Abstract
Borna disease virus (BDV) is characterized by highly neurotropic infection. BDV enters its target cells using virus surface glycoprotein (G), but the cellular molecules mediating this process remain to be elucidated. We demonstrate here that the N-terminal product of G, GP1, interacts with the 78-kDa chaperone protein BiP. BiP was found at the surface of BDV-permissive cells, and anti-BiP antibody reduced BDV infection as well as GP1 binding to the cell surface. We also reveal that BiP localizes at the synapse of neurons. These results indicate that BiP may participate in the cell surface association of BDV.
Borna disease virus (BDV) belongs to the Bornaviridae family of nonsegmented, negative-strand RNA viruses and is characterized by highly neurotropic and noncytopathic infection (18, 33). BDV infects a wide variety of host species and causes central nervous system (CNS) diseases in animals, which are frequently associated with behavioral disorders (14, 19, 29, 31). BDV cell entry is mediated by endocytosis, following the attachment of viral envelope glycoprotein (G) to the cellular receptor (2, 7, 8). BDV G is translated as a precursor protein, GP, which is posttranslationally cleaved by the cellular protease furin to generate two functional subunits of the N (GP1) and C (GP2) termini (28). Recent studies revealed that GP1 is involved in virus interaction with as-yet-unidentified cell surface receptor(s) and that GP2 mediates a pH-dependent fusion event between viral and cell membranes (2, 7, 27). In addition, a previous work using a hippocampal culture system suggested that BDV G is required for viral dissemination in neurons (2); however, cellular factors involved in BDV cell entry, especially cell surface association, remain to be elucidated.
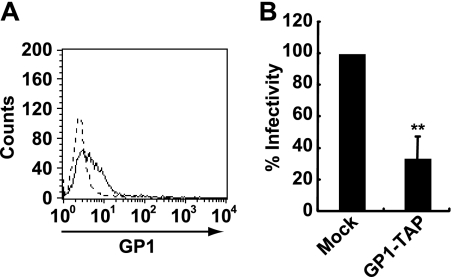
To extend our understanding of the role of BDV G in the interaction with the cell plasma membrane, we transfected GP1 fused with hemagglutinin-tobacco etch virus protease cleavage site-FLAG tags (GP1-TAP) into human oligodendroglioma OL cells. GP1-TAP was purified using anti-FLAG M2 affinity gel (Sigma). To verify that GP1-TAP binds to OL cells, the cells were incubated with 4 μg/ml GP1-TAP, and binding was detected by anti-FLAG M2 antibody (Sigma). A flow cytometric analysis indicated that GP1-TAP binds to OL cells (Fig. 1A). To further validate the binding of GP1-TAP, we tested whether GP1-TAP inhibits BDV infection. OL cells were pretreated with 4 μg/ml GP1-TAP for 30 min. Proteins purified from mock-transfected cells using an anti-FLAG M2 affinity gel served as a control. The cells were then mixed with cell-free BDV. After 1 h of absorption, the supernatants were removed and fresh medium was added. At 3 days postinfection, the viral antigens were stained with anti-nucleoprotein (N) monoclonal and anti-matrix (M) polyclonal antibodies. As shown in Fig. 1B, GP1-TAP reduced BDV infection by 40% compared to levels for mock-treated cells. This result was consistent with earlier reports showing that recombinant GP1 protein binds to the cell surface and inhibits BDV infection (6, 20).
FIG. 1.
BDV GP1 binds to the cell surface. (A) Binding of BDV GP1 to OL cells. OL cells were incubated with GP1-TAP (solid line), and its binding was detected using anti-FLAG M2 antibody and flow cytometry. As a control, cells incubated with proteins purified from mock-transfected cells were detected by an anti-FLAG M2 antibody (dotted line). (B) Inhibition of BDV infection by GP1. OL cells pretreated with GP1-TAP were inoculated with the BDV huP2br strain. Values are the means + standard deviations (SD) from three independent experiments. **, P < 0.01.
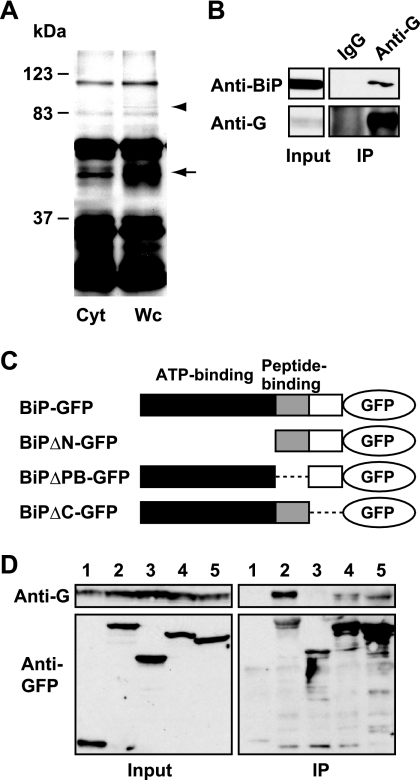
To investigate the host factor(s) that mediates the interaction of GP1 with the cell surface, a combination of tandem affinity purification (TAP) and liquid chromatography tandem mass spectrometry analyses was designed (13). We transfected GP1-TAP into OL cells and then purified GP1 from cell homogenates using a TAP strategy. We compared the purified proteins from the whole-cell and cytosol fractions (Fig. 2A), and the bands detected only in the whole-cell fraction were determined as GP1-binding proteins in the membrane and/or nuclear fractions. In addition to GP1 protein (Fig. 2A, arrow), we identified a specific band around 80 kDa in the whole-cell homogenate, but not in the cytosol fraction (Fig. 2A, arrowhead), and determined that the band corresponded to the BiP (immunoglobulin heavy chain-binding protein) molecular chaperone, also called glucose-regulated protein 78 (GRP78), by mass spectrometry analysis. We confirmed the specific interaction between endogenous BiP and BDV G in infected cells by immunoprecipitation analysis (Fig. 2B). To map the binding domain on BiP to GP1, we constructed a series of deletion mutants of the green fluorescent protein (GFP)-tagged BiP plasmid (Fig. 2C). We transfected the mutant plasmids into BDV-infected OL cells and then performed an immunoprecipitation assay using anti-GFP antibody (Invitrogen). As shown in Fig. 2D, BDV G was coimmunoprecipitated with truncated BiP mutants, except for BiPΔN-GFP, which lacks the ATP-binding domain of BiP (lane 3), suggesting that BiP interacts with GP1 via its N-terminal region.
FIG. 2.
BDV GP1 interacts with BiP molecular chaperone. (A) TAP analysis of BDV GP1. Proteins coimmunoprecipitated with GP1-TAP in OL cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by silver staining. Cyt, cytosol fraction; Wc, whole-cell homogenate. Arrow, GP1-TAP; arrowhead, BiP. (B) Coimmunoprecipitation (IP) of BDV G and endogenous BiP. BDV G was immunoprecipitated from BDV-infected OL cells by anti-BDV G polyclonal antibody. Endogenous BiP was then detected by anti-BiP monoclonal antibody (Becton Dickinson). IgG, immunoglobulin G. (C) Schematic representation of deletion mutants of recombinant BiP-GFP. The known functional regions are indicated. (D) Immunoprecipitation analysis of BiP-GFP mutants in BDV-infected OL cells. The deletion plasmids were transfected and immunoprecipitated by anti-GFP antibody. Specific binding was detected using anti-BDV G antibody. Lane 1, GFP; lane 2, BiP-GFP; lane 3, BiPΔN-GFP; lane 4, BiPΔPB-GFP; lane 5, BiPΔC-GFP.
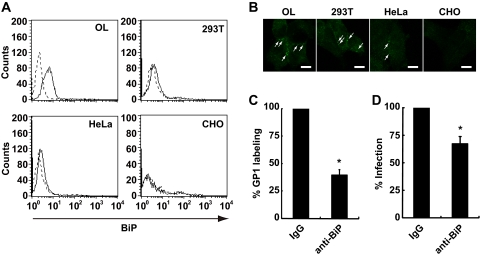
BiP is known to be resident primarily in the endoplasmic reticulum and functions as a molecular chaperone involved in the folding process of nascent proteins, mostly through interaction with its peptide-binding domain (12, 17, 21). On the other hand, BiP has been reported to serve as a coreceptor of certain viruses at the plasma membrane (15, 34). Recent studies also revealed that cell surface BiP mediates the internalization of its ligands into cells (1, 10). We first investigated whether BiP is expressed on the cell surface of BDV-permissive OL and 293T cells using an anti-BiP polyclonal antibody (H-129; Santa Cruz Biotechnology, Inc.). As shown in Fig. 3A, BiP expression is detected on the surface of both cell lines. This result is in agreement with recent observations that BiP is expressed on the surface of various types of cells (9, 10, 15, 23, 24, 34). We also investigated whether BiP is expressed on the cell surface of BDV-nonpermissive cell lines, such as HeLa and CHO cells. As shown in Fig. 3A, we detected BiP expression on the surface of HeLa, but not CHO, cells. These observations were confirmed by immunofluorescence analysis (Fig. 3B). Note that BiP is clearly detected at the endoplasmic reticulum in the permeabilized CHO cells by the antibody (see Fig. S1 in the supplemental material), suggesting that BiP is expressed at a very low level, if at all, on the surface of CHO cells. We next examined whether cell surface BiP serves as a binding molecule of BDV GP1. To test this, we performed an inhibition assay using an anti-BiP polyclonal antibody (N-20; Santa Cruz Biotechnology, Inc.) which recognizes the N terminus of BiP. As shown in Fig. 3C, the antibody inhibited GP1 binding to the cell surface by 40%. Furthermore, BDV infection was found to decrease by 70% when cells were treated with the antibody (Fig. 3D).
FIG. 3.
Cell surface BiP mediates cell association of BDV. (A) Flow cytometric analysis was performed with anti-BiP antibody (H-129) in BDV-permissive (OL and 293T) and -nonpermissive (HeLa and CHO) cells (solid lines). Cells stained with normal rabbit immunoglobulin G were used as a control (dotted lines). (B) Immunofluorescence analysis was performed by using anti-BiP antibody (H-129) with BDV-permissive and -nonpermissive cells. Arrows indicate BiP staining at the membrane. Scale bars, 10 μm. (C) Inhibition of GP1 binding by anti-BiP antibody (N-20). OL cells were pretreated with anti-BiP antibody, followed by labeling with GP1. GP1 binding on the cell surface was detected using flow cytometry. Values are the means + SD from three independent experiments. *, P < 0.05. (D) Inhibition of BDV infection by anti-BiP antibody. OL cells were incubated with 10 μg/ml anti-BiP antibody or normal goat immunoglobulin G and then the cells were mixed with cell-free BDV. After 1 h absorption, the supernatants were replaced with fresh medium. Virus infection was measured by immunofluorescence analysis using anti-N and -M antibodies at 3 days postinfection. Values are the means + SD from three independent experiments. *, P < 0.05. IgG, immunoglobulin G.
To investigate the role of cell surface BiP in the infection of BDV, the BiP expression was inhibited by short interfering RNA (siRNA) in OL cells (see Fig. S2A in the supplemental material). We selected an siRNA (Hs_HSPA5_4; Qiagen, Inc.) which could partially downregulate the cell surface expression of BiP (see Fig. S2B in the supplemental material). However, siRNA treatment of BiP did not influence the infectivity of BDV in OL cells (see Fig. S2C in the supplemental material). This may be due to an incomplete reduction of BiP expression on the cell surface. Alternatively, while BiP mediates at least in part the cell surface association of BDV particles, this result may exhibit the presence of another, as-yet-unidentified BDV G-binding protein that is involved in the binding and subsequent cell entry of BDV.
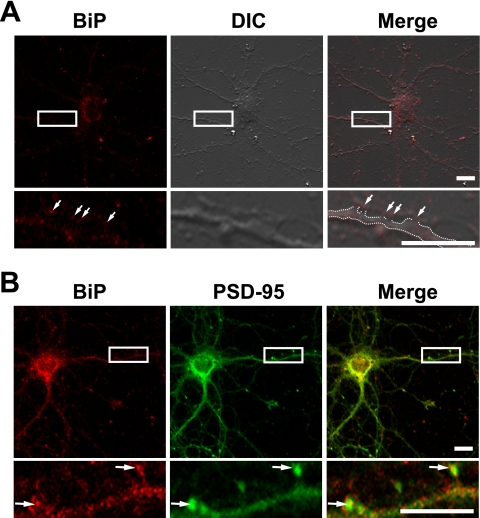
Previous studies demonstrated that BDV can be traced centripetally and transsynaptically after olfactory, ophthalmic, or intraperitoneal inoculation (3, 25). Migration of BDV to the CNS after footpad infection can be prevented by sciatic nerve transection (3). These observations suggest that BDV may disseminate primarily via neural networks. Recently, it has been demonstrated that BDV G was expressed at the termini of neurites or at contact sites of neurites (2), suggesting that local assembly of BDV may take place at the presynaptic terminals of synapses, similar to assembly of other neurotropic viruses (22, 26, 32). If BiP localizes at synapse sites, BiP may efficiently participate in the transmission of BDV particles at the synapses. To evaluate this hypothesis, we examined BiP localization in primary culture of mouse hippocampal neurons. After in vitro culture for 17 days, BiP localization was determined by an immunofluorescence assay without permeabilization. As shown in Fig. 4A, BiP signals were clearly detected at neurites, including the contact sites between dendrites and axons, as punctate staining (arrows), suggesting that BiP is expressed at the neuronal surface, most likely at the synapses. We next examined the localization of BiP with postsynaptic density 95 (PSD-95), a marker of postsynaptic density (5). Although BiP signals were detected mainly in the perinuclear area of the hippocampal neurons, punctate staining was also found at neurites colocalized with PSD-95 (Fig. 4B, arrows). Taken together, these observations suggested that BiP is distributed at the synaptic surface, including the postsynaptic membrane, of neurons, a possible site for BDV budding and entry (2).
FIG. 4.
BiP localizes at the synaptic surface of hippocampus neurons. (A) Localization of BiP at synaptic surface. Hippocampal neurons were immunostained with anti-BiP antibody (N-20) without permeabilization. A differential interference contrast (DIC) image is shown. Dotted lines in the Merge panel indicate the dendrite outline. Arrows indicate BiP staining at the contact sites between axons and dendrites. (B) Colocalization between BiP and a postsynaptic protein. Hippocampal neurons were immunostained with anti-BiP (N-20) and anti-PSD-95 (Millipore) antibodies. Arrows indicate colocalized signals of BiP and PSD-95 at neurites. Scale bars, 10 μm.
In summary, this study demonstrates that BiP is a GP1-binding protein at the synaptic surface. This is the first report showing the BDV G-binding factor on the cell surface. The first step of BDV entry might be mediated by the interaction of GP1 with as-yet-unidentified cell surface receptors, which may form a complex with other molecules, such as BiP. We showed that treatment with anti-BiP antibody affects BDV infection as well as GP1 binding to the cell surface (Fig. 3). Furthermore, synaptic distribution of BiP was found in hippocampal primary neurons (Fig. 4). These findings strongly suggest that BiP plays critical roles in BDV association with the neuronal surface via interaction with GP1. On the other hand, a BDV-nonpermissive cell line, HeLa, appeared to express BiP on the cell surface, suggesting that the cell surface BiP may not be necessarily involved in the infectivity of BDV. A recent study by Clemente et al. (6) revealed that following initial attachment to the cell surface, BDV is recruited to the plasma membrane lipid raft (LR) prior to internalization of the particles. The study suggested that BDV may use the LR as a platform to interact with additional host cell factor(s) required for efficient BDV internalization. Because BiP does not contain transmembrane regions, BiP needs another host protein(s) with transmembrane regions on the cell surface. It has been reported that cell surface BiP interacts with diverse proteins, such as major histocompatibility complex class I molecules (34), the voltage-dependent anion channel (9), and the DnaJ-like protein MTJ-1 (4), all of which associate with LR in the plasma membrane (16, 24, 35). Once BDV has attached to the cell surface, it might utilize such BiP-associated LR proteins for efficient cell surface attachment or internalization. Previously, it has been proposed that kainate 1 (KA-1) receptor might represent the BDV receptor within the CNS (11). Because some glutamate receptors are shown to bind to BiP (30), KA-1 receptors might interact with BiP and serve as a receptor complex for BDV. Further studies are required for a full understanding of the cell association processes, especially receptor binding, of BDV.
Supplementary Material
Acknowledgments
We thank K. Saito of DNA-chip Development Center for Infectious Diseases (BIKEN, Osaka University) for mass spectrometry analysis.
This work was supported by a Ministry of Education, Culture, Sports, Science and Technology Grant-in-Aid for Science Research on Priority Areas (Infection and Host Responses; Matrix of Infection Phenomena) (K.T.) and a PRESTO grant from the Japan Science and Technology Agency (K.T.).
Footnotes
Published ahead of print on 23 September 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Arap, M. A., J. Lahdenranta, P. J. Mintz, A. Hajitou, A. S. Sarkis, W. Arap, and R. Pasqualini. 2004. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 6:275-284. [DOI] [PubMed] [Google Scholar]
- 2.Bajramovic, J. J., S. Munter, S. Syan, U. Nehrbass, M. Brahic, and D. Gonzalez-Dunia. 2003. Borna disease virus glycoprotein is required for viral dissemination in neurons. J. Virol. 77:12222-12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone, K. M., C. S. Duchala, J. W. Griffin, A. L. Kincaid, and O. Narayan. 1987. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J. Virol. 61:3431-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chevalier, M., H. Rhee, E. C. Elguindi, and S. Y. Blond. 2000. Interaction of murine BiP/GRP78 with the DnaJ homologue MTJ1. J. Biol. Chem. 275:19620-19627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho, K. O., C. A. Hunt, and M. B. Kennedy. 1992. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 9:929-942. [DOI] [PubMed] [Google Scholar]
- 6.Clemente, R., A. de Parseval, M. Perez, and J. C. de la Torre. 2009. Borna disease virus requires cholesterol in both cellular membrane and viral envelope for efficient cell entry. J. Virol. 83:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Dunia, D., B. Cubitt, and J. C. de la Torre. 1998. Mechanism of Borna disease virus entry into cells. J. Virol. 72:783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Dunia, D., B. Cubitt, F. A. Grasser, and J. C. de la Torre. 1997. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J. Virol. 71:3208-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Gronow, M., S. J. Kaczowka, S. Payne, F. Wang, G. Gawdi, and S. V. Pizzo. 2007. Plasminogen structural domains exhibit different functions when associated with cell surface GRP78 or the voltage-dependent anion channel. J. Biol. Chem. 282:32811-32820. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Gronow, M., M. A. Selim, J. Papalas, and S. V. Pizzo. 2009. GRP78: a multifunctional receptor on the cell surface. Antioxid. Redox Signal. 11:2299-2306. [DOI] [PubMed] [Google Scholar]
- 11.Gosztonyi, G., and H. Ludwig. 2001. Interactions of viral proteins with neurotransmitter receptors may protect or destroy neurons. Curr. Top. Microbiol. Immunol. 253:121-144. [DOI] [PubMed] [Google Scholar]
- 12.Haas, I. G. 1994. BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia 50:1012-1020. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, Y., M. Horie, T. Daito, T. Honda, K. Ikuta, and K. Tomonaga. 2009. Heat shock cognate protein 70 controls Borna disease virus replication via interaction with the viral non-structural protein X. Microbes Infect. 11:394-402. [DOI] [PubMed] [Google Scholar]
- 14.Ikuta, K., M. S. Ibrahim, T. Kobayashi, and K. Tomonaga. 2002. Borna disease virus and infection in humans. Front. Biosci. 7:d470-d495. [DOI] [PubMed] [Google Scholar]
- 15.Jindadamrongwech, S., C. Thepparit, and D. R. Smith. 2004. Identification of GRP 78 (BiP) as a liver cell expressed receptor element for dengue virus serotype 2. Arch. Virol. 149:915-927. [DOI] [PubMed] [Google Scholar]
- 16.Kim, K. B., J. W. Lee, C. S. Lee, B. W. Kim, H. J. Choo, S. Y. Jung, S. G. Chi, Y. S. Yoon, G. Yoon, and Y. G. Ko. 2006. Oxidation-reduction respiratory chains and ATP synthase complex are localized in detergent-resistant lipid rafts. Proteomics 6:2444-2453. [DOI] [PubMed] [Google Scholar]
- 17.Lee, A. S. 2001. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem. Sci. 26:504-510. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig, H., and L. Bode. 2000. Borna disease virus: new aspects on infection, disease, diagnosis and epidemiology. Rev. Sci. Tech. 19:259-288. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig, H., L. Bode, and G. Gosztonyi. 1988. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 35:107-151. [PubMed] [Google Scholar]
- 20.Makino, A., T. Horimoto, and Y. Kawaoka. 2009. Binding properties of GP1 protein of Borna disease virus. J. Vet. Med. Sci. 71:243-246. [DOI] [PubMed] [Google Scholar]
- 21.McKay, D. B. 1993. Structure and mechanism of 70-kDa heat-shock-related proteins. Adv. Protein Chem. 44:67-98. [DOI] [PubMed] [Google Scholar]
- 22.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misra, U. K., M. Gonzalez-Gronow, G. Gawdi, and S. V. Pizzo. 2005. The role of MTJ-1 in cell surface translocation of GRP78, a receptor for alpha 2-macroglobulin-dependent signaling. J. Immunol. 174:2092-2097. [DOI] [PubMed] [Google Scholar]
- 24.Misra, U. K., and S. V. Pizzo. 2008. Heterotrimeric Gα11 co-immunoprecipitates with surface-anchored GRP78 from plasma membranes of α2M*-stimulated macrophages. J. Cell. Biochem. 104:96-104. [DOI] [PubMed] [Google Scholar]
- 25.Morales, J. A., S. Herzog, C. Kompter, K. Frese, and R. Rott. 1988. Axonal transport of Borna disease virus along olfactory pathways in spontaneously and experimentally infected rats. Med. Microbiol. Immunol. 177:51-68. [DOI] [PubMed] [Google Scholar]
- 26.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez, M., M. Watanabe, M. A. Whitt, and J. C. de la Torre. 2001. N-terminal domain of Borna disease virus G (p56) protein is sufficient for virus receptor recognition and cell entry. J. Virol. 75:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richt, J. A., T. Furbringer, A. Koch, I. Pfeuffer, C. Herden, I. Bause-Niedrig, and W. Garten. 1998. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J. Virol. 72:4528-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 30.Rubio, M. E., and R. J. Wenthold. 1999. Calnexin and the immunoglobulin binding protein (BiP) coimmunoprecipitate with AMPA receptors. J. Neurochem. 73:942-948. [DOI] [PubMed] [Google Scholar]
- 31.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 32.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 33.Tomonaga, K., T. Kobayashi, and K. Ikuta. 2002. Molecular and cellular biology of Borna disease virus infection. Microbes Infect. 4:491-500. [DOI] [PubMed] [Google Scholar]
- 34.Triantafilou, K., D. Fradelizi, K. Wilson, and M. Triantafilou. 2002. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J. Virol. 76:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triantafilou, K., and M. Triantafilou. 2003. Lipid raft microdomains: key sites for coxsackievirus A9 infectious cycle. Virology 317:128-135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.