Abstract
3,4-Methylenedioxymethamphetamine (MDMA) is a drug of abuse world-wide and a selective serotonin (5-HT) neurotoxin. An important factor in the risk of drug abuse and relapse is stress. Although multiple parallels exist between MDMA abuse and stress including effects on 5-HTergic neurotransmission, few studies have investigated the consequences of combined exposure to MDMA and chronic stress. Therefore, rats were pretreated with MDMA and exposed 7 days later to 10 days of mild chronic unpredictable stress (CUS). MDMA pretreatment was hypothesized to enhance the effects of CUS leading to enhanced 5-HT transporter (SERT) depletion in the hippocampus and increased anxiety and cognitive impairment. While MDMA alone increased anxiety-like behavior on the elevated plus maze, CUS alone or in combination with MDMA pretreatment did not increase anxiety-like behavior. In contrast, MDMA pretreatment led to CUS-induced learning impairment in the Morris water maze but not an enhanced depletion of hippocampal SERT protein. These results show that prior exposure to MDMA leads to stress-induced impairments in learning behavior that is not otherwise observed with stress alone and appear unrelated to an enhanced depletion of SERT.
Keywords: MDMA, Chronic Unpredictable Stress, elevated plus maze, Morris water maze, hippocampus
Introduction
3,4-Methylenedioxymethamphetamine (MDMA, Ecstasy), a synthetic amphetamine analog, is a recreational drug of abuse often consumed among adolescent youth during social gatherings known as “raves” (Gross, Barrett, Shestowsky, & Pihl, 2002). MDMA causes the rapid release of many neurotransmitters including serotonin (5-HT), dopamine, and norepinephrine (Schmidt, 1987; Yamamoto & Spanos, 1988; Stone, Stahl, Hanson, & Gibb, 1986; Stone, Merchant, Hanson, & Gibb, 1987; Fitzgerald & Reid, 1990). Although its acute effects involve multiple neurotransmitters, the neurotoxic profile of MDMA is selective to the 5-HTergic system and is characterized by long-term decreases in 5-HT tissue content, the 5-HT transporter (SERT), tryptophan hydroxylase (TPH), and damage to the 5-HT nerve terminals and fibers (Stone et al., 1986; Stone et al., 1987; Schmidt, 1987). This selective damage is particularly evident in the hippocampus, the brain area most commonly associated with learning, memory, and anxiety (Marr, 1971; McNaughton & Gray, 2000). In fact, hippocampal damage in part may explain the reported cognitive deficits and increased anxiety-like behavior in rats exposed to MDMA (Sprague, Preston, Leifheit, & Woodside, 2003; Gurtman, Morley, Li, Hunt, & McGregor, 2002).
Several reports have shown that stress enhances the risk of abuse and relapse to a number of drugs of abuse including the amphetamines (Sinha, 2001). Moreover, stress may enhance the reinforcing effects of psychostimulant drug use (Shaham & Stewart, 1994). These findings suggest that stress, in particular the emotional response to stress (Sinha, Catapano, & O'Malley, 1999), may enhance the rewarding effects and probability of drug use. Stress is a common experience that influences a host of physiological responses necessary to maintain homeostasis in response to a stimulus. Serotonin (5-HT) is a particularly important neurotransmitter in the adaptation and response to stress (Chaouloff, Berton, & Mormede, 1999). Studies in rodents have shown that, similar to MDMA, acute stress increases TPH activity (Singh, Corley, Phan, & Boadle-Biber, 1990) and causes the rapid release of 5-HT in several brain areas, including the hippocampus (Fujino et al., 2002; Amat, Matus-Amat, Watkins, & Maier, 1998).
Several studies have shown that chronic stress may have deleterious effects on hippocampal 5-HT neurotransmission and morphology. Long-term exposure to chronic predictable stress (CPS) and chronic unpredictable stress (CUS), have both been shown to decrease 5-HT levels and increase 5-HT metabolites in the hippocampus (Torres, Gamaro, Vasconcellos, Silveira, & Dalmaz, 2002; Li et al., 2003). Additionally, several reports suggest that CPS can induce dendritic atrophy on hippocampal pyramidal neurons (Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002; Magarinos & McEwen, 1995). These data suggest that long-term exposure to CPS or CUS is similar to the long-term effects of MDMA by producing damage to the hippocampus with associated learning and memory deficits (Luine, Villegas, Martinez, & McEwen, 1994; Bodnoff et al., 1995; Sousa, Lukoyanov, Madeira, Almeida, & Paula-Barbosa, 2000) as well as an increase in anxiety-like behavior (Vyas & Chattarji, 2004; Tannenbaum, Tannenbaum, Sudom, & Anisman, 2002).
Although these striking parallels exist and stress enhances the probability of drug use, few studies have investigated the additive or synergistic interactions resulting from the combined exposure to MDMA and stress. Therefore, the present study was designed to examine the effects CUS in rats pre-exposed to a neurotoxic regimen of MDMA. This mild stress regimen was initially developed as a model of depression (Willner, 1997; Katz, Roth, & Carroll, 1981) and may be more effective than CPS in increasing the probability of drug use (Haile, GrandPre, & Kosten, 2001). Although mild CUS alone is not associated with 5-HT depletions (Harro, Tonissaar, Eller, Kask, & Oreland, 2001), does not affect hippocampal cell morphology, and does not enhance anxiety (Vyas et al., 2004; Vyas et al., 2002), it was hypothesized that prior exposure to MDMA would enhance the effects of CUS and augment SERT depletions in the hippocampus. Furthermore, it was posited that mild CUS after neurotoxic MDMA would lead to enhanced cognitive impairments in the Morris water maze (MWM) and anxiety-like behavior in the elevated plus maze (EPM).
Materials and Methods
Subjects
Adult male Sprague-Dawley rats (175–200 g, Harlan Sprague Dawley, IN, USA) were used in all experiments. Upon arrival, rats were group housed and allowed 2–3 days to acclimate to the animal colony. The rats were maintained on a 12:12-h light dark cycle (0700–1900h light:1900-0700h dark) in a temperature- and humidity-controlled room. Food and water were available ad libitum. All procedures and housing conditions were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Boston University.
Drug Administration
MDMA was obtained from the National Institutes of Drug Abuse (NIDA, Research Triangle). Rats were injected intraperitoneally (ip) with 0.9% saline (1 ml/kg) or MDMA (7.5mg/kg) dissolved in saline once every 2 hr for a total of four injections (i.e., 7.5 mg/kg every 2 hr × 4, ip). Based on preliminary findings, the dose of MDMA was chosen to achieve sub-maximal serotonin neurotoxicity and avoid hyperthermia-induced death. Temperatures were measured via a rectal probe digital thermometer (Thermalert TH-8; Physitemp Instruments Inc., Clifton, NJ, USA) an hour after each injection. Rats that became excessively hyperthermic (above 42 °C) were briefly cooled indirectly by placing their cages on ice packs and/or wet ice.
Chronic Unpredictable Stress (CUS)
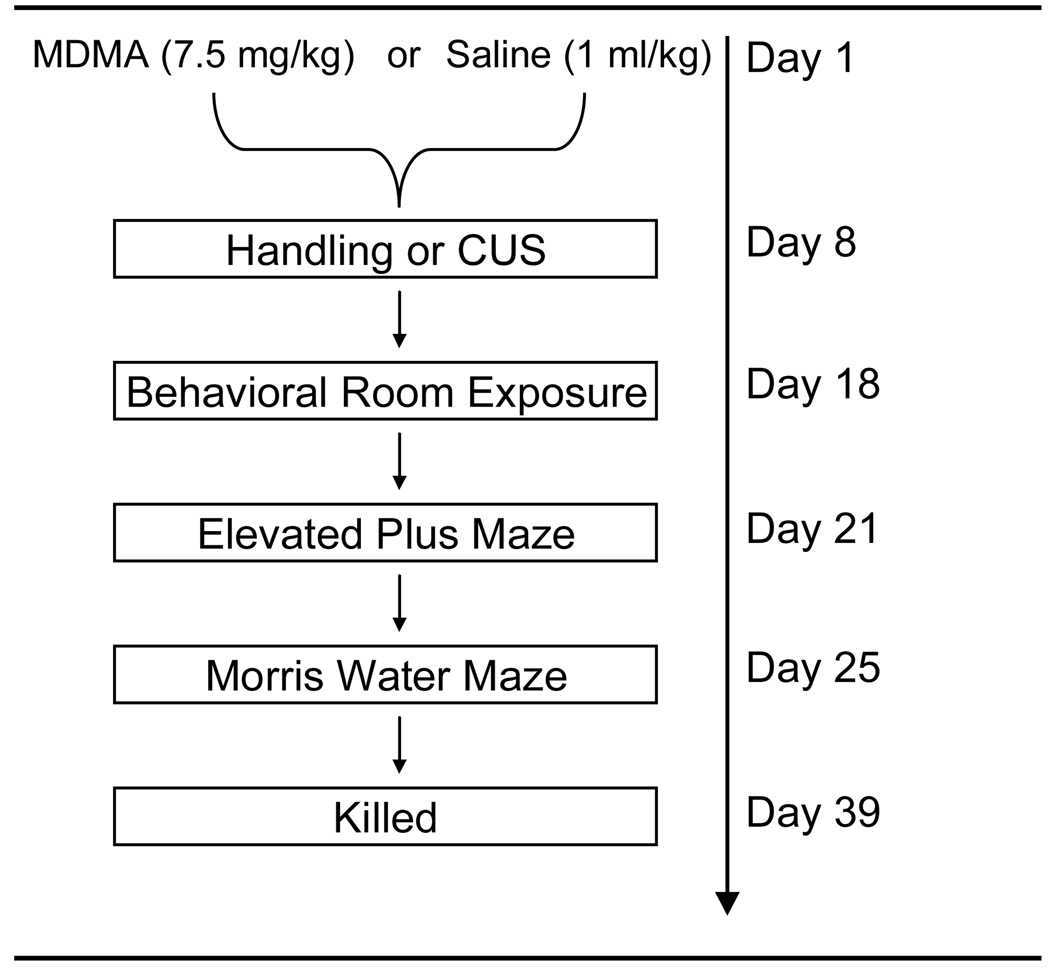
As shown in Figure 1, seven days after MDMA or saline administration, rats were either handled or exposed to 10 days of various stressors as previously described (Matuszewich & Yamamoto, 2004) with modification. The procedure was as follows; Day 1: 10:00 30 min cage rotation, 16:00 Isolation overnight; Day 2: 10:00 3hr lights off, 18:00 Food & water deprivation overnight; Day 3: 10:00 30 min restraint, 18:00 Lights on overnight; Day 4: 11:00 Wet Bedding, 15:00 Social stress; Day 5: 9:00 60 min restraint, 18;00 Food & water deprivation overnight; Day 6: 15:00 2hr lights off, 17:00 Isolation overnight; Day 7: 10:00 60 min cage agitation, 18:00 60 min restraint stress; Day 8: 11:00 Wet Bedding, 18:00 Food & water deprivation overnight; Day 9: 9:00 3hr lights off , 18:00 Lights on overnight (PM); Day 10: 10:00 30 min cage agitation, 18:00 Isolation overnight.
Figure 1.
Schematic diagram of the treatment and behavioral testing schedule. Briefly, rats were treated with MDMA (7.5 mg/kg every 2 hr × 4, ip) or Saline (1 ml/kg). Seven days later rats were either handled or exposed to 10 d of mild CUS. Three days after the last stressor, rats were tested for anxiety behavior in the EPM. Four days later learning and behavior was assessed in the MWM. Three days after the MWM testing, rats were killed for SERT and actin immunoreactivity.
Elevated Plus Maze (EPM)
Following the CUS procedure, rats were allowed to habituate to the testing room for three days before being tested on the EPM. Behavior was measured using a wooden maze consisting of two open arms (50 cm long × 10 cm wide) opposite each other, crossed by two enclosed arms (50 cm long × 10 cm wide × 40 cm high) with an open roof. A camera mounted above the center of the maze recorded rat behavior (tracks) which was analyzed using Spontaneous Motor Activity Recording and Tracking (SMART) software (San Diego Instruments, San Diego, USA). During testing, rats were placed in the center of the maze facing an open arm and allowed to explore for 10 min during which time the experimenter remained outside the room. During this period, the following behaviors were collected in two 5 min bins (0–5 min and 6–10 min); entries into open and closed arms, time spent in each arm and distance traveled in the closed arms. Anxiety behavior was defined as a decrease in overall exploration and decreased time spent in the open arms. After testing, rats were immediately returned to home cages and the maze was wiped down with a 10% isopropyl alcohol solution. Based on previous studies, rats were only included if the saline non-stressed rats within the testing groups spent at least 20% of the test time on the open arm (Bondi, Rodriguez, Gould, Frazer, & Morilak, 2007; Vyas et al., 2004; Matuszewich et al., 2007).
Spatial Navigation Procedure (Morris Water Maze Task, MWM)
Four days after the EPM, hippocampal-dependent learning and memory was assessed using a 1.5 m diameter and 45 cm deep white plastic Morris maze in combination with SMART software which monitored and recorded swim paths for later analysis. The maze was filled to a depth of 27 cm with 25 °C (±1 °C) water rendered opaque with approximately 1.5 L of 2% milk. A circular platform, 25 cm high and 12 cm in diameter, was submerged below the surface of the water in one of four quadrants (south west (SW), north west (NW), south east (SE), north east (NE)). Rats were required to learn to navigate to the platform using distal cues in the vicinity of the tank. To measure acquisition learning behavior, three swim trials were given per day for five consecutive days where rats were launched from one of three randomized start positions adjacent to the wall in the center of the three quadrants not containing the escape platform. Distance to the platform (cm) and latency to escape (s) were recorded. Rats were allowed a maximum time of 1 min to find the platform and were guided to the platform if they failed to locate the platform within the specified time. During the 16th swim trial (probe trial), corresponding to the 4th trial on the day five, the platform was removed from the pool and the rats launched from the quadrant diagonal to the previous platform position. The probe trial, designed to test spatial memory, lasted for 1 min and the distance and permanence time in each quadrant were recorded. To measure reversal learning and memory behavior, trials 1–16 were repeated with the escape platform located in the diagonal quadrant. Unpublished data from our laboratory has demonstrated that by day five rats reach the shortest latencies and distances traveled to reach the escape platform. Based on this, prior to the start of the experiments, we selected day five as the criteria day where rats were expected to have learned the MWM task. Previous data with a visible platform have confirmed that neither MDMA nor CUS affect visual or motor performance in the water maze.
Biochemical Measurements (Western Blot)
Thirty-nine days after MDMA or saline administration and following behavioral assessment, rats were killed via rapid decapitation. Brains were rapidly extracted and the hippocampus from each side of the brain was dissected on wet ice, frozen on dry ice and stored at −80 °C until further analysis. The hippocampus from either the left or right side of the brain from each rat was homogenized in 20:1 (vol:wt; µL/mg) buffer that contained 10 mM Tris (pH 7.4), 10 mM EGTA, 250 mM sucrose, 2 µg/mL aprotinin, 10 µg/mL leupeptin, 2 µg/mL pepstatin A and 1 mM phenylmethylsulfonyl fluoride (PMSF). Protein content was determined by the Bradford method using Bradford protein dye (Bio-Rad, Hercules, CA). Homogenates were diluted 1:1 (vol:vol; µL/ µL) using 2x sodium dodecyl sulfate (SDS) loading buffer (Invitrogen, Grand Island, NY, USA). Samples were heated at 85 °C for 5 min and stored at −80 °C until assayed.
Western blot analysis for SERT was performed using 30 µg of protein. Samples were loaded onto 10% Tris-glycine gels and transferred to polyvinylidine difluoride (PVDF) membranes in 1X Tris-glycine transfer buffer containing 20% methanol and 0.02% SDS. Membranes were rinsed in TBS-T (10 mM Tris, 150 mM NaCl, 0.5% Tween 20) and blocked for 1 h at room temperature (RT) in TBS-T containing 5% powdered milk. Membranes were then incubated overnight at 4 °C with appropriate primary antibody (SERT; dilution 1:1250, Santa Cruz, CA, USA). The following day, membranes were washed with TBS-T and probed with the appropriate secondary antibody (anti-goat; dilution 1:2500, Santa Cruz: anti-rabbit: dilution 1:2500, Chemicon) for 1 hr at RT. Membranes were rinsed and visualized using chemilumincescence reagents and hyperfilm (Amersham Biosciences, NJ, USA). For actin immunoreactivity, SERT membranes were washed 2X in TBS-T, blocked for 30min at RT in blocking buffer and probed with actin primary antibody (dilution 1:10,000; Millipore, MA, USA) for 30min at RT. Membranes were then rinsed 3X in TBS-T, probed with HRP-conjugated anti-mouse secondary antibody (dilution 1:2500; Santa Cruz, CA, USA) for 30min at RT and visualized. The SERT bands were measured as relative optical density units and normalized to actin. The data were analyzed and presented as the percent of non-stressed saline controls.
Data Analysis
The effects of MDMA and CUS on behavior in the EPM are represented as percent of time spent on open arms and percent of distance traveled on the closed arms. The effects in the MWM are represented by latency and distance traveled to find the escape platform during acquisition and permanence time in quadrants for probe trials. The overall effects of MDMA and CUS on SERT expression in the hippocampus are represented as percent of saline no stress controls.
Temperature data were analyzed with a repeated measures two-way analysis of variance (ANOVA). For behavioral analysis, a two-way ANOVA was used to compare the effects of saline or MDMA and stress or no stress on EPM behavior. A one-way ANOVA was used to compare the effects of saline or MDMA pretreatment on percent of time spent on the open arm in the EPM. A two-way ANOVA and a two-way ANOVA with repeated measures were used to compare MWM behavioral effects of rats pretreated with saline or MDMA, stress or no stress, and time or quadrant. To compare the effect of MDMA or saline within stressed rats on acquisition learning on day five, a one-way analysis of variance (ANOVA) was performed. For neurochemical analysis, a two-way ANOVA was used to compare SERT expression in rats pretreated with saline of MDMA and stress or no stress. A Tukey’s test was used for all post hoc analyses for any significant differences. For all conditions and experiments, statistical significance was set at p<0.05. Data are presented as ± standard error of the mean.
Results
The effects of MDMA on body temperature
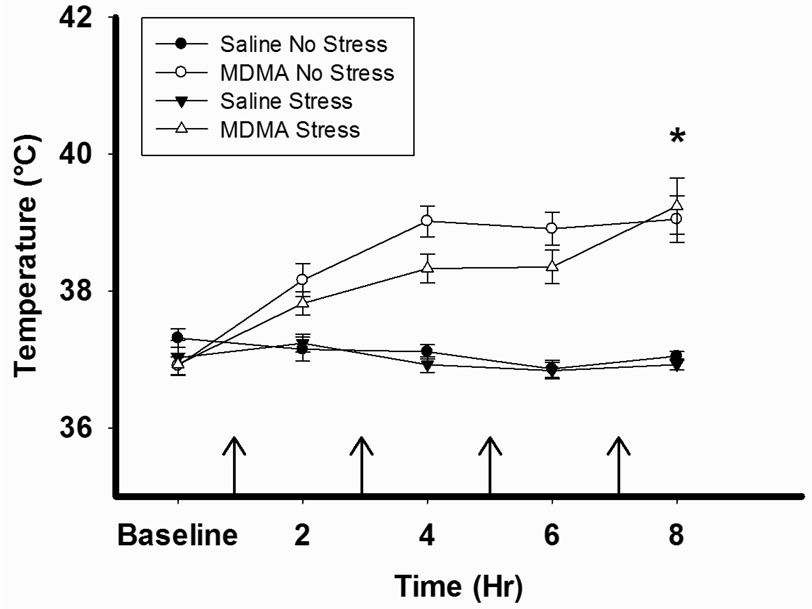
Figure 2 illustrates that MDMA pretreatment significantly increased body temperature over time during the treatment regimen when measured one hour after each injection (MDMA × Time interaction, F12,167=4.229, p<0.001). There was no difference between MDMA/No Stress and MDMA/Stress rats.
Figure 2.
MDMA-induced hyperthermia. Rectal temperatures were measured prior to and one hour after each injection of either MDMA (7.5 mg/kg every 2hr ×4, ip) or saline. MDMA pretreatment significantly increased body temperature during the treatment regimen, *p<0.001. The arrows indicate the time of MDMA or Saline injection. Saline No Stress n= 8, MDMA No Stress n=10, Saline Stress n=8, MDMA Stress n=10.
The effects of MDMA and CUS pretreatment on behavior measured in the EPM
One subset of rats, consisting of Saline/No Stress n=2, MDMA/No stress n=4, Saline/Stress n=4, and MDMA/Stress n=4, was excluded from the EPM data analysis because the control Saline/No Stress rats within the set did not meet the open arm time criteria. In addition, an outlier was removed from both the Saline/No Stress and the MDMA/Stress groups respectively. No differences were observed between the first (0–5 min) and second (6–10) 5 min bins. Therefore, the data were pooled and analyzed across the 10 min test.
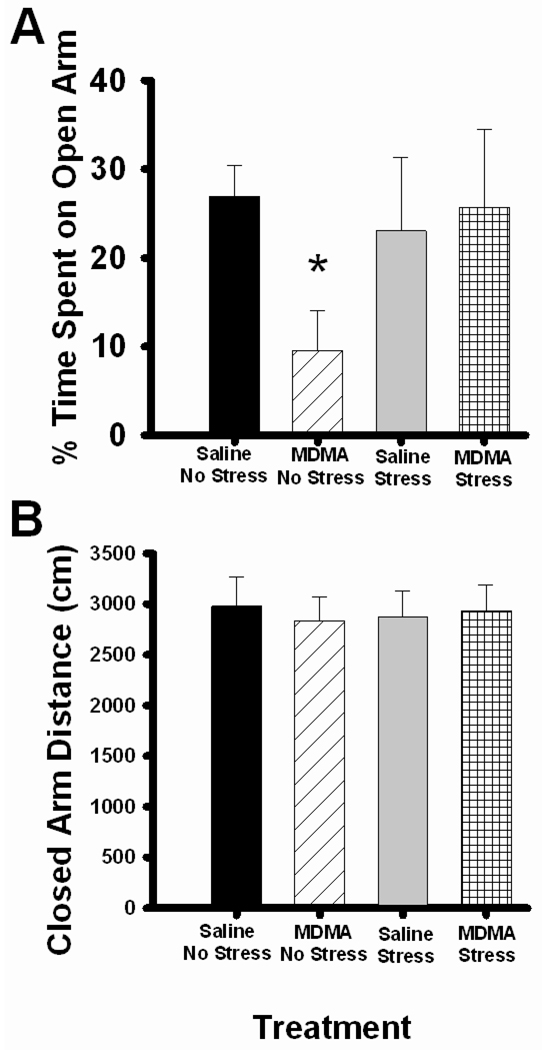
Non-stressed rats pretreated with saline spent about 30% of the test time in the open arms. No differences were observed in stressed rats or in stressed rats pretreated with MDMA. Interestingly, when compared by a one-way ANOVA, MDMA pretreatment alone decreased time spent in the open arms in non-stressed rats as noted by a significant main effect of MDMA, F1,9 =8.754, p<0.05(Figure 3A). CUS did not affect time spent in the open arm in rats pre-exposed to saline and MDMA. No differences in locomotor activity were observed across groups as evidenced by no significant differences in distance traveled within the closed arms (Figure 3B) or in the number of closed arm entries (data not shown).
Figure 3.
Anxiety behavior testing in the EPM. Rats were pretreated with saline or MDMA (7.5 mg/kg every 2 hr × 4, ip) followed by handling or CUS 7 days later. Saline No Stress n= 5, MDMA No Stress n=6, Saline Stress n=6, MDMA Stress n=5. (A) The percent of time spent on the open arm during 10 min testing on the EPM. MDMA No Stress rats demonstrated a significant decrease in open arm time compared to Saline No Stress, *p<0.05. (B) The percent of distance traveled within the closed arms during 10 min testing on the EPM. There were no differences seen in total distance on the closed arms between any groups.
The effects of MDMA and CUS pretreatment on latency to find the escape platform in the MWM
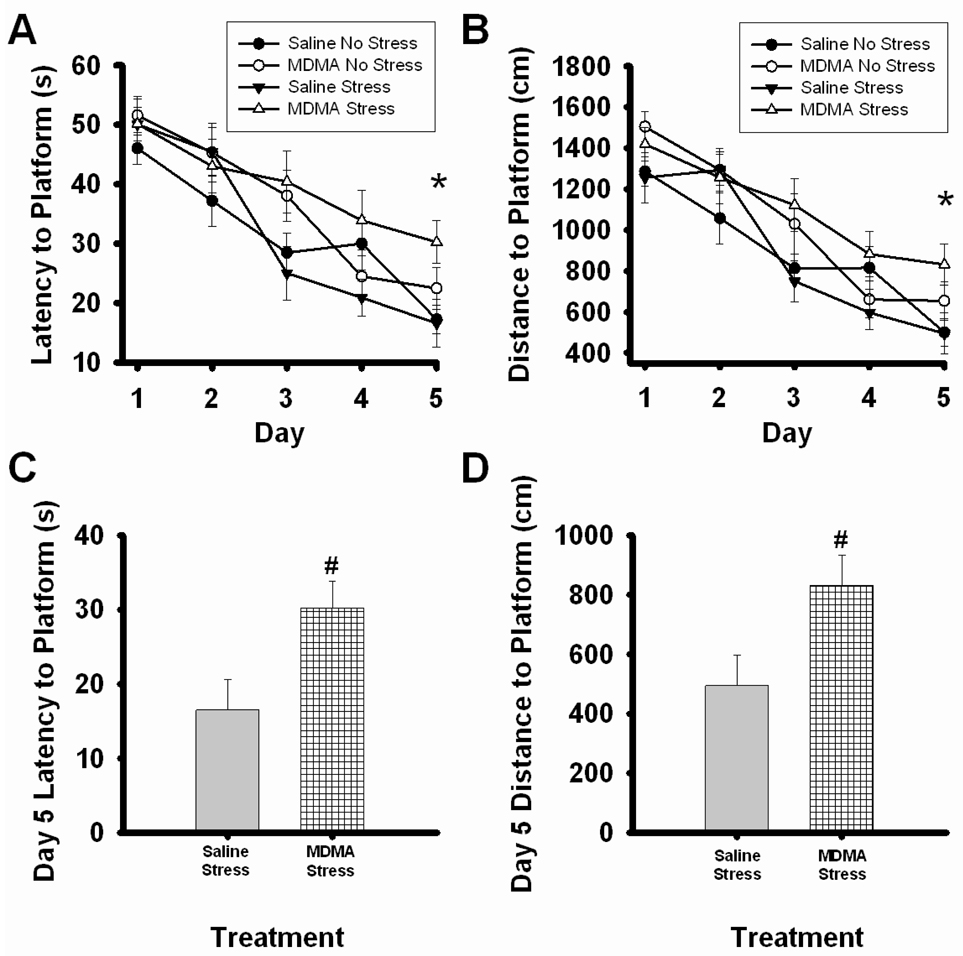
During acquisition training, a significant main effect of day, F4,173=40.18, p=<0.001, revealed that latency to find the escape platform decreased across days in all treatment groups (Figure 4A). Analysis on the final day of acquisition revealed a main effect of MDMA, F1,34=7.258, p=<0.05, demonstrating that MDMA pretreated animals showed higher latencies to find the escape platform. Interestingly MDMA pretreatment in stressed rats significantly increased the latency to find the escape platform as noted by a significant effect of MDMA (F1,16= 6.44, p<0.05, Figure 4C).
Figure 4.
Acquisition learning in the MWM. Rats were pretreated with saline or MDMA (7.5 mg/kg every 2 hr × 4, ip) followed by handling or CUS 7 days later. Saline No Stress n=8, MDMA No Stress n=10, Saline Stress n=8, MDMA Stress n=10. (A) The latency and (B) distance traveled to find the escape platform across days in the MWM. Rats were giving 3 trials per day for 5 days and a significant effect of day was noted, p<0.001 significant effect of day (data not indicated). MDMA rats showed significant increases in latency and distance to find the escape platform, *p<0.05. (C) The latency and (D) distance traveled to find the platform on criteria day 5. MDMA Stress rats had significantly higher latencies and distances traveled to the escape platform than Saline Stress rats, #p<0.05.
The effects of MDMA and CUS pretreatment on distance to find the escape platform in the MWM
Similar to the effects on latency to the platform, a significant main effect of day, F4,173=50.285, p=<0.001, revealed that the distance traveled to the platform decreased across days during acquisition in all treatment groups (Figure 4B). Analysis of the final day of acquisition revealed that MDMA pretreated animals traveled greater distances to find the platform as noted by a main effect of MDMA (F1,34=6.752, p=<0.05). In corroboration with the latency data, a significant main effect of MDMA, F1,16=5.429, p=<0.05, revealed that MDMA pretreatment in stressed animals increased the distance traveled to find the escape platform.
The effects of MDMA and CUS pretreatment on spatial retention in the MWM
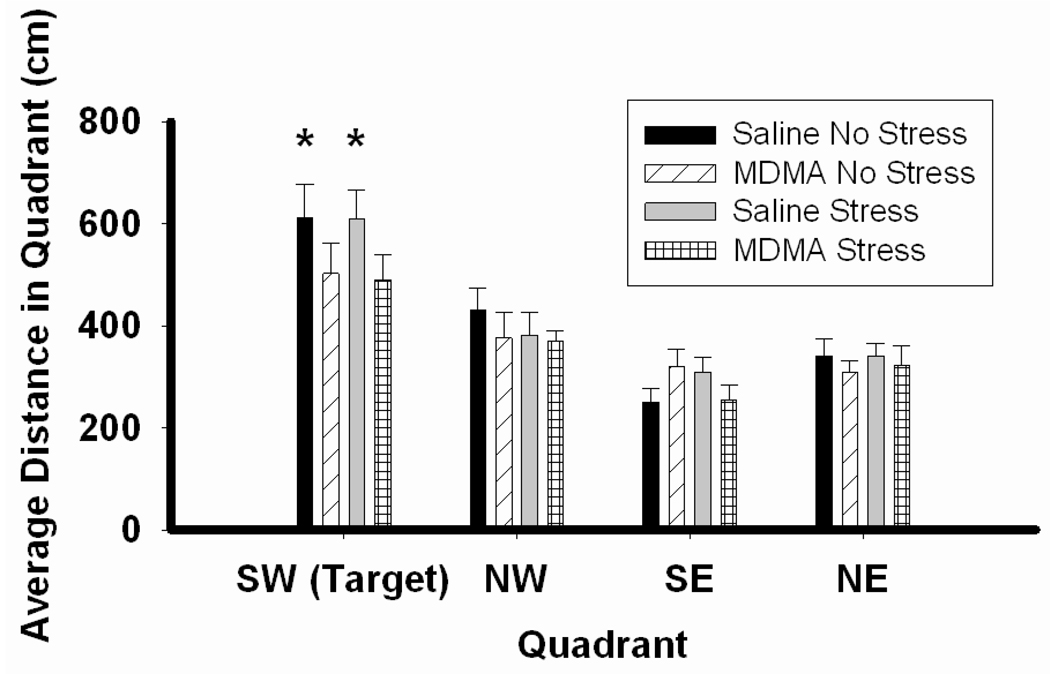
During acquisition probe trials, all groups traveled the greatest distance in the quadrant (SW) which previously housed the escape platform, as revealed by a main effect of quadrant F3,140=37.20, p<0.001 (Figure 5). Post hoc analysis revealed that only saline pretreated rats traveled more distance in the SW quadrant than any other quadrant in the maze ( p<0.001 for SW compared to all other quadrants in saline pretreated groups).
Figure 5.
Acquisition probe trials in the MWM. Rats were pretreated with saline or MDMA (7.5 mg/kg every 2 hr × 4, ip) followed by handling or CUS 7 days later. Saline No Stress n=8, MDMA No Stress n=10, Saline Stress n=8, MDMA Stress n=10. All groups traveled a significant amount of distance in the correct quadrant (SW) compared to the remaining quadrants (SE, NW, NE). Post hoc analysis revealed that only rats pretreated with saline traveled more distance in the correct quadrant (SW) than any other quadrant. SW quadrant in Saline No Stress and Saline/Stress rats, *p<0.001 compared to remaining quadrants.
The effects of MDMA and CUS pretreatment on reversal learning and spatial retention in the MWM
During reversal training, similar to acquisition, latency decreased across days, F4,173=21.62, p<0.001. No significant differences were seen in latency to find the platform on day five of reversal learning (data not shown). No group differences in permanence time in the correct quadrant (NW) were seen in reversal spatial retention (data not shown).
SERT tissue content in the hippocampus after MDMA and CUS
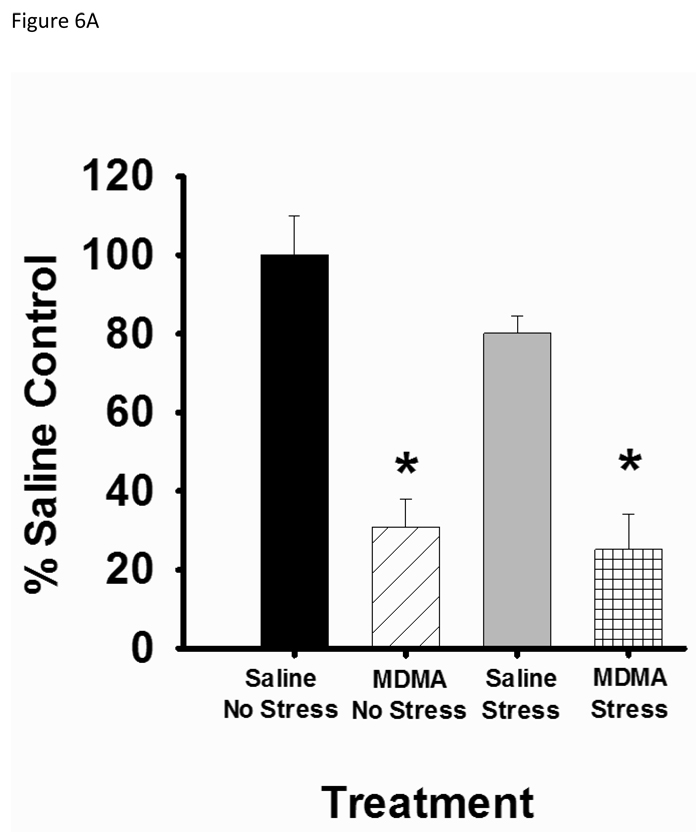
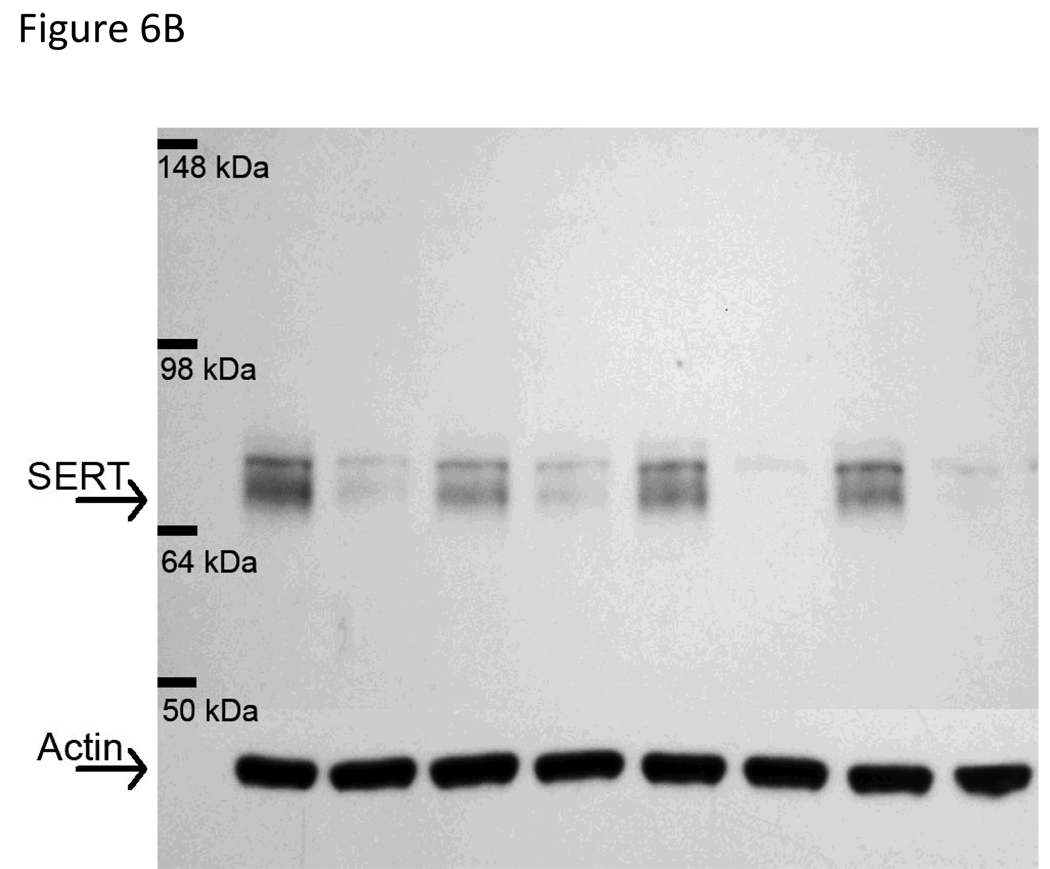
SERT immunoreactivity presented as two bands, one at 63–68 kDa and another at 70kDa (Xie, Tong, Mclane, Hatzidmitriou, Yuan, McCann & Ricarte, 2006; Quinton & Yamamoto, 2007; Tata & Yamamoto, 2008). In our results, both the 63–68 kDa and the 70 kDa band were decreased in MDMA pretreated animals. Based on the results from Xie et al. (2006) however, we quantified the lower 63–68 kDa band for SERT immunoreactivity. Pretreatment with MDMA produced a significant depletion of SERT protein in the hippocampus as noted by a main effect of MDMA, F1,34=58.889, p<0.001 (Figure 6A). CUS alone did not affect SERT protein and pretreatment with MDMA followed by CUS did not enhance SERT depletion.
Figure 6.
Hippocampal SERT protein analysis. Rats were pretreated with saline or MDMA (7.5 mg/kg every 2 hr × 4, ip) followed by handling or CUS 7 days later. Saline No Stress n=8, MDMA No Stress n=10, Saline Stress n=8, MDMA Stress n=10. SERT content was assessed 39 days after pretreatment. (A) Rats pretreated with MDMA showed significant decreases in SERT protein in the hippocampus, *p<0.05 saline versus MDMA pretreatment. (B) Representative western blot image. The arrow at 63–68 kDa indicates the lower SERT band doublet and the arrow at 43 kDA labels the actin band used as a loading control. MDMA significantly decreases the SERT immunoreactive band without affecting actin immunoreactivity. The order of lanes from left to right are Saline/No Stress, MDMA/No Stress, Saline/Stress, MDMA/Stress, Saline/No Stress, MDMA/No Stress, Saline/Stress, MDMA/Stress.
Discussion
The main finding of the present study is that MDMA pretreatment leads to spatial learning deficits in stressed rats. In addition, MDMA pretreatment alone impaired spatial reference memory in both stressed and non-stressed groups. In contrast to the effects on spatial learning and memory behavior, the combination of MDMA and CUS did not enhance anxiety-like behavior in the EPM.
A comparison of MDMA versus Saline pretreated controls in non-stressed rats revealed that MDMA decreases time spent on the open arm in the EPM. This finding is consistent with Gurtman et al., (2002) suggesting that MDMA alone increases anxiety-like behavior in rats. The effect of decreased open-arm time was not due to differences in locomotor or exploratory behavior since no significant differences in total distance traveled on the closed arms or in the number of closed arm entries were measured. Ten days of CUS alone did not affect open arm time in the EPM and corroborates the findings by Vyas et al. (2002). However, a longer exposure of 14 days of CUS decreased open arm exploration and enhanced anxiety in the EPM (Bondi et al., 2007). Moreover, the effects of mild CUS may be more specific to learned anxiety tasks such as defensive burying behavior compared innate anxiety behavior measured by the EPM (Matuszewich et al., 2007). These distinctions highlight the importance of duration/type of stressors, the amount of time between stress and the method of assessing anxiety-like behaviors in rats.
The combination of MDMA and CUS did not enhance anxiety-like behavior in the EPM. Although it appears that CUS attenuated MDMA-induced anxiety-like behavior, there was no significant difference between stress and no stress in MDMA pretreated rats. This finding is surprising in light of a recent study demonstrating that exposure to a more potent chronic restraint stress increased anxiety-like behavior in rats pretreated with para-chloroamphetamine (PCA), a 5-HT neurotoxin (Zhou et al., 2008). The mild nature of the current stress procedure where rats are exposed to novel environments and novel stressors may have added to the variable response on the EPM. Moreover, rats exposed to mild CUS showed increased variability in open arm time and latency to enter the open arm in the EPM when tested one day after the last stressor (Matuszewich et al., 2007). Overall, the present data suggest that a mild form of CUS has no effect on behavior in the EPM.
The results from the MWM showed that both the latency and distance traveled to reach the platform decreased across days during training indicating that all rats were able to learn the spatial task. Throughout acquisition trials, MDMA pretreated rats trended towards increased latency and distance traveled to find the escape platform. Interestingly by day five, when the rats were expected to have learned the task, MDMA rats performed significantly worse in the MWM. This indicates that MDMA pretreated rats were not as able to acquire the learning task as well as saline pretreated rats. These results are inconsistent with Sprague et al. (2003) who showed no changes in latency to find the platform in rats pretreated with MDMA (20 mg/kg every 12 hr × 2; sc) and tested one week later. In the present study, rats were tested in the MWM three to four weeks after MDMA administration and therefore, MDMA-induced spatial impairments in the MWM appear to exhibit a delayed appearance and develop slowly over time after neurotoxic drug administration.
In contrast to the effects of MDMA and CUS on anxiety behavior, our results are the first to demonstrate that MDMA pretreatment leads to mild CUS-induced deficits in MWM spatial learning. When compared to stressed rats pretreated with saline, rats treated with MDMA and exposed to mild CUS demonstrated increased latencies and distances traveled to find the escape platform. Overall, these findings confirm the hypothesis that MDMA pretreatment leads to mild CUS-induced impairments in learning behavior and further suggest that the effects of mild CUS in MDMA pretreated rats are more specific to learning and memory impairments than increases in anxiety-like behavior.
Neurotoxic doses of MDMA alone slightly decreased spatial memory, as assessed by the probe trials during acquisition and are consistent with the findings of Sprague et al. (2003). When compared to saline pretreated rats, MDMA pretreated rats traveled less distance in the correct quadrant (SW) which previously housed the platform. Decreased distance in the correct quadrant suggests that MDMA pretreated rats were less accurate in remembering the previous position of the platform. Mild CUS alone did not have an effect during probe testing although a much longer 40 day exposure to CUS has been shown to increase latency to reach the original platform position (Vasconcellos, Tabajara, Ferrari, Rocha, & Dalmaz, 2003). Therefore, unlike MDMA, mild CUS alone or in combination with MDMA pretreatment did not significantly affect spatial memory during MWM acquisition probe trials.
In contrast to effects during acquisition learning, MDMA did not affect reversal learning or reversal memory. Similarly, neither CUS nor the combination of MDMA and CUS affected reversal learning and memory. These data indicate that all groups were able to learn a new platform position. Therefore, the changes in learning and memory induced by MDMA and mild CUS may be specific to acquisition learning and memory in the MWM. A higher dose of MDMA however has been shown to affect reversal performance. Rats treated with MDMA (15 mg/kg every 2 hr × 4, subcutaneously) have shown increased latency and distance to the platform on reversal but not acquisition learning (Cohen et al., 2005). Similarly, exposure to CPS for 21 d has been shown to selectively affect reversal learning and not acquisition (Hill et al., 2005). Clearly the mechanisms behind both MDMA and CUS-induced impairments in MWM reversal learning and memory are sensitive to dosing regimen, stress type/duration and the amount of time between the last stressor and the MWM testing.
Given the role of 5-HT in both learning, memory and anxiety (Meneses, 1999; Handley, 1995), MDMA- and mild CUS-induced changes in hippocampal SERT expression were hypothesized to underlie behavioral changes in the MWM and EPM. SERT is not only responsible for the maintenance of synaptic 5-HT but several preclinical studies suggest that alterations in SERT function may also lead to an enhanced vulnerability to stress (Champoux et al., 2002; Adamec, Burton, Blundell, Murphy, & Holmes, 2006). In addition, genetic polymorphisms and mutations in the SERT gene are associated with increased anxiety in the both humans and rodents (Lesch et al., 1996; Holmes, Lit, Murphy, Gold, & Crawley, 2003; Zhao et al., 2006). Therefore it was hypothesized that decreases in SERT expression or function may lead to increased anxiety and cognitive impairments.
In the current study, pretreatment with neurotoxic MDMA caused a severe SERT depletion in non-stressed rats. This depletion may explain the apparent increase in anxiety-like behavior in MDMA pretreated non-stressed rats in the EPM. This finding is consistent with evidence showing that SERT depletions are associated with MDMA-induced anxiety behavior (McGregor et al., 2003). Contrary to the hypothesis, CUS did not enhance MDMA-induced SERT depletion. There was still however a significant decrease in SERT protein in MDMA pretreated stressed rats. The lack of enhanced anxiety-like behavior with the combination of MDMA and CUS suggests that changes in SERT alone cannot account for changes in anxiety behavior. This is supported by data showing that rats exposed to low dose MDMA (5 mg/kg × 1; ip) show enhanced anxiety in the absence of SERT depletions (McGregor et al., 2003). In addition, while SERT depletions in MDMA pretreated rats may explain the impairments in acquisition learning and reference memory in the MWM, enhanced SERT depletions alone cannot explain the enhanced deficits in acquisition learning in the MWM in rats pretreated with MDMA and exposed to mild CUS.
Interestingly, the present data also suggest that mild CUS may produce localized damage to the hippocampus after neurotoxic MDMA. Several reports suggest regional dissociation in hippocampal regulation of memory and anxiety behavior (Bannerman et al., 2004). Lesions to the dorsal hippocampus led to impairments in spatial behavior whereas ventral hippocampal lesion affected anxiety behavior (Hock, Jr. & Bunsey, 1998; Bertoglio, Joca, & Guimaraes, 2006; Bannerman et al., 2003). Thus, the effects of mild CUS in MDMA pretreated rats may be specific to the dorsal hippocampus since mild CUS after MDMA pretreatment led to spatial impairments but not enhanced anxiety behavior in the present study. Future studies will examine the regional effects of MDMA and mild CUS in the hippocampus.
In conclusion, several reports have identified the comorbid properties of drug abuse and stress yet few studies have investigated the potential synergistic and/or additive properties of their combined exposure. The present study highlights the interactions between MDMA and mild stress on learning behavior. While stress alone may not produce learning impairment, prior exposure to MDMA may enhance the effects stress by producing cognitive deficits. Furthermore, the present data suggests that the normal response to stress may be compromised after exposure to MDMA.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
Reference List
- Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behavioral Brain Research. 2006;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Research. 1998;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioral Brain Research. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience & Biobehavioral Reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Joca SR, Guimaraes FS. Further evidence that anxiety and memory are regionally dissociated within the hippocampus. Behavioral Brain Research. 2006;175:183–188. doi: 10.1016/j.bbr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. Journal of Neuroscience. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic Unpredictable Stress Induces a Cognitive Deficit and Anxiety-Like Behavior in Rats that is Prevented by Chronic Antidepressant Drug Treatment. Neuropsychopharmacology. 2007;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Cohen MA, Skelton MR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (ecstasy) in rats: interaction with exposure in adulthood. Synapse. 2005;57:148–159. doi: 10.1002/syn.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JL, Reid JJ. Effects of methylenedioxymethamphetamine on the release of monoamines from rat brain slices. European Journal of Pharmacology. 1990;191:217–220. doi: 10.1016/0014-2999(90)94150-v. [DOI] [PubMed] [Google Scholar]
- Fujino K, Yoshitake T, Inoue O, Ibii N, Kehr J, Ishida J, et al. Increased serotonin release in mice frontal cortex and hippocampus induced by acute physiological stressors. Neuroscience Letters. 2002;320:91–95. doi: 10.1016/s0304-3940(02)00029-0. [DOI] [PubMed] [Google Scholar]
- Gross SR, Barrett SP, Shestowsky JS, Pihl RO. Ecstasy and drug consumption patterns: a Canadian rave population study. Canadian Journal of Psychiatry. 2002;47:546–551. doi: 10.1177/070674370204700606. [DOI] [PubMed] [Google Scholar]
- Gurtman CG, Morley KC, Li KM, Hunt GE, McGregor IS. Increased anxiety in rats after 3,4-methylenedioxymethamphetamine: association with serotonin depletion. European Journal of Pharmacology. 2002;446:89–96. doi: 10.1016/s0014-2999(02)01820-4. [DOI] [PubMed] [Google Scholar]
- Haile CN, GrandPre T, Kosten TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology. 2001;154:213–220. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Handley SL. 5-Hydroxytryptamine pathways in anxiety and its treatment. Pharmacology & Therapeutics. 1995;66:103–148. doi: 10.1016/0163-7258(95)00004-z. [DOI] [PubMed] [Google Scholar]
- Harro J, Tonissaar M, Eller M, Kask A, Oreland L. Chronic variable stress and partial 5-HT denervation by parachloroamphetamine treatment in the rat: effects on behavior and monoamine neurochemistry. Brain Research. 2001;899:227–239. doi: 10.1016/s0006-8993(01)02256-9. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hock BJ, Jr, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. Journal of Neuroscience. 1998;18:7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes, Brain and Behavior. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neuroscience & Biobehavioral Reviews. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li JM, Kong LD, Wang YM, Cheng CH, Zhang WY, Tan WZ. Behavioral and biochemical studies on chronic mild stress models in rats treated with a Chinese traditional prescription Banxia-houpu decoction. Life Sciences. 2003;74:55–73. doi: 10.1016/j.lfs.2003.06.030. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philosophical Transactions of the Royal Society Lond B Biological Sciences. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Karney JJ, Carter SR, Janasik SP, O'brien JL, Friedman RD. The delayed effects of chronic unpredictable stress on anxiety measures. Physiology & Behavior. 2007;90:674–681. doi: 10.1016/j.physbeh.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience. 2004;124:637–646. doi: 10.1016/j.neuroscience.2003.12.007. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Clemens KJ, Van der PG, Li KM, Hunt GE, Chen F, et al. Increased anxiety 3 months after brief exposure to MDMA ("Ecstasy") in rats: association with altered 5-HT transporter and receptor density. Neuropsychopharmacology. 2003;28:1472–1484. doi: 10.1038/sj.npp.1300185. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. Journal of Affective Disorders. 2000;61:161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- Meneses A. 5-HT system and cognition. Neuroscience & Biobehavioral Reviews. 1999;23:1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Neurotoxic effects of chronic restraint stress in the striatum of methamphetamine-exposed rats. Pyschopharmacology. 2007;193 doi: 10.1007/s00213-007-0796-x. 3431-350. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. Journal of Pharmacology and Experimental Therapeutics. 1987;240:1–7. [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology. 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- Singh VB, Corley KC, Phan TH, Boadle-Biber MC. Increases in the activity of tryptophan hydroxylase from rat cortex and midbrain in response to acute or repeated sound stress are blocked by adrenalectomy and restored by dexamethasone treatment. Brain Research. 1990;516:66–76. doi: 10.1016/0006-8993(90)90898-l. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Preston AS, Leifheit M, Woodside B. Hippocampal serotonergic damage induced by MDMA (ecstasy): effects on spatial learning. Physiology & Behavior. 2003;79:281–287. doi: 10.1016/s0031-9384(03)00092-1. [DOI] [PubMed] [Google Scholar]
- Stone DM, Merchant KM, Hanson GR, Gibb JW. Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in brain of rat. Neuropharmacology. 1987;26:1677–1683. doi: 10.1016/0028-3908(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. European Journal of Pharmacology. 1986;128:41–48. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- Tannenbaum B, Tannenbaum GS, Sudom K, Anisman H. Neurochemical and behavioral alterations elicited by a chronic intermittent stressor regimen: implications for allostatic load. Brain Research. 2002;953:82–92. doi: 10.1016/s0006-8993(02)03273-0. [DOI] [PubMed] [Google Scholar]
- Tata DA, Yamamoto BK. Chronic stress enhances methamphetamine-induced extracellular glutamate and excitotoxicity in the rat striatum. Synapse. 2008;62:325–336. doi: 10.1002/syn.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochemical Research. 2002;27:519–525. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- Vasconcellos AP, Tabajara AS, Ferrari C, Rocha E, Dalmaz C. Effect of chronic stress on spatial memory in rats is attenuated by lithium treatment. Physiology & Behavior. 2003;79:143–149. doi: 10.1016/s0031-9384(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behavioral Neuroscience. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Xie T, Tong L, McLane MW, Hatzidmitriou G, Yuan J, McCann U, Ricarte G. Loss of serotonin transporter protein after MDMA and other ring-substituted amphetamines. Neuropsychopharmacology. 2006;31:2639–2651. doi: 10.1038/sj.npp.1301031. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Spanos LJ. The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. European Journal of Pharmacology. 1988;148:195–203. doi: 10.1016/0014-2999(88)90564-x. [DOI] [PubMed] [Google Scholar]
- Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jaing C, et al. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006;140:321–334. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Zhou J, Li L, Tang S, Cao X, Li Z, Li W, et al. Effects of serotonin depletion on the hippocampal GR/MR and BDNF expression during the stress adaptation. Behavioral Brain Research. 2008;195:129–138. doi: 10.1016/j.bbr.2008.06.009. [DOI] [PubMed] [Google Scholar]