Abstract
Antibodies against the extracellular virion (EV or EEV) form of vaccinia virus are an important component of protective immunity in animal models and likely contribute to the protection of immunized humans against poxviruses. Using fully human monoclonal antibodies (MAbs), we now have shown that the protective attributes of the human anti-B5 antibody response to the smallpox vaccine (vaccinia virus) are heavily dependent on effector functions. By switching Fc domains of a single MAb, we have definitively shown that neutralization in vitro—and protection in vivo in a mouse model—by the human anti-B5 immunoglobulin G MAbs is isotype dependent, thereby demonstrating that efficient protection by these antibodies is not simply dependent on binding an appropriate vaccinia virion antigen with high affinity but in fact requires antibody effector function. The complement components C3 and C1q, but not C5, were required for neutralization. We also have demonstrated that human MAbs against B5 can potently direct complement-dependent cytotoxicity of vaccinia virus-infected cells. Each of these results was then extended to the polyclonal human antibody response to the smallpox vaccine. A model is proposed to explain the mechanism of EV neutralization. Altogether these findings enhance our understanding of the central protective activities of smallpox vaccine-elicited antibodies in immunized humans.
The smallpox vaccine, live vaccinia virus (VACV), is frequently considered the gold standard of human vaccines and has been enormously effective in preventing smallpox disease. The smallpox vaccine led to the worldwide eradication of the disease via massive vaccination campaigns in the 1960s and 1970s, one of the greatest successes of modern medicine (30). However, despite the efficacy of the smallpox vaccine, the mechanisms of protection remain unclear. Understanding those mechanisms is key for developing immunologically sound vaccinology principles that can be applied to the design of future vaccines for other infectious diseases (3, 101).
Clinical studies of fatal human cases of smallpox disease (variola virus infection) have shown that neutralizing antibody titers were either low or absent in patient serum (24, 68). In contrast, neutralizing antibody titers for the VACV intracellular mature virion (MV or IMV) were correlated with protection of vaccinees against smallpox (68). VACV immune globulin (VIG) (human polyclonal antibodies) is a promising treatment against smallpox (47), since it was able to reduce the number of smallpox cases ∼80% among variola-exposed individuals in four case-controlled clinical studies (43, 47, 52, 53, 69). In animal studies, neutralizing antibodies are crucial for protecting primates and mice against pathogenic poxviruses (3, 7, 17, 21, 27, 35, 61, 66, 85).
The specificities and the functions of protective antipoxvirus antibodies have been areas of intensive research, and the mechanics of poxvirus neutralization have been debated for years. There are several interesting features and problems associated with the antibody response to variola virus and related poxviruses, including the large size of the viral particles and the various abundances of many distinct surface proteins (18, 75, 91, 93). Furthermore, poxviruses have two distinct virion forms, intracellular MV and extracellular enveloped virions (EV or EEV), each with a unique biology. Most importantly, MV and EV virions share no surface proteins (18, 93), and therefore, there is no single neutralizing antibody that can neutralize both virion forms. As such, an understanding of virion structure is required to develop knowledge regarding the targets of protective antibodies.
Neutralizing antibodies confer protection mainly through the recognition of antigens on the surface of a virus. A number of groups have discovered neutralizing antibody targets of poxviruses in animals and humans (3). The relative roles of antibodies against MV and EV in protective immunity still remain somewhat unclear. There are compelling data that antibodies against MV (21, 35, 39, 66, 85, 90, 91) or EV (7, 16, 17, 36, 66, 91) are sufficient for protection, and a combination of antibodies against both targets is most protective (66). It remains controversial whether antibodies to one virion form are more important than those to the other (3, 61, 66). The most abundant viral particles are MV, which accumulate in infected cells and are released as cells die (75). Neutralization of MV is relatively well characterized (3, 8, 21, 35). EV, while less abundant, are critical for viral spread and virulence in vivo (93, 108). Neutralization of EV has remained more enigmatic (3).
B5R (also known as B5 or WR187), one of five known EV-specific proteins, is highly conserved among different strains of VACV and in other orthopoxviruses (28, 49). B5 was identified as a protective antigen by Galmiche et al., and the available evidence indicated that the protection was mediated by anti-B5 antibodies (36). Since then, a series of studies have examined B5 as a potential recombinant vaccine antigen or as a target of therapeutic monoclonal antibodies (MAbs) (1, 2, 7, 17, 40, 46, 66, 91, 110). It is known that humans immunized with the smallpox vaccine make antibodies against B5 (5, 22, 62, 82). It is also known that animals receiving the smallpox vaccine generate antibodies against B5 (7, 20, 27, 70). Furthermore, previous neutralization assays have indicated that antibodies generated against B5 are primarily responsible for neutralization of VACV EV (5, 83). Recently Chen at al. generated chimpanzee-human fusion MAbs against B5 and showed that the MAbs can protect mice from lethal challenge with virulent VACV (17). We recently reported, in connection with a study using murine monoclonal antibodies, that neutralization of EV is highly complement dependent and the ability of anti-B5 MAbs to protect in vivo correlated with their ability to neutralize EV in a complement-dependent manner (7).
The focus of the study described here was to elucidate the mechanisms of EV neutralization, focusing on the human antibody response to B5. Our overall goal is to understand underlying immunobiological and virological parameters that determine the emergence of protective antiviral immune responses in humans.
MATERIALS AND METHODS
Ethics statement.
Informed consent was obtained for all human work, and the human studies were Institutional Review Board approved. All animal experiments were conducted using Institutional Animal Care and Use Committee-approved animal protocols.
Sera.
Plasma samples from a nonimmune donor and a Dryvax-immunized donor at 4 weeks postvaccination were stored at −80°C. Rabbit-anti-L1 sera were generated by immunizing two rabbits with recombinant L1 protein as described previously (7). Human plasma and rabbit sera were heat inactivated prior to use to eliminate complement activity (56°C; 30 and 60 min, respectively). Human VACV immune globulin (50 mg/ml VIG; Cangene Corp., Winnipeg, Canada) was stored at −80°C.
Mice.
KM mice were generated by crossbreeding double transchromosomic mice and transgenic mice (50, 97). KM mice possess the human chromosome fragments containing the entire human immunoglobulin heavy-chain locus and the YAC transgene for 50% of the human immunoglobulin kappa light-chain locus. KM mice are engineered to express neither endogenous immunoglobulin heavy chain (μMT−/−) nor kappa light chain (K−/−). KM mice are used to obtain human monoclonal antibodies. Female BALB/cByJ mice (Jackson Laboratory), 8 to 12 weeks of age, were used in all in vivo protection experiments.
Viruses.
VACVWR (Western Reserve) was used unless otherwise stated. VACVIHDJ was obtained from Stuart Isaacs (University of Pennsylvania). Recombinant VACVWR B5-green fluorescent protein (GFP) (VACV/B5-GFP) was obtained from Bernard Moss (NIH) (104).
VACV MV stocks were grown on HeLa cells in D-10 (Dulbecco's modified Eagle medium plus 10% fetal calf serum plus penicillin-streptomycin-glutamine) as described previously (7). Fetal calf serum used in all experiments was heat inactivated (56°C, 30 min) prior to use to eliminate complement activity. Purified VACVWR, VACV/B5-GFP, or VACVIHDJ stocks were made via centrifugation through a sucrose cushion as described previously (7). Virus was stored at −80°C.
EV stocks of VACV were prepared as described previously (7). Briefly, HeLa cells were used, and the medium containing EV was harvested at 2 days after infection. Virus was isolated by centrifugation (450 × g, 8 min) twice to remove cells. Clarified supernatant was used immediately or stored at 4°C for a maximum of 3 to 4 weeks (1). Titers of EV VACVWR stocks (∼5 × 105 PFU/ml) were determined on VeroE6 cells after 30 min of incubation in the presence of rabbit anti-L1 antibodies (1:50 final dilution) to block MV present in the EV stock (1, 36, 98).
Generation of anti-B5 monoclonal antibodies.
Recombinant B5 protein (rB5) was produced and purified as described previously (7). The sequence encoding the extracellular domain of VACV B5 (amino acids 1 to 275) was amplified by reverse transcription-PCR. The PCR amplification product of the VACVNYCBOH sequence was identical to ACAM2000 B5 except at amino acid positions 55 and 82, where the sequence was identical to the MVA and Lister B5 sequences.
KM mice were immunized subcutaneously with the rB5 protein plus adjuvant and boosted by intravenous injection with the rB5 protein alone, similar to the procedure described in reference 7. Spleens were harvested, and single-cell suspensions were fused to a myeloma cell line (SP2/O-Ag14) (ATCC, Rockville, MD) to generate hybridomas according to the procedure described previously (7). Crude hybridoma supernatants were screened for B5 specificity by using an rB5 enzyme-linked immunosorbent assay (ELISA). Cells from positive wells were expanded and subjected to two to three rounds of limiting-dilution cloning to obtain monoclonal antibodies. Antibodies were purified as described previously (7). The purified antibody concentration was determined by the Lowry method. Quantitative ELISAs were performed as described previously (7).
Antibody gene cloning.
Total RNA from hybridoma cells was isolated (RNeasy mini kit; Qiagen) and used as a template in the rapid amplification of 5′ cDNA ends (SMART-RACE kit; Clontech) (with SuperScriptII reverse transcriptase; Invitrogen Corp., Carlsbad, CA) coding for the antibody heavy- and light-chain variable domains. For MAb h104, these were cloned into the vector pCR-BluntII-TOPO (Invitrogen) and then subcloned into the antibody expression vectors N5KG1 and N5KG4PE (Biogen IDEC, Cambridge, MA) for expression as an immunoglobulin G1 (IgG1) or IgG4PE isotype, respectively. G4PE is a variant of human IgG4 that contains two mutations: Ser228Pro to prevent the half-antibody formation (4) and Leu235Glu to reduce residual antibody-dependent cellular cytotoxicity (ADCC) (87). The H104 IgG1 and h104 IgG4 antibodies were expressed by transient expression in 293-F cells (Invitrogen) and were subsequently purified from supernatants.
VACV EV neutralization.
Briefly, VeroE6 cells were used, and EV neutralization was performed as described previously (7) using diluted antibody samples in the absence or the presence of 10% (final concentration) sterile baby rabbit complement (Cedarlane Laboratories, Ontario, Canada) as described previously (7). Plasma was heat inactivated prior to use to eliminate complement function. Rabbit anti-L1 (1:25 to 1:100, final concentration) was used to block the MV present in the EV stock in all experiments. VACV EV supplemented with anti-L1 antibody alone was regularly used in each assay with or without baby rabbit complement as a negative control.
Comet-tail assays were performed using the VACVIHDJ strain and 20 μg/ml of anti-B5 MAbs, 100 μg/ml of human VIG (the polyclonal human antibody), or a 1/100 dilution of immune or nonimmune human plasma as described previously (7).
VACV intranasal infection protection studies.
Age-matched female BALB/c mice were used in all experiments as described previously (7, 21, 91). Briefly, for B5 monoclonal protection studies and isotype specificity of MAbs, mice were treated intraperitoneally (i.p.) with 100 μg of the anti-B5 MAb h101, h102, h106, or h104 1 day before intranasal infection with 5 × 104 PFU VACVWR. Control mice received phosphate-buffered saline (PBS) alone or an irrelevant MAb.
Complement pathway.
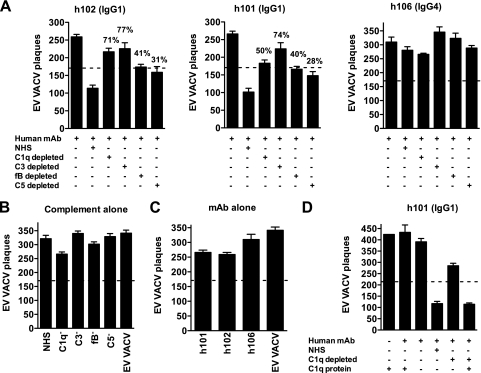
VeroE6 cells were seeded at 5 × 105 cells/well in six-well Costar plates and used the following day (75 to 90% confluence). For Fig. 6, human monoclonal antibodies against B5 (h101 IgG1, h102 IgG1, or h106 IgG4; 10 μg/ml, final concentration) in D-10 medium were used in the absence or the presence of 10% of normal human serum complement (NHS) or C1q-, C3-, fB-, or C5-depleted human serum (Quidel, San Diego, CA). For Fig. 6D, C1q-purified protein (10 μg/ml; Quidel, San Diego, CA) was used. A 100-μl amount of MAb plus complement samples were incubated in an equal volume of EV VACVWR (1:100 dilution plus a 1:50 final concentration of anti-L1 antibody) for 30 min at 37°C in 5% CO2. EV plaque assays were performed as described above. The percentage of EV neutralization activity loss was calculated according to the following formula: 100 − [(PNMAb − PNMAb + C′-depleted)/(PNMAb − PNMAb + NHS) × 100], where PNMAb is the plaque count after treatment with MAb alone, and PNMAb + C′-depleted is the plaque count after treatment with MAb and complement component-depleted human sera; PNMAb + NHS is the plaque count after treatment with MAb plus NHS.
FIG. 6.
. Complement activation pathways. (A to C) VACV EV neutralization by the anti-B5 MAb at 10 μg/ml (h101 IgG1, h102 IgG1, or h106 IgG4 isotype) in the presence of human complement (NHS) or C1q-, C3-, fB- or C5-depleted human sera (10%). The dashed line indicates 50% neutralization based on that for VACV EV alone without antibody and complement. Significant decreases in anti-B5 MAb (h101 or h102 IgG1 isotype) VACV EV neutralization were observed when the complement component C1q, C3, C5, or fB was depleted. Percent EV neutralization activity loss in the absence of C1q or C3 was higher than that in the absence of fB or C5. (B) EV neutralization by human complement (NHS) or C1q-, C3-, fB-, or C5-depleted sera alone. (C) Anti-B5 MAb h101 IgG1, h102 IgG1, or h106 IgG4 neutralization in the absence of complement. (D) EV neutralization by anti-B5 MAb (h101 IgG1) at 10 μg/ml in the presence of human complement (NHS), C1q-depleted sera, or C1q-purified protein. The addition of the C1q protein (10 μg/ml) to C1q-depleted sera restored EV neutralization activity. Data are representative of three experiments. Error bars indicate the SEM in each group.
Surface staining of B5.
For the immunofluorescence assay, confluent monolayers of VeroE6 cells seeded in eight-well chambered cover-glass slides (Lab-Tek; Nalge Nunc International, Rochester, NY) were infected overnight with 8 PFU/cell of VACV/B5-GFP. Infected cells were washed with PBS and then blocked in 1% fetal bovine serum in PBS and subsequently incubated for 1 h at room temperature with 10 μg/ml of a human anti-B5 monoclonal antibody (h101). Cells were washed three times, and the anti-B5 antibody was detected using donkey anti-human IgG antibody conjugated to allophycocyanin (Jackson Immunoresearch, West Grove, PA) at a 1:200 dilution for 1 h at room temperature, followed by fixation. Slides were examined using a Nikon Eclipse E1000 immunofluorescence microscope (Mariana).
The flow cytometry assay was carried out using the human anti-B5 MAb h101 (20 μg/ml) and a 1:100 dilution of allophycocyanin-conjugated donkey anti-human IgG(H+L) antibody (Jackson Immunoresearch, West Grove, PA) to detect the expression of B5 on the surfaces of infected cells as described previously (7). Data were analyzed, and the mean fluorescence intensity of surface B5 expression was determined using the FlowJo software program.
Complement lysis assay.
Vero E6 cell monolayers (1 × 106 cells/well) were infected with VACVWR at a multiplicity of infection of 8 and incubated for 12 h at 37°C in 5% CO2. Infected cells were washed twice and pretreated with D-10 medium with or without 10 μg/ml anti-B5 MAb h101, h102, or h104 IgG1, IgG3, or IgG4 isotype (final concentration), irrelevant MAb (antidinitrophenol [anti-DNP] IgG1, IgG3, or IgG4 isotype), a 1/10 dilution of immune or nonimmune human plasma, or 1 mg/ml of VIG for 30 min at 37°C in 5% CO2. After the preincubation, cells were treated with plain Dulbecco's modified Eagle medium with or without 20% rabbit complement (final concentration) for 40 min at 37°C in 5% CO2. Medium was removed, and cells were fixed and stained with 0.1% crystal violet in 20% reagent alcohol. Representative areas in each well were examined using a Nikon Eclipse E1000 microscope at magnification ×40. Quantitation of cell numbers was carried out using the ImarisFile Converter 6.1.3 software program (2008; Bitplane AG).
Statistical analysis.
Tests were performed using the Prism 4.0 or 5.0 software program (GraphPad, San Diego, CA). Statistics were determined using a two-tailed, unpaired t test with 95% confidence bounds unless otherwise indicated. Bar graph error bars are ± 1 standard error of the mean (SEM). Statistical analysis of mouse weight loss after intranasal infection with VACVWR was done by tabulating the weight nadir (30% for dead mice, which was the maximum weight loss before euthanization) for each mouse, and the maximum weight loss significance was calculated using a two-tailed, unpaired t test without assuming a normal distribution (Welch's correction). Survival curve significance was calculated using Kaplan-Meier statistical analysis.
RESULTS
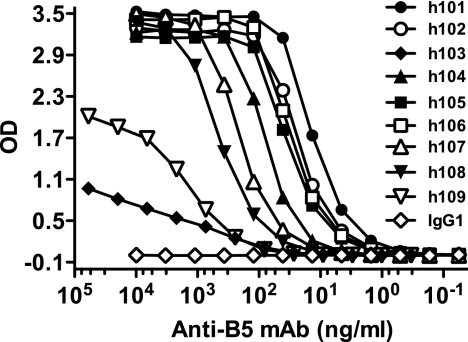
To facilitate a better understanding of neutralization of poxvirus by human antibodies, we developed nine fully human MAbs against the B5 protein, using transchromosomic KM mice engineered to express human immunoglobulin. These mice contain the full human immunoglobulin heavy-chain gene locus and the majority of the kappa light-chain gene locus (50, 97). The mice are bred on an endogenous immunoglobulin heavy chain knockout, kappa light chain knockout background, eliminating all murine immunoglobulin production. Seven human anti-B5 monoclonal antibodies obtained from KM mice were shown to have high relative binding affinities in the nanomolar and subnanomolar range, and two antibodies displayed low binding affinities (Table 1; Fig. 1).
TABLE 1.
Human anti-B5 monoclonal IgG binding affinities
| Clone | Isotype | EC50a (nM) |
|---|---|---|
| h101 | IgG1 | 0.082 |
| h102 | IgG1 | 0.140 |
| h103 | IgG4 | 14.25 |
| h104 | IgG3 | 0.476 |
| h105 | IgG1 | 0.203 |
| h106 | IgG4 | 0.186 |
| h107 | IgG1 | 1.317 |
| h108 | IgG2 | 2.731 |
| h109 | IgG1 | 9.0 |
EC50, halt maximal binding concentration.
FIG. 1.
Binding affinities of human anti-B5 MAbs. Titration of anti-B5 MAbs was done using rB5 ELISA. Data are representative of three independent experiments. OD, optical density.
EV neutralization in vitro by human anti-B5 MAbs was complement dependent, and protection in vivo was affinity and isotype dependent.
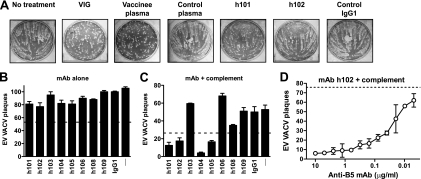
Hybridomas selected in the primary rB5 ELISA screening were tested for EV neutralization potency. Initially, we utilized the most commonly used in vitro EV neutralization assay, comet tail inhibition, which measures a reduction of VACVIHDJ spread in the culture supernatant (36, 60). This assay required high antibody concentrations for effective virus neutralization (20 μg/ml). Out of nine top anti-B5 human antibodies tested in this assay, only a single candidate (h101) (Fig. 2A) showed strong inhibition, and two candidates (h102) [Fig. 2A] and h108 [data not shown]) showed partial inhibition. VIG or plasma from a human smallpox vaccinee that had high anti-B5 IgG titers (data not shown) showed complete inhibition of comet tail formation, while control human plasma or IgG1 or IgG3 MAb had no inhibitory effect on comet tail formation (Fig. 2A and data not shown).
FIG. 2.
Complement-dependent human MAb neutralization of VACV EV. (A) Human anti-B5 MAbs, VIG, and immune human plasma exhibited comet tail inhibition activity in vitro. Vero E6 cells were infected with VACVIHDJ and then cultured in the absence of antibody (no treatment), with human VIG (100 μg/ml), with human plasma samples from a smallpox vaccine recipient or unvaccinated people (1/100 dilution), with anti-B5 MAbs (20 μg/ml; h101 or h102), or with irrelevant MAb (20 μg/ml; IgG1). (B to D) VACV EV virion neutralization by human anti-B5 MAbs is dependent on complement. VACV EV neutralization activity of the full panel of monoclonal antibodies against B5 at 10 μg/ml in the absence (B) or the presence (C) of 10% rabbit complement. VACV EV alone (—) and an irrelevant human IgG1 MAb plus EV (IgG1) were negative controls. The dashed line indicates 50% neutralization based on VACV EV alone (B) or in the presence of complement without antibody (C). (D) Titrated VACV EV neutralization activity of human anti-B5 MAb h102 (IgG1) in the presence of 10% of rabbit complement. The dashed line indicates the plaque number of VACV EV in the presence of complement without antibody. All data are representative of three or more experiments.
Direct EV neutralization with human MAbs alone was poor, with maximal neutralization of ∼20% even at high antibody concentrations (Fig. 2B and 4B and D). This was consistent with the literature showing the difficulty of neutralizing EV (7, 16, 17, 34, 36, 66, 67, 98, 99). Recently we developed a complement-dependent EV neutralization assay that is convenient, provides a high signal-to-noise ratio, and has biological relevance since it accurately predicted protective capacities of murine MAbs against lethal VACV challenges in vivo (7). We refer to this complement-dependent neutralization assay as a “physiological EV neutralization assay.” The physiological EV neutralization assay was applied to screen these B5-specific human IgGs. This assay revealed four strongly neutralizing antibodies: h101, h102, h104, and h105 (Fig. 2C) and one antibody with partial activity (h108). Neutralization was observed only among the high-affinity MAbs (clone h101, h102, h104, or h105) and not the lower-affinity MAbs (clone h103 or h109). Representative high-affinity monoclonal h102 potently neutralized EV in a dose-dependent manner, with a 50% plaque neutralization titer (PRNT50) of 27 ng/ml (Fig. 2D).
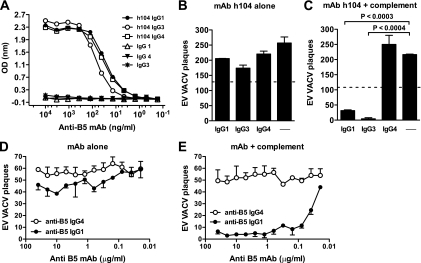
FIG. 4.
Isotype and complement-dependent human antibody neutralization of VACV EV. (A) Titration of anti-B5 MAb h104 IgG1, IgG3, or IgG4 isotype by rB5 ELISA. (B to C) VACV EV neutralization activity with or without different MAb h104 isotypes at 10 μg/ml in the absence (B) or the presence (C) of 10% of rabbit complement. VACV EV alone (B) or plus complement (C) (—) was a negative control. The dashed line indicates 50% neutralization based on VACV EV alone (B) or in the presence of complement without antibody (C). (D and E) Titrated VACV EV neutralization activity of human anti-B5 MAbs in the absence (D) or the presence (E) of 10% of rabbit complement. All data are representative of three or more experiments.
Neutralization also correlated with isotype, since the IgG1 and IgG3 antibodies neutralized (clones h101, h102, h104, and h105) and IgG4 did not (clone h106). IgG2 (clone h108) partially neutralized EV. This is consistent with the effective complement-fixing properties of human IgG1 and IgG3. Human IgG2 fixes complement poorly, and human IgG4 is completely unable to fix complement. The human IgG subclasses (IgG1, IgG2, IgG3, and IgG4) have different hinge region sequences, which determines the flexibility of the molecule, the accessibility of the complement binding sites to C1q, and the ability to fix and activate complement (73, 95).
Our findings show that MAb affinity, concentration, and isotype can be correlated with virus neutralization activity in vitro. Only complement-fixing MAbs with high affinity had the ability to neutralize VACV EV in the presence of complement.
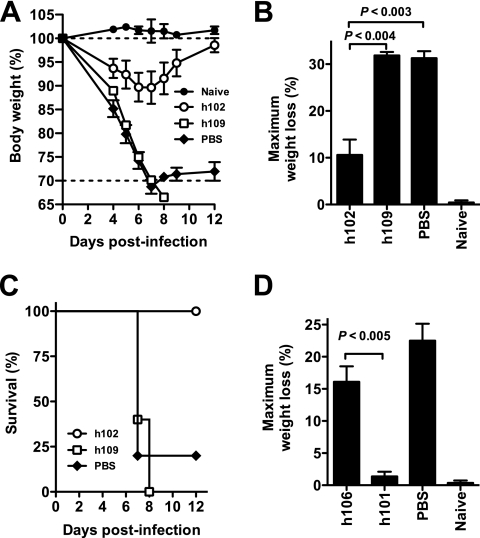
From the physiological EV neutralization assay, we predicted that only high-affinity anti-B5 MAbs of the IgG1 or IgG3, but not IgG4, isotype would be protective in vivo. To test these predictions, we first tested high- and low-affinity anti-B5 IgG1 MAbs (h102 and h109, respectively) for in vivo efficacy using the VACVWR intranasal challenge model with mice. This is an established small-animal model for testing vaccinations and therapeutic treatments related to human inhalation smallpox (6, 7, 17, 66, 109). Here, mice were treated with individual human anti-B5 MAbs followed by a lethal dose of VACVWR via intranasal challenge. As shown in Fig. 3A to C, mice treated with low- affinity h109 human IgG1 MAb lost weight after infection similarly to untreated mice (Fig. 3A and B) and died by day 8 postinfection (Fig. 3C). In contrast, the high-affinity h102 human IgG1 MAb was highly protective (P < 0.004, h102 versus h109; P < 0.003, h102 versus PBS) (Fig. 3B). All h102-treated mice survived (P < 0.02, h102 versus PBS; P < 0.004, h102 versus h109) (Fig. 3C). Furthermore, in separate challenge experiments, we tested a high-affinity h106 IgG4 antibody that had shown no in vitro physiological EV neutralization activity. Anti-B5 h106 IgG4 was not protective in vivo (P < 0.005, h106 versus h101; P ≫ 0.05, h106 versus PBS) (Fig. 3D). Therefore, the physiological neutralization assay is a good predictor of efficacy of human antibodies in vivo, and the protective capacity of human antibody responses against VACV EV is affinity and isotype dependent, reflecting the importance of complement.
FIG. 3.
Protection against a lethal VACVWR infection. High-affinity human anti-B5 MAb (h102) exhibited protection in vivo. (A to C) BALB/c mice were inoculated i.p. with 100 μg of anti-B5 MAb (h102 or h109) or an equivalent volume of PBS. One day later, mice were challenged intranasally with 5 × 104 PFU of purified VACVWR. Naive mice were unchallenged (no infection; “Naive”). (A) Body weight was tracked daily. Dotted lines indicate starting body weight (upper line) or terminal body weight (bottom). (B) Weight loss nadirs for each group are quantitated. (C) Survival after intranasal challenge. H102 MAb-treated mice were protected from death compared to results for mock-treated mice (P < 0.02, MAb h102 versus PBS) and compared to results with low-affinity MAb h109 (P < 0.003). Group average ± SEM from one of three independent experiments is shown. (D) BALB/c mice were inoculated i.p. with a high-affinity anti-B5 MAb (h101 or h106) or PBS at day −1 and challenged intranasally with 5 × 104 PFU of purified VACVWR at day 0. Maximum weight loss (%) is shown. H101-treated mice exhibited no significant weight loss compared to results for uninfected mice (P ≫ 0.05) and were significantly better protected than mice treated with h106 or given PBS treatment (P < 0.005 or P < 0.002, respectively; n = 5/group).
EV neutralization in vitro and protection in vivo by isotype-switched human anti-B5 MAbs.
To formally test the importance of the Fc domain in VACV EV neutralization, we switched the isotype of human anti-B5 monoclonal h104 from IgG3 to either IgG1 or IgG4 by genetic engineering. These three isotypes of MAb h104 displayed comparable high-affinity binding to B5 (Fig. 4A; relative affinities for IgG1, IgG3, and IgG4: 0.16 nM, 0.42 nM, and 0.19 nM, respectively), and none of the h104 isotypes exhibited direct EV neutralization activity in the absence of complement (Fig. 4B). The complement-fixing IgG1 and IgG3 h104 MAbs possessed strong EV-neutralizing activity in vitro (P < 0.0003 and P < 0.0004, respectively; Fig. 4C). Anti-B5 IgG1 possessed a PRNT50 of 29 ng/ml (Fig. 4D and E), and anti-B5 IgG3 exhibited comparable activity in dose titrations (PRNT50 = 15 ng/ml). Importantly, while MAb h104 IgG4 bound B5 with high affinity, it did not neutralize the virus (P ≫ 0.05; Fig. 4C and E), consistent with the essential role of complement in EV neutralization and the inability of IgG4 to fix complement.
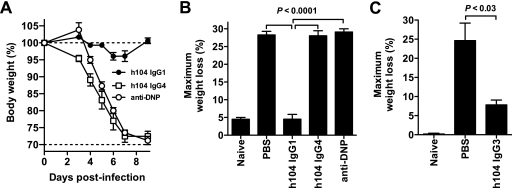
Using the isotype-switched h104 clones, we then directly tested which IgG isotype provided the best protection against VACV in vivo and whether the in vivo protection correlated with in vitro complement-mediated neutralization. Mice treated with anti-B5 h104 IgG1 lost no weight after lethal challenge with VACVWR (P < 0.0001 versus results with PBS or h104 IgG4; P ≫ 0.05 versus results with naive mice) (Fig. 5A and B), comparable to our results with h101 and h102 (Fig. 3). Strikingly, mice treated with anti-B5 h104 IgG4 lost weight identically to mice given an irrelevant MAb (P ≫ 0.05 versus results with irrelevant IgG1; P ≫ 0.05 versus results with PBS) (Fig. 5B). In separate experiments, h104 IgG3 exhibited good protection (P < 0.03 versus results with PBS) (Fig. 5C). Our results demonstrate that the anti-B5 MAb IgG1 and IgG3 isotypes are highly protective against EV and that human IgG4 has no measurable protective efficacy in vivo. The results correlate well with in vitro complement-dependent EV neutralization activity (Fig. 2B to D and 4B to E). These results demonstrate that isotype and antibody effector functions can be crucial components of protection against VACV EV and that antibody binding by itself is insufficient for neutralization and effective protection in vivo by these anti-B5 MAbs.
FIG. 5.
Anti-B5 protection in vivo is antibody isotype dependent. Anti-B5 MAbs h104 IgG1 and h104 IgG3 were protective in vivo, while IgG4 was not protective. (A and B) BALB/c mice were inoculated i.p. with 100 μg of anti-B5 MAb h104 IgG1, h104 IgG4, a negative control human MAb (anti-DNP IgG1) or PBS at day −1 and challenged intranasally with 5 × 104 PFU of purified VACVWR at day 0. Naive mice were unchallenged (no infection; “Naive”). Weight loss kinetics for each group (A) and maximum weight loss (weight nadir) (B) are shown. Dotted lines indicated the starting body weight (upper line) or terminal body weight (bottom line). H104 IgG1 isotype-treated mice exhibited no significant weight loss compared to results for uninfected mice (P ≫ 0.05) and were significantly better protected than mice treated with the h104 IgG4 isotype, human anti-DNP, or PBS (P < 0.0001; n = 6/group). The group average ± SEM from one of two independent experiments is shown. (C) In an independent experiment, BALB/c mice were inoculated i.p. with 100 μg of the anti-B5 MAb h104 IgG3 isotype or PBS at day −1 and challenged intranasally with 5 × 104 PFU of purified VACVWR at day 0. Maximum weight loss (weight nadir) is shown. H104 IgG3 isotype-treated mice were significantly better protected than mice treated with PBS (P < 0.03; n = 6/group). The group average ± SEM from one of three independent experiments is shown.
Complement components involved in VACV EV neutralization.
Complement can be activated via three pathways: classical, alternative, and lectin. Given that complement-fixing human IgG isotypes were required for VACV EV neutralization, we examined which complement factors were involved by using C1q-, C3-, C5- and fB-depleted human sera. Experiments revealed a strong dependence on C1q (71% VACV EV neutralization activity loss) and C3 (77% loss), whereas the absence of fB or C5 had little effect on EV neutralization (Fig. 6A, left panel). Human anti-B5 IgG4 had no neutralization activity under any condition (h106; Fig. 6A, right panel). None of the human anti-B5 IgGs exhibited significant EV neutralization activity in the absence of complement (Fig. 6B and C). Neutralization by human IgG anti-B5 required C1q (Fig. 6A); however, anti-B5 h101 IgG1 was unable to neutralize EV VACV when supplemented with purified C1q protein alone (P < 0.02, results for C1q protein plus h101 versus results for NHS plus h101) (Fig. 6D). The addition of purified C1q to C1q- depleted human serum fully restored neutralization activity, demonstrating that the purified C1q protein was fully active (P < 0.006, results for C1q-depleted serum plus the C1q protein plus h101 versus results for C1q-depleted serum plus h101) (Fig. 6D). These experiments suggest that the primary mechanism of neutralization was opsonization via coating of the EV particles with antibody bound to C1q and subsequent activation and fixation of complement C3, sterically preventing EV from binding to cells (See Discussion and Fig. 10). Given that C5 was dispensable (Fig. 6A), complement-mediated lysis of EV viral particles (virolysis) did not appear to be a major pathway of neutralization by human anti-B5 antibodies.
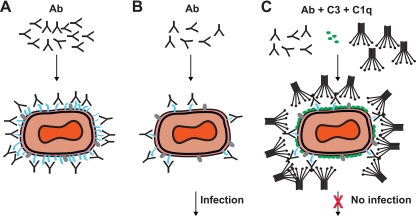
FIG. 10.
Model of VACV EV neutralization. Schematic diagrams of potential virion neutralization pathways. B5 is drawn in blue. Another representative EV surface antigen is drawn in gray. Ab, anti-B5 antibody. (A) Basic occupancy model. Antibodies against B5 could completely coat the virion surface and thereby neutralize the virus. This model failed. (B) Model 2. VACV EV escape neutralization by anti-B5 antibody binding due to low density of the B5 protein on the surface of EV. Direct occupancy of B5 with anti-B5 IgG is insufficient to block infection of target cells. (C) Complement-assisted coating of VACV EV (opsonization). Antibody-mediated protection against VACV EV is dependent on antibody recruitment of complement C1q and covalent attachment of C3 to the surface of the virus. See Discussion for details.
Complement-fixing B5 antibodies mediated complement-dependent cytotoxicity destruction of VACV-infected cells.
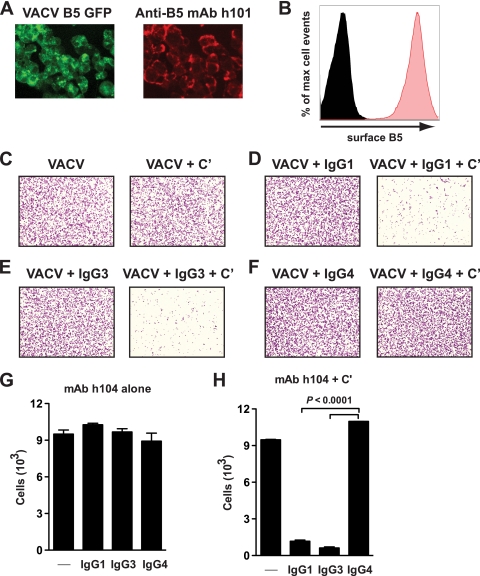
Complement can function in multiple ways, and we recently showed that murine anti-B5 antibodies are effective at directing complement-mediated destruction of VACV-infected cells, utilizing the membrane attack complex (7). EV are secreted from the plasma membrane, and B5 is expressed on the surface of VACV-infected cells (7, 18, 49, 75, 93, 104). Human anti-B5 MAb h101 efficiently labeled B5 on the surface of VACV-infected cells (Fig. 7A and B). We examined the ability of h104 IgG1, IgG3, or IgG4 to mediate complement-dependent cell killing of VACV-infected cells. Treatment of infected cells with complement alone had no effect (Fig. 7C). Treatment with h104 IgG1, IgG3, or IgG4 alone also had no effect (Fig. 7D to G). In contrast, addition of anti-B5 h104 IgG1 (Fig. 7D) or IgG3 (Fig. 7E) with complement resulted in rapid and complete killing of VACV-infected cells (P < 0.0001, results with IgG1 or IgG3 versus results with IgG4 or complement alone) (Fig. 7H). Treatment with the non-complement-fixing anti-B5 h104 human IgG4 plus complement did not direct any cell lysis, again highlighting the importance of the antibody isotype in this antiviral function (P ≫ 0.05, IgG4 versus complement alone) (Fig. 7F and H). Our finding here shows clearly that human antibody-mediated killing of virus-infected cells was specific. Killing was only observed in the presence of complement-fixing anti-B5 MAb, was dependent on the presence of complement, was active only against virally infected cells, and was completely absent when the MAb Fc domain was switched to IgG4 even though anti-B5 IgG4 had identical specificity and affinity to B5 (Fig. 4A). These results and their correlation with the other in vitro and in vivo data shown indicate that anti-B5 antibody killing of VACV-infected cells in vivo is likely an important protective mechanism of the anti-EV humoral immune response induced by the smallpox vaccine.
FIG. 7.
Human IgG1 or IgG3 anti-B5 antibodies are able to direct complement lysis of VACV-infected cells. (A) Vero E6 cell monolayers were infected with VACVWR B5-GFP (green, multiplicity of infection = 8), and surface expression of B5 (red) was determined 12 h postinfection by surface staining with anti-B5 MAb h101 and immunofluorescence microscopy. (B) Surface expression of B5 was tested (red filled curve) after infection with VACVWR by surface staining of infected cells with anti-B5 MAb and performance of flow cytometry. Uninfected cells, negative control (black filled curve). (C to H) Anti-B5-directed complement lysis of infected cells. Virus-infected cells stained with crystal violet are shown at magnification ×40. VACV-infected cells were treated with media (C) (left) or 20% rabbit complement (+ C′) (C to F) (right) in the absence (C) or presence (D to F) of anti-B5 MAb h104 isotypes. (G and H) Quantitation of live cell numbers (cells/image field) from experiment shown in panels C to F. Destruction of VACV-infected cells was statistically significant in the presence of complement plus anti-B5 IgG1 or IgG3 isotype versus results for anti-B5 IgG1 or IgG3 alone (P < 0.0001), complement alone (P < 0.001), or complement plus anti-B5 IgG4 (P < 0.0001). No killing was observed for anti-B5 IgG4 in the absence or presence of complement (P ≫ 0.05). Error bars indicate the SEM in each group. One of two independent experiments is shown.
Functional attributes of human polyclonal anti-B5 IgG from vaccinees.
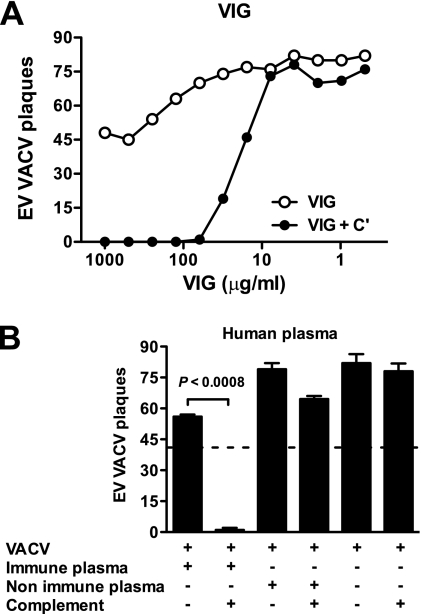
VIG is purified polyclonal human antibody from individuals immunized with DryVax (47). There is evidence that the ability of VIG to neutralize EV is due largely to the presence of anti-B5 IgG (5, 83). As shown in Fig. 8A, VIG alone has only modest EV neutralization activity in vitro even at very high concentrations (0.5 to 1 mg/ml). However, neutralization by VIG was vastly improved by the presence of complement (Fig. 8A). Comparable EV neutralization activity was obtained using plasma from human smallpox vaccinees (Fig. 8B). Nonimmune human plasma had no activity (Fig. 8B).
FIG. 8.
EV neutralization by antibodies from human vaccinees is complement dependent. VACV EV neutralization activity of VIG (A) or human plasma (B) in the presence or absence of complement. (A) Titrated VACV EV neutralization activity of VIG in the absence (VIG) or the presence of 10% of rabbit complement (VIG + C′). VIG possessed strong EV-neutralizing activity in the presence of complement. (B) Plasma samples used are from a single human vaccinee (“immune”) or nonimmune human plasma. The dashed line indicates 50% neutralization based on results for VACV EV alone without plasma samples and complement. EV neutralization was highly statistically significant in the presence of immune human plasma plus complement (P < 0.0008). Error bars indicate SEM in each group. One of two (A) or one of three (B) independent experiments is shown.
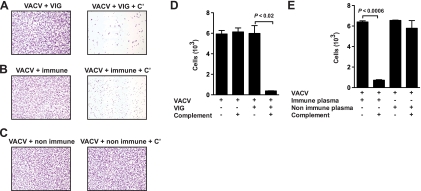
VIG was very effective at lysing VACV-infected cells in vitro in the presence of complement (Fig. 9A and D). Plasma from individual human vaccinees was also very efficient at killing VACV-infected cells in the presence of the complement (Fig. 9B and E). Together, these results demonstrate the physiological relevance of this study and that significant EV neutralization activity (>90%) by human antibodies is observed only in the presence of complement.
FIG. 9.
VIG or plasma from individual human vaccinees directs complement lysis of VACV-infected cells. (A to C) Virus-infected Vero E6 cells stained with crystal violet at magnification ×40. VACV-infected cells were treated with VIG, plasma from a single human vaccinee (immune), or nonimmune human plasma alone (A to C, left) or with complement (+ C′) (A to C, right). (D and E) Quantitation of live cell numbers (cells/image field) after cell lysis in the presence of VIG (D) or human plasma (E). Destruction of VACV-infected cells was highly statistically significant in the presence of VIG plus complement (P < 0.02) or immune human plasma plus complement (P < 0.0006). No killing was observed for nonimmune human plasma in the absence or presence of complement (P ≫ 0.05; not significant). Error bars indicate the SEM for each group. One of two independent experiments is shown.
DISCUSSION
It is important to have a clear understanding of the immunological mechanisms involved in the protection mediated by the smallpox vaccine. Understanding those mechanisms is key to developing immunologically sound vaccinology principles that can be applied to the design of future vaccines. Antibodies play a central role in protecting humans, primates, or mice against pathogenic poxviruses (3, 5, 7, 8, 21, 23, 29, 61, 66, 91). The functions and specificities of the protective antibodies have constituted an area of intensive research, and the mechanisms of poxvirus neutralization have been long debated.
EV neutralization has been a conundrum for decades. While it has been demonstrated in multiple studies that antibodies against B5 antigen protect against a lethal intranasal VACV challenge in animal models (7, 17, 34, 36, 66, 91) and antibodies against B5 are present in human vaccinees (5, 22, 62, 82, 83), the mechanism(s) of protection have been unknown. Recently we clarified several key features of protective murine anti-B5 antibodies (7). The relationship between EV neutralization by human monoclonal and polyclonal antibodies and protection in vitro and in vivo remained unclear. We generated a new panel of human anti-B5 MAbs to facilitate these studies. These human MAbs can also potentially be used as human therapeutic agents for protection against vaccinia virus, monkeypox virus, or variola virus.
Using three isotype-switched versions of the same antibody (h104 IgG1, IgG3, and IgG4), we have demonstrated that only the human IgG1 or IgG3 MAbs neutralize VACV EV and the neutralization is complement dependent. Only human MAbs possessing complement-fixing Fc domains (IgG1 or IgG3) and having high affinity had the ability to neutralize EV virions in the presence of complement. Furthermore, only those MAbs were protective in vivo. Dramatically, human IgG4 anti-B5 of the exact same epitope specificity showed no neutralization in vitro or protection in vivo. Human IgG1 and IgG3 are potent activators of the classical complement pathway, while IgG4 is incapable of activating the classical complement pathway (26, 54, 73, 95, 105). Therefore, the antiviral effects of the antibodies are heavily dependent on Fc-mediated effector functions and are not simply dependent on binding an appropriate VACV virion antigen. These are the most definitive experiments to date showing that antibody effector functions can be key to protective immunity against EV. Our study further shows the direct relevance to human immunology, both by using human monoclonal antibodies and antibodies from smallpox vaccine-immunized humans.
Interestingly, we observed that human anti-B5 IgG1 MAb had an inhibitory effect on comet tail formation in vitro while IgG3 and IgG4 anti-B5 MAbs had no effect on comet tails (data not shown). The comet tail reduction assay therefore appears to measure some complement fixation capacity, as well as affinity, and possibly other attributes of immunoglobulins, such as an ability to block virion release (98).
Mechanism of neutralization.
A complete understanding of virus neutralization by antibodies still remains challenging, and several models for different viruses have been proposed (41, 55-57, 65, 72, 81, 107). A basic occupancy model posits that a virion is neutralized at the point at which the surface antigens are sufficiently coated with antibody, leading to inhibition of viral attachment to target cells or interference with the entry/fusion process (79). This model has successfully predicted neutralizing antibody activities against many small viruses (33, 56, 65, 79, 81, 88). However, VACV is a very large virus. Furthermore, it does not appear to have a symmetrical surface made up of regular repeating units. According to the basic occupancy model of virus neutralization, we hypothesized that high/full occupancy of the B5 protein on the virion surface by anti-B5 antibody should neutralize the virus (Fig. 10A). We found that even high concentrations of anti-B5 IgG were insufficient to neutralize the virus (Fig. 4D), indicating that the coating model is not applicable to the EV of poxviruses. We postulated that the coating model fails because EV escape direct neutralization by anti-B5 IgG binding because the B5 protein is present at relatively low density on the surface of EV and direct occupancy of all B5 binding sites is insufficient to fully cover the surface of the virus (Fig. 10B). Therefore, the neutralization activity of anti-B5 MAbs is observed only when complement is present (Fig. 10C). Alternatively, the double membrane of poxviruses poses unique challenges for virus neutralization by immunoglobulins and may be an important aspect of the complement dependence of EV neutralization (67). Complement plays important roles in antibody-mediated protection against several viral infections (9, 14, 19, 42, 48, 71, 77, 92, 107). In the present study, complement component depletion experiments with C1q or C3 show that the classical pathway of the complement system is required for antibody-mediated neutralization in vitro (Fig. 6). We propose in our neutralization model that C1q increases the footprint of the immunoglobulin, occupying a much larger surface area of the virion, sterically hindering infection (Fig. 10C). Furthermore, covalent attachment of many C3bs to the virion obstructs binding to target cells (Fig. 10C) (10, 79).
The EV neutralization model we propose (Fig. 10) also posits that B5 is not essential for EV virion infectivity, since our data show that saturating levels of anti-B5, presumably occupying all B5 proteins, still does not block the infectivity of EV (Fig. 4D and 10B). This indicates that viral binding and entry can be mediated by other EV virion antigens or components.
High concentrations of some human anti-B5 antibodies exhibited modest EV neutralization (≤30%) in the absence of the complement. This limited neutralization likely is via aggregation and cross-linking of EV. This potential activity may provide a modest degree of protection in vivo, as illustrated by the non-complement-fixing human MAb h106 (Fig. 3). These results are consistent with findings in the literature showing that murine IgG1 antibodies can provide protection in vivo against VACV (1, 2, 7). Alternatively, murine IgG1 does possess some antibody-dependent cell-mediated cytotoxicity (ADCC) activity, such that the modest in vivo protection of murine IgG1s may instead be due to ADCC effector function.
The in vitro complement-mediated VACV neutralization assay is both a direct assay and a surrogate assay for in vivo mechanisms of antibody protection against VACV. Since both the human IgG1 and IgG3 subclasses are capable not only of fixing complement but also of exerting effector functions through binding Fc receptors, we cannot and do not exclude ADCC as part of the protection mechanism in vivo (86). Our data strongly suggest that the potent protective efficacy of human anti-B5 IgG1 and IgG3 MAbs in vivo was due at least in part to their ability to fix and activate complement. Furthermore, depletion of complement in mice abrogated most of the protective efficacy provided by a murine anti-B5 MAb (7). Recently Moulton et al. (76) showed that natural antibody neutralizes ectomelia virus, the causative agent of mousepox, in a complement-dependent manner via activation of both classical and alternative pathways (76).
While C5-mediated virion destruction (virolysis) occurs in some viral infections (equine arteritis virus, lymphocytic choriomeningitis virus, Sindbis virus, and avian bronchitis virus infections) (9, 84, 94, 107) and may occur with poxviruses, virolysis is not necessary for VACV neutralization (7) (Fig. 6). Moulton et al. also recently found that C5 is not necessary for ectomelia virus neutralization in vitro, suggesting that opsonization is the predominant mechanism of neutralization (76). We have also shown that complement-mediated neutralization of EV does not require the presence of anti-MV immunoglobulin (7). This is consistent with the human antibody results reported here. Interestingly, previous studies have demonstrated C1 through C3 were responsible for enhancement of IgG antibody-mediated neutralization of VACV MV in the absence of virolysis, indicating that neutralization of both VACV MV and EV virions may occur through the same pathway in vivo (63). A similar mechanism has been proposed for vesicular stomatitis virus (51, 63).
Human complement deficiencies are frequently associated with autoimmune diseases such as systematic lupus erythematosus, arthritis, and others (11, 12, 38, 64, 102, 103). There is evidence that human complement deficiencies also result in serious health problems after viral or microbial infection. C3 deficiency in humans typically results in recurrent pyogenic bacterial infections (13, 32, 78). Several reports now indicate that a genetic deficiency in C1q in humans results in an enhanced susceptibility to pathogens (15, 32, 96, 106), and if individuals survive these infections, they develop lupus-like autoimmune disease (12, 74, 80, 102, 103).
VACV encodes the VACV complement control protein, VCP (also known as C3L or WR025), which binds C3b and C4b (58, 59, 89) and can also form a covalent heterodimer with the EV surface protein A56 (37, 100). VCP is a known virulence factor of VACV (58). VACVWR, which expresses VCP, was used in our in vitro and in vivo experiments. Therefore the inhibitory effects of VCP are only partial and can be overcome. The observation that VCP deletion VACV mutants have only a moderate loss of virulence is consistent with these findings (48).
Protective anti-EV antibodies in vaccinees.
The smallpox vaccine generates strong antibody responses to the EV form in immunized humans, and the antibody responses are mostly directed against the B5, A33, and A56 viral proteins (5, 22, 25, 62, 82, 83). Mice infected with VACVWR also generate strong antibody responses against multiple EV proteins, and A33 or B5 immunization can be protective in mice (7, 44, 45). The complement-dependent EV neutralization findings using VIG or plasma from human smallpox vaccinees (Fig. 8) are consistent with the human anti-B5 MAb data presented (Fig. 2 to 6) and consistent with the observation of anti-B5 antibody responses in human vaccinees (5, 83). However, our recent finding that the redundancy and diversity of anti-MV neutralizing antibody responses against multiple viral proteins are a defining characteristic of the human response to smallpox vaccination (8) may also apply to EV-neutralizing antibody responses. We hypothesize that human vaccinees may also possess anti-A33 and/or anti-A56 complement-dependent EV-neutralizing antibodies. This would be consistent with studies that show titers of anti-A33 and -A56 antibodies in human vaccinees (62, 83) and that a chimpanzee/human fusion MAb against A33 is protective in vivo (16).
In summary, we show that human antibody neutralization of VACV EV virions is not simply dependent on antigen binding but is actually heavily dependent on antibody effector functions. Altogether, our results indicate that the complement pathway is a crucial component of the success of the smallpox vaccine in immunized humans.
Acknowledgments
We thank Lindsay Crickard and Sacha Garcia for technical assistance.
This work was supported in part by Kirin Pharma, NIH NIAID grant no. AI63107, NIH NIAID grant no. AI077953, a Pew Scholar Award, and a Cancer Research Institute Award to S.C.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 79:6260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldaz-Carroll, L., Y. Xiao, J. C. Whitbeck, M. P. de Leon, H. Lou, M. Kim, J. Yu, E. L. Reinherz, S. N. Isaacs, R. J. Eisenberg, and G. H. Cohen. 2007. Major neutralizing sites on vaccinia virus glycoprotein B5 are exposed differently on variola virus ortholog B6. J. Virol. 81:8131-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211:320-337. [DOI] [PubMed] [Google Scholar]
- 4.Angal, S., D. J. King, M. W. Bodmer, A. Turner, A. D. Lawson, G. Roberts, B. Pedley, and J. R. Adair. 1993. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol. Immunol. 30:105-108. [DOI] [PubMed] [Google Scholar]
- 5.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benhnia, M. R., M. M. McCausland, J. Moyron, J. Laudenslager, S. Granger, S. Rickert, L. Koriazova, R. Kubo, S. Kato, and S. Crotty. 2009. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 83:1201-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benhnia, M. R., M. M. McCausland, H. P. Su, K. Singh, J. Hoffmann, D. H. Davies, P. Felgner, S. Head, A. Sette, D. N. Garboczi, and S. Crotty. 2008. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J. Virol. 82:3751-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry, D. M., and J. D. Almeida. 1968. The morphological and biological effects of various antisera on avian infectious bronchitis virus. J. Gen. Virol. 3:97-102. [DOI] [PubMed] [Google Scholar]
- 10.Bindon, C. I., G. Hale, M. Bruggemann, and H. Waldmann. 1988. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J. Exp. Med. 168:127-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botto, M., C. Dell'Agnola, A. E. Bygrave, E. M. Thompson, H. T. Cook, F. Petry, M. Loos, P. P. Pandolfi, and M. J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56-59. [DOI] [PubMed] [Google Scholar]
- 12.Botto, M., and M. J. Walport. 2002. C1q, autoimmunity and apoptosis. Immunobiology 205:395-406. [DOI] [PubMed] [Google Scholar]
- 13.Botto, M., and M. J. Walport. 1993. Hereditary deficiency of C3 in animals and humans. Int. Rev. Immunol. 10:37-50. [DOI] [PubMed] [Google Scholar]
- 14.Brier, A. M., C. Wohlenberg, J. Rosenthal, M. Mage, and A. L. Notkins. 1971. Inhibition or enhancement of immunological injury of virus-infected cells. Proc. Natl. Acad. Sci. USA 68:3073-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celik, I., C. Stover, M. Botto, S. Thiel, S. Tzima, D. Kunkel, M. Walport, W. Lorenz, and W. Schwaeble. 2001. Role of the classical pathway of complement activation in experimentally induced polymicrobial peritonitis. Infect. Immun. 69:7304-7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, I. Gorshkova, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2007. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J. Virol. 81:8989-8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, Y. H. Zhou, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, J. Svitel, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2006. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc. Natl. Acad. Sci. USA 103:1882-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Condit, R. C., N. Moussatche, and P. Traktman. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 66:31-124. [DOI] [PubMed] [Google Scholar]
- 19.Da Costa, X. J., M. A. Brockman, E. Alicot, M. Ma, M. B. Fischer, X. Zhou, D. M. Knipe, and M. C. Carroll. 1999. Humoral response to herpes simplex virus is complement-dependent. Proc. Natl. Acad. Sci. USA 96:12708-12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, D. H., X. Liang, J. E. Hernandez, A. Randall, S. Hirst, Y. Mu, K. M. Romero, T. T. Nguyen, M. Kalantari-Dehaghi, S. Crotty, P. Baldi, L. P. Villarreal, and P. L. Felgner. 2005. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA 102:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies, D. H., M. M. McCausland, C. Valdez, D. Huynh, J. E. Hernandez, Y. Mu, S. Hirst, L. Villarreal, P. L. Felgner, and S. Crotty. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies, D. H., D. Molina, J. Wrammert, J. Miller, S. Hirst, Y. Mu, J. Pablo, B. Unal, R. Nakajima-Sasaki, X. Liang, S. Crotty, K. Karem, I. Damon, R. Ahmed, L. Villarreal, and P. Felgner. 2007. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics 7:1678-1686. [DOI] [PubMed] [Google Scholar]
- 23.Davies, D. H., L. S. Wyatt, F. K. Newman, P. L. Earl, S. Chun, J. E. Hernandez, D. Molina, S. Hirst, B. Moss, S. E. Frey, and P. L. Felgner. 2008. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus Ankara is comparable to that of Dryvax. J. Virol. 82:652-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downie, A. W., and K. McCarthy. 1958. The antibody response in man following infection with viruses of the pox group. III. Antibody response in smallpox. J. Hyg. (London) 56:479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duke-Cohan, J. S., K. Wollenick, E. A. Witten, M. S. Seaman, L. R. Baden, R. Dolin, and E. L. Reinherz. 2009. The heterogeneity of human antibody responses to vaccinia virus revealed through use of focused protein arrays. Vaccine 27:1154-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duncan, A. R., and G. Winter. 1988. The binding site for C1q on IgG. Nature 332:738-740. [DOI] [PubMed] [Google Scholar]
- 27.Edghill-Smith, Y., H. Golding, J. Manischewitz, L. R. King, D. Scott, M. Bray, A. Nalca, J. W. Hooper, C. A. Whitehouse, J. E. Schmitz, K. A. Reimann, and G. Franchini. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11:740-747. [DOI] [PubMed] [Google Scholar]
- 28.Engelstad, M., S. T. Howard, and G. L. Smith. 1992. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 188:801-810. [DOI] [PubMed] [Google Scholar]
- 29.Fang, M., H. Cheng, Z. Dai, Z. Bu, and L. J. Sigal. 2006. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology 345:231-243. [DOI] [PubMed] [Google Scholar]
- 30.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 31.Reference deleted.
- 32.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flamand, A., H. Raux, Y. Gaudin, and R. W. Ruigrok. 1993. Mechanisms of rabies virus neutralization. Virology 194:302-313. [DOI] [PubMed] [Google Scholar]
- 34.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogg, C. N., J. L. Americo, P. L. Earl, W. Resch, L. Aldaz-Carroll, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2008. Disparity between levels of in vitro neutralization of vaccinia virus by antibody to the A27 protein and protection of mice against intranasal challenge. J. Virol. 82:8022-8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71-80. [DOI] [PubMed] [Google Scholar]
- 37.Girgis, N. M., B. C. Dehaven, X. Fan, K. M. Viner, M. Shamim, and S. N. Isaacs. 2008. Cell surface expression of the vaccinia virus complement control protein is mediated by interaction with the viral A56 protein and protects infected cells from complement attack. J. Virol. 82:4205-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glass, D., D. Raum, D. Gibson, J. S. Stillman, and P. H. Schur. 1976. Inherited deficiency of the second component of complement. Rheumatic disease associations. J. Clin. Investig. 58:853-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He, Y., J. Manischewitz, C. Meseda, M. Merchlinsky, R. Vassell, L. Sirota, I. Berkower, H. Golding, and C. Weiss. 2007. Antibodies to the A27 protein of vaccinia virus neutralize and protect against infection but represent a minor component of Dryvax vaccine-induced immunity. J. Infect. Dis. 196:1026-1032. [DOI] [PubMed] [Google Scholar]
- 40.Heraud, J. M., Y. Edghill-Smith, V. Ayala, I. Kalisz, J. Parrino, V. S. Kalyanaraman, J. Manischewitz, L. R. King, A. Hryniewicz, C. J. Trindade, M. Hassett, W. P. Tsai, D. Venzon, A. Nalca, M. Vaccari, P. Silvera, M. Bray, B. S. Graham, H. Golding, J. W. Hooper, and G. Franchini. 2006. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 177:2552-2564. [DOI] [PubMed] [Google Scholar]
- 41.Hessell, A. J., L. Hangartner, M. Hunter, C. E. Havenith, F. J. Beurskens, J. M. Bakker, C. M. Lanigan, G. Landucci, D. N. Forthal, P. W. Parren, P. A. Marx, and D. R. Burton. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101-104. [DOI] [PubMed] [Google Scholar]
- 42.Hicks, J. T., F. A. Ennis, E. Kim, and M. Verbonitz. 1978. The importance of an intact complement pathway in recovery from a primary viral infection: influenza in decomplemented and in C5-deficient mice. J. Immunol. 121:1437-1445. [PubMed] [Google Scholar]
- 43.Hobday, T. L. 1962. Antivaccinial gamma-globulin in the control of smallpox. Lancet i:907-908. [DOI] [PubMed] [Google Scholar]
- 44.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329-339. [DOI] [PubMed] [Google Scholar]
- 45.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hopkins, R. J., and J. M. Lane. 2004. Clinical efficacy of intramuscular vaccinia immune globulin: a literature review. Clin. Infect. Dis. 39:819-826. [DOI] [PubMed] [Google Scholar]
- 48.Isaacs, S. N., G. J. Kotwal, and B. Moss. 1992. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. USA 89:628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isaacs, S. N., E. J. Wolffe, L. G. Payne, and B. Moss. 1992. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J. Virol. 66:7217-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishida, I., K. Tomizuka, H. Yoshida, T. Tahara, N. Takahashi, A. Ohguma, S. Tanaka, M. Umehashi, H. Maeda, C. Nozaki, E. Halk, and N. Lonberg. 2002. Production of human monoclonal and polyclonal antibodies in TransChromo animals. Cloning Stem Cells 4:91-102. [DOI] [PubMed] [Google Scholar]
- 51.Johnson, J. B., G. A. Capraro, and G. D. Parks. 2008. Differential mechanisms of complement-mediated neutralization of the closely related paramyxoviruses simian virus 5 and mumps virus. Virology 376:112-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kempe, C. H. 1960. Studies on smallpox and complications of smallpox vaccination. Pediatrics 25:176-189. [PubMed] [Google Scholar]
- 53.Kempe, C. H., T. O. Berge, and B. England. 1956. Hyperimmune vaccinial gamma globulin. Pediatrics 18:177-188. [PubMed] [Google Scholar]
- 54.Kishore, U., and K. B. Reid. 2000. C1q: structure, function, and receptors. Immunopharmacology 49:159-170. [DOI] [PubMed] [Google Scholar]
- 55.Klasse, P. J. 2007. Modeling how many envelope glycoprotein trimers per virion participate in human immunodeficiency virus infectivity and its neutralization by antibody. Virology 369:245-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klasse, P. J., and D. Burton. 2007. Antibodies to West Nile virus: a double-edged sword. Cell Host Microbe 1:87-89. [DOI] [PubMed] [Google Scholar]
- 57.Klasse, P. J., and J. P. Moore. 1996. Quantitative model of antibody- and soluble CD4-mediated neutralization of primary isolates and T-cell line-adapted strains of human immunodeficiency virus type 1. J. Virol. 70:3668-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotwal, G. J., S. N. Isaacs, R. McKenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250:827-830. [DOI] [PubMed] [Google Scholar]
- 59.Kotwal, G. J., and B. Moss. 1988. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335:176-178. [DOI] [PubMed] [Google Scholar]
- 60.Law, M., R. Hollinshead, and G. L. Smith. 2002. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 83:209-222. [DOI] [PubMed] [Google Scholar]
- 61.Law, M., M. M. Pütz, and G. L. Smith. 2005. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J. Gen. Virol. 86:991-1000. [DOI] [PubMed] [Google Scholar]
- 62.Lawrence, S. J., K. R. Lottenbach, F. K. Newman, R. M. Buller, C. J. Bellone, J. J. Chen, G. H. Cohen, R. J. Eisenberg, R. B. Belshe, S. L. Stanley, Jr., and S. E. Frey. 2007. Antibody responses to vaccinia membrane proteins after smallpox vaccination. J. Infect. Dis. 196:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leddy, J. P., R. L. Simons, and R. G. Douglas. 1977. Effect of selective complement deficiency on the rate of neutralization of enveloped viruses by human sera. J. Immunol. 118:28-34. [PubMed] [Google Scholar]
- 64.Lewis, M. J., and M. Botto. 2006. Complement deficiencies in humans and animals: links to autoimmunity. Autoimmunity 39:367-378. [DOI] [PubMed] [Google Scholar]
- 65.Li, L., K. L. Coelingh, and W. J. Britt. 1995. Human cytomegalovirus neutralizing antibody-resistant phenotype is associated with reduced expression of glycoprotein H. J. Virol. 69:6047-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lustig, S., C. Fogg, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 79:13454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30-35. [DOI] [PubMed] [Google Scholar]
- 68.Mack, T. M., J. Noble, Jr., and D. B. Thomas. 1972. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 21:214-218. [DOI] [PubMed] [Google Scholar]
- 69.Marennikova, S. S. 1962. The use of hyperimmune antivaccinia gamma-globulin for the prevention and treatment of smallpox. Bull. W. H. O. 27:325-330. [PMC free article] [PubMed] [Google Scholar]
- 70.Marriott, K. A., C. V. Parkinson, S. I. Morefield, R. Davenport, R. Nichols, and T. P. Monath. 2008. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine 26:581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mehlhop, E., and M. S. Diamond. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 203:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M. S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 79:7466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miletic, V. D., and M. M. Frank. 1995. Complement-immunoglobulin interactions. Curr. Opin. Immunol. 7:41-47. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell, D. A., M. C. Pickering, J. Warren, L. Fossati-Jimack, J. Cortes-Hernandez, H. T. Cook, M. Botto, and M. J. Walport. 2002. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J. Immunol. 168:2538-2543. [DOI] [PubMed] [Google Scholar]
- 75.Moss, B. 2006. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe, P. Howley, D. Giriffin, and R. Lamb (ed.), Fundamental virology, 5th ed. Lippincott Williams & Wilkins, Hagerstown, MD.
- 76.Moulton, E. A., J. P. Atkinson, and R. M. Buller. 2008. Surviving mousepox infection requires the complement system. PLoS Pathog. 4:e1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, M. C. Carroll, H. Hengartner, and R. M. Zinkernagel. 1999. Protective T cell-independent antiviral antibody responses are dependent on complement. J. Exp. Med. 190:1165-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Osofsky, S. G., B. H. Thompson, T. F. Lint, and H. Gewurz. 1977. Hereditary deficiency of the third component of complement in a child with fever, skin rash, and arthralgias: response to transfusion of whole blood. J. Pediatr. 90:180-186. [DOI] [PubMed] [Google Scholar]
- 79.Parren, P. W., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77:195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pickering, M. C., P. Macor, J. Fish, P. Durigutto, F. Bossi, F. Petry, M. Botto, and F. Tedesco. 2008. Complement C1q and C8β deficiency in an individual with recurrent bacterial meningitis and adult-onset systemic lupus erythematosus-like illness. Rheumatology (Oxford) 47:1588-1589. [DOI] [PubMed] [Google Scholar]
- 81.Pierson, T. C., Q. Xu, S. Nelson, T. Oliphant, G. E. Nybakken, D. H. Fremont, and M. S. Diamond. 2007. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1:135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pütz, M. M., I. Alberini, C. M. Midgley, I. Manini, E. Montomoli, and G. L. Smith. 2005. Prevalence of antibodies to vaccinia virus after smallpox vaccination in Italy. J. Gen. Virol. 86:2955-2960. [DOI] [PubMed] [Google Scholar]
- 83.Pütz, M. M., C. M. Midgley, M. Law, and G. L. Smith. 2006. Quantification of antibody responses against multiple antigens of the two infectious forms of vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 12:1310-1315. [DOI] [PubMed] [Google Scholar]
- 84.Radwan, A. I., and D. Burger. 1973. The complement-requiring neutralization of equine arteritis virus by late antisera. Virology 51:71-77. [DOI] [PubMed] [Google Scholar]
- 85.Ramirez, J. C., E. Tapia, and M. Esteban. 2002. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J. Gen. Virol. 83:1059-1067. [DOI] [PubMed] [Google Scholar]
- 86.Ravetch, J. V., and J. P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9:457-492. [DOI] [PubMed] [Google Scholar]
- 87.Reddy, M. P., C. A. Kinney, M. A. Chaikin, A. Payne, J. Fishman-Lobell, P. Tsui, P. R. Dal Monte, M. L. Doyle, M. R. Brigham-Burke, D. Anderson, M. Reff, R. Newman, N. Hanna, R. W. Sweet, and A. Truneh. 2000. Elimination of Fc receptor-dependent effector functions of a modified IgG4 monoclonal antibody to human CD4. J. Immunol. 164:1925-1933. [DOI] [PubMed] [Google Scholar]
- 88.Roden, R. B., E. M. Weissinger, D. W. Henderson, F. Booy, R. Kirnbauer, J. F. Mushinski, D. R. Lowy, and J. T. Schiller. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 68:7570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sahu, A., S. N. Isaacs, A. M. Soulika, and J. D. Lambris. 1998. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 160:5596-5604. [PubMed] [Google Scholar]
- 90.Sakhatskyy, P., S. Wang, T. H. Chou, and S. Lu. 2006. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology 355:164-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sette, A., M. Moutaftsi, J. Moyron-Quiroz, M. M. McCausland, D. H. Davies, R. J. Johnston, B. Peters, M. Rafii-El-Idrissi Benhnia, J. Hoffmann, H. P. Su, K. Singh, D. N. Garboczi, S. Head, H. Grey, P. Felgner, and S. Crotty. 2008. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity 28:847-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sissons, J. G., and M. B. Oldstone. 1980. Killing of virus-infected cells: the role of antiviral antibody and complement in limiting virus infection. J. Infect. Dis. 142:442-448. [DOI] [PubMed] [Google Scholar]
- 93.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 94.Stollar, V. 1975. Immune lysis of Sindbis virus. Virology 66:620-624. [DOI] [PubMed] [Google Scholar]
- 95.Tao, M. H., R. I. Smith, and S. L. Morrison. 1993. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J. Exp. Med. 178:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor, P. R., E. Seixas, M. J. Walport, J. Langhorne, and M. Botto. 2001. Complement contributes to protective immunity against reinfection by Plasmodium chabaudi chabaudi parasites. Infect. Immun. 69:3853-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomizuka, K., T. Shinohara, H. Yoshida, H. Uejima, A. Ohguma, S. Tanaka, K. Sato, M. Oshimura, and I. Ishida. 2000. Double trans-chromosomic mice: maintenance of two individual human chromosome fragments containing Ig heavy and kappa loci and expression of fully human antibodies. Proc. Natl. Acad. Sci. USA 97:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1997. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J. Gen. Virol. 78:2041-2048. [DOI] [PubMed] [Google Scholar]
- 99.Vanderplasschen, A., E. Mathew, M. Hollinshead, R. B. Sim, and G. L. Smith. 1998. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc. Natl. Acad. Sci. USA 95:7544-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wagenaar, T. R., and B. Moss. 2007. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J. Virol. 81:6286-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walker, B. D., and D. R. Burton. 2008. Toward an AIDS vaccine. Science 320:760-764. [DOI] [PubMed] [Google Scholar]
- 102.Walport, M. J. 2002. Complement and systemic lupus erythematosus. Arthritis Res. 4(Suppl. 3):S279-S293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walport, M. J., K. A. Davies, and M. Botto. 1998. C1q and systemic lupus erythematosus. Immunobiology 199:265-285. [DOI] [PubMed] [Google Scholar]
- 104.Ward, B. M., and B. Moss. 2000. Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein. J. Virol. 74:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ward, E. S., and V. Ghetie. 1995. The effector functions of immunoglobulins: implications for therapy. Ther. Immunol. 2:77-94. [PubMed] [Google Scholar]
- 106.Warren, J., P. Mastroeni, G. Dougan, M. Noursadeghi, J. Cohen, M. J. Walport, and M. Botto. 2002. Increased susceptibility of C1q-deficient mice to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 70:551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Welsh, R. M., Jr., P. W. Lampert, P. A. Burner, and M. B. Oldstone. 1976. Antibody-complement interactions with purified lymphocytic choriomeningitis virus. Virology 73:59-71. [DOI] [PubMed] [Google Scholar]
- 108.Wolffe, E. J., S. N. Isaacs, and B. Moss. 1993. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J. Virol. 67:4732-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiao, Y., L. Aldaz-Carroll, A. M. Ortiz, J. C. Whitbeck, E. Alexander, H. Lou, H. L. Davis, T. J. Braciale, R. J. Eisenberg, G. H. Cohen, and S. N. Isaacs. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 25:1214-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]