE footpad or intranasal challenge, and the average survival time of mice challenged intracranially with VEE was significantly extended.
Protection from VEE infection has typically been associated with the presence of neutralizing antibody (11, 24, 29, 49, 55). However, nonspecific protection against VEE has been suggested, including the involvement of the innate immune response (10, 26, 28, 33, 61, 73). In one instance, mice “vaccinated” with an attenuated clone of VEE were protected against lethal VEE challenge administered just 24 h after vaccination (26). In separate studies, the complete attenuation of a VEE mutant harboring a single noncoding nucleotide change was attributed to a heightened sensitivity of the virus to the host antiviral state (73). Additionally, mice with severe combined immunodeficiency survive longer than immunocompetent mice (9 days as opposed to 6 days) following infection with virulent VEE (12). These findings firmly indicate that the nonspecific host response to VEE is a critical component of controlling the earliest stages of infection.
While IFN and the IFN-induced antiviral state are undoubtedly key mediators of the initial response to VRP infection in vivo, they may not solely be responsible for a rapidly induced protective state. In the challenge model presented here, VRP pretreatment was unable to protect mice from death following heterologous challenge with another IFN-sensitive virus, vesicular stomatitis virus (VSV). However, VRP pretreatment successfully protected mice from lethal challenge with influenza virus. Collectively, our results raise at least three important implications. First, the innate host response is rapidly mobilized following infection with VRP/VEE, at areas both proximal and distal to the site of active replication. Second, there exist components of the innate immune response to VEE that remain uncharacterized. Third, viruses are specifically and differentially sensitive to unique innate immune response profiles. These data provide new insight into the rapid mobilization of the host response to viral infection and present an effective pretreatment/challenge model to further investigate specific components of the innate response critical to protection against infectious pathogens.
MATERIALS AND METHODS
Virus and replicon particles.
The construction of the full-length VEE cDNA clone pV3000, derived from the Trinidad donkey isolate of VEE, has been described previously (15, 17, 27). Virus stocks of wild-type virulent VEE (V3000) were produced by electroporating infectious RNA into BHK-21 cells (12, 27). Virus particles were harvested from the supernatant at 24 h postinfection, when significant cytopathic effect was evident, and were clarified by centrifugation, and virus stocks were further concentrated by being pelleted through 20% (wt/vol) sucrose in low-endotoxin phosphate-buffered saline (PBS). Virus titers were determined by standard plaque assay on BHK-21 cells, and stocks were stored in single-use aliquots at −70°C.
The construction and packaging of VRP using a split helper system have been described previously (51). Replicon plasmid constructs that do not encode any functional transgene sequence downstream of the 26S promoter were utilized throughout this study. The resulting particles, termed “null VRP,” contain the VEE nonstructural genes, 14 nucleotides (nt) of VEE sequence downstream of the 26S mRNA transcription start site, and the 43-nt multiple cloning site (69). The null VRP genome also includes the authentic viral 5′ and 3′ untranslated regions. All replicon particles used in this study were packaged in the wild-type (V3000) VEE envelope, with the absence of propagating recombinant virus confirmed by blind passage in BHK-21 cells, as described previously (51). BHK-21 titers were determined by immunocytochemistry using mouse sera containing antibody to the VEE nonstructural proteins.
For VSV challenge experiments, the Orsay strain (Indiana-1 serotype) of VSV was the generous gift of Douglas Lyles and John Connor (Wake Forest University, Winston-Salem, NC). Strain A/PR/8/34 was used for influenza virus challenge experiments.
Cells.
BHK-21 cells (ATCC CCL-10) and murine L929 fibroblasts (ATCC CCL-1) were maintained in alpha-minimum essential medium (Gibco) supplemented with 10% donor calf serum, 10% tryptose phosphate broth, 0.29 mg of l-glutamine/ml, 100 U of penicillin/ml, and 0.05 mg of streptomycin/ml (37°C, 5% CO2).
Mice.
Specific-pathogen-free BALB/c mice were obtained commercially (Charles River Laboratories). Unless otherwise noted, 10- to 12-week-old female BALB/c mice were utilized. Breeding pairs of 129Sv/Ev mice deficient for the IFN-α/β receptor (IFN-αβR−/− mice) were kindly provided by Barbara Sherry (North Carolina State University, Raleigh). Mice were bred under specific-pathogen-free conditions in the Department of Laboratory Animal Medicine breeding colony facilities at the University of North Carolina, Chapel Hill (UNC-CH). Age-matched wild-type 129Sv/Ev (129S6/SvEvTac) mice were obtained commercially from Taconic (Germantown, NY). Animal housing and care were in accordance with all UNC-CH Institutional Animal Care and Use Committee (IACUC) guidelines. All mouse studies were performed in an environmentally controlled room in a biosafety level 3 facility, allowing mice to acclimate for 3 to 5 days in the facility before experimental manipulation.
Mice pretreated with VRP were inoculated in the right rear footpad (RRFP) with 2 × 106 infectious units (IU) of null VRP (unless otherwise stated) in 10 μl of low-endotoxin PBS diluent containing 1% donor calf serum. Mock-pretreated animals received diluent alone. When footpad was the route of challenge, mice received 10 PFU of wild-type virulent VEE (V3000) inoculated in the opposing left rear footpad (LRFP) (10-μl total volume). Mice challenged intranasally with VEE received a dose of 103 PFU of wild-type VEE (V3000) in a 10-μl volume, with 5 μl administered into each nare. Mice challenged intracranially were anesthetized with isoflurane and inoculated with 103 PFU of wild-type virulent VEE in 10 μl diluent.
Mice challenged intranasally with VSV received a dose of 2 × 106 PFU of VSV diluted in 20 μl RPMI medium (Gibco), with 10 μl administered into each nare. Male BALB/c mice (5 to 7 weeks of age) were utilized for VSV challenge experiments to adhere to established VSV intranasal challenge protocols and avoid the reported natural resistance to lethal VSV infection in female mice (4, 20, 31, 32, 53). Mice challenged intranasally with influenza virus received a dose of 12 PFU/mouse, delivering 25 μl per nostril.
For morbidity and mortality studies, mice were monitored for clinical signs of disease and weighed every 24 h for 14 to 18 days. Morbidity was defined as greater than 10% weight loss and/or signs of clinical disease for 2 or more consecutive days. Clinical signs of disease included ruffled fur, hunching, ataxia, paresis (dragging of hind limb), paralysis (complete loss of hind limb function), and/or a moribund state. In the interest of animal welfare and in accordance with UNC-CH IACUC guidelines, mice experiencing a loss in weight of more than 20% of starting weight while showing clinical signs of disease were euthanized. To determine viral titers in the serum and brain, mice were euthanized by anesthesia overdose, followed by cardiac puncture and exsanguination to collect blood samples. Serum was separated in Microtainer tubes, aliquoted, and stored at −70°C. Each animal was perfused with 1× PBS, and the brain was removed by dissection, weighed, and stored at −80°C in a 20% (wt/vol) suspension of 1× PBS containing 1% donor calf serum, 110 mM Ca2+, and 50 mM Mg2+. After one freeze-thaw cycle, the samples were homogenized and clarified by centrifugation, and viral titers were assessed by standard BHK-21 plaque assay.
RNA isolation and cDNA synthesis.
At the indicated times postinfection, mice were euthanized and perfused with PBS. The draining popliteal lymph node, liver, and brain were dissected and stored at −80°C in RNAlater (RNA stabilization reagent; Ambion). According to the manufacturer's protocol, tissue homogenates were prepared using a plastic pestle and handheld motor, as well as passage through an 18-gauge needle. Total cellular RNA was isolated using the RNeasy Protect kit (Qiagen).
A one-tube DNase treatment and reverse transcription protocol was used to generate cDNA, using the SuperScript III reverse transcriptase first-strand cDNA kit (Invitrogen). Total RNA (0.75 to 1.0 μg in 10 μl) was combined with 1 μl 10 mM deoxynucleoside triphosphate (dNTP) mix (Amersham Biosciences), 4 μl 5× SuperScript III reverse transcriptase buffer, 1 μl 0.1 mM dithiothreitol, 1 μl 40 U/μl RNaseOUT (Invitrogen), and 1 μl RQ1 RNase-free DNase (Promega). The samples were DNase treated at 37°C for 30 min, followed by the addition of RQ1 stop solution (Promega) and heat inactivation of the samples. Following the addition of random hexamer primers (150 ng; Invitrogen), reverse transcription of the samples was continued in the same tube, according to the SuperScript III protocol (Invitrogen).
Real-time PCR and analysis.
Real-time PCR was performed to determine the relative abundance of specific cellular mRNAs in tissues isolated from mock- and VRP-infected animals (3 mice per group). TaqMan gene expression primer probe sets (Applied Biosystems) for various target host messages were used, with each reaction performed in a 25-μl total volume, including 5 μl cDNA. For all samples, an equivalent amount of RNA was reverse transcribed and an internal reference control of 18S rRNA was included. The default amplification profile was performed in the ABI Prism 7000 real-time PCR system. Using 7000 sequence detection software (v1.2.3, Applied Biosystems), the results were converted into cycle threshold (CT) values corresponding to the cycle number at which the fluorescence of the PCR product reached significant levels above the background level. Results are presented as gene expression (fold) in the infected samples over that in the mock samples, analyzed using the well-established 2−ΔΔCT method (ABI Prism 7000 sequence detection system user bulletin; Applied Biosystems).
IFN bioassay.
The levels of type I IFN present in the serum of infected mice were measured by a standard biological assay on L929 cells, as described previously (63, 73). Briefly, serum samples were diluted 1:10 in medium and acidified to a pH of 2.0 for 24 h. Following neutralization to pH 7.4, the samples were titrated by twofold dilutions and added to confluent monolayers of L929 murine fibroblasts. Twenty-four hours after the addition of the serum, IFN-sensitive encephalomyocarditis virus (2 × 105 PFU) was added to each well and incubated at 37°C. At 18 to 24 h postinfection, 3-[4,5-dimethylthylthiazol-2yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma), an indicator of viable cells, was added to each well. Absorbance was read on a microplate reader at 570 nm. Each plate contained twofold dilutions of an IFN standard (Chemicon or R&D Systems), ranging from 500 to 0.49 IU/ml. The concentration of type I IFN in each serum sample was based on the standard curves generated with the IFN standard. End-point titers were measured as the dilution at which an optical density reading of 0.5 was reached, corresponding to ∼50% protection of the cell monolayer from encephalomyocarditis virus-induced cell death.
RESULTS
VRP infection rapidly induces a robust antiviral state at the initial site of replication in vivo, as well as in remote downstream tissues.
Several studies have highlighted the importance of the DLN during VEE infection (26, 27, 36, 39, 44, 68, 69, 73). Following footpad inoculation, Langerhans cells at the site of inoculation are the initial cells infected, rapidly migrating to the lymph node draining the injection site. As such, it is the DLN that serves as the earliest site for viral replication, not the tissue surrounding the inoculation site (40, 44). Consequently, the earliest host response to VEE infection is established within the DLN, as demonstrated by the robust induction of the host antiviral gene response within the infected cells of the DLN (26, 39). Accordingly, it has been hypothesized that early events within the DLN set the stage for a specific pattern of virus replication and subsequent host response.
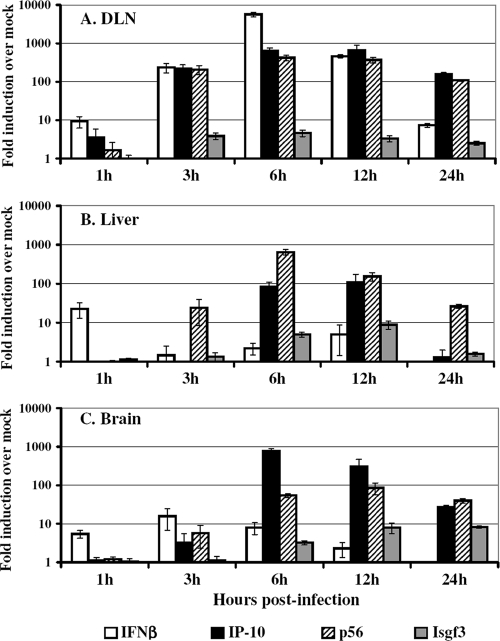
To determine whether the antiviral state established early in the DLN leads to a systemic response throughout the animal, mice were inoculated in the RRFP with 2 × 106 IU of null VRP. The VRP system effectively isolates the active infection to the DLN, allowing us to separately examine the early impact of infection at the site of primary replication and within distal uninfected tissues. At 1, 3, 6, 12, and 24 h postinoculation, serum was collected and each animal was perfused with PBS. The proximal popliteal DLN, liver, and brain (including olfactory bulbs) were dissected from each animal, and total RNA was isolated. The expression levels of several host antiviral genes (coding for IFN-β, IP-10, p56, and Isgf3γ) were measured from each tissue sample by real-time PCR.
The results shown in Fig. 1 demonstrate a robust activation of the innate immune response not only in the DLN (Fig. 1A), but also in tissues remote from the site of active VRP RNA replication (liver [Fig. 1B] and brain [Fig. 1C]). As early as 1 to 3 h following footpad inoculation, high levels of antiviral gene induction were detected in the DLN, including IFN-β, IP-10, and p56 gene expression at levels of 100-fold over that in mock-infected mice. This response peaked in the DLN at 6 to 12 h following footpad inoculation, with expression levels of IP-10, p56, and IFN-β climbing to 400- to 5,000-fold over that in mock-infected animals. By 24 h, this response within the DLN appeared to be waning. Interestingly, in studies monitoring the migration of VEE-infected dendritic cells to the DLN, the majority of dendritic cells reached the DLN from the inoculation site within 30 min and appeared to plateau by 2 h postinfection (44). Therefore, the VRP-induced antiviral response observed in the DLN may correlate with the rapid kinetics of dendritic cell migration, viral gene expression, and eventual clearance of infected cells from the DLN.
FIG. 1.
Rapid, systemic activation of the host antiviral response following VRP footpad inoculation. Adult BALB/c mice (3 mice per group) were inoculated in the RRFP with 2 × 106 IU of null VRP or were mock infected with diluent. At 1, 3, 6, 12, or 24 h following inoculation, the mice were euthanized and then perfused with PBS, and tissues were resected. Total cellular RNA was isolated, cDNA was synthesized, and the expression of a panel of IFN-stimulated genes (coding for IFN-β, IP-10, p56, and Isgf3γ) was assessed by TaqMan real-time PCR in the DLN (A), liver (B), and brain (C). The induction (fold) of each gene is represented as expression in VRP-infected animals relative to expression in mock-infected animals. Bars represent mean values ± the standard error of the mean.
The kinetics of the antiviral response in distal, uninfected tissues were similar to those observed in the DLN: antiviral gene responses were initially detected at 1 to 3 h postinfection in the liver and brain, peaked at 6 to 12 h, and waned by 24 h. Peak antiviral responses in the liver and brain reached levels of 10- to nearly 1,000-fold that in mock-pretreated animals, suggesting that within mere hours the entire infected animal was alerted to the presence of the invading pathogen and mounted a systemic antiviral response reaching far beyond the site of active replication. It is important to note that these distal tissues were analyzed for the presence of VRP message and subsequently found to be completely devoid of replicon RNA, as measured by real-time PCR for VEE nsP1 message (data not shown). Lymph nodes other than the popliteal DLN are also consistently absent of viral antigen following VRP infection of mice, including the contralateral DLN (40, 44).
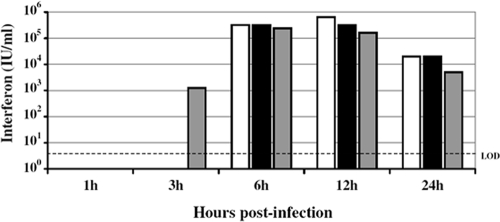
Although these remote organs did not encounter VRP, they nevertheless were exposed to soluble, systemically circulating immune mediators that were initially induced by active infection in the DLN. In fact, high levels of biologically active IFN were detected in the serum of VRP-pretreated mice (Fig. 2). Serum IFN levels reached nearly 400,000 IU/ml by 6 h postinfection and correlated with a peak in antiviral gene response. This intense IFN response following VRP infection is comparable to that observed following infection with VEE virus, with peak serum IFN levels following footpad inoculation with VEE reaching up to 80,000 IU/ml (73). In addition, peak cytokine gene induction (e.g., IFN-γ, interleukin-6, and interluekin-10) in the DLN is reported to reach 10- to 100-fold over mock expression by ≤24 h after footpad inoculation with VEE (26), mirroring the kinetics of the VRP-induced innate response observed here.
FIG. 2.
High levels of biologically active IFN are present in the serum of VRP-infected mice. Adult BALB/c mice were inoculated in the RRFP with 2 × 106 IU of null VRP. At 1, 3, 6, 12, or 24 h following inoculation, the mice were euthanized, followed by cardiac puncture and exsanguination to collect blood samples. Type I IFN present in the serum was measured by standard IFN biological assay on L929 cells, and the results are presented as IU per milliliter. Each bar represents an individual animal, with the limit of detection (LOD) for the assay indicated by a dotted line. The data in this and the previous figure correspond to the same group of mice.
Short-duration VRP pretreatment protects mice from challenge with virulent VEE.
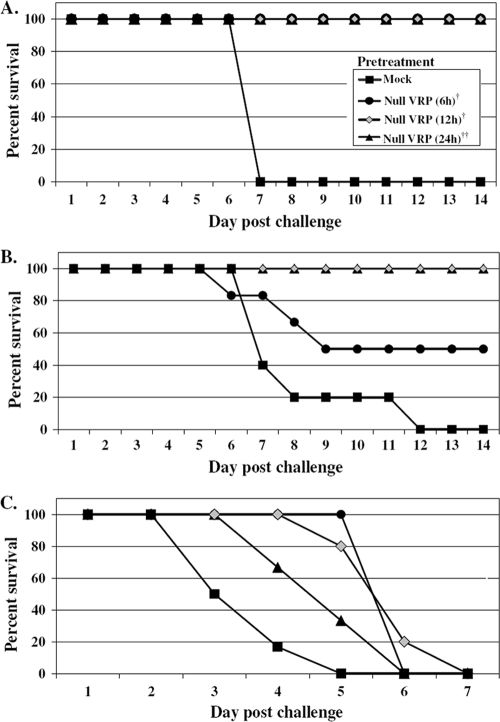
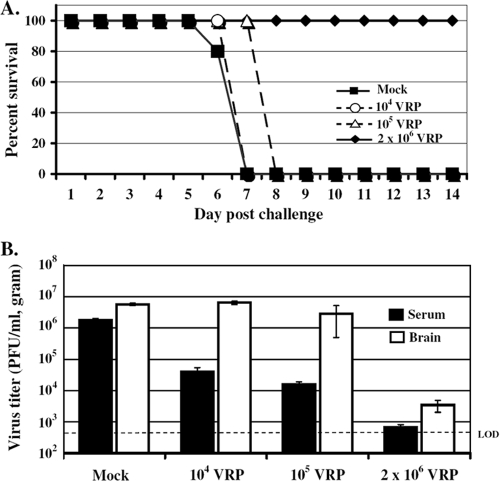
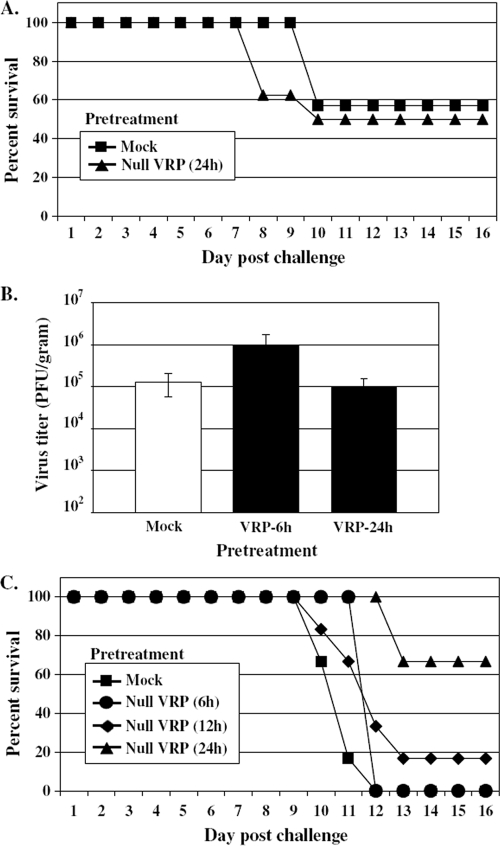
The magnitude and rapidity of the systemic antiviral state induced following VRP inoculation prompted studies to investigate the protective capacity of this response. To address whether VRP inoculation rapidly induced a protective state, adult female BALB/c mice were inoculated with 2 × 106 IU of null VRP in the RRFP at 6, 12, or 24 h prior to lethal challenge with VEE (10 PFU) administered into the opposing LRFP. Mice were weighed and observed every 24 h for signs of clinical disease, comparing morbidity and mortality to mice receiving a mock (diluent only) pretreatment prior to virulent VEE challenge. The results (Fig. 3A) demonstrate that pretreatment with 2 × 106 IU of VRP inoculated into the footpad, even just 6 h prior to a lethal VEE challenge, can completely protect animals from death. All animals receiving VRP pretreatment survived, including all pretreatment durations tested, thereby eradicating the 100% mortality observed following VEE challenge. The incidence of morbidity observed in VRP-treated animals was largely reduced as well, falling from 100% in mock-pretreated mice to a range of 0 to 33% morbidity in mice receiving VRP, and generally was limited to ruffling of the fur. Allowing even a short 6-h window between VRP inoculation and VEE challenge was sufficient to effectively protect animals from death, a testament to the effectiveness of the early antiviral response.
FIG. 3.
VRP pretreatment prior to peripheral and intranasal challenge with virulent VEE protects mice from death and extends the average survival time of mice challenged intracranially. Adult BALB/c mice (5 to 6 mice per group) were pretreated by inoculation in the RRFP with 2 × 106 IU null VRP for 6, 12, or 24 h prior to peripheral challenge with 10 PFU of VEE in the opposing LRFP (A), intranasal challenge with 103 PFU of VEE (a dose necessary to achieve 100% mortality in mock-pretreated animals) (B), or intracranial challenge with 103 PFU of VEE (C). Mock-pretreated mice received diluent in the RRFP 6 h prior to challenge. Animals were monitored daily for morbidity and mortality. The ASTs following intracranial challenge were as follows (mean ± standard error): mock-pretreated mice, 2.7 ± 0 days; VRP-pretreated mice (6 h), 5.0 ± 0 days (P < 0.001); VRP-pretreated mice (12 h), 5.0 ± 0.3 days (P < 0.001); and VRP-pretreated mice (24 h), 4.0 ± 0.4 days (P < 0.05). One-way analysis of variance was used for statistical analysis performed on AST values following intracranial challenge of VRP-pretreated animals compared to mock-pretreated animals.
To further assess the protective nature of this VRP pretreatment, more rigorous routes of VEE challenge were examined. Inoculated peripherally, VEE induces an initial lymphotropic phase characterized by a high serum viremia that seeds infection of the olfactory neuroepithelium, followed by invasion of the central nervous system leading to fatal encephalitis (13, 27). By granting direct access to the olfactory neuroepithelium, intranasal delivery of VEE bypasses the necessity of a high serum viremia for establishment of the neurotropic disease phase. When BALB/c mice were pretreated with a VRP footpad inoculation (2 × 106 IU) for 12 or 24 h prior to virulent intranasal VEE challenge (103 PFU), they were completely protected from death (Fig. 3B). Pretreatment administered 6 h prior to intranasal challenge protected 50% of mice from death. This is in contrast to the 100% mortality resulting from the same intranasal challenge of mock-pretreated animals.
Direct intracranial inoculation of VEE bypasses the requirement for viral neuroinvasion and serves as a means to directly measure viral neurovirulence. In our studies, intracranial VEE inoculation served as the most rigorous challenge route examined. As demonstrated in Fig. 3C, VRP footpad pretreatment failed to protect animals from death following intracranial challenge with 103 PFU of VEE. Intracranial challenge with a lower dose of VEE (10 PFU) also resulted in 100% mortality of both mock- and VRP-pretreated animals (data not shown). However, VRP-pretreated mice did exhibit a statistically significant extension in average survival time compared to mock-pretreated mice (Fig. 3C).
Taken together, the VEE challenge experiments demonstrate that a remarkably-short-duration pretreatment with VRP effectively protects animals from peripheral (footpad) or mucosal (intranasal) challenge with virulent VEE. While the same pretreatment regimen cannot protect animals against direct intracranial administration of VEE, it does result in an extension in survival time following challenge.
VRP pretreatment reduces viral load in the serum and brain of mice challenged with VEE.
To determine whether VRP pretreatment functionally reduced or limited VEE replication following challenge, virus titers in the serum and brain were determined by standard plaque assay. At 6, 12, or 24 h following the standard pretreatment of 2 × 106 IU null VRP in the RRFP, mice were challenged in the LRFP with 10 PFU of VEE. At 24 h postchallenge, serum was collected from the tail vein, followed by PBS perfusion and resection of the brain (including the olfactory bulbs) at 88 h postchallenge. Titers from VRP-pretreated animals were compared to those from mock-pretreated animals.
The results shown in Fig. 4 demonstrate that VRP pretreatment dramatically reduces the viral load in serum and brain following VEE footpad challenge. By 24 h postchallenge, serum viremia in mock-pretreated animals had reached 6.5 × 105 PFU/ml, while the viral load in the serum of VRP-pretreated animals was reduced by over 2 logs to 2.1 × 103 PFU/ml. Furthermore, viral titers in the brain of mock-pretreated animals had reached nearly 7.5 × 106 PFU/g by 88 h postchallenge, while in VRP-pretreated animals the amount of virus in the brain was at or below the limit of detection. Therefore, short-duration pretreatment with VRP effectively reduces challenge virus load in both serum and the brain, thereby controlling viral replication and spread following VEE challenge.
FIG. 4.
VRP pretreatment dramatically reduces the viral load in the serum and brain of animals challenged with VEE. Adult BALB/c mice (3 mice per group) were pretreated by inoculation in the RRFP with 2 × 106 null VRP for 6, 12, or 24 h prior to challenge with 10 PFU of virulent VEE in the opposing LRFP. Mock-pretreated mice received diluent 6 h prior to challenge. At 24 h postchallenge, serum was collected by tail vein bleed. At 88 h postchallenge, mice were euthanized and then perfused with PBS, and the brain (including the olfactory bulbs) was resected. Challenge virus titers were determined by standard plaque assay on BHK cells. Bars represent mean values ± the standard error of the mean. The assay limit of detection (LOD) is indicated by the dotted line.
Protection from virulent VEE challenge is dependent on the dose of VRP pretreatment.
To elucidate factors fundamental to the protection bestowed by VRP pretreatment, we first addressed the parameter of pretreatment dose. Adult BALB/c mice were pretreated with decreasing doses of null VRP in the RRFP and then challenged 6 h later in the opposing LRFP with 10 PFU of virulent VEE. Consistent with our previous results, mice that received the standard pretreatment dose of 2 × 106 IU of VRP were completely protected from death. When the VRP pretreatment dose was reduced to 105 or 104 IU, protection against mortality was lost, suggesting a fairly narrow dose threshold for the protective effect (Fig. 5A). However, it is possible that protection may have been observed if pretreatment with a lower dose was extended beyond 6 h.
FIG. 5.
VRP pretreatment dose is a critical parameter in mediating protection against VEE challenge. Adult BALB/c mice (5 mice per group) were pretreated with decreasing doses of null VRP (2 × 106 IU, 105 IU, or 104 IU) inoculated in the RRFP for 6 h prior to challenge with 10 PFU of virulent VEE (LRFP). Mock-pretreated mice received diluent 6 h prior to challenge. (A) Animals were monitored daily for morbidity and mortality. (B) At 24 h postchallenge, serum was collected by tail vein bleed. At 88 h postchallenge, mice were euthanized and then perfused with PBS, and the brain (including the olfactory bulbs) was resected. At each dose of VRP pretreatment, subsequent challenge virus titers were determined by standard plaque assay on BHK cells. Bars represent mean values ± the standard error of the mean. The assay limit of detection (LOD) is indicated by the dotted line.
Challenge virus titers in the serum and brain were also determined in mice receiving lower VRP pretreatment doses (Fig. 5B). Titers following the standard VRP pretreatment of 2 × 106 IU remained below a threshold of 700 PFU/ml in serum and 3.5 × 103 PFU/g in brain. However, consistent with the lack of protection observed, challenge virus titers in the serum and brain remained high in animals receiving less than the standard pretreatment dose. Therefore, a dose-dependent trend in the ability of pretreatment to control challenge virus replication does exist.
Signaling through IFN-α/βR is necessary for induction of the downstream antiviral gene response and protection from virulent VEE challenge.
We have demonstrated large amounts of biologically active IFN circulating in the serum of VRP-pretreated mice by 6 h postinoculation (Fig. 2). The kinetics of this serum IFN response correlate with the induction of a robust host antiviral gene response in pretreated animals (Fig. 1). We hypothesize that this rapidly induced innate antiviral state mediates protection from death upon virulent virus challenge and used IFN-α/βR−/− mice to investigate the role of IFN signaling in our model.
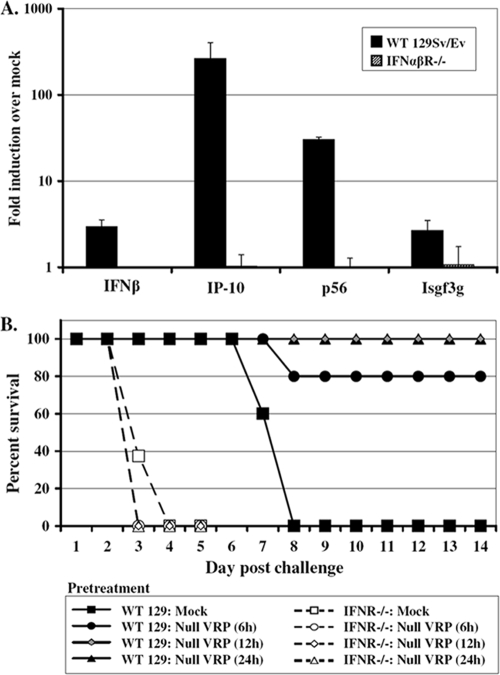
Wild-type 129 or IFN-α/βR−/− mice were inoculated with 2 × 106 IU of null VRP in the RRFP. At 6 h postinoculation, the mice were euthanized and then perfused with PBS, and liver and brain tissue were resected. Total RNA extracted from these tissues was used to investigate whether signaling through IFN-α/βR was required for the rapidly induced VRP antiviral response in downstream tissues. The expression of the same panel of four IFN-stimulated genes (coding for IFN-β, IP-10, p56, and Isgf3γ) was measured by real-time PCR and compared to levels in mock-infected mice.
The results shown in Fig. 6A demonstrate a near complete elimination of the downstream antiviral gene response at 6 h in mice lacking IFN-α/βR. This is in stark contrast to the robust responses measured in the same tissues isolated from wild-type 129 (IFN-α/βR+/+) mice. Therefore, the lack of IFN-α/βR greatly impairs the early, systemic antiviral response that is induced in the downstream tissues of wild-type animals.
FIG. 6.
VRP-induced antiviral gene induction in the brain is diminished and VRP-mediated protection from challenge is abolished in IFN-α/βR knockout mice. Adult wild-type 129 Sv/Ev or IFN-α/βR knockout (IFN-α/βR−/−) mice were inoculated in the RRFP with 2 × 106 IU of null VRP or were mock infected with diluent. (A) At 6 h following inoculation, the mice were euthanized and then perfused with PBS, and brain tissue was resected. Total cellular RNA was isolated, and the expression of a panel of IFN-stimulated genes (coding for IFN-β, IP-10, p56, and Isgf3γ) was assessed by TaqMan real-time PCR. Black bars represent gene expression in wild-type 129 mice, and striped bars represent gene expression in IFN-α/βR−/− mice. The induction (fold) of each gene is represented by expression in VRP-infected animals relative to expression in mock-infected animals. Bars represent mean values (3 animals per group) ± the standard error of the mean. (B) Adult wild-type (WT) 129 Sv/Ev (5 mice per group) or IFN-α/βR−/− mice (8 mice per group) were pretreated by inoculation in the RRFP with 2 × 106 IU null VRP for 6, 12, or 24 h prior to challenge with 10 PFU of virulent VEE in the opposing footpad. Mock-pretreated mice received diluent 6 h prior to challenge. Animals were monitored for morbidity and mortality every 6 h for the first 48 h and every 24 h thereafter.
Concordantly, protection against virulent VEE challenge was also lost in IFN-α/βR−/− mice following this short-duration VRP pretreatment (Fig. 6B). We have previously reported equivalent levels of serum IFN circulating in wild-type 129 and IFN-α/βR−/− mice at early times after VRP footpad inoculation, including at 6 h (73). As such, we utilized the VRP pretreatment duration of 6 h in our studies comparing host responses and challenge outcomes in wild-type 129 and IFN-α/βR−/− mice. It is important to note that by 12 h post-VRP infection, serum IFN levels in IFN-α/βR−/− mice have been reported as being ∼1 log lower than that in wild-type mice (73). Thus, the possibility exists that the kinetics of systemic antiviral responses may be shifted in IFN-α/βR−/− mice.
Nonetheless, the sensitivity of alphaviruses to the host IFN response has been well documented, in fact—most convincingly in studies utilizing mice deficient for the IFN-α/βR (28, 57, 61, 73). IFN-α/βR−/− mice infected with VEE experience a significantly shorter average survival time (AST) (30 h) than wild-type mice (7.7 days), indicating that viral replication in control mice is significantly restricted by IFN (73). This intrinsic sensitivity of VEE to IFN ultimately makes it difficult to determine whether the lack of protection observed in our IFN-α/βR−/− experiment is truly due to a dampened or impaired pretreatment response or is merely a function of the general increased susceptibility of these mice to infection with VEE.
VRP pretreatment differentially protects mice from challenge with heterologous viruses.
To address whether the protection imparted by short-duration VRP pretreatment extends to other viruses, two heterologous challenge models were employed. VSV and influenza virus served as attractive heterologous challenge models as they have been extensively used in studies of viral pathogenesis and the IFN response to infection.
The pathogenesis of VSV has been well described in mice (7, 46, 47, 53, 56, 58, 65). Mortality can be difficult to induce when delivering VSV by subcutaneous inoculation, while the mortality rate following intranasal inoculation of VSV averages 30 to 60% when a high challenge dose (e.g., >106 PFU) is administered to adult mice (19, 20, 41). Intranasal inoculation of VSV delivers virus to the neuroepithelium, where it replicates and spreads to the olfactory bulb (31, 32, 53), similar to intranasal infection with VEE. To address whether VRP pretreatment could protect mice following a heterologous VSV challenge, 6- to 7-week-old male BALB/c mice were inoculated in the RRFP with 2 × 106 IU of null VRP and then intranasally challenged 24 h later with 2 × 106 PFU of VSV. The mice were monitored daily, and their weight and clinical scores were recorded.
As demonstrated in Fig. 7A, pretreatment with VRP was not sufficient to protect animals from death following intranasal challenge with VSV. Furthermore, VRP pretreatment 6 or 24 h prior to VSV intranasal challenge did not prevent VSV from reaching the brain nor did it limit the level of challenge virus replication (Fig. 7B). A challenge dose of 2 × 106 PFU of VSV was required to induce a modest level of mortality in mock-pretreated animals (Fig. 7A), reflecting the relatively high dose of VSV that is traditionally required to induce mortality when delivered to adult mice intranasally (7, 20, 31, 32). Nonetheless, our results suggest that the magnitude or kinetics of the host response were either not ideal or not sufficient to control viral replication and bestow protection from this VSV challenge.
FIG. 7.
VRP pretreatment differentially protects mice from challenge with heterologous viruses. (A) VRP pretreatment does not protect mice against heterologous challenge with VSV. Adult BALB/c mice (7 or 8 mice per group) were pretreated by inoculation in the RRFP with 2 × 106 IU of null VRP for 24 h prior to intranasal challenge with 2 × 106 PFU of VSV. Mock-pretreated mice received diluent 24 h prior to challenge. Animals were monitored daily for morbidity and mortality. (B) Adult BALB/c mice (3 mice per group) were pretreated by inoculation in the RRFP with 2 × 106 IU of null VRP for 6 or 24 h prior to intranasal challenge with 2 × 106 PFU of VSV. Mock-pretreated mice received diluent 24 h prior to challenge. At 88 h postchallenge, mice were euthanized and then perfused with PBS, and the brain (including the olfactory bulbs) was resected. Challenge virus titers were determined by standard plaque assay on BHK cells. Bars represent mean values ± the standard error of the mean. (C) VRP pretreatment is capable of protecting mice from death following lethal influenza virus challenge. Adult BALB/c mice (6 mice per group) were pretreated by inoculation in the RRFP with 2 × 106 IU null VRP for 6, 12, or 24 h prior to intranasal challenge with 12 PFU of influenza virus. Mock-pretreated mice received diluent 6 h prior to challenge. Animals were monitored daily for morbidity and mortality.
Experimental infection of mice with influenza virus has also long provided a model for studying viral pathogenesis (18, 21, 72, 74), with replication occurring in the upper and lower respiratory tract following intranasal inoculation. In contrast to the VSV studies, VRP pretreatment was capable of protecting mice challenged heterologously with influenza virus (Fig. 7C). Mice receiving a VRP footpad inoculation 24 h prior to lethal intranasal challenge with influenza virus (12 PFU) were protected from death (66.7% protection) (Fig. 7C). Interestingly, this pretreatment was less effective if administered 12 h prior to challenge (16.7% protection) and was ineffective when delivered 6 h prior to challenge. These results demonstrate that VRP pretreatment is capable of providing protection against lethal challenge with a heterologous virus. However, the mechanism of this protection may involve specific elements or compartments of the host response to which not all viruses are universally sensitive to the same extent.
DISCUSSION
Countless studies have documented the specific changes induced as an invading pathogen is sensed and the host response is mounted during viral infection. However, few studies describe, on a molecular level, how the entire infected animal is affected systemically, particularly in tissues that are distal to the site(s) of active viral replication. Using VRP, we examined the host response at the initial site of active viral replication in vivo, while simultaneously monitoring the downstream response in distal uninfected tissues within the same animal, without the complication of viral spread. A unique, whole-animal profile of the host response to primary infection resulted, including the induction of several IFN-stimulated genes, namely those coding for Isgf3γ, IP-10, p56, and IFN-β, all with well-documented roles in innate immunity (30, 45, 47, 62, 67, 70, 71). This rapid, systemic induction of a diverse range of antiviral genes suggests that the invading pathogen was sensed and an innate immune response initiated on an animal-wide scale almost immediately.
This innate antiviral state induced by peripheral infection with VRP rapidly promoted a protective response, as short-duration VRP pretreatment protected animals from death following virulent VEE challenge. Future studies will explore whether any individual gene product is solely responsible for the observed protective innate response or whether a particular panel of antiviral activity is crucial for VRP-induced immunity. While the exact mechanisms underlying the protection mediated by VRP pretreatment remain to be fully defined, our study outlines several critical parameters.
There is a temporal correlation between the capacity of VRP pretreatment to bestow protection and the systemic innate response induced in pretreated animals. Mice challenged with VEE at times during which strong antiviral and IFN responses were demonstrated (e.g., at 6, 12, and 24 h post-VRP pretreatment) were completely protected from death. In contrast, when animals were challenged with VEE at times during which the antiviral gene response and serum IFN levels were relatively low (e.g., at 1 h and 3 h post-VRP pretreatment), they were not protected from death (100% and 50% mortality rates, respectively [data not shown]). Taken together, these results suggest that the rapidly induced innate response observed in VRP-pretreated animals may largely contribute to protection.
Indeed the most obvious candidate responsible for inducing this systemic response may be IFN itself, particularly given the rapidity with which the protective state is established. Numerous studies have described the sensitivity of VEE to the host IFN response (2, 10, 25, 34, 37, 64), including the dramatic acceleration of VEE-induced mortality in mice deficient for IFN-α/βR with VEE (28, 57, 61, 73). Here, we observed a complete loss of the early antiviral gene response following VRP pretreatment observed in the brain of mice deficient for the IFN-α/βR, further implicating the IFN response as a key mediator of the initial response to VRP infection.
However, IFN alone may not solely be responsible. While IFN is necessary for protection against lethal VEE infection, past studies have demonstrated that the administration of IFN or IFN-inducing agents—e.g., double-stranded RNA, poly(I-C), or lipopolysaccharide—prior to VEE challenge is not sufficient to protect animals from death (8, 28, 42, 48). Furthermore, a recent study comparing the innate host responses to VRP infection in control and IFN-α/βR−/− dendritic cells suggested that IFN-mediated signaling may not be the primary paracrine mediator of the innate response in bystander cells (39). Future studies designed to transfer serum from VRP-pretreated mice to naïve animals (including IFN-α/βR−/− mice) prior to VEE challenge should define the soluble serum components critical to the observed protection.
While VRP pretreatment was not sufficient to protect animals from death upon intracranial challenge with VEE, it did extend the average survival time of these animals. Interestingly, the shortest VRP pretreatment duration (6 h) led to the most significant extension in survival time. This phenomenon may reflect a relatively tight temporal window following intracranial challenge with VEE during which the antiviral response can be effective in limiting or delaying peak VEE growth within this specific tissue compartment. Accordingly, a direct relationship may exist between challenge virus replication kinetics and the protective capability of VRP pretreatment.
The substantial reduction in challenge virus titers in the serum and brain of pretreated animals suggests that viral invasion of the brain following peripheral challenge was successfully blocked or largely limited as a result of the VRP pretreatment. In previous studies, a critical threshold of at least 104 PFU/ml of VEE in the serum was required for VEE to effectively seed infection of the neuroepithelium and invade the central nervous system (K. A. Bernard and R. E. Johnston, unpublished data). Therefore, it remains unclear whether the reduction in challenge virus titers in the brains of VRP-pretreated animals was the result of a protective state induced in the neuroepithelium that limited neuroinvasion, a consequence of the overall reduction in serum viremia, or perhaps a combination of both. Further experimentation exploring mortality, antiviral gene responses, and challenge virus titers in animals receiving a range of VRP pretreatment doses should further elucidate the mechanism(s) by which the brain is protected within this system.
A potential trivial explanation for VRP-mediated protection in this model is direct homologous interference between replicon and challenge virus particles; however, such a mechanism is not supported by our studies. The VRP pretreatments and subsequent VEE challenge were either administered in opposing footpads or separated completely between the footpad and nasal cavity, thereby functionally isolating the path of initial replication. Elements of the adaptive immune response are also unlikely to play a major role in our protection model, as the time required to mount a protective antibody response strongly argues against involvement of VEE-specific antibody. In fact, detectable levels of VEE-specific serum immunoglobulin G are not evident until 5 days postinoculation, even at high viral doses (5). Nonetheless, we are currently investigating the contribution, if any, of the early adaptive response utilizing mice deficient in B and T cells.
Our results demonstrate that VRP pretreatment is capable of providing protection against both homologous VEE challenge and heterologous challenge with influenza virus. However, the same pretreatment regimen was incapable of protecting mice from a virulent challenge with VSV. The differential capability of VRP pretreatment to protect against heterologous viral challenge may largely be due to the magnitude or kinetics of the antiviral response. The course of VSV pathogenesis in vivo is generally extended in comparison to that of VEE. Therefore, the rapidly established VRP antiviral response may have already begun to resolve at times critical to suppressing VSV replication. In contrast, VEE and influenza virus tend to replicate and disseminate quickly in vivo. For example, peak serum viremia is established by 12 to 24 h following peripheral VEE inoculation. Therefore, administering the VRP pretreatment at 6, 12, or 24 h prior to challenge in effect allows the antiviral response to reach the peak state at the time of VEE inoculation and/or during the period most critical to inhibiting VEE replication and spread.
It is also plausible that VEE, influenza virus, and VSV are differentially sensitive to the particular antiviral response profile induced by VRP pretreatment. The action of IFN-induced genes can be quite virus specific, and a particular gene product or combination of responses may be required for complete protection (38, 59, 62). Alternatively, VRP-pretreated animals may lapse into a temporary state of IFN unresponsiveness during a critical point in VSV replication, a phenomenon that has been described to enhance susceptibility to secondary infection through transient immunosuppression (1). Future studies exploring different parameters of pretreatment and challenge models will aid in further defining the specific antiviral parameters that are most effective against alphavirus infection as well as other heterologous challenge viruses.
The rapid and robust, yet transient, activation of a systemic antiviral response in animals receiving VRP pretreatment sheds new light on the dynamics of virus replication and the host response. Within just a few short hours following infection of the first cells, the animal begins to respond and is instantly changed. The initial infection leads to the induction of immune modulators that act in an autocrine fashion to amplify this response. Paracrine mediators then act to rapidly induce a response in surrounding bystander cells, leading to the production of soluble mediators that act at a distance to alter transcription in remote uninfected tissues. By just 6 h postinfection, and under conditions in which the infection is limited to the first round of infected cells, the innate response is activated throughout the body. These results suggest a new paradigm for acute viral disease in which the ultimate pathogenesis of the virus is largely determined by events in the first few moments after infection. This response is capable of alerting the entire infected animal to the presence of the invading pathogen and largely determines the outcome of infection. In addition, considering that several alphavirus family members are in development as vaccine vectors (9, 16, 43, 50, 52, 54), insights gained from this model may influence the design of future preventative or therapeutic vaccines.
Acknowledgments
We gratefully thank the entire Carolina Vaccine Institute for stimulating discussions, including Nancy Davis and Clayton Beard for critical reading of the manuscript. We thank Mark Heise for generous assistance with experimental design and Melissa Parsons for excellent animal care. We also express our appreciation to John Connor and Doug Lyles for supplying VSV and for generous assistance with VSV-associated protocols.
This work was supported by National Institutes of Health (NIH) Public Health Service grant R01-AI51990 (to R.E.J.) and NIH Predoctoral Training grant T32-AI07419 (to J.L.K.).
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Alsharifi, M., M. Regner, R. Blanden, M. Lobigs, E. Lee, A. Koskinen, and A. Mullbacher. 2006. Exhaustion of type I interferon response following an acute viral infection. J. Immunol. 177:3235-3241. [DOI] [PubMed] [Google Scholar]
- 2.Anishchenko, M., S. Paessler, I. P. Greene, P. V. Aguilar, A.-S. Carrara, and S. C. Weaver. 2004. Generation and characterization of closely related epizootic and enzootic infectious cDNA clones for studying interferon sensitivity and emergence mechanisms of Venezuelan equine encephalitis virus. J. Virol. 78:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson, J. F., F. B. Grieder, N. L. Davis, P. C. Charles, T. Knott, K. Brown, and R. E. Johnston. 2000. A single-site mutant and revertants arising in vivo define early steps in the pathogenesis of Venezuelan equine encephalitis virus. Virology 270:111-123. [DOI] [PubMed] [Google Scholar]
- 4.Barna, M., T. Komatsu, Z. Bi, and C. S. Reiss. 1996. Sex differences in susceptibility to viral infection of the central nervous system. J. Neuroimmunol. 67:31-39. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, A. M., S. J. Elvin, A. J. Wright, S. M. Jones, and R. J. Phillpotts. 2000. An immunological profile of Balb/c mice protected from airborne challenge following vaccination with a live attenuated Venezuelan equine encephalitis virus vaccine. Vaccine 19:337-347. [DOI] [PubMed] [Google Scholar]
- 6.Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276:93-103. [DOI] [PubMed] [Google Scholar]
- 7.Bi, Z., M. Barna, T. Komatsu, and C. S. Reiss. 1995. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J. Virol. 69:6466-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradish, C. J., and D. Titmuss. 1981. The effects of interferon and double-stranded RNA upon the virus-host interaction: studies with togavirus strains in mice. J. Gen. Virol. 53:21-30. [DOI] [PubMed] [Google Scholar]
- 9.Brave, A., K. Ljungberg, B. Wahren, and M. A. Liu. 2007. Vaccine delivery methods using viral vectors. Mol. Pharm. 4:18-32. [DOI] [PubMed] [Google Scholar]
- 10.Casals, J., S. M. Buckley, and D. W. Barry. 1973. Resistance to arbovirus challenge in mice immediately after vaccination. Appl. Microbiol. 25:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles, P. C., K. W. Brown, N. L. Davis, M. K. Hart, and R. E. Johnston. 1997. Mucosal immunity induced by parenteral immunization with a live attenuated Venezuelan equine encephalitis virus vaccine candidate. Virology 228:153-160. [DOI] [PubMed] [Google Scholar]
- 12.Charles, P. C., J. Trgovcich, N. L. Davis, and R. E. Johnston. 2001. Immunopathogenesis and immune modulation of Venezuelan equine encephalitis virus-induced disease in the mouse. Virology 284:190-202. [DOI] [PubMed] [Google Scholar]
- 13.Charles, P. C., E. Walters, F. Margolis, and R. E. Johnston. 1995. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology 208:662-671. [DOI] [PubMed] [Google Scholar]
- 14.Davis, N. L., F. B. Grieder, J. F. Smith, G. F. Greenwald, M. L. Valenski, D. C. Sellon, P. C. Charles, and R. E. Johnston. 1994. A molecular genetic approach to the study of Venezuelan equine encephalitis virus pathogenesis. Arch. Virol. Suppl. 9:99-109. [DOI] [PubMed] [Google Scholar]
- 15.Davis, N. L., N. Powell, G. F. Greenwald, L. V. Willis, B. J. Johnson, J. F. Smith, and R. E. Johnston. 1991. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology 183:20-31. [DOI] [PubMed] [Google Scholar]
- 16.Davis, N. L., A. West, E. Reap, G. MacDonald, M. Collier, S. Dryga, M. Maughan, M. Connell, C. Walker, K. McGrath, C. Cecil, L. H. Ping, J. Frelinger, R. Olmsted, P. Keith, R. Swanstrom, C. Williamson, P. Johnson, D. Montefiori, and R. E. Johnston. 2002. Alphavirus replicon particles as candidate HIV vaccines. IUBMB Life 53:209-211. [DOI] [PubMed] [Google Scholar]
- 17.Davis, N. L., L. V. Willis, J. F. Smith, and R. E. Johnston. 1989. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology 171:189-204. [DOI] [PubMed] [Google Scholar]
- 18.Durbin, J. E., A. Fernandez-Sesma, C.-K. Lee, T. D. Rao, A. B. Frey, T. M. Moran, S. Vukmanovic, A. Garcia-Sastre, and D. E. Levy. 2000. Type I IFN modulates innate and specific antiviral immunity. J. Immunol. 164:4220-4228. [DOI] [PubMed] [Google Scholar]
- 19.Durbin, R. K., S. E. Mertz, A. E. Koromilas, and J. E. Durbin. 2002. PKR protection against intranasal vesicular stomatitis virus infection is mouse strain dependent. Viral Immunol. 15:41-51. [DOI] [PubMed] [Google Scholar]
- 20.Forger, J. M., III, R. T. Bronson, A. S. Huang, and C. S. Reiss. 1991. Murine infection by vesicular stomatitis virus: initial characterization of the H-2d system. J. Virol. 65:4950-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Sastre, A., R. K. Durbin, H. Zheng, P. Palese, R. Gertner, D. E. Levy, and J. E. Durbin. 1998. The role of interferon in influenza virus tissue tropism. J. Virol. 72:8550-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleiser, C. A., W. S. Gochenour, Jr., T. O. Berge, and W. D. Tigertt. 1962. The comparative pathology of experimental Venezuelan equine encephalomyelitis infection in different animal hosts. J. Infect. Dis. 110:80-97. [DOI] [PubMed] [Google Scholar]
- 23.Greene, I. P., S. Paessler, L. Austgen, M. Anishchenko, A. C. Brault, R. A. Bowen, and S. C. Weaver. 2005. Envelope glycoprotein mutations mediate equine amplification and virulence of epizootic Venezuelan equine encephalitis virus. J. Virol. 79:9128-9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenway, T. E., J. H. Eldridge, G. Ludwig, J. K. Staas, J. F. Smith, R. M. Gilley, and S. M. Michalek. 1998. Induction of protective immune responses against Venezuelan equine encephalitis (VEE) virus aerosol challenge with microencapsulated VEE virus vaccine. Vaccine 16:1314-1323. [DOI] [PubMed] [Google Scholar]
- 25.Gresser, I. 1984. Role of interferon in resistance to viral infection in vivo, p. 221-247. In J. Vilcek and E. De Maeyer (ed.), Interferon: interferons and the immune system, vol. 2. Elsevier Science, Amsterdam, The Netherlands. [Google Scholar]
- 26.Grieder, F. B., B. K. Davis, X. D. Zhou, S. J. Chen, F. D. Finkelman, and W. C. Gause. 1997. Kinetics of cytokine expression and regulation of host protection following infection with molecularly cloned Venezuelan equine encephalitis virus. Virology 233:302-312. [DOI] [PubMed] [Google Scholar]
- 27.Grieder, F. B., N. L. Davis, J. F. Aronson, P. C. Charles, D. C. Sellon, K. Suzuki, and R. E. Johnston. 1995. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology 206:994-1006. [DOI] [PubMed] [Google Scholar]
- 28.Grieder, F. B., and S. N. Vogel. 1999. Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology 257:106-118. [DOI] [PubMed] [Google Scholar]
- 29.Hart, M. K., W. Pratt, F. Panelo, R. Tammariello, and M. Dertzbaugh. 1997. Venezuelan equine encephalitis virus vaccines induce mucosal IgA responses and protection from airborne infection in BALB/c, but not C3H/HeN mice. Vaccine 15:363-369. [DOI] [PubMed] [Google Scholar]
- 30.Hui, D. J., F. Terenzi, W. C. Merrick, and G. C. Sen. 2005. Mouse p56 blocks a distinct function of eukaryotic initiation factor 3 in translation initiation. J. Biol. Chem. 280:3433-3440. [DOI] [PubMed] [Google Scholar]
- 31.Huneycutt, B. S., Z. Bi, C. J. Aoki, and C. S. Reiss. 1993. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J. Virol. 67:6698-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huneycutt, B. S., I. V. Plakhov, Z. Shusterman, S. M. Bartido, A. Huang, C. S. Reiss, and C. Aoki. 1994. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis. Brain Res. 635:81-95. [DOI] [PubMed] [Google Scholar]
- 33.Huprikar, J., M. C. Dal Canto, and S. G. Rabinowitz. 1990. Protection against lethal Venezuelan equine encephalitis (VEE) virus infection by cell-free supernatant obtained from immune spleen cells. J. Neurol. Sci. 97:143-153. [DOI] [PubMed] [Google Scholar]
- 34.Jahrling, P. B., E. Navarro, and W. F. Scherer. 1976. Interferon induction and sensitivity as correlates to virulence of Venezuelan encephalitis viruses for hamsters. Arch. Virol. 51:23-35. [DOI] [PubMed] [Google Scholar]
- 35.Johnson, B. J., R. M. Kinney, C. L. Kost, and D. W. Trent. 1986. Molecular determinants of alphavirus neurovirulence: nucleotide and deduced protein sequence changes during attenuation of Venezuelan equine encephalitis virus. J. Gen. Virol. 67:1951-1960. [DOI] [PubMed] [Google Scholar]
- 36.Johnston, L. J., G. M. Halliday, and N. J. C. King. 2000. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J. Investig. Dermatol. 114:560-568. [DOI] [PubMed] [Google Scholar]
- 37.Jordan, G. W. 1973. Interferon sensitivity of Venezuelan equine encephalomyelitis virus. Infect. Immun. 7:911-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 39.Konopka, J. L., L. O. Penalva, J. M. Thompson, L. J. White, C. W. Beard, J. D. Keene, and R. E. Johnston. 2007. A two-phase innate host response to alphavirus infection identified by mRNP-tagging in vivo. PLoS Pathog. 3:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laust, A. K., B. W. Sur, K. Wang, B. Hubby, J. F. Smith, and E. L. Nelson. 2007. VRP immunotherapy targeting neu: treatment efficacy and evidence for immunoediting in a stringent rat mammary tumor model. Breast Cancer Res. Treat. 106:371-382. [DOI] [PubMed] [Google Scholar]
- 41.Letchworth, G. J., L. L. Rodriguez, and J. Del cbarrera. 1999. Vesicular stomatitis. Vet. J. 157:239-260. [DOI] [PubMed] [Google Scholar]
- 42.Lukaszewski, R. A., and T. J. G. Brooks. 2000. Pegylated alpha interferon is an effective treatment for virulent Venezuelan equine encephalitis virus and has profound effects on the host immune response to infection. J. Virol. 74:5006-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundstrom, K. 2003. Alphavirus vectors for vaccine production and gene therapy. Expert Rev. Vaccines 2:447-459. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malmgaard, L. 2004. Induction and regulation of IFNs during viral infections. J. Interferon Cytokine Res. 24:439-454. [DOI] [PubMed] [Google Scholar]
- 46.Marcus, P. I., L. L. Rodriguez, and M. J. Sekellick. 1998. Interferon induction as a quasispecies marker of vesicular stomatitis virus populations. J. Virol. 72:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry, A. K., G. Chen, D. Zheng, H. Tang, and G. Cheng. 2005. The host type I interferon response to viral and bacterial infections. Cell Res. 15:407-422. [DOI] [PubMed] [Google Scholar]
- 48.Pinto, A. J., P. S. Morahan, and M. A. Brinton. 1988. Comparative study of various immunomodulators for macrophage and natural killer cell activation and antiviral efficacy against exotic RNA viruses. Int. J. Immunopharmacol. 10:197-209. [DOI] [PubMed] [Google Scholar]
- 49.Pittman, P. R., R. S. Makuch, J. A. Mangiafico, T. L. Cannon, P. H. Gibbs, and C. J. Peters. 1996. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine 14:337-343. [DOI] [PubMed] [Google Scholar]
- 50.Polo, J. M., J. P. Gardner, Y. Ji, B. A. Belli, D. A. Driver, S. Sherrill, S. Perri, M. A. Liu, and T. W. Dubensky, Jr. 2000. Alphavirus DNA and particle replicons for vaccines and gene therapy. Dev. Biol. (Basel) 104:181-185. [PubMed] [Google Scholar]
- 51.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 52.Rayner, J. O., S. A. Dryga, and K. I. Kamrud. 2002. Alphavirus vectors and vaccination. Rev. Med. Virol. 12:279-296. [DOI] [PubMed] [Google Scholar]
- 53.Reiss, C. S., I. V. Plakhov, and T. Komatsu. 1998. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann. N. Y. Acad. Sci. 855:751-761. [DOI] [PubMed] [Google Scholar]
- 54.Riezebos-Brilman, A., A. de Mare, L. Bungener, A. Huckriede, J. Wilschut, and T. Daemen. 2006. Recombinant alphaviruses as vectors for anti-tumour and anti-microbial immunotherapy. J. Clin. Virol. 35:233-243. [DOI] [PubMed] [Google Scholar]
- 55.Rivas, F., L. A. Diaz, V. M. Cardenas, E. Daza, L. Bruzon, A. Alcala, O. De la Hoz, F. M. Caceres, G. Aristizabal, J. W. Martinez, D. Revelo, F. De la Hoz, J. Boshell, T. Camacho, L. Calderon, V. A. Olano, L. I. Villarreal, D. Roselli, G. Alvarez, G. Ludwig, and T. Tsai. 1997. Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. J. Infect. Dis. 175:828-832. [DOI] [PubMed] [Google Scholar]
- 56.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. M. Knipe, B. N. Fields, and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 57.Ryman, K. D., L. J. White, R. E. Johnston, and W. B. Klimstra. 2002. Effects of PKR/RNase L-dependent and alternative antiviral pathways on alphavirus replication and pathogenesis. Viral Immunol. 15:53-76. [DOI] [PubMed] [Google Scholar]
- 58.Sabin, A. B., and P. K. Olitsky. 1937. Influence of host factors on neuroinvasiveness of vesicular stomatitis virus. I. Effect of age on the invasion of the brain by virus instilled in the nose. J. Exp. Med. 66:15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samuel, C. E. 1991. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology 183:1-11. [DOI] [PubMed] [Google Scholar]
- 60.Schoneboom, B. A., K. M. K. Catlin, A. M. Marty, and F. B. Grieder. 2000. Inflammation is a component of neurodegeneration in response to Venezuelan equine encephalitis virus infection in mice. J. Neuroimmunol. 109:132-146. [DOI] [PubMed] [Google Scholar]
- 61.Schoneboom, B. A., J. S. Lee, and F. B. Grieder. 2000. Early expression of IFN-alpha/beta and iNOS in the brains of Venezuelan equine encephalitis virus-infected mice. J. Interferon Cytokine Res. 20:205-216. [DOI] [PubMed] [Google Scholar]
- 62.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 63.Shabman, R. S., T. E. Morrison, C. Moore, L. White, M. S. Suthar, L. Hueston, N. Rulli, B. Lidbury, J. P.-Y. Ting, S. Mahalingam, and M. T. Heise. 2007. Differential induction of type I interferon responses in myeloid dendritic cells by mosquito and mammalian-cell-derived alphaviruses. J. Virol. 81:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spotts, D. R., R. M. Reich, M. A. Kalkhan, R. M. Kinney, and J. T. Roehrig. 1998. Resistance to alpha/beta interferons correlates with the epizootic and virulence potential of Venezuelan equine encephalitis viruses and is determined by the 5′ noncoding region and glycoproteins. J. Virol. 72:10286-10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinhoff, U., U. Muller, A. Schertler, H. Hengartner, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J. Virol. 69:2153-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 67.Terenzi, F., S. Pal, and G. C. Sen. 2005. Induction and mode of action of the viral stress-inducible murine proteins, P56 and P54. Virology 340:116-124. [DOI] [PubMed] [Google Scholar]
- 68.Thompson, J. M., M. G. Nicholson, A. C. Whitmore, M. Zamora, A. West, A. Iwasaki, H. F. Staats, and R. E. Johnston. 2008. Nonmucosal alphavirus vaccination stimulates a mucosal inductive environment in the peripheral draining lymph node. J. Immunol. 181:574-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson, J. M., A. C. Whitmore, J. L. Konopka, M. L. Collier, E. M. B. Richmond, N. L. Davis, H. F. Staats, and R. E. Johnston. 2006. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc. Natl. Acad. Sci. USA 103:3722-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsunoda, I., T. E. Lane, J. Blackett, and R. S. Fujinami. 2004. Distinct roles for IP-10/CXCL10 in three animal models, Theiler's virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult. Scler. 10:26-34. [DOI] [PubMed] [Google Scholar]
- 71.Wacher, C., M. Müller, M. J. Hofer, D. R. Getts, R. Zabaras, S. S. Ousman, F. Terenzi, G. C. Sen, N. J. C. King, and I. L. Campbell. 2007. Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses. J. Virol. 81:860-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ward, A. C. 1997. Virulence of influenza A virus for mouse lung. Virus Genes 14:187-194. [DOI] [PubMed] [Google Scholar]
- 73.White, L. J., J.-G. Wang, N. L. Davis, and R. E. Johnston. 2001. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. J. Virol. 75:3706-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wright, P. F., and R. G. Webster. 2001. Orthomyxoviruses, p. 1533-1579. In D. M. Knipe, B. N. Fields, and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.