Abstract
A vector based on Semliki Forest virus (SFV) expressing high levels of interleukin-12 (SFV-enhIL-12) has previously demonstrated potent antitumoral efficacy in small rodents with hepatocellular carcinoma (HCC) induced by transplantation of tumor cells. In the present study, the infectivity and antitumoral/antiviral effects of SFV vectors were evaluated in the clinically more relevant woodchuck model, in which primary HCC is induced by chronic infection with woodchuck hepatitis virus (WHV). Intratumoral injection of SFV vectors expressing luciferase or IL-12 resulted in high reporter gene activity within tumors and cytokine secretion into serum, respectively, demonstrating that SFV vectors infect woodchuck tumor cells. For evaluating antitumoral efficacy, woodchuck tumors were injected with increasing doses of SFV-enhIL-12, and tumor size was measured by ultrasonography following treatment. In five (83%) of six woodchucks, a dose-dependent, partial tumor remission was observed, with reductions in tumor volume of up to 80%, but tumor growth was restored thereafter. Intratumoral treatment further produced transient changes in WHV viremia and antigenemia, with ≥1.5-log10 reductions in serum WHV DNA in half of the woodchucks. Antitumoral and antiviral effects were associated with T-cell responses to tumor and WHV antigens and with expression of CD4 and CD8 markers, gamma interferon, and tumor necrosis factor alpha in peripheral blood mononuclear cells, suggesting that immune responses against WHV and HCC had been induced. These experimental observations suggest that intratumoral administration of SFV-enhIL-12 may represent a strategy for treatment of chronic HBV infection and associated HCC in humans but indicate that this approach could benefit from further improvements.
Hepatocellular carcinoma (HCC) is a major public health problem worldwide, representing the fifth most common type of cancer. HCC is also the third leading cause of cancer-related death, mainly because only surgical and local ablative therapeutic options have shown efficacy in patients with this type of cancer (21). Approximately 80% of all HCC cases are attributed to chronic infection with hepatitis C virus and/or hepatitis B virus (HBV). Chronic carriers of HBV have a greater than 100-fold-increased relative risk of developing HCC compared to HBV-uninfected humans, with an annual incidence rate of 2 to 6% in cirrhotic patients. The high incidence of HCC, together with its poor prognosis and limited therapeutic options, warrants the development of new treatment strategies for this disease.
There is increasing evidence that stimulation of the immune system for subsequent recognition and killing of tumor cells may be a valuable treatment option for liver cancer. In general, HCC appears to be an attractive target for immunotherapy because cases of spontaneous tumor regression have been reported, HCC is often infiltrated with lymphocytes, and HCC-associated proteins such as alpha-fetoprotein may be used as targets for immune-mediated killing of tumors (5, 49).
A promising strategy to stimulate the deficient antitumoral immune response is based on the transfer and subsequent expression of immunostimulatory genes in tumor cells using viral or nonviral delivery vectors. One of the most effective immunostimulatory cytokines is interleukin-12 (IL-12), a protein usually expressed by macrophages and dendritic cells. IL-12 has been demonstrated to induce strong antitumoral effects that are mediated by the stimulation of T-helper cell type 1 (Th1) responses, including the activation of cytolytic T lymphocytes (CTL) and natural killer cells, and by the inhibition of angiognesis (48, 50). All of these effects are dependent on the production of gamma interferon (IFN-γ). Viral vectors that are based on adenovirus have been used to deliver IL-12 into several animal models with transplantable HCC, resulting in a localized expression of this cytokine and usually leading to antitumoral effects (3, 14, 37). However, and despite successful treatment of HCC in preclinical studies, a phase I clinical trial with a first-generation adenoviral vector for delivery and expression of IL-12 in patients with primary and metastatic liver cancer produced only a modest antitumoral effect (41). This poor response was probably due to the low and transient IL-12 expression in tumors. These results in humans indicated a need for vectors with higher potency and for preclinical testing in relevant models of HCC (i.e., large animals with spontaneous tumors).
Vectors based on Semliki Forest virus (SFV), a member of the alphavirus group, are highly efficient in inducing antitumoral responses in a variety of animal models (2, 9, 10, 39, 44, 53). The SFV vector used in the present study is based on a viral RNA genome in which the region coding for the structural proteins has been replaced by a heterologous gene (24). Recombinant SFV RNA can be transcribed in vitro and transfected into cells, resulting in viral replication and subsequent production of a subgenomic RNA from which the heterologous protein is expressed at very high levels. Recombinant SFV RNA can be packaged into viral particles (vp) by cotransfecting it into cells together with two helper RNAs coding for the capsid and the envelope proteins (43). Compared to adenoviral vectors expressing IL-12, tumor treatment with SFV vectors expressing the same cytokine resulted in greater antitumoral effects in a murine colon adenocarcinoma model and also in a rat orthotopic HCC model (16, 39). The greater antitumoral effect mediated by SFV vectors has been attributed to the higher expression of IL-12 and to the induction of apoptosis caused by SFV replication within tumor cells. Apoptosis leads to the release of tumor antigens that can be taken up by antigen-presenting cells, thereby potentiating the antitumoral response induced by IL-12 (54). Furthermore, SFV vectors have low immunogenicity when delivered intratumorally, allowing repetitive administrations into the same tumor, which is not possible with adenoviral vectors (38).
In the present study, the antitumoral efficacy of an SFV vector expressing IL-12 (SFV-enhIL-12) was investigated in woodchucks with HCC. The Eastern woodchuck (Marmota monax) is frequently infected with the woodchuck hepatitis virus (WHV), which is closely related to the human HBV in its structure, genomic organization, mechanism of replication, and course of infection (29). The woodchuck has been used as a mammalian model for research on HBV, including the pathogenesis of acute and chronic HBV infection, and for preclinical evaluation of the safety and efficacy of candidate antiviral drugs and therapeutic immunomodulators for the treatment of chronic HBV infection (29) and prevention of HCC (47).
All woodchucks chronically infected with WHV as neonates develop HCC, and the median time for tumor appearance is 24 months of age (34, 47). After identification of HCC, the median survival time of woodchucks is 6 months, a situation similar to that for patients with HCC. In addition, WHV-induced hepatocarcinogenesis shows strong similarity to HBV-induced carcinogenesis in humans (34, 47). These features of HCC that are associated with persistent hepatitis virus infection make the woodchuck model unique compared to other animal models, in which HCC is induced by a chemical carcinogen or by transplantation of established tumor cell lines into immune-deficient or immune-compatible hosts. Woodchucks with large liver tumors that acquire malignant characteristics in a stepwise process similar to HCC in humans are an attractive and suitable model for the preclinical evaluation of new treatment strategies for HBV-induced HCC in humans (47).
The antitumoral efficacy of a SFV vector expressing high levels of IL-12 (SFV-enhIL-12) was investigated in six woodchucks with established chronic WHV infection and primary HCC. The results demonstrate that SFV-delivered IL-12 expression produced a dose-dependent, partial tumor remission that was associated with a general activation of cellular immune responses against HCC. The antitumoral activity, in addition to an antiviral activity against WHV, and the favorable safety profile in woodchucks suggest that a therapeutic approach based on SFV-enhIL-12 may represent a treatment strategy for HCC in patients with chronic HBV infection, but the overall results also indicate that this approach needs further improvement for inducing a complete tumor remission.
MATERIALS AND METHODS
Cell lines and animals.
The BHK-21 cell line (ATCC CCL-10) was cultured in Glasgow minimum essential medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (Invitrogen) as described previously (39). The woodchuck HCC-derived cell line WCH17 (ATCC CRL-2082) and the human HCC-derived cell lines HepB3 (ATCC HB-8064) and Huh7 (our laboratory stock) were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen).
All experimental procedures involving woodchucks were performed under protocols approved by the Cornell University Institutional Animal Care and Use Committee. Woodchucks were born to WHV-negative females and reared in environmentally controlled laboratory animal facilities at Cornell University. Woodchucks were inoculated at 3 days of age by subcutaneous inoculation with 5 × 106 50% woodchuck infectious doses of a standardized WHV inoculum (cWHV7P2) (11). Persistence of WHV infection was based on the consecutive detection of WHV DNA and WHV surface antigen (WHsAg) in serum from 3 months of age. All chronic WHV carrier woodchucks had developed HCC at the beginning of the study as determined by hepatic ultrasound examination and elevated serum of γ-glutamyl-transferase (GGT) activity (≥11 IU/liter, compared to 3 to 4 IU/liter in chronic WHV carrier woodchucks without HCC).
Cell transfection and virus production.
Plasmids pSFV-Luc and pSFV-enhIL-12 and the generation of recombinant SFV vp in BHK-21 cells have been described previously (39). Plasmid pSFV3-LacZ was kindly provided by P. Liljeström (Karolinska Institute, Stockholm, Sweden) (24). RNA synthesis from SFV plasmids, transfection into BHK-21 cells by electroporation, and packaging of recombinant RNA into SFV vp were performed as described previously (39, 43). Briefly, BHK-21 cells were coelectroporated with the recombinant RNA, together with two helper RNAs (i.e., SFV-helper-C-S219A and SFV-helper-S2 RNAs), which provided in trans the capsid and the spike proteins. SFV vp were harvested and purified by ultracentrifugation as described previously (15). Indirect immunofluorescence was applied on SFV-infected BHK-21 cells to determine the titer of SFV-Luc, SFV-LacZ, and SFV-enhIL-12 recombinant virus stocks as described previously (40). A rabbit polyclonal antiserum specific for the nsp2 subunit of the SFV replicase was used as the primary antibody (6).
Analysis of SFV-LacZ infectivity and β-galactosidase expression in vitro.
BHK-21 or HCC-derived cell lines were infected in duplicate plates with serial dilutions of the same SFV-LacZ virus stock. Twenty-four hours later, cells from one dish were lysed and the amount of β-galactosidase protein present in the lysate was measured as described previously (23). Cells from the duplicate dish were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Invitrogen), and the number of cells infected with SFV-LacZ was determined. The amount of β-galactosidase protein produced per cell was calculated for each virus dilution by dividing the amount of β-galactosidase protein detected per dish by the number of SFV-LacZ-infected cells in each dish.
Analysis of luciferase and IL-12 expression in vivo.
Four woodchucks with HCC were used to determine that the expression of luciferase and IL-12 in hepatic tumors is dependent on the SFV dose and to verify that intratumoral IL-12 expression mediates increases in the levels of type I and II IFNs. Animals were anesthetized by intramuscular injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). A blood sample was obtained, followed by laparotomy to expose the liver. Vector SFV-Luc or SFV-enhIL-12, each in a total volume of 0.6 ml, was administered intratumorally into one hepatic tumor at 10 separate sites. The first woodchuck received 1 × 109 vp of SFV-Luc, whereas the second woodchuck was administered a threefold-higher dose of SFV-Luc (i.e., 3 × 109 vp). The latter woodchuck also received 3 × 109 vp of SFV-enhIL-12 into another hepatic tumor. The third woodchuck was administered a fourfold-higher dose of SFV-enhIL-12 (i.e., 1.2 × 1010 vp). The fourth woodchuck was left untreated, and the hepatic tumor from this animal served as a control. Twenty-four hours later, woodchucks were euthanized, a blood sample was obtained, and tissues (hepatic tumors, adjacent liver tissues, and other organs) were excised and snap-frozen in liquid nitrogen. Following homogenization in lysis buffer (phosphate-buffered saline containing 0.05% [vol/vol] Tween20 and protease inhibitor cocktail tablets [Complete; Roche, Basel, Switzerland]), samples were centrifuged twice for 10 min at 9,300 × g. Luciferase activity in the supernatants was determined using a conventional luminometer. IL-12 concentrations were determined in supernatants and in serum using a mouse p70-specific enzyme-linked immunosorbent assay kit (BD Bioscience, Franklin Lakes, NJ). The amount of total protein present in tissue homogenates was quantified using a standard Bradford assay. The expression of mRNAs for IFN-α and IFN-γ in hepatic tumor tissues was determined by a real-time reverse transcription-PCR-based assay (see below).
Antitumoral study.
Nine woodchucks of both sexes, 20 to 30 months of age, with one or more hepatic tumors between 1.3 and 3.4 cm in diameter were used in this study. Two weeks prior to the start of the study, hepatic tumors for intratumoral administration of SFV-enhIL-12 or saline placebo were identified by ultrasonography (US) and confirmed by computed tomography (CT) while woodchucks were under general anesthesia (ketamine [50 mg/kg] and xylazine [5 mg/kg] intramuscularly). On the day of treatment (week 0), median laparotomies were performed on anesthetized woodchucks, and grossly identifiable tumors were located, photographed, and measured with calipers before the injection of vector or placebo. Woodchucks then received a single dose of 3 × 109 vp (SFV-1 and SFV-2), 6 × 109 vp (SFV-3 and SFV-4), or 1.2 × 1010 vp (SFV-5 and SFV-6) of SFV-enhIL-12 or the same volume (0.6 ml) of saline (control-1, control-2, and control-3) by intratumoral injections into 10 separate sites of one tumor. Woodchucks were followed for 23 to 24 weeks and were euthanized thereafter. During necropsy, the liver was exposed, and hepatic tumors were photographed and measured.
Analysis of tumor size.
The sizes of tumors treated with SFV-enhIL-12 or placebo were determined by US and direct caliper measurements. For US, the Aloka ProSound model SSD-3500 high-frequency ultrasound system (Wallingford, CT) was used. Before each imaging session, the woodchuck abdomen was shaved to remove any hair. US imaging gel was applied to the abdomen to improve the contact with the transducer and to obtain a clearer image. US is strongly reflected by the ribcage, which hinders imaging of any tissue located beneath the ribs, such as the lungs and a portion of the liver. Thus, the liver tissue accessible for US imaging may vary between woodchucks and between imaging sessions for the same woodchuck. In general, the left lateral, left medial, and right medial liver lobes were routinely accessible for imaging. During imaging, two-dimensional images were acquired in the transverse (cranial/caudal [Cr/C]), coronal (dorsal/ventral [D/V]), and sagittal (left/right [L/R]) planes.
Tumor size was determined by US at week 0 prior to the administration of SFV-enhIL-12 or placebo and then at 2, 4, 6, 10, 14, 18, and 23 to 24 weeks posttreatment. Tumor diameter measurements were obtained from the digital images captured on the hard drive of the US machine and printed on the integral image printer at the time of the study. The transducer was moved until the image showing the greatest tumor diameter was located. The image was frozen, and two points on opposite sides along the circumference of the tumor were marked. The integrated software then calculated the distance connecting the two points. Two additional points along the circumference of the tumor which describe a diameter perpendicular to the first diameter were then located and marked. The integrated software then calculated the distance connecting these two points. In this way, measurements of the Cr/C and D/V diameters were made. The transducer was then rotated 90 degrees and the procedure repeated. The second set of measurements included the L/R and a repeat of the D/V diameters. For calculating the mean tumor diameter, the two separate measurements of the D/V diameter were averaged, followed by averaging the mean D/V diameter and the measurements of the Cr/C and L/R diameters.
Tumor size also was determined by direct caliper measurements during laparotomy at week 0 and during necropsy at the end of the study. From woodchucks that were euthanized because of seizures, tumor measurements were also available during subsequent necropsy. The Cr/C, D/V, and L/R diameters were measured and averaged to calculate the mean tumor diameter. Tumor size also was confirmed by CT prior to the start of the study, whenever possible between 6 and 10 weeks posttreatment, and at the end of the study at weeks 23 to 24.
The tumor volume was calculated based on the diameters obtained by US using the formula V = πabc/6, where a is the mean D/V diameter, b is the Cr/C diameter, and c is the L/R diameter. This formula was chosen because most large liver tumors (i.e., ≥1.5 cm in diameter) in woodchucks are irregular and closely approximate an ellipsoid form.
Analysis of WHV serum markers.
Blood samples were obtained for WHV DNA analysis and serological testing prior to the start of the study; at week 0 prior to the administration of SFV-enhIL-12 or placebo; and then at 2, 4, 6, 10, 14, 18, and 23 to 24 weeks posttreatment. WHV DNA in serum was determined quantitatively by dot blot hybridization (assay sensitivity, ≥1.0 × 107 WHV genome equivalents per ml (28). WHsAg in serum was determined qualitatively by enzyme-linked immunosorbent assay using dilutions of serum. (12) The cutoff value of this assay was defined as ≥0.05 optical density unit.
Analysis of T-cell responses.
For determining T-cell responses against WHV, in vitro stimulators were used at concentrations previously determined as optimal for cultures of woodchuck peripheral blood mononuclear cells (PBMCs) (31, 32). Viral antigen stimulators consisted of native 22-nm WHsAg (2 μg/ml) and synthetic peptides of WHsAg and WHcAg. WHs peptide S18 (10 μg/ml; Sigma-Genosys, The Woodlands, TX) corresponded to amino acids 341 to 360 of WHsAg starting at the N terminus of the large surface protein (32). WHc peptide C100-119 (10 μg/ml; Sigma-Genosys) corresponded to amino acids 100 to 119 of WHcAg starting at the N terminus of WHcAg (31). Culture medium and a peptide unrelated to WHV (10 μg/ml; Sigma-Genosys) were used as negative controls.
Recombinant proteins that present tumor antigens in woodchuck liver were not available for determining antitumoral T-cell responses. To circumvent this limitation and because other studies have used soluble protein fractions of tumor tissues for the detection of antitumoral T-cell responses, (see, e.g., references 8, 17, and 42), cell lysates of tumor (neoplastic) and normal (nonneoplastic) liver tissues from five chronic WHV carrier woodchucks with HCC were used as stimulators. Briefly, tissues were cut with sterile scalpels into small pieces, placed in plastic vials, and homogenized using plastic pestles and buffer (phosphate-buffered saline containing 0.001% [vol/vol] β-mercaptoethanol [Sigma], 5% [wt/vol] collagenase [Sigma], and 50 units/ml DNase I [Invitrogen]). Homogenates were filtered using a 70-μm filter (BD Bioscience) and centrifuged twice for 15 min at 9,300 × g. Following the last centrifugation, clear supernatant from neoplastic or nonneoplastic liver tissue samples was combined, and protein concentrations were determined using a standard Bradford assay (Pierce, Rockford, IL).
Preliminary testing of these cell lysates at concentrations ranging from 0.5 to 10.0 μg total protein per ml using PBMCs from three WHV-negative woodchucks indicated that background proliferation following stimulation with nonneoplastic or neoplastic liver cell lysates was low compared to that for medium alone (e.g., the mean counts per minute following stimulation with nonneoplastic or neoplastic liver cell lysates at a concentration of 1.0 μg total protein per ml were only 1.3- and 1.5-fold higher, respectively, than those obtained with medium alone).
Neoplastic liver cell lysate at concentrations of 0.5, 1.0, and 2.0 μg total protein per ml then was used as a tumor antigen stimulator for testing antitumoral T-cell responses in woodchucks following treatment with SFV-enhIL-12 or placebo. Nonneoplastic liver cell lysate at concentrations of 0.5, 1.0, and 2.0 μg total protein per ml was used as a stimulator to control for nonspecific (negative) T-cell responses to tumor antigens.
The in vitro proliferation assay using woodchuck PBMCs is comparable to those performed in human studies, except that dividing cells were labeled with [2-3H]adenine (Amersham Pharmacia Biotech, Inc., Arlington Heights, IL) rather than [3H]thymidine because of a deficiency in woodchuck thymidine kinase 1 transcription (26). Woodchuck PBMCs were isolated from whole blood and stimulated as described previously (32). The cpm of triplicate PBMC cultures were averaged and expressed as a stimulation index (SI) by dividing the average sample cpm in the presence of the stimulator by that observed in the absence of stimulator (six replicates). A SI of ≥3.1 was considered to represent a positive, specific T-cell response (32).
Analysis of leukocyte surface marker and cytokine expression.
The expression of mRNAs for the woodchuck leukocyte surface markers CD3, CD4, and CD8 and the cytokines IFN-α, IFN-γ, tumor necrosis factor alpha (TNF-α), IL-6, and IL-12 was determined in vitro by a real-time reverse transcription-PCR-based assay (13, 30, 32). Hepatic tumor tissues were excised, snap-frozen in liquid nitrogen, and homogenized, and total RNA was isolated using the RNeasy kit from Qiagen (Valencia, CA). PBMCs were cultured in the presence of medium, WHsAg (2 μg/ml), tumor (neoplastic) cell lysate (1 μg/ml), or normal (nonneoplastic) liver cell lysate (1 μg/ml) and collected after 2 1/2 days. PBMCs then were lysed and total RNA isolated using the RNeasy kit (Qiagen). Following treatment with DNase I (Invitrogen), RNA was reverse transcribed into cDNA with MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA) using random hexamers. Triplicates of cDNA were amplified on an ABI Prism 7000 sequence detection instrument (Applied Biosystems) using SYBR green master mix (Applied Biosystems) and woodchuck-specific primers. Woodchuck β-actin mRNA expression was used to normalize target gene expression (13, 30). Transcription levels of target genes were determined by the formula 2ΔCT, where ΔCT indicates the difference in the threshold cycle between β-actin and target gene expression. Results were represented as fold change of the transcription level in unstimulated PBMC cultures at each time point. A fold change of ≥3.1 was considered to represent positive, specific expression (30).
Evaluation of liver toxicity.
The general health of woodchucks was evaluated daily by observation of appearance, general behavior, and food and water intake. Each time woodchucks were anesthetized and bled, body weight was recorded. Complete blood counts and serum chemistry measurements were performed prior to the start of the study, at week 0 prior to the administration of SFV-enhIL-12 or placebo, at 6 and 14 weeks posttreatment, and at the end of the study. Serum chemistry measurements included GGT, alkaline phosphatase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), sorbitol dehydrogenase (SDH), total bilirubin, albumin, blood urea nitrogen, creatinine, Na+, K+, Cl−, bicarbonate, total serum iron, iron binding capacity, and percent iron saturation (28). Serum AST, ALT, and SDH activities are markers of hepatocellular injury in woodchucks. Serum GGT is a marker of HCC.
RESULTS
Infection of woodchuck hepatic tumor cells with SFV vectors results in reporter gene expression and IL-12 secretion.
Although SFV vectors have a broad host and cell tropism, their infectivity in woodchucks has not been determined. The infectivity of an SFV vector carrying the gene for β-galactosidase (SFV-LacZ) was first tested in vitro using the woodchuck HCC-derived cell line WCH17. SFV infectivity and β-galactosidase protein expression in woodchuck cells were compared to those in the human HCC-derived cell lines Huh7 and HepB3. BHK-21 cells were used as a positive control because they are infected efficiently by SFV. As shown in Table 1, the SFV-LacZ vector was able to infect WCH17 cells, but the infection efficiency was 22- to 35-fold lower than that in Huh7 and HepB3 cells. In addition, β-galactosidase protein expression per cell was 3- to 10-fold lower in the woodchuck HCC-derived cell line than in the human HCC-derived cell lines (Table 1).
TABLE 1.
In vitro infectivity of SFV-LacZ and β-galactosidase protein expression in woodchuck and human HCC-derived cell lines
| Cell line | Cell origin | Infectivity (%)a | β-Galactosidase protein expression (pg/cell) |
|---|---|---|---|
| BHK-21 | Kidney fibroblast (hamster) | 100.0 | 55.9 |
| WCH17 | HCC (woodchuck) | 0.3 | 1.4 |
| Huh7 | HCC (human) | 6.7 | 14.4 |
| HepB3 | HCC (human) | 10.6 | 5.0 |
SFV infectivity in HCC-derived cell lines is presented as a percentage of the SFV infectivity in BHK-21 cells, which was set at 100%.
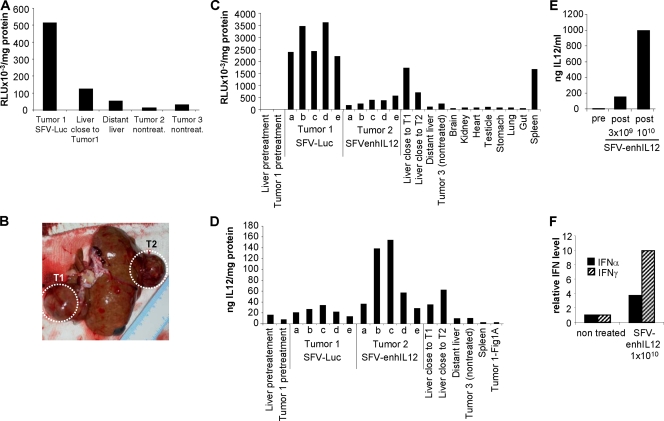
In order to test if SFV vectors can infect in vivo woodchuck hepatic tumor cells, two chronic WHV carrier woodchucks with HCC were selected, each having three tumors with sizes between 1 and 3 cm in diameter. The first woodchuck received intratumorally 1 × 109 vp of an SFV vector expressing luciferase (SFV-Luc) into one tumor (tumor 1), whereas the other two tumors (tumors 2 and 3) were left untreated (Fig. 1A). This woodchuck was euthanized 24 h later, and luciferase activity in the treated and untreated tumors and in surrounding liver tissue was measured. Luciferase activity was detected at high levels only in the treated tumor, demonstrating that transgene expression was mainly confined to the area of injection.
FIG. 1.
Infectivity of SFV vectors in chronic WHV carrier woodchucks with HCC. (A) One woodchuck received intratumorally 1 × 109 vp of SFV-Luc into one tumor with a size of approximately 3 cm in diameter (tumor 1). Two other tumors with sizes between approximately 1 and 3 cm in diameter were left untreated (tumors 2 and 3). Luciferase activity in tumor and liver homogenates was determined 24 h later. (B) A second woodchuck received intratumorally 3 × 109 vp of SFV-Luc into one tumor (tumor 1 [T1]) and 3 × 109 vp of SFV-enhIL-12 into another tumor (T2). The picture shows the liver at necropsy, and SFV-treated tumors (T1 and T2) are indicated by dotted circles. Both tumors had sizes of approximately 2 cm in diameter. A third tumor (tumor 3) with a size of 3 cm was left untreated (not visible in the picture). (C and D) Luciferase activity (C) and IL-12 protein concentrations (D) in homogenates of tumors, liver, and organs from the second woodchuck were determined 24 h later as indicated. Tumors 1 and 2 were divided into five sections (a to e), and each section was analyzed separately for luciferase activity and IL-12 protein concentration. SFV-Luc-treated tumor 1 from the first woodchuck (A) was used as an additional negative control for the analysis of intratumoral IL-12 expression (D). (E) The IL-12 protein concentrations in serum in the second woodchuck and in a third woodchuck that received 1.2 × 1010 vp of SFV-enhIL-12 into one tumor with a size of approximately 0.7 cm in diameter were determined 24 h later. (F) Tumor homogenates from the third woodchuck and from an untreated control woodchuck with one tumor with a size of approximately 1 cm in diameter were analyzed 24 h later for the expression of IFN-α and IFN-γ mRNAs. Pre, pretreatment; post, posttreatment.
The second woodchuck received intratumorally 3 × 109 vp of SFV-Luc into one tumor (tumor 1) and 3 × 109 vp of an SFV vector expressing murine IL-12 (SFV-enhIL-12) into a second tumor (tumor 2), whereas a third tumor was left untreated (Fig. 1B). Following euthanasia 24 h later, luciferase activity and IL-12 expression in treated and untreated tumors, adjacent liver tissues, and several other organs were measured, as presented in Fig. 1C and D. Luciferase activity was elevated in all sections of tumor 1 and was five- to sevenfold higher than in the first woodchuck, which had received a threefold-lower dose of SFV-Luc (Fig. 1A and C). Luciferase activity was low in all other tissues analyzed, with the exception of liver tissue surrounding tumor 1 and spleen tissue. In this woodchuck, IL-12 protein was detected at high concentrations in two sections of tumor 2 treated with SFV-enhIL-12 (Fig. 1D). Lower IL-12 concentrations were also detected in other sections of tumor 2, in proximal liver tissue, and in tumor 1 treated with SFV-Luc. The SFV-Luc-treated tumor 1 from the first woodchuck was used as an additional negative control, and IL-12 protein was detectable only at background level (Fig. 1A and D). These results and the additional observation that IL-12 was detected in serum of the second woodchuck at a concentration of 152 ng/ml (Fig. 1E) suggest that this cytokine was secreted from neoplastic cells of tumor 2 following infection with SFV-enhIL-12.
For establishing a correlation between the dose of SFV-enhIL-12 administered into a hepatic tumor and the IL-12 concentration detected thereafter in serum, a third woodchuck received intratumorally 1.2 × 1010 vp of SFV-enhIL-12 into one tumor with a size of approximately 0.7 cm in diameter (Fig. 1E). The serum IL-12 concentration determined 24 h later was 993 ng/ml. This cytokine level was approximately sevenfold higher than that in the second woodchuck, which had received a fourfold-lower dose of SFV-enhIL-12, suggesting that the IL-12 level in serum is dependent on the SFV dose administered.
In order to verify that IL-12 expression from SFV-enhIL-12 induces increases in the levels of type I and II IFNs, tumor tissue from the third woodchuck was also analyzed for the expression of IFN-α and IFN-γ mRNAs (Fig. 1F). Tumor tissue from a fourth woodchuck with one untreated tumor with a size of approximately 1.0 cm in diameter was used as a control. Twenty-four hours after intratumoral administration of SFV-enhIL-12, the IFN-α and IFN-γ mRNA expression levels in the SFV-treated tumor were 4- and 10-fold higher, respectively, than those in the control tumor, suggesting that the activation of any antitumoral or antiviral immune responses by SFV-enhIL-12 could be mediated via the induction of both cytokines.
HCC treatment with SFV-enhIL-12 results in partial tumor remission.
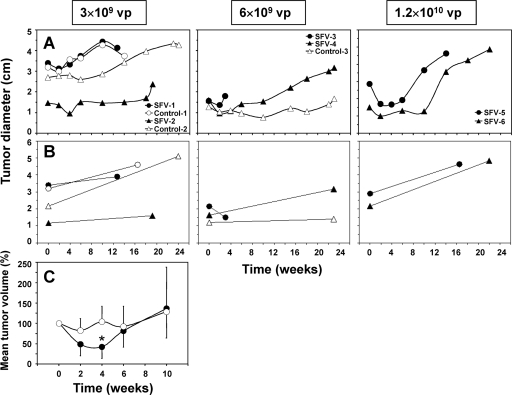
For evaluating the antitumoral efficacy of a SFV vector expressing murine IL-12 on established, nontransplanted liver tumors, pairs of woodchucks were treated intratumorally with single doses of 3 × 109, 6 × 109, or 1.2 × 1010 vp of SFV-enhIL-12. Three other woodchucks were injected intratumorally with saline and served as placebo-treated controls. Tumor size was determined by US every 2 to 4 weeks (Fig. 2A). Tumor size was also measured with calipers at the day of treatment during laparotomy and again at the end of the study during necropsy (Fig. 2B).
FIG. 2.
Tumor sizes in chronic WHV carrier woodchucks following intratumoral treatment with increasing doses of SFV-enhIL-12. Pairs of woodchucks received intratumorally single doses of 3 × 109 vp (SFV-1 and SFV-2), 6 × 109 vp (SFV-3 and SFV-4), or 1.2 × 1010 vp (SFV-5 and SFV-6) of SFV-enhIL-12 into one hepatic tumor of between 1.3 and 3.4 cm in diameter. Control woodchucks received intratumorally a single dose of saline as placebo into one hepatic tumor of between 1.3 and 3.2 cm in diameter (Control-1, Control-2, and Control-3). (A and B) Tumor sizes were determined by US every 2 to 4 weeks (A) and by direct caliper measurements at the day of treatment during laparotomy and at the end of the study during necropsy (B). Woodchuck SFV-3 was euthanized 3 weeks after treatment because of seizures. (C) Mean changes in tumor volumes in SFV- and saline-treated woodchucks based on US measurements between weeks 0 and 10. Changes are expressed as a percentage of the tumor volume at pretreatment, which was set at 100%. Mean percentages and standard deviations are displayed. The asterisk indicates that the mean tumor volume in woodchucks treated with SFV-enhIL-12 was statistically different from that in placebo-treated control woodchucks at 4 weeks posttreatment (P < 0.05; two-tailed Student's t test).
A dose-dependent, transient reduction in tumor size was observed by US in all woodchucks treated with SFV-enhIL-12. Reductions started immediately following treatment, were maximal between 2 and 4 weeks posttreatment, and lasted for another 2 to 8 weeks thereafter. Tumor remission in SFV-treated woodchucks, however, was only partial, and tumor growth was restored between 6 and 14 weeks posttreatment. One of two woodchucks (SFV-3) treated with the intermediate dose of SFV-enhIL-12 was euthanized at 3 weeks posttreatment due to seizures, but this animal also demonstrated a remarkable reduction in tumor size (Fig. 2B). On average, treatment with SFV resulted in a reduction of tumor volume of 58% at 4 weeks posttreatment (Fig. 2C), with four of six animals demonstrating reductions of >70%. The difference in mean tumor volume between SFV-treated and control woodchucks was statistically significant at 4 weeks posttreatment (P < 0.05; two-tailed Student's t test).
Partial tumor remission is associated with activation of antitumoral immune responses.
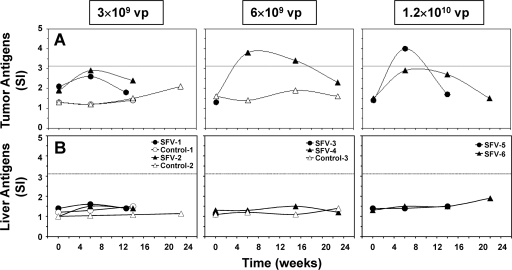
Cellular immune responses elicited in woodchucks following treatment of HCC with SFV-enhIL-12 were determined in vitro by proliferation of T cells and expression of leukocyte surface markers and Th1 cytokines in PBMC cultures in response to stimulation with tumor antigens. Recombinant proteins that present tumor antigens in woodchuck liver were not available; therefore, a combined cell lysate of neoplastic liver tissues from five chronic WHV carrier woodchucks with HCC was used for cell stimulation.
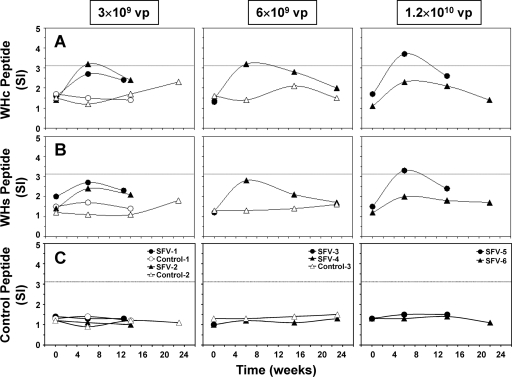
Prior to treatment with SFV-enhIL-12, T-cell responses to tumor antigens were essentially undetectable in chronic WHV carrier woodchucks with HCC, because SIs were below the assay cutoff value of ≥3.1 (Fig. 3A). Following treatment with SFV-enhIL-12, all woodchucks had increases in their T-cell response to tumor antigens, and in two, T-cell response became positive, with SIs of ≥3.1. The T-cell response was transient, peaked around the time of maximum tumor remission, and declined thereafter (Fig. 2 and 3). In contrast, none of the control woodchucks had comparable changes in the tumor antigen-specific T-cell response. Liver antigens (i.e., a combined cell lysate of nonneoplastic liver tissues from the same woodchucks described above) were used as a control stimulator, and T-cell responses against these antigens were not detected in any woodchuck, demonstrating the specificity of the antitumoral T-cell response following treatment with SFV-enhIL-12 (Fig. 3B).
FIG. 3.
Antitumoral T-cell responses of chronic WHV carrier woodchucks following intratumoral treatment with increasing doses of SFV-enhIL-12. (A) T-cell responses to tumor antigens (i.e., clear supernatant of a combined cell lysate of neoplastic liver tissues from five chronic WHV carrier woodchucks with HCC at a concentration of 1.0 μg/ml). (B) T-cell responses to liver antigens (i.e., clear supernatant of a combined cell lysate of nonneoplastic liver tissues from the same woodchucks described above at a concentration of 1.0 μg/ml). The T-cell response was considered positive if the SI was ≥3.1, as indicated by the dotted line.
The results for antitumoral T-cell responses were extended by determining the mRNA expression of leukocyte surface markers and cytokines in PBMC cultures stimulated with tumor antigens (Table 2). Compared to the pretreatment expression, treatment with SFV-enhIL-12 resulted in increased expression (≥2.1-fold above the mRNA expression in unstimulated PBMC cultures but below the assay cutoff value of ≥3.1) or positive expression (≥3.1) of CD3, CD4, and CD8 in all woodchucks analyzed at 6 weeks posttreatment. At this time point the expression of IFN-γ was positive in tumor antigen-stimulated PBMC cultures from all SFV-treated woodchucks analyzed. Elevated IFN-γ expression correlated with increases in the expression of TNF-α, IL-6, and woodchuck IL-12, and expression of these cytokines was positive in both woodchucks treated with the highest dose of SFV-enhIL-12. Expression of leukocyte surface markers and cytokines was transient, peaked around the time of maximum tumor remission, and declined thereafter (Fig. 2 and Table 2). Comparable changes in leukocyte surface marker and cytokine expression were not observed in tumor antigen-stimulated PBMC cultures from control woodchucks and in liver antigen-stimulated PBMC cultures from all woodchucks (Table 2), demonstrating again the specific activation of antitumoral immune responses following treatment with SFV-enhIL-12.
TABLE 2.
Tumor and liver antigen-specific expression of leukocyte surface markers and cytokines in woodchucks following intratumoral administration of SFV-enhIL-12 at increasing doses
| Antigen | Woodchuck | SFV dose (vp) | Wk | Fold change in mRNA expression in antigen-stimulated PBMC culturesa |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD3 | CD4 | CD8 | IFN-γ | TNF-α | IL-6 | IL-12 | ||||
| Tumor | SFV-2 | 3 × 109 | 0 | 1.2 | 1.1 | 1.2 | 1.4 | 1.3 | 1.5 | 1.3 |
| 6 | 3.0 | 2.2 | 3.1 | 10.7 | 4.1 | 2.4 | 3.4 | |||
| 14 | 2.1 | 1.7 | 2.0 | 4.5 | 2.2 | 2.6 | 2.3 | |||
| SFV-4 | 6 × 109 | 0 | 1.4 | 1.4 | 1.7 | 1.6 | 1.4 | 1.4 | 1.5 | |
| 6 | 2.6 | 2.1 | 2.7 | 3.7 | 2.8 | 2.9 | 2.7 | |||
| 15 | 1.8 | 1.8 | 1.4 | 2.0 | 1.7 | 1.7 | 1.8 | |||
| SFV-5 | 1.2 × 1010 | 0 | 1.7 | 1.2 | 1.5 | 1.4 | 1.2 | 1.1 | 1.4 | |
| 6 | 6.3 | 6.9 | 5.2 | 9.0 | 4.4 | 3.9 | 4.0 | |||
| 14 | 1.6 | 1.1 | 1.2 | 1.4 | 1.3 | 1.3 | 1.6 | |||
| SFV-6 | 1.2 × 1010 | 0 | 1.2 | 1.4 | 1.2 | 1.1 | 1.0 | ND | ND | |
| 6 | 3.7 | 3.3 | 3.8 | 4.1 | 3.2 | 4.0 | 4.5 | |||
| 14 | 1.7 | 1.8 | 2.0 | 1.6 | 2.0 | 1.8 | 2.2 | |||
| 22 | 1.5 | 1.5 | 1.5 | 1.1 | 1.7 | 1.3 | 1.4 | |||
| Control-2 | 0 | 0 | 0.9 | 1.0 | 1.0 | ND | 1.2 | 1.0 | 1.4 | |
| 6 | 1.0 | 1.2 | 1.0 | 1.1 | 1.0 | 1.8 | 1.1 | |||
| 14 | 1.8 | 1.7 | 1.3 | 2.4 | 1.6 | 1.6 | 1.6 | |||
| Control-3 | 0 | 0 | 1.3 | 1.3 | 1.1 | 1.5 | 1.2 | 1.2 | 1.3 | |
| 6 | 1.3 | 1.2 | 1.3 | 1.3 | 1.3 | 1.5 | 1.3 | |||
| 15 | 1.3 | 1.3 | 1.3 | 1.6 | 1.2 | 1.6 | 1.4 | |||
| Liver | SFV-2 | 3 × 109 | 0 | 1.2 | 1.3 | 1.1 | 1.5 | 1.0 | 1.1 | 1.3 |
| 6 | 1.8 | 1.6 | 1.6 | 2.4 | 1.9 | 1.6 | 1.8 | |||
| 14 | 1.2 | 1.1 | 1.1 | 1.9 | 1.4 | 1.5 | 1.4 | |||
| SFV-4 | 6 × 109 | 0 | 1.2 | 1.1 | 1.3 | 1.3 | 1.1 | 1.4 | 1.4 | |
| 6 | 1.6 | 1.5 | 1.8 | 1.9 | 1.7 | 1.6 | 1.9 | |||
| 15 | 1.6 | 1.4 | 1.3 | 1.4 | 1.5 | 1.5 | 1.6 | |||
| SFV-5 | 1.2 × 1010 | 0 | 1.2 | 1.5 | 1.1 | 1.0 | 1.1 | 1.0 | 1.1 | |
| 6 | 1.4 | 1.4 | 1.1 | 1.7 | 1.5 | 1.2 | 1.6 | |||
| 14 | 1.2 | 1.0 | 1.1 | 1.1 | 1.2 | 1.2 | 1.2 | |||
| SFV-6 | 1.2 × 1010 | 0 | 1.3 | 1.1 | 1.1 | 1.0 | 1.1 | 1.2 | 1.1 | |
| 6 | 1.4 | 1.3 | 1.1 | 1.5 | 1.4 | 1.2 | 1.1 | |||
| 14 | 1.2 | 1.3 | 1.4 | 1.6 | 1.1 | 1.2 | 1.4 | |||
| 22 | 1.0 | 1.1 | 1.2 | 0.9 | 1.0 | 1.3 | ND | |||
| Control-2 | 0 | 0 | 0.9 | 1.0 | 1.0 | ND | 1.3 | 1.0 | 1.1 | |
| 6 | 1.6 | 1.3 | 1.3 | 1.5 | 1.5 | 1.6 | 1.7 | |||
| 14 | 1.5 | 1.1 | 1.2 | 1.6 | 1.4 | 1.6 | 1.6 | |||
| Control-3 | 0 | 0 | 1.4 | 1.2 | 1.1 | 1.3 | 1.2 | 1.4 | 1.2 | |
| 6 | 1.2 | 1.3 | 1.3 | 1.6 | 1.5 | 1.3 | 1.1 | |||
| 15 | 1.6 | 1.3 | 1.5 | ND | 1.5 | 1.8 | ND | |||
The mRNA expression of leukocyte surface markers (i.e., CD3, CD4, and CD8) and cytokines (IFN-γ, TNF-α, IL-6, and IL-12) in PBMC cultures stimulated with tumor antigens, or with liver antigens as a control stimulator, was compared to that in unstimulated PBMC cultures from the same woodchuck at each time point. Values in bold were above the assay cutoff of ≥3.1 and were considered positive for antigen-specific mRNA expression. Underlined values were below the assay cutoff but were ≥2.1-fold above the mRNA expression in unstimulated PBMC cultures. ND, undetectable.
Intratumoral SFV treatment results in antiviral effects and antiviral immune responses.
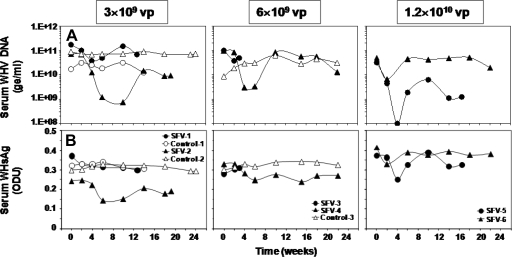
Following treatment of HCC with SFV-enhIL-12, all woodchucks demonstrated a transient reduction in serum WHV DNA concentrations ranging between 0.4 and 2.5 logs10 units from pretreatment concentrations (Fig. 4A). Reductions in WHV DNA were associated with a 1.2- to 1.7-fold decline in serum WHsAg from pretreatment levels in most woodchucks (Fig. 4B), although the transient changes in WHs antigenemia were less pronounced than those in WHV viremia. Decreases in WHV DNA occurred immediately following treatment and were maximal between 2 and 10 weeks posttreatment. This was followed by recrudescence of viral replication in all woodchucks. Declines in serum WHsAg followed a kinetic similar to that of WHV DNA, with maximum reductions between 2 and 6 weeks posttreatment. Comparable changes in WHV markers were not observed in sera of control woodchucks.
FIG. 4.
Antiviral effects in chronic WHV carrier woodchucks following intratumoral treatment with increasing doses of SFV-enhIL-12. (A) Changes in serum WHV DNA concentrations. (B) Changes in serum WHsAg levels. ge, WHV genomic equivalents (virion- or WHV DNA-containing virus particles).
The antiviral effects induced by treatment with SFV-enhIL-12 were associated with the development of cellular immune responses to WHV (Fig. 5A to C). Prior to treatment with SFV-enhIL-12, T-cell responses to WHV peptides were undetectable in woodchucks, with SIs below 3.1. Following treatment, SFV-treated woodchucks had increases in their WHV-specific T-cell responses, and three of them developed a positive T-cell response to WHc peptide C100-110, with SIs of ≥3.1. One woodchuck treated with the highest SFV dose (SFV-5) also had a positive T-cell response to WHs peptide S18. WHV-specific T-cell responses were transient, peaked around the time of maximum reduction in WHV viremia and WHs antigenemia, and declined thereafter. Control woodchucks had no comparable changes in their WHV-specific T-cell responses, and T-cell responses to a control peptide unrelated to WHV were not detected in any woodchuck. Furthermore, all SFV-treated woodchucks had increased (SI of ≥2.1) or positive (SI of ≥3.1) expression of CD3, CD4, and CD8 in PBMC cultures stimulated with WHsAg (Table 3). IFN-γ expression was positive in PBMC cultures from most SFV-treated woodchucks and correlated with increases in the expression of TNF-α, IL-6, and IL-12. Leukocyte surface marker and cytokine expression was transient, with a peak around the time of maximum reduction in serum WHV markers. Comparable changes in expression were not observed in WHsAg-stimulated PBMC cultures from control woodchucks, demonstrating the specific activation of antiviral immune responses by SFV-enhIL-12.
FIG. 5.
Antiviral T-cell responses of chronic WHV carrier woodchucks following intratumoral treatment with increasing doses of SFV-enhIL-12. (A) T-cell responses to a synthetic peptide of the WHV core protein (WHc peptide C100-119; 10 μg/ml). (B) T-cell responses to a synthetic peptide of WHsAg (WHs peptide S18; 10 μg/ml). (C) T-cell responses to a synthetic control peptide unrelated to WHV (10 μg/ml). The T-cell response was considered positive if the SI was ≥3.1, as indicated by the dotted line.
TABLE 3.
WHV antigen-specific expression of leukocyte surface markers and cytokines in woodchucks following intratumoral administration of SFV-enhIL-12 at increasing doses
| Woodchuck | SFV dose (vp) | Wk | Fold change in mRNA expression in WHsAg-stimulated PBMC culturesa |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CD3 | CD4 | CD8 | IFN-γ | TNF-α | IL-6 | IL-12 | |||
| SFV-2 | 3 × 109 | 0 | 1.4 | 1.2 | 1.2 | ND | 1.4 | 1.3 | ND |
| 6 | 4.5 | 3.3 | 2.7 | 7.4 | 3.4 | 3.1 | 4.7 | ||
| 14 | 2.7 | 2.4 | 2.2 | 3.1 | 2.4 | 2.1 | 2.5 | ||
| SFV-4 | 6 × 109 | 0 | 1.2 | 1.4 | 1.1 | 1.3 | 1.2 | 1.0 | 1.2 |
| 6 | 2.4 | 2.4 | 2.3 | 2.9 | 2.7 | 2.7 | 2.9 | ||
| 15 | 2.2 | 1.8 | 2.0 | 2.2 | 1.8 | 1.9 | 1.9 | ||
| SFV-5 | 1.2 × 1010 | 0 | 1.4 | 1.3 | 1.4 | 1.1 | 1.2 | 1.1 | 1.3 |
| 6 | 5.4 | 5.6 | 4.0 | 12.3 | 4.5 | 3.3 | 3.9 | ||
| 14 | 2.2 | 2.2 | 2.3 | 2.3 | 2.0 | 2.2 | 2.4 | ||
| SFV-6 | 1.2 × 1010 | 0 | 1.1 | 1.1 | 1.0 | 1.2 | 1.1 | 1.0 | 1.1 |
| 6 | 2.4 | 2.5 | 2.6 | 3.5 | 2.5 | 2.8 | 2.6 | ||
| 14 | 1.5 | 1.2 | 1.4 | 1.6 | 1.7 | 1.1 | 1.4 | ||
| 22 | 1.2 | 1.3 | 1.4 | 1.3 | 1.6 | 1.5 | 1.3 | ||
| Control-2 | 0 | 0 | 1.6 | 1.4 | 1.3 | ND | 1.2 | 1.1 | 1.4 |
| 6 | 1.4 | 1.2 | 1.1 | 1.1 | 1.6 | 1.6 | 1.4 | ||
| 14 | 1.4 | 1.3 | 1.2 | 1.2 | 1.7 | 1.2 | 1.7 | ||
| Control-3 | 0 | 0 | 1.1 | 1.2 | 1.0 | 1.1 | 1.1 | 1.1 | 1.1 |
| 6 | 1.4 | 1.3 | 1.2 | 1.5 | 1.5 | 1.3 | 1.3 | ||
| 15 | 1.1 | 1.0 | 1.2 | 1.2 | 1.2 | 1.4 | 1.1 | ||
The mRNA expression of leukocyte surface markers (i.e., CD3, CD4, and CD8) and cytokines (IFN-γ, TNF-α, IL-6, and woodchuck IL-12) in PBMC cultures stimulated with WHsAg was compared to that in unstimulated PBMC cultures from the same woodchuck at each time point. Values in bold were above the assay cutoff of ≥3.1 and were considered positive for WHsAg-specific mRNA expression. Underlined values were below the assay cutoff but were ≥2.1-fold above the mRNA expression in unstimulated PBMC cultures. ND, undetectable.
Intratumoral SFV treatment induces no systemic toxicity.
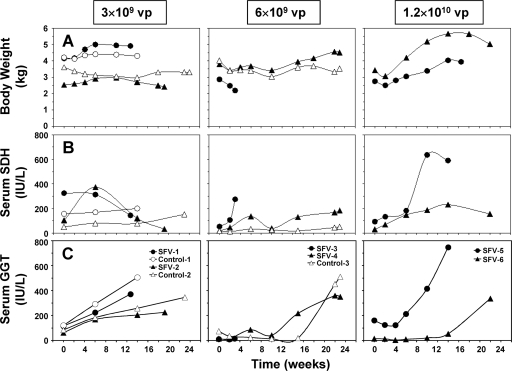
No clinical signs of toxicity were observed in woodchucks following treatment of HCC with SFV-enhIL-12 (Fig. 6A to C). Changes in body weights of woodchucks treated with low and intermediate doses of SFV-enhIL-12 were similar to those in control woodchucks, except in woodchuck SFV-3, which was euthanized at 3 weeks posttreatment due to seizures. A subsequent necropsy of this woodchuck revealed end-stage HCC but also indicated signs of brain damage, both of which could be the cause for the seizures. Encephalopathy in this woodchuck induced by SFV-enhIL-12 was excluded, as immunohistochemical staining for the nsp2 subunit of the SFV replicase demonstrated the absence of replicating SFV in brain tissue (data not shown). On the other hand, encephalopathy is frequently found in patients with acute and chronic liver disease (18), and although there are no data available for woodchucks with chronic hepatitis, it is possible that a certain percentage of woodchucks with chronic liver disease may develop hepatic encephalopathy. Following administration of the highest SFV dose, a modest loss in body weight was observed at 2 weeks posttreatment, but changes were transient and both woodchucks gained weight thereafter. The hematological and biochemical profiles of SFV-treated woodchucks were similar to those of control woodchucks following treatment, except for blood Na+ and Cl− levels, which were transiently reduced in most SFV-treated woodchucks at 6 weeks posttreatment (data not shown). Most SFV-treated woodchucks had moderate increases in serum SDH activity at 6 weeks posttreatment (and later), but changes were transient. Increases in SDH activity during this time were almost always associated with proportional elevations in ALT and AST activities (data not shown), suggesting increased hepatic injury. Serum activity of GGT, a marker of HCC development in woodchucks, continuously increased in most woodchucks following treatment, except in woodchucks treated with the highest SFV dose, with a transient reduction at 4 weeks posttreatment. At the end of the study, serum GGT activity reached the highest level in all SFV- and saline-treated woodchucks.
FIG. 6.
Body weight and liver enzymes of chronic WHV carrier woodchucks following intratumoral treatment with increasing doses of SFV-enhIL-12. (A) Changes in body weight. (B) Changes in serum SDH activity. (C) Changes in serum GGT activity. IU, international units.
DISCUSSION
In previous studies it was shown that SFV-enhIL-12 induced sustained antitumoral responses in several transplantable liver tumor models, including a rat orthotopic HCC model (16, 39). Although these results suggested that SFV vectors could have potential application for the treatment of HCC, the efficacy and safety of SFV-enhIL-12 need to be evaluated in more relevant models of HCC before being applied to humans. The woodchuck model of HBV infection and associated liver diseases was chosen because all woodchucks chronically infected with WHV ultimately develop HCC, which is comparable to HBV-induced hepatocarcinogenesis in humans (34, 47). Additional advantages of the woodchuck model are the outbred nature of the animals; the heterogeneity of liver tumors, resembling the situation in humans; and that tumors of relatively large size can be subjected to anticancer treatment.
Although SFV vectors have a broad tropism, their infectivity in woodchuck hepatic tumor cells was unknown. An initial study demonstrated that the in vitro infectivity of SFV-LacZ in the woodchuck HCC-derived cell line WCH17 was about 300-fold lower than that observed in BHK-21 cells, which are the standard cells for testing SFV infectivity (Table 1). Furthermore, SFV infectivity and β-galactosidase protein expression in WCH17 cells were much lower than in the human HCC-derived cell lines HepB3 and Huh7 (Table 1), suggesting that SFV vectors could be efficient for gene delivery to human HCC.
Despite the rather low infectivity and reporter gene expression in vitro, the intratumoral inoculation of SFV-Luc into two woodchucks resulted in relatively high luciferase activity in vivo, which was dose dependent and mainly confined to the infected tumor, in addition to the secretion of IL-12 at high levels expressed from SFV-enhIL-12 that was administered in parallel (Fig. 1). However, luciferase activity was about 10- to 100-fold lower than that observed in mouse tumors inoculated with an equivalent dose of SFV-Luc (38), suggesting that SFV vectors have only moderate infectivity in woodchuck tumor cells.
Despite the low infectivity in woodchuck HCC, SFV-enhIL-12 induced a dose-dependent antitumoral effect in woodchucks. The effect on tumor growth was most pronounced following treatment with the highest dose of SFV-enhIL-12, resulting in tumor volume reductions of 71% and 80% in woodchucks SFV-5 and SFV-6, respectively (Fig. 2). Partial tumor remission was transient, and tumors started to grow again between 6 and 14 weeks posttreatment. As a consequence of this regrowth, most SFV-treated tumors reached a size at the end of the study that was comparable in some cases to the tumor sizes observed in control woodchucks. Direct caliper measurement of tumor size during the transient antitumoral response was possible in only one of the woodchucks treated with the intermediate dose of SFV-enhIL-12, which was euthanized because of seizures. In this woodchuck (SFV-3), a 66% reduction in tumor volume was observed within 3 weeks, demonstrating the efficacy of the applied treatment. Overall, the tumor sizes determined by direct caliper measurement at laparatomy or necropsy (and by CT measurement [data not shown]) correlated with those determined by US at the same time points, suggesting that US measurements are a simple and reliable method to evaluate tumor growth in woodchucks.
Two studies so far have tested viral vectors for treatment of HCC using woodchucks. Both studies were based on intratumoral injection of a first-generation adenoviral vector for the expression of murine IL-12 and the coestimulatory factor B7.1 (35) or herpes simplex virus thymidine kinase (4). Intratumoral expression of IL-12 and B7.1 resulted in an average tumor size reduction of 80%, which was observed in four woodchucks between 1 and 2 weeks posttreatment (35), an antitumoral efficacy comparable to that observed in woodchucks treated with the highest dose of SFV-enhIL-12 in our study. In the previous study (35) tumor reduction was maintained for 7 weeks in one woodchuck, but the other woodchucks were euthanized shortly after maximum tumor remission, making it difficult to estimate the sustainability of the induced antitumoral effect. In contrast, treatment with herpes simplex virus thymidine kinase followed by administration of ganciclovir did not induce tumor regression, although necrotic areas were present in tumors at 1 week posttreatment (4).
Partial tumor remission following treatment with SFV-enhIL-12 was associated with the induction of an antitumoral immune response, as demonstrated by T-cell proliferation and expression of leukocyte surface markers and cytokines in response to tumor antigens (Fig. 3 and Table 2). Antitumoral immune responses peaked at week 6 posttreatment, around the time of maximal tumor remission, suggesting that tumor antigens were released, most probably by SFV-induced apoptosis. The apoptotic bodies generated following SFV infection could be subjected to cross-priming by host dendritic cells that would capture, process, and present tumor-associated cellular antigens to naive T cells, thereby inducing tumor antigen-specific CTL responses (1). The increased expression of CD8 in tumor antigen-stimulated PBMC cultures of SFV-treated woodchucks suggests that tumor-specific CTL responses were induced. Increased CD8 marker expression was also observed in woodchucks following HCC treatment with an adenovirus expressing IL-12/B7.1 (35).
Although the induction of apoptosis in liver tumor cells by replicating SFV itself could be partially responsible for the antitumoral immune responses observed in woodchucks, due to the limited number of animals that were available and suitable for the present study, it was not possible to control for this effect by treating additional woodchucks with an SFV vector expressing a reporter gene. However, studies with a mouse model have shown that the antitumoral effects mediated by SFV are dependent on the (high) expression of IL-12 (39), because treatment of subcutaneous tumors with SFV-LacZ in mice following transplantation of MC38 cells induced tumor regression in only 9% of animals. Tumor regression increased significantly to 92% when mice were treated with the same dose of SFV-enhIL-12, demonstrating that an SFV vector expressing a reporter gene has very limited antitumoral efficacy.
In addition to the antitumoral effect, HCC treatment with SFV-enhIL-12 also resulted in a transient reduction of WHV viremia in all woodchucks that was associated in some cases with a transient decline in WHs antigenemia (Fig. 4). Although changes in WHV DNA were variable among individual woodchucks, a greater-than-30-fold reduction was observed in half of the woodchucks. Maximum reduction in WHV viremia was observed between 2 and 10 weeks posttreatment, followed by viral recrudescence in all woodchucks. Sustained antiviral effects have been observed previously in woodchucks without HCC following long-term treatment with an adenoviral vector expressing IL-12 (13). Because WHV replication appears to be minimal or undetectable in woodchuck HCC compared to normal liver tissue using in situ hybridization and immunohistochemistry for the detection of WHV DNA and proteins (22, 27, 36, 47, 51), SFV-mediated apoptosis of tumor cells seems less likely to be responsible for the transient reduction in WHV viremia. However, the antiviral effect was associated with the induction of an antiviral immune response as demonstrated by T-cell responses to WHV antigens and by expression of leukocyte surface markers and cytokines in WHsAg-stimulated PBMC cultures (Fig. 5 and Table 3). This suggests that CTL-mediated killing of WHV-infected hepatocytes and/or cytokine-mediated downregulation of WHV replication in liver cells was most likely responsible for the transient reduction in WHV viremia as indicated by increased expression of CD8 and IFN-γ in PBMC cultures. It was not possible to study directly the induction of type I and II IFNs in tumors of SFV-enhIL-12-treated woodchucks following expression of IL-12 because such tissues were not available until the end of the study (i.e., up to 6 months following treatment). For investigating a possible role of type I and II IFNs, an additional woodchuck received intratumorally the highest dose of SFV-enhIL-12, and the expression of IFN-α and IFN-γ mRNAs in hepatic tumor tissues was determined 24 h later (Fig. 1F). Because IFN-α and IFN-γ levels were increased in the SFV-treated tumor compared to an untreated control tumor, this result suggests that the antiviral (and antitumoral) responses observed in the SFV-treated woodchucks could be mediated by the induction of type I and II cytokines.
The parallel induction of antitumoral and antiviral immune responses was also observed in HBV-infected patients with HCC following intratumoral treatment with an oncolytic poxvirus expressing granulocyte-monocyte colony-stimulating factor (25). The antitumoral and antiviral effects observed in this study were attributed to the expression of granulocyte-monocyte colony-stimulating factor and to virus-mediated killing of tumor cells that resulted in the release of tumor and viral antigens and uptake by antigen-presenting cells. The latter effects are also mediated by SFV vectors, which are not oncolytic but can replicate and induce apoptosis in tumor cells (33, 54).
Treatment of HCC in woodchucks with SFV-enhIL-12 demonstrated a favorable safety profile with moderate increases in the serum activity of liver enzymes and a modest, transient reduction in body weight following administration of the highest SFV dose (Fig. 6). However, no survival benefit was observed in SFV-treated woodchucks, which was most likely due to the tumor regrowth following partial tumor remission and to the appearance of several new tumor nodules during the end stage of HCC (data not shown).
The observation that transient rather than sustained antitumoral and antiviral effects were achieved in woodchucks following intratumoral administration of a single dose of SFV-enhIL-12 indicates that this approach to HCC treatment could benefit from further improvements. Both effects may likely be sustained in a future woodchuck study by administering a dose of SFV-enhIL-12 exceeding 1.2 × 1010 vp, which was the highest dose used in the present study; by using a SFV vector that expresses woodchuck IL-12 instead of mouse IL-12; and by administering multiple vector doses over time into one or separate hepatic tumors, all of which could be performed by US-guided intratumoral injections, thereby eliminating the need for exposing the liver during laparotomy. The kinetics of partial tumor remission and regrowth in woodchucks also suggest that the timing for additional vector injections might be critical and that SFV-enhIL-12 should be readministered when maximal reductions in tumor growth following initial treatment are observed (i.e., before tumor growth is restored). In addition, administration of a combination of vectors for the expression of IL-12 and other cytokines such as IL-15 and IL-18 could be considered for the treatment of HCC. Because long-term treatment with an adenoviral vector expressing IL-12 resulted in sustained antiviral effects in chronic WHV carrier woodchucks (13), repeated intratumoral administration of SFV-enhIL-12 for the short-term expression of IL-12 in combination with peritumoral administration of the adenoviral vector for the long-term expression of IL-12 could be tested as a treatment strategy for HCC in woodchucks. The antitumoral efficacy of IL-15 and IL-18 has been demonstrated in several animal models of cancer (7, 19, 20, 45, 46, 52), and a combination of SFV and adenoviral vectors expressing IL-12, IL-15, or IL-18 could be also tested in the woodchuck model for inducing sustained antitumoral effects.
In summary, the present study demonstrates that the antitumoral efficacy mediated by an SFV vector expressing IL-12 is not limited to transplantable models in small rodents and therefore may represent a strategy for the treatment of chronic HBV infection and associated HCC and, by analogy, of other types of HCCs in humans.
Acknowledgments
This work was supported by an unrestricted gift from FIMA and by FIS grants from the Spanish Health Department (PI052100) and from the Government of Navarra (GNE-BIODISTRIBUTION). Woodchucks used in the antitumoral response study were bred, infected with WHV, and maintained as chronic WHV carriers under contract N01-AI-05399 (College of Veterinary Medicine, Cornell University) from the National Institute of Allergy and Infectious Diseases (NIAID) until the development of HCC.
We gratefully acknowledge the expert assistance of Erkuden Casales from the Center for Applied Medical Research (CIMA); of Mercedes Fernandez and Yolanda Azcona from the University Clinic (UNAV, Pamplona, Spain); and of Betty Baldwin, Lou Ann Graham, and Erin Graham from Cornell University.
Footnotes
Published ahead of print on 9 September 2009.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Asselin-Paturel, C., N. Lassau, J. M. Guinebretiere, J. Zhang, F. Gay, F. Bex, S. Hallez, J. Leclere, P. Peronneau, F. Mami-Chouaib, and S. Chouaib. 1999. Transfer of the murine interleukin-12 gene in vivo by a Semliki Forest virus vector induces B16 tumor regression through inhibition of tumor blood vessel formation monitored by Doppler ultrasonography. Gene Ther. 6:606-615. [DOI] [PubMed] [Google Scholar]
- 3.Barajas, M., G. Mazzolini, G. Genove, R. Bilbao, I. Narvaiza, V. Schmitz, B. Sangro, I. Melero, C. Qian, and J. Prieto. 2001. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology 33:52-61. [DOI] [PubMed] [Google Scholar]
- 4.Bilbao, R., R. Gerolami, M. P. Bralet, C. Qian, P. L. Tran, B. Tennant, J. Prieto, and C. Brechot. 2000. Transduction efficacy, antitumoral effect, and toxicity of adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir therapy of hepatocellular carcinoma: the woodchuck animal model. Cancer Gene Ther. 7:657-662. [DOI] [PubMed] [Google Scholar]
- 5.Blondon, H., L. Fritsch, and D. Cherqui. 2004. Two cases of spontaneous regression of multicentric hepatocellular carcinoma after intraperitoneal rupture: possible role of immune mechanisms. Eur. J. Gastroenterol. Hepatol. 16:1355-1359. [DOI] [PubMed] [Google Scholar]
- 6.Casales, E., J. R. Rodriguez-Madoz, M. Ruiz-Guillen, N. Razquin, Y. Cuevas, J. Prieto, and C. Smerdou. 2008. Development of a new noncytopathic Semliki Forest virus vector providing high expression levels and stability. Virology 376:242-251. [DOI] [PubMed] [Google Scholar]
- 7.Chang, C. Y., J. Lee, E. Y. Kim, H. J. Park, C. H. Kwon, J. W. Joh, and S. J. Kim. 2007. Intratumoral delivery of IL-18 naked DNA induces T-cell activation and Th1 response in a mouse hepatic cancer model. BMC Cancer 7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, G. C., H. C. Lan, S. H. Juang, Y. C. Wu, H. C. Lee, Y. M. Hung, H. Y. Yang, J. Whang-Peng, and K. J. Liu. 2005. A pilot clinical trial of vaccination with dendritic cells pulsed with autologous tumor cells derived from malignant pleural effusion in patients with late-stage lung carcinoma. Cancer 103:763-771. [DOI] [PubMed] [Google Scholar]
- 9.Chikkanna-Gowda, C. P., B. J. Sheahan, M. N. Fleeton, and G. J. Atkins. 2005. Regression of mouse tumours and inhibition of metastases following administration of a Semliki Forest virus vector with enhanced expression of IL-12. Gene Ther. 12:1253-1263. [DOI] [PubMed] [Google Scholar]
- 10.Colmenero, P., M. Chen, E. Castanos-Velez, P. Liljestrom, and M. Jondal. 2002. Immunotherapy with recombinant SFV-replicons expressing the P815A tumor antigen or IL-12 induces tumor regression. Int. J. Cancer 98:554-560. [DOI] [PubMed] [Google Scholar]
- 11.Cote, P. J., B. E. Korba, R. H. Miller, J. R. Jacob, B. H. Baldwin, W. E. Hornbuckle, R. H. Purcell, B. C. Tennant, and J. L. Gerin. 2000. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 31:190-200. [DOI] [PubMed] [Google Scholar]
- 12.Cote, P. J., C. Roneker, K. Cass, F. Schodel, D. Peterson, B. Tennant, F. De Noronha, and J. Gerin. 1993. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 6:161-169. [DOI] [PubMed] [Google Scholar]
- 13.Crettaz, J., I. Otano, L. Ochoa, A. Benito, A. Paneda, I. Aurrekoetxea, P. Berraondo, J. R. Rodriguez-Madoz, A. Astudillo, F. Kreppel, S. Kochanek, J. Ruiz, S. Menne, J. Prieto, and G. Gonzalez-Aseguinolaza. 2009. Treatment of chronic viral hepatitis in woodchucks by prolonged intrahepatic expression of interleukin-12. J. Virol. 83:2663-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drozdzik, M., C. Qian, X. Xie, D. Peng, R. Bilbao, G. Mazzolini, and J. Prieto. 2000. Combined gene therapy with suicide gene and interleukin-12 is more efficient than therapy with one gene alone in a murine model of hepatocellular carcinoma. J. Hepatol. 32:279-286. [DOI] [PubMed] [Google Scholar]
- 15.Fleeton, M. N., B. J. Sheahan, E. A. Gould, G. J. Atkins, and P. Liljestrom. 1999. Recombinant Semliki Forest virus particles encoding the prME or NS1 proteins of louping ill virus protect mice from lethal challenge. J. Gen. Virol. 80:1189-1198. [DOI] [PubMed] [Google Scholar]
- 16.Guan, M., J. R. Rodriguez-Madoz, P. Alzuguren, C. Gomar, M. G. Kramer, S. Kochanek, J. Prieto, C. Smerdou, and C. Qian. 2006. Increased efficacy and safety in the treatment of experimental liver cancer with a novel adenovirus-alphavirus hybrid vector. Cancer Res. 66:1620-1629. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi, S. M., Z. M. Hassan, S. Soudi, and S. Shahabi. 2008. The effect of vaccination with the lysate of heat-shocked tumor cells on nitric oxide production in BALB/c mice with fibrosarcoma tumor. Cell Biol. Int. 32:835-840. [DOI] [PubMed] [Google Scholar]
- 18.Haussinger, D., and F. Schliess. 2008. Pathogenetic mechanisms of hepatic encephalopathy. Gut 57:1156-1165. [DOI] [PubMed] [Google Scholar]
- 19.Hwang, K. S., W. K. Cho, J. Yoo, Y. R. Seong, B. K. Kim, S. Kim, and D. S. Im. 2004. Adenovirus-mediated interleukin-18 mutant in vivo gene transfer inhibits tumor growth through the induction of T cell immunity and activation of natural killer cell cytotoxicity. Cancer Gene Ther. 11:397-407. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, H., S. Dubois, N. Sato, H. Sabzevari, Y. Sakai, T. A. Waldmann, and Y. Tagaya. 2005. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 105:721-727. [DOI] [PubMed] [Google Scholar]
- 21.Lau, W. Y., and E. C. Lai. 2008. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat. Dis. Int. 7:237-257. [PubMed] [Google Scholar]
- 22.Li, Y., H. Hacker, A. Kopp-Schneider, U. Protzer, and P. Bannasch. 2002. Woodchuck hepatitis virus replication and antigen expression gradually decrease in preneoplastic hepatocellular lineages. J. Hepatol. 37:478-485. [DOI] [PubMed] [Google Scholar]
- 23.Liljestrom, P., and H. Garoff. 1994. Expression of proteins using Semliki Forest virus vectors, p. 16.20.11-16.20.16. In F. M. Ausubel et al. (ed.), Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, NY.
- 24.Liljestrom, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology 9:1356-1361. [DOI] [PubMed] [Google Scholar]
- 25.Liu, T. C., T. Hwang, B. H. Park, J. Bell, and D. H. Kirn. 2008. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol. Ther. 16:1637-1642. [DOI] [PubMed] [Google Scholar]
- 26.Maschke, J., S. Menne, J. R. Jacob, E. Kreuzfelder, B. C. Tennant, M. Roggendorf, and H. Grosse-Wilde. 2001. Thymidine utilization abnormality in proliferating lymphocytes and hepatocytes of the woodchuck. Vet. Immunol. Immunopathol. 78:279-296. [DOI] [PubMed] [Google Scholar]
- 27.Mason, W. S., A. R. Jilbert, and J. Summers. 2005. Clonal expansion of hepatocytes during chronic woodchuck hepatitis virus infection. Proc. Natl. Acad. Sci. USA 102:1139-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menne, S., S. D. Butler, A. L. George, I. A. Tochkov, Y. Zhu, S. Xiong, J. L. Gerin, P. J. Cote, and B. C. Tennant. 2008. Antiviral effect of lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate, adminstered orally alone and in combination, to woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 52:3617-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menne, S., and P. J. Cote. 2007. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J. Gastroenterol. 13:104-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menne, S., P. J. Cote, S. D. Butler, I. A. Toshkov, J. L. Gerin, and B. C. Tennant. 2007. Immunosuppression reactivates viral replication long after resolution of woodchuck hepatitis virus infection. Hepatology 45:614-622. [DOI] [PubMed] [Google Scholar]
- 31.Menne, S., C. A. Roneker, M. Roggendorf, J. L. Gerin, P. J. Cote, and B. C. Tennant. 2002. Deficiencies in the acute-phase cell-mediated immune response to viral antigens are associated with development of chronic woodchuck hepatitis virus infection following neonatal inoculation. J. Virol. 76:1769-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menne, S., B. C. Tennant, J. L. Gerin, and P. J. Cote. 2007. Chemoimmunotherapy of chronic hepatitis B virus infection in the woodchuck model overcomes immunologic tolerance and restores T-cell responses to pre-S and S regions of the viral envelope protein. J. Virol. 81:10614-10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, A. M., M. M. Morris-Downes, B. J. Sheahan, and G. J. Atkins. 2000. Inhibition of human lung carcinoma cell growth by apoptosis induction using Semliki Forest virus recombinant particles. Gene Ther. 7:1477-1482. [DOI] [PubMed] [Google Scholar]
- 34.Popper, H., L. Roth, R. H. Purcell, B. C. Tennant, and J. L. Gerin. 1987. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc. Natl. Acad. Sci. USA 84:866-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putzer, B. M., T. Stiewe, F. Rodicker, O. Schildgen, S. Ruhm, O. Dirsch, M. Fiedler, U. Damen, B. Tennant, C. Scherer, F. L. Graham, and M. Roggendorf. 2001. Large nontransplanted hepatocellular carcinoma in woodchucks: treatment with adenovirus-mediated delivery of interleukin 12/B7.1 genes. J. Natl. Cancer Inst. 93:472-479. [DOI] [PubMed] [Google Scholar]
- 36.Radaeva, S., Y. Li, H. J. Hacker, V. Burger, A. Kopp-Schneider, and P. Bannasch. 2000. Hepadnaviral hepatocarcinogenesis: in situ visualization of viral antigens, cytoplasmic compartmentation, enzymic patterns, and cellular proliferation in preneoplastic hepatocellular lineages in woodchucks. J. Hepatol. 33:580-600. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez, M. M., S. M. Ryu, C. Qian, M. Geissler, C. Grimm, J. Prieto, H. E. Blum, and L. Mohr. 2008. Immunotherapy of murine hepatocellular carcinoma by alpha-fetoprotein DNA vaccination combined with adenovirus-mediated chemokine and cytokine expression. Hum. Gene Ther. 19:753-759. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Madoz, J. R., J. Prieto, and C. Smerdou. 2007. Biodistribution and tumor infectivity of Semliki Forest virus vectors in mice: effects of re-administration. Mol. Ther. 15:2164-2171. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Madoz, J. R., J. Prieto, and C. Smerdou. 2005. Semliki forest virus vectors engineered to express higher IL-12 levels induce efficient elimination of murine colon adenocarcinomas. Mol. Ther. 12:153-163. [DOI] [PubMed] [Google Scholar]
- 40.Salminen, A., J. M. Wahlberg, M. Lobigs, P. Liljestrom, and H. Garoff. 1992. Membrane fusion process of Semliki Forest virus. II. Cleavage-dependent reorganization of the spike protein complex controls virus entry. J. Cell Biol. 116:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangro, B., G. Mazzolini, J. Ruiz, M. Herraiz, J. Quiroga, I. Herrero, A. Benito, J. Larrache, J. Pueyo, J. C. Subtil, C. Olague, J. Sola, B. Sadaba, C. Lacasa, I. Melero, C. Qian, and J. Prieto. 2004. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J. Clin. Oncol. 22:1389-1397. [DOI] [PubMed] [Google Scholar]
- 42.Schott, M., J. Feldkamp, D. Schattenberg, T. Krueger, C. Dotzenrath, J. Seissler, and W. A. Scherbaum. 2000. Induction of cellular immunity in a parathyroid carcinoma treated with tumor lysate-pulsed dendritic cells. Eur. J. Endocrinol. 142:300-306. [DOI] [PubMed] [Google Scholar]
- 43.Smerdou, C., and P. Liljestrom. 1999. Two-helper RNA system for production of recombinant Semliki forest virus particles. J. Virol. 73:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smyth, J. W., M. N. Fleeton, B. J. Sheahan, and G. J. Atkins. 2005. Treatment of rapidly growing K-BALB and CT26 mouse tumours using Semliki Forest virus and its derived vector. Gene Ther. 12:147-159. [DOI] [PubMed] [Google Scholar]
- 45.Subleski, J. J., V. L. Hall, T. C. Back, J. R. Ortaldo, and R. H. Wiltrout. 2006. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 66:11005-11012. [DOI] [PubMed] [Google Scholar]
- 46.Tang, F., L. T. Zhao, Y. Jiang, N. Ba de, L. X. Cui, and W. He. 2008. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cell Mol. Immunol. 5:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tennant, B. C., I. A. Toshkov, S. F. Peek, J. R. Jacob, S. Menne, W. E. Hornbuckle, R. D. Schinazi, B. E. Korba, P. J. Cote, and J. L. Gerin. 2004. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology 127:S283-S293. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 49.Unitt, E., A. Marshall, W. Gelson, S. M. Rushbrook, S. Davies, S. L. Vowler, L. S. Morris, N. Coleman, and G. J. Alexander. 2006. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J. Hepatol. 45:246-253. [DOI] [PubMed] [Google Scholar]
- 50.Voest, E. E., B. M. Kenyon, M. S. O'Reilly, G. Truitt, R. J. D'Amato, and J. Folkman. 1995. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 87:581-586. [DOI] [PubMed] [Google Scholar]
- 51.Xu, C., T. Yamamoto, T. Zhou, C. E. Aldrich, K. Frank, J. M. Cullen, A. R. Jilbert, and W. S. Mason. 2007. The liver of woodchucks chronically infected with the woodchuck hepatitis virus contains foci of virus core antigen-negative hepatocytes with both altered and normal morphology. Virology 359:283-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yajima, T., H. Nishimura, W. Wajjwalku, M. Harada, H. Kuwano, and Y. Yoshikai. 2002. Overexpression of interleukin-15 in vivo enhances antitumor activity against MHC class I-negative and -positive malignant melanoma through augmented NK activity and cytotoxic T-cell response. Int. J. Cancer 99:573-578. [DOI] [PubMed] [Google Scholar]
- 53.Yamanaka, R. 2004. Alphavirus vectors for cancer gene therapy. Int. J. Oncol. 24:919-923. [PubMed] [Google Scholar]
- 54.Ying, H., T. Z. Zaks, R. F. Wang, K. R. Irvine, U. S. Kammula, F. M. Marincola, W. W. Leitner, and N. P. Restifo. 1999. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 5:823-827. [DOI] [PMC free article] [PubMed] [Google Scholar]