Abstract
Histidine residues have been hypothesized to function as sensors of environmental pH that can trigger the activity of viral fusion proteins. We investigated a requirement for histidine residues in the envelope (E) protein of West Nile virus during pH-dependent entry into cells. Each histidine was individually replaced with a nonionizable amino acid and tested functionally. In each instance, mutants capable of orchestrating pH-dependent infection were identified. These results do not support a requirement for any single histidine as a pH-sensing “switch,” and they suggest that additional features of the E protein are involved in triggering pH-dependent steps in the flavivirus life cycle.
Flaviviruses are enveloped RNA viruses that cause a spectrum of illnesses in humans ranging from fever to encephalitis and hemorrhagic disease (20). These small (∼50 nM) spherical virions incorporate 180 envelope (E) proteins that orchestrate the process of virus entry (22). Flaviviruses bind cells via poorly characterized receptors, are internalized by clathrin-mediated endocytosis (10), and fuse with the membranes of endosomal compartments in a pH-dependent fashion (18, 33). Upon exposure to mildly acidic conditions (∼pH 6.5), E proteins undergo extensive changes in conformation and oligomeric state that serve to tether viral and cellular membranes and pull them into the close apposition required to promote lipid mixing (12, 29). While this transformation has been detailed using biochemical and structural approaches (14, 22, 29), how an acidic environment triggers these events is less clear. The “histidine switch” hypothesis identifies a critical role for histidine residues as sensors that trigger the activity of viral fusion proteins that direct pH-dependent entry into cells (16, 21). The rationale for this theory is that histidine is unique among amino acids in that it becomes protonated and charged upon exposure to acidic environments similar to those that support viral fusion (the pKa of histidine in proteins is ∼6.4) (32). Evidence in support of a required role for histidine residues in the conformational changes of viral proteins that direct membrane fusion has been obtained using several groups of viruses that enter cells in a pH-dependent fashion (4, 5, 9, 15, 27, 31).
West Nile virus (WNV) is a mosquito-borne encephalitic flavivirus that has emerged during the last decade as a threat to public health in the Western hemisphere (3). To investigate a requirement for the ionization of histidine residues during WNV entry, we constructed a panel of mutants with histidine substitutions in the E protein and evaluated their capacity to mediate infection. The E protein is composed of three distinct domains (DI to DIII) that are connected to the viral membrane by a helical region called the stem-anchor (17, 24). Each of these protein regions contains histidine residues that could be involved in triggering conformational changes that drive pH-dependent membrane fusion (Fig. 1A). Five of the 13 histidine residues in the E protein are completely conserved among flaviviruses. Each individual histidine was mutated to two different amino acids using QuikChange mutagenesis (Stratagene, La Jolla, CA). Alanine and glutamine substitutions were selected because the side chains of these amino acids are nonionizable (Fig. 1B to D).
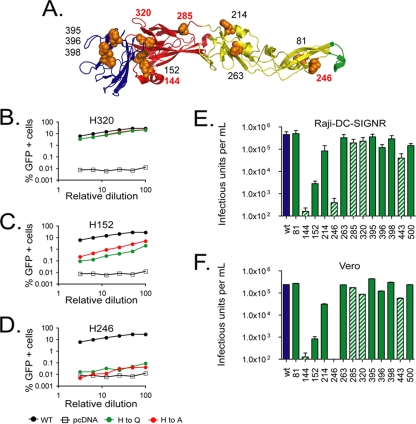
FIG. 1.
Impact of mutations at individual histidine residues in the WNV E protein on virus infectivity. (A) The WNV E protein is composed of three structurally distinct domains (DI-DIII). There are 13 histidine residues in the E protein; 3 of these are located in DI (red ribbons), 4 in DII (yellow ribbons), 4 in DIII (blue ribbons), and 2 in the stem-anchor region that anchors the E protein to the viral membrane (not pictured). Five of these histidine residues are conserved among flaviviruses (labeled in red). (B to D) RVPs composed of E proteins incorporating alanine (red) or glutamine (green) histidine substitutions were produced by genetic complementation. The infectious titer of each preparation was determined following infection of Raji-DC-SIGNR cells with serial twofold dilutions of RVPs. Infection was scored as a function of reporter gene expression 48 h postinfection and was measured using flow cytometry. (E) The infectious titer of each glutamine substitution mutant in Raji-DC-SIGNR cells was calculated for multiple independent RVP preparations using data from linear portions of the virus dose/infectivity curves. Hatched bars show results for mutants with substitutions at conserved histidine residues. The means of the results for four or five independent RVP preparations are shown; error bars identify the standard errors. (F) The titers of glutamine substitution mutants were determined following infection of Vero cells with serial twofold dilutions of RVPs. The infectious titer of each mutant was calculated as described above. Hatched bars show results for mutants with substitutions at conserved histidine residues. The means of titers from four independent experiments are shown; error bars represent the standard errors.
WNV reporter virus particles (RVPs) that incorporate each E protein mutant were produced by complementation as described previously (25, 26). Briefly, BHK-21 cells that propagate a subgenomic WNV replicon encoding green fluorescent protein were transfected with plasmids encoding WNV capsid and precursor to membrane (prM)-E proteins, followed by incubation for 2 days at 37°C. The titers of RVP preparations in Raji cells expressing the attachment factor DC-SIGNR were determined following infection with six serial twofold dilutions of virions (7). Because RVPs are capable of only a single round of infection and do not encapsidate a genome that encodes the E protein, there is no opportunity for reversion to the wild-type (WT) sequence during production. Analysis of the panel of mutants revealed three patterns (Fig. 1B to D and data not shown). Near-WT levels of infectivity were observed for mutants with both substitutions at four different histidine residues (H81, H320, H395, and H398) (Fig. 1B). Analysis of multiple independent RVP preparations with glutamine substitutions indicated that, on average, the titer of RVPs incorporating these mutants was reduced by only ∼25% (n = 4 or 5 preparations) in comparison to the titer in preparations with WT E protein (Fig. 1E). In contrast, neither substitution at position H144 or H246 yielded infectious virus particles (Fig. 1D and E). A third group of mutants displayed an intermediate phenotype with the degree of attenuation dependent upon the amino acid substitution (Fig. 1C and E). A similar pattern was observed when Vero cells were used to determine RVP titers (Fig. 1F). While in several instances (H144 and H246) a glutamine substitution resulted in a significant reduction in the number of virus particles released from transfected cells, quantitative analysis of the E protein content of RVP preparations indicated that a simple failure to release virus particles is not sufficient to explain the reduction in infectious titer observed with each mutant (data not shown). A limitation of any mutagenesis approach is that negative results must be interpreted with caution. While the identification of functional substitutions that encode nonionizable amino acids rules out a requirement for histidine protonation at a given position during virus entry, mutations may also subtly affect E protein structure or functions not relating to sensing changes in endosomal pH.
We next expanded our efforts to identify functional substitutions at positions H144 and H246. Additional mutants were constructed by site-directed mutagenesis employing primers encoding a random sequence (NNN) at the codons of H144 or H246. Because mutations may affect virus infectivity by modulating the efficiency of virion maturation, RVPs were produced in cells cotransfected with a plasmid expressing human furin, a condition shown previously to dramatically enhance the efficiency of prM cleavage (7, 23). Augmented furin expression in RVP-producing cells resulted in complete maturation of each mutant tested (data not shown), suggesting that the low-pH-mediated conformational changes that regulate exposure of the furin cleavage site are not dependent upon a single histidine residue. RVPs were also produced at both 37°C and 28°C; experiments at the latter temperature were performed to minimize the potential impact of mutations on E protein folding and stability. For substitutions at most histidine residues, overexpression of furin in producer cells had only a modest effect on the release of infectious RVPs relative to the level in control experiments with WT E proteins (data not shown; n = 3 or 4 preparations). Notably, conditions that promoted more efficient maturation resulted in a significant increase in the production of infectious RVPs incorporating the H144Q substitution (25-fold, P < 0.05), albeit at very low titers (Fig. 2A and data not shown; ∼1.4 × 103 infectious units/ml at 37°C, n = 9). Analysis of seven additional H144 mutants identified asparagine (Fig. 2A and B) and methionine (data not shown) substitutions that could be incorporated into infectious virions. RVPs composed of the H144N mutant were produced at ∼1.4% and 31% (37°C and 28°C, respectively; n = 4) of the level observed in paired experiments with WT RVPs, and the levels were not significantly different in the presence or absence of furin overexpression despite relatively inefficient cleavage of prM of H144N in the absence of furin expression (data not shown). All 19 amino acid substitutions at H246 were analyzed, and substitutions with aromatic side chains (tryptophan, phenylalanine, and tyrosine) were identified as capable of orchestrating virus entry (Fig. 2C and D and data not shown). When produced at 28°C, the titer of H246F RVPs was reduced only ∼twofold relative to the titers in studies with WT RVPs. In agreement with these findings, a neutralization escape mutant of WNV encoding an H246Y mutation has recently been described (35). Altogether, these studies identified individual substitutions with nonionizable side chains at each of the 13 E protein histidine residues that can be incorporated into RVPs with significant infectious titers.
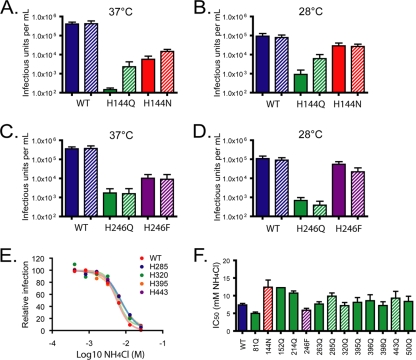
FIG. 2.
Identification of functional substitutions for H144 and H246. WNV RVPs incorporating E protein histidine substitution mutants were produced by complementation in the presence or absence of a plasmid expressing human furin, followed by incubation at 37°C (A and C) or 28°C (B and D) (1, 7, 23). The infectious titers of RVP populations were determined as described in the Fig. 1 legend. All titer experiments were performed at 37°C, even when lower temperatures were used to produce the RVPs. The titers of RVPs produced in the presence of exogenous furin expression are shown by hatched bars. The means of the results of three or four independent experiments are displayed; error bars identify the standard errors. (E and F) Entry of WNV RVPs incorporating histidine mutants is pH dependent. Raji-DC-SIGNR cells were treated with serial dilutions of the weak base NH4Cl (from 0.2 to 50 mM) for 5 to 10 min at room temperature prior to infection with RVPs. Cells were washed at 12 h postinfection and cultured for an additional 36 h in fresh medium. Infectivity was measured by flow cytometry at 48 h postinfection. The concentration of NH4Cl required to inhibit infection by 50% (IC50) was calculated by nonlinear regression. (F) The means of two or three independent measurements are shown; error bars represent the standard errors. Hatched bars show results for mutants with substitutions at conserved histidine residues.
To confirm that virions composed of each functional mutant entered cells in a pH-dependent fashion, we next established that infectious entry could be inhibited by neutralization of endosomal compartments. Ammonium chloride is a lysosomotropic agent shown to block membrane fusion by pH-dependent viruses, including flaviviruses (10, 11, 13, 28, 34). Infection by each mutant was completely inhibited by NH4Cl; the concentrations required to inhibit 50% of infection were calculated by nonlinear regression and found to be similar for WT WNV and histidine mutants (Fig. 2E and F).
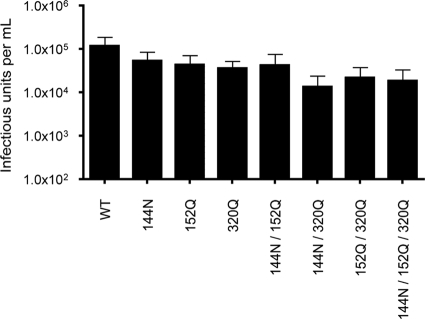
While our data suggest that no single histidine residue is required for E protein-mediated entry of WNV, it is possible that several act in concert. For example, ionization of multiple histidine residues is thought to trigger pH-dependent conformational changes in the fusion proteins of vesicular stomatitis virus and baculovirus (4, 15). The antiparallel arrangement of E proteins on the virion clusters H144, H152, and H320 at the hinge between DI and DII along the dimer interface of the E protein. Previous structural studies speculated that ionization of these three residues may trigger dimer disassociation following exposure to an acidic environment (2, 9, 24). Therefore, we constructed double and triple mutants containing substitutions in histidine residues at the DI-DII hinge and were able to identify combinations of mutations that allow for the production of infectious RVPs with significant titers (Fig. 3). While these data indicate that this cluster of histidine residues hypothesized previously to function as a pH sensor are not required for infection, it is certainly possible that other combinations of histidine (and nonhistidine) residues may coordinate to function in this regard.
FIG. 3.
Production of infectious RVPs incorporating multiple histidine substitutions at the DI-DII hinge. RVPs incorporating substitutions at one or more histidine positions clustered around the DI-DII hinge were produced by genetic complementation. The infectious titers of multiple independent preparations of RVPs were calculated as described in the Fig. 1 legend. The means of two or three independent measurements are shown; error bars represent the standard errors.
Recent studies of tick-borne encephalitis virus (TBEV) identified the conserved histidine at position 323 (corresponding to H320 of WNV) as the critical pH sensor required for the initiation of viral membrane fusion (9). Using a recombinant subviral particle (SVP) system (6, 8, 30), Fritz and colleagues found that TBEV SVPs incorporating a H323A mutation form unstable E protein trimers and are unable to fuse with synthetic liposomes when exposed to low pH (9). SVPs are small (∼30 nM), noninfectious virus-like particles composed of 60 E proteins that have been used extensively as a model of flavivirus fusion (29). Because our studies with WNV RVPs and a replication-competent infectious clone (data not shown) indicate that this residue is dispensable for pH-dependent infection, we explored this discrepancy by introducing mutations into the E protein of Langat virus (LGTV), a flavivirus that shares roughly 80% amino acid identity with TBEV. A panel of H146 and H323 mutants were produced using degenerate primers as described above and incorporated into RVPs composed of the replicon RNA and capsid protein of WNV. LGTV RVPs composed of H323L and H146R mutants were produced with titers similar to or greater than the titer of those incorporating WT LGTV E proteins (1.1 and 4.6 times the WT titer, respectively) (Fig. 4A). In each case, RVP infection remained pH dependent (Fig. 4B). How to reconcile the differences between our findings with WNV and LGTV and those reported for TBEV is unclear. One possibility is that the sensor of acidic pH differs between SVPs and infectious virions, perhaps due to differences in the geometry of these two classes of virus particles (T = 1 and pseudo-T = 3, respectively) (8, 19). Alternatively, the lipid-mixing assay used in vitro may be differentially sensitive to factors that affect the requirements for and efficiency of viral membrane fusion.
FIG. 4.
Mutation of conserved histidine residues in the tick-borne flavivirus LGTV. (A) A panel of mutants at positions H146 and H323 in LGTV was produced by QuikChange mutagenesis employing redundant primers. Mutants were assayed for their capacity to direct virus entry of RVPs produced by complementation. Multiple substitutions for positions H146 and H323 were capable of mediating entry of RVPs (data not shown). The titers of RVPs composed of H146R and H323L were calculated as described in the Fig. 1 legend. The mean titers from three independent RVP preparations are shown; error bars represent the standard errors. (B) The pH dependence of LGTV RVP entry was confirmed as described in the Fig. 2 legend. The means of two or three independent measurements are shown; error bars represent the standard errors.
Histidine residues acquire a positive charge when exposed to the mildly acidic conditions known to trigger the fusion machinery of flaviviruses. In principle, these residues are uniquely suited to serve as sensors of environmental pH. However, our results indicate that there is no requirement for protonation of a particular “histidine switch” during the infectious entry of WNV, as mutagenesis studies identified viable substitutions with nonionizable side chains at all 13 histidine residues within the E protein. While not all the mutants identified were as infectious as WT RVPs, this is not particularly surprising, as any change away from the naturally selected WT sequence has the potential to affect protein structure or function. How the E protein senses a low-pH environment remains unclear and appears to require the contribution of additional amino acids. Because amino acid side-chain pKa is influenced locally by protein structure, it is possible that nonhistidine residues are coordinated such that they are ionized at ∼pH 6.5. Resolving this complexity awaits further study.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Institutes of Allergy and Infectious Diseases (NIAID).
We thank members of our laboratory for useful discussions and their comments on the manuscript.
Footnotes
Published ahead of print on 23 September 2009.
REFERENCES
- 1.Ansarah-Sobrinho, C., S. Nelson, C. A. Jost, S. S. Whitehead, and T. C. Pierson. 2008. Temperature-dependent production of pseudoinfectious dengue reporter virus particles by complementation. Virology 381:67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bressanelli, S., K. Stiasny, S. L. Allison, E. A. Stura, S. Duquerroy, J. Lescar, F. X. Heinz, and F. A. Rey. 2004. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 23:728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briese, T., and K. A. Bernard. 2005. West Nile virus—an old virus learning new tricks? J. Neurovirol. 11:469-475. [DOI] [PubMed] [Google Scholar]
- 4.Carneiro, F. A., F. Stauffer, C. S. Lima, M. A. Juliano, L. Juliano, and A. T. Da Poian. 2003. Membrane fusion induced by vesicular stomatitis virus depends on histidine protonation. J. Biol. Chem. 278:13789-13794. [DOI] [PubMed] [Google Scholar]
- 5.Chanel-Vos, C., and M. Kielian. 2004. A conserved histidine in the ij loop of the Semliki Forest virus E1 protein plays an important role in membrane fusion. J. Virol. 78:13543-13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corver, J., A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269:37-46. [DOI] [PubMed] [Google Scholar]
- 7.Davis, C. W., H. Y. Nguyen, S. L. Hanna, M. D. Sanchez, R. W. Doms, and T. C. Pierson. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80:1290-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferlenghi, I., M. Clarke, T. Ruttan, S. L. Allison, J. Schalich, F. X. Heinz, S. C. Harrison, F. A. Rey, and S. D. Fuller. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593-602. [DOI] [PubMed] [Google Scholar]
- 9.Fritz, R., K. Stiasny, and F. X. Heinz. 2008. Identification of specific histidines as pH sensors in flavivirus membrane fusion. J. Cell Biol. 183:353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gollins, S. W., and J. S. Porterfield. 1985. Flavivirus infection enhancement in macrophages: an electron microscopic study of viral cellular entry. J. Gen. Virol. 66(Pt. 9):1969-1982. [DOI] [PubMed] [Google Scholar]
- 11.Gollins, S. W., and J. S. Porterfield. 1984. Flavivirus infection enhancement in macrophages: radioactive and biological studies on the effect of antibody on viral fate. J. Gen. Virol. 65(Pt. 8):1261-1272. [DOI] [PubMed] [Google Scholar]
- 12.Gollins, S. W., and J. S. Porterfield. 1986. pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J. Gen. Virol. 67:157-166. [DOI] [PubMed] [Google Scholar]
- 13.Guirakhoo, F., A. R. Hunt, J. G. Lewis, and J. T. Roehrig. 1993. Selection and partial characterization of dengue 2 virus mutants that induce fusion at elevated pH. Virology 194:219-223. [DOI] [PubMed] [Google Scholar]
- 14.Harrison, S. C. 2008. Viral membrane fusion. Nat. Struct. Mol. Biol. 15:690-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadlec, J., S. Loureiro, N. G. Abrescia, D. I. Stuart, and I. M. Jones. 2008. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat. Struct. Mol. Biol. 15:1024-1030. [DOI] [PubMed] [Google Scholar]
- 16.Kampmann, T., D. S. Mueller, A. E. Mark, P. R. Young, and B. Kobe. 2006. The role of histidine residues in low-pH-mediated viral membrane fusion. Structure 14:1481-1487. [DOI] [PubMed] [Google Scholar]
- 17.Kanai, R., K. Kar, K. Anthony, L. H. Gould, M. Ledizet, E. Fikrig, W. A. Marasco, R. A. Koski, and Y. Modis. 2006. Crystal structure of West Nile virus envelope glycoprotein reveals viral surface epitopes. J. Virol. 80:11000-11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan, M. N., B. Sukumaran, U. Pal, H. Agaisse, J. L. Murray, T. W. Hodge, and E. Fikrig. 2007. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J. Virol. 81:4881-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindenbach, B. D., H. J. Thiel, and C. M. Rice. 2007. Flaviviridae: the viruses and their replication, p. 1101-1152. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 1. Lippincott-Williams & Wilkins, Philadelphia, PA.
- 21.Mueller, D. S., T. Kampmann, R. Yennamalli, P. R. Young, B. Kobe, and A. E. Mark. 2008. Histidine protonation and the activation of viral fusion proteins. Biochem. Soc. Trans. 36:43-45. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay, S., R. J. Kuhn, and M. G. Rossmann. 2005. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 3:13-22. [DOI] [PubMed] [Google Scholar]
- 23.Nelson, S., C. A. Jost, Q. Xu, J. Ess, J. E. Martin, T. Oliphant, S. S. Whitehead, A. P. Durbin, B. S. Graham, M. S. Diamond, and T. C. Pierson. 2008. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 4:e1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nybakken, G. E., C. A. Nelson, B. R. Chen, M. S. Diamond, and D. H. Fremont. 2006. Crystal structure of the West Nile virus envelope glycoprotein. J. Virol. 80:11467-11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierson, T. C., M. D. Sanchez, B. A. Puffer, A. A. Ahmed, B. J. Geiss, L. E. Valentine, L. A. Altamura, M. S. Diamond, and R. W. Doms. 2006. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology 346:53-65. [DOI] [PubMed] [Google Scholar]
- 26.Pierson, T. C., Q. Xu, S. Nelson, T. Oliphant, G. E. Nybakken, D. H. Fremont, and M. S. Diamond. 2007. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1:135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin, Z. L., Y. Zheng, and M. Kielian. 2009. Role of conserved histidine residues in the low-pH dependence of the Semliki Forest virus fusion protein. J. Virol. 83:4670-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph, V. B., and V. Stollar. 1990. Low pH-induced cell fusion in flavivirus-infected Aedes albopictus cell cultures. J. Gen. Virol. 71(Pt. 8):1845-1850. [DOI] [PubMed] [Google Scholar]
- 29.Stiasny, K., and F. X. Heinz. 2006. Flavivirus membrane fusion. J. Gen. Virol. 87:2755-2766. [DOI] [PubMed] [Google Scholar]
- 30.Stiasny, K., C. Koessl, and F. X. Heinz. 2003. Involvement of lipids in different steps of the flavivirus fusion mechanism. J. Virol. 77:7856-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thoennes, S., Z. N. Li, B. J. Lee, W. A. Langley, J. J. Skehel, R. J. Russell, and D. A. Steinhauer. 2008. Analysis of residues near the fusion peptide in the influenza hemagglutinin structure for roles in triggering membrane fusion. Virology 370:403-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurlkill, R. L., G. R. Grimsley, J. M. Scholtz, and C. N. Pace. 2006. pK values of the ionizable groups of proteins. Protein Sci. 15:1214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Schaar, H. M., M. J. Rust, C. Chen, H. van der Ende-Metselaar, J. Wilschut, X. Zhuang, and J. M. Smit. 2008. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 4:e1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Schaar, H. M., M. J. Rust, B. L. Waarts, H. van der Ende-Metselaar, R. J. Kuhn, J. Wilschut, X. Zhuang, and J. M. Smit. 2007. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J. Virol. 81:12019-12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogt, M. R., B. Moesker, J. Goudsmit, M. Jongeneelen, S. K. Austin, T. Oliphant, S. Nelson, T. C. Pierson, J. Wilschut, M. Throsby, and M. S. Diamond. 2009. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J. Virol. 83:6494-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]