Abstract
Background
Coronary vasospasms have been reported in the early stages of cardiomyopathy in the Syrian cardiomyopathic hamster (CM, BIO-TO2 strain). It has been proposed these alterations could lead to ischemic heart disease and heart failure. However, the cause of these coronary abnormalities has not been established. In this study, we evaluated coronary hemodynamic to assess the role of Ang-II, ROS, and NO in the development of these alterations in CM of 1, 2, and 6 months of age.
Methods and Results
Excised hearts from control (CT) and CM were retroperfused with KRB, and coronary resistance (CR) was determined. The experimental protocol involved sequential infusions of the thromboxane analog U46619 (THX, 0.1 μmol/L), bradykinin (BKN, 10 μmol/L), and sodium nitroprusside (SNP, 10 μmol/L). Similar experiments were conducted after treatment of hearts with Nω-nitro-L-arginine methyl ester (L-NAME, 10 μmol/L). Basal CR increased with age, but no significant differences were observed between CT and CM. Reactivity to THX was increased (69%, P<0.05) in 2-month-old CM when compared with CT. This effect was observed concomitantly with a significant reduction (53%, P<0.05) in BKN-induced relaxation. The reduction in BKN-dependent relaxation was prevented by treatment for 1-month with the antioxidant N-acetylcysteine (1 g/kg/day), or losartan, an AT-1 receptor blocker (10 mg/kg/day). Losartan also prevented the THX-induced increased reactivity in 2-month-old CM. The BKN-induced relaxation occurred through an L-NAME-sensitive pathway that was impaired with age. SNP dilation was preserved in all animal groups.
Conclusions
Our results strongly implicate vascular RAS and oxidative stress in endothelial dysfunction and increased reactivity in the early stages of cardiomyopathy in CM. These findings could be relevant to understand the etiology of cardiovascular disorders, in particular, in patients with sarcoglycanopathies.
Keywords: Cardiomyopathic Hamsters, Coronary Hyperreactivity, Endothelial Dysfunction, Nitric Oxide, Vascular RAS
Introduction
Animal studies conducted mainly with Syrian hamsters,1–10 a genetically inbred strain that spontaneously develops cardiomyopathy and muscular dystrophy, have been critical in understanding the etiology of heart dysfunction due to focal myocytolytic necrosis. The development and progression of cardiomyopathy in these animals undergo similar stages to the disease in humans. These stages consist of a prenecrotic phase (<30 days after birth) in which no histological evidence of the disease exists, and a necrotic phase (30–60 days) where acute focal myolysis develops. Following these early phases there is fibrosis and calcification of necrotic patches (60–90 days), and ventricular hypertrophy (BIO 14.6 strain) or dilation (BIO-T02 strain) from 90–150 days. After this stage (>150 days), heart failure develops. Therefore, studies of the initial stages of the disease in hamsters could be relevant to understand the etiology of idiopathic cardiomyopathy in humans.
Early studies in the cardiomyopathic hamster (CM) indicated that the necrosis observed in the heart showed an infarct-like pattern (discrete patches) starting at 1 month of age.3,8,11 Factor et al.,1,12–13 first suggested that these necrotic patches result from coronary vasospasms that cause ischemia, reperfusion injury, and focal myolysis. The relevance of coronary spasms as a critical event in ischemic heart disease in human patients has been long recognized.14 A fundamental role of vasospasms in experimental cardiomyopathy is revealed by the beneficial effect of the Ca2+ channel antagonist and vasodilator verapamil, which prevents the occurrence of coronary vasospasms and the histological and functional evidence of cardiomyopathy in various animal models including the BIO-T02 strain.8, 12–13, 15–20 The action of verapamil also indicates the critical role of Ca2+ in promoting coronary smooth muscle and cardiomyocyte dysfunction in the hamster. Indeed, Ca2+-overload and abnormal T-type Ca2+ channels have been reported in cardiomyocytes from CM.20–22 It has been proposed23 that mutations in the δ-sarcoglycan gene lead to altered Ca2+ homeostasis making cardiomyocytes prone to ischemic damage from transient coronary vasospasms. However, the genesis and nature of coronary vasospasms in CM has not been fully elucidated. Some investigators15–16 favor a primary mechanism for spasms developing in the vessel from disruption of the vascular smooth muscle (VSM) sarcoglycan complex. Others19,24 have presented evidence of coronary spasms in γ-sarcoglycan mutant mice with cardiomyocyte perturbations despite normal sarcoglycan complex in VSM. This suggests that vasospasms originate from events secondary to the damage induced by the δ-sarcoglycan deficit in cardiomyocytes.19 However, the δ-sarcoglycan downstream cellular mechanisms leading to coronary vasospasms in CM have not been established.
The presence of vasospasms in the coronary circulation during the necrotic stage of cardiomyopathy in the hamster has been associated with increased coronary reactivity to agonists such as arginine vasopressin.25–26 Our group27–29 has also documented hyperreactivity of the aorta to Ang II during this phase of development in CM, and Conway et al.,30 reported increased microvascular reactivity in cremaster muscle from young myopathic hamsters. In addition, evidence has been presented suggesting increased susceptibility of young hamsters to stress-induced coronary hyperreactivity and stress-related death in mature animals.31 These findings suggest that endothelial dysfunction (ED) is a fundamental alteration of the arterial vasculature at this early stage.32 However, the relationship of ED, increased reactivity, and vasospasms of coronary arteries has not been established.
Our group has suggested27–29,33 that Ang II-dependent reactive oxygen species (ROS) generation could be the pathway leading to ED and hyperreactivity of the vasculature in young CM hamsters (BIO-T02). Indeed, we have reported upregulation of ACE activity, augmented Ang II-type-1 (AT-1) receptor number, ED, increased Ang II-dependent superoxide generation, and hyperreactivity of the aorta to Ang II during the necrotic stage of CM. Blockade of the AT-1 receptor with losartan, reduced superoxide generation, improved endothelial function, and the hyperreactivity of the aorta in CM. Furthermore, the antioxidant N-acetylcysteine (NAC) was effective in inhibiting ROS production and improving ED. A recent study34 confirmed the presence of the Ang II-dependent ROS generation in the aorta of 10-month-old CM. Similar mechanisms involving Ang II-dependent ROS generation appear to be present in porcine35, and rat coronary arteries.36–37 For these reasons, we postulate that ROS generation by an Ang II-dependent mechanism could contribute to generate ED, hyperreactivity, and consequently, vasospasms in coronary arteries during the necrotic stage in CM. However, while there is a report demonstrating the presence of endothelial dysfunction in isolated coronary arteries of CM38, evidence is lacking concerning its presence in the coronary circulation in situ, its nature, the time-course of development, and relationship to coronary hyperreactivity and resistance in CM. Furthermore, the effects of AT-1 receptor blockade and antioxidant treatment in vivo on these alterations have not been evaluated.
In this work, we report studies of coronary hemodynamic in CM during the transition phase of pre-necrotic (1 month of age) to necrotic phase (2 months of age), and in adult animals (6-months of age). The results presented here confirm the presence of hyperreactivity in the coronary circulation during the necrotic stage in CM, and suggest that Ang II-dependent, ROS-mediated, endothelial dysfunction is a critical element of this vascular alteration.
Methods
Male Golden Syrian control (F1-B strain = CT) and cardiomyopathic (BIO-T02 strain = CM) hamsters of 1, 2, and 6 months of age were obtained from Bio breeders (Fitchburg, MA) and housed individually in the University of Puerto Rico-Medical Sciences Campus animal care facilities. The animals were housed in a temperature-controlled room (12-hour light/dark cycle) and acclimatized for a period of 1 week following transportation from the supplier. Commercial rat chow and tap water were available ad libitum. After the quarantine period, the animals were grouped and used for the proposed studies. The proposed investigation conforms to the Guide and Care for the Use of Laboratory Animals published by NIH.
General procedures
Coronary hemodynamic was studied using a beating heart preparation in a Langendorff setup.39–40 Briefly, the animals were anesthetized using sodium pentobarbital (50 mg/kg BW, ip), treated with heparin 850U, and the heart rapidly excised and transfused using a cannula (0.3mm bore diameter) placed immediately distal to the aortic valve. Hearts were perfused at constant pressure (70 mmHg) with Krebs-Heinseleit buffer (mmol/L: 118 NaCl, 5 KCl, 1.1 MgSO4, 1.2 KH2PO4, 10 glucose, 25 NaHCO3, 2.5 CaCl2) equilibrated with 95% O2 and 5% CO2 in a temperature-controlled chamber (37°C). Coronary flow (mL/min x g) and coronary pressure (mmHg) were continuously determined using a magnetic flow meter module (Transonic Systems, Inc., Model D-79232) placed in the perfusion line upstream to the heart. Coronary resistance (CR, mmHg x min x g / mL) was calculated from pressure and flow measurements. The perfused heart in zero-load conditions was allowed to stabilize for 30 minutes before the initiation of studies. When examining the effect of the thromboxane analog U46619 (THX) 0.1 μmol/L, bradykinin (BKN)10 μmol/L, and sodium nitroprusside (SNP) 10 μmol/L, the drugs were injected into the perfusion line (flow at about 4 mL/min) as a bolus. When used, Nω-nitro-L-arginine methyl ester (L-NAME, 10 μmol/L) was infused (1μL/ml) into the perfusion line for 30 minutes previous to the addition of THX, BKN, and SNP. In these studies, the drugs were tested in the same preparation before and after L-NAME infusion. The data was collected using a custom data logger (LabView). Heart rate was allowed to change spontaneously and was determined from 15-second segments of stable pressure tracings before and following the addition of drugs. At the end of every experiment, the heart was dried at 80°C for 48 hours and weighed to normalize the data. Heart to body weight (HW/BW) ratios was expressed as mg dry heart weight per gram of body weight.
N-acetylcysteine and losartan studies
NAC (1 gm/kg/day) and losartan (10 mg/kg/day) were administered in the drinking water to 1 month old CT (n=8) and CM (n=8) for 30 days. Following treatment, the animals were weighed and processed as indicated for coronary hemodynamic studies. The results from these studies were compared with those from similar experiments conducted with untreated 2-month-old CT and CM. The doses of NAC and losartan used were based on previous studies.41–42
Lucigenin chemiluminiscence
Superoxide activity in aortic rings was determined using a modification of the method described by Rajagopalan et al.43 Aortic segments (2–3 cm) were placed in a chilled buffer containing (mmol/L): NaCl, 99.01; KCl 4.69; CaCl2, 1.87; MgSO4, 1.20; K2PO4, 1.03; NaHCO3, 25.0; Na-Hepes, 20.0; and glucose, 11.1, pH 7.4, and the periadventitial tissue was removed. The vessels were washed to remove adherent blood cells. To determine superoxide activity, aortic rings (5 mm in length) were added to glass scintillation vials containing 2 mL of Krebs bicarbonate buffer (mmol/L): 118 NaCl, 2.5 CaCl2, 5 KCl, 1.1 MgSO4, 25 NaHCO3, 1.2 KH2PO4, and 10 glucose, pH = 7.4) The samples were placed in a scintillation counter (Beckman LS 9800) switched to single photon monitor for an equilibration period of 5 minutes in the dark. The reaction was started by the addition of 5 μmol/L lucigenin44 as the electron acceptor, and chemiluminiscence was monitored for 5 minutes at 30 second intervals. The basal activity produced by aortic rings without lucigenin, or with lucigenin containing vials in the absence of aortic rings was subtracted from sample readings. Chemiluminiscence was normalized by using the dry weight of tissue samples and expressed as cpm per mg dry weight.
Drugs and Chemicals
All chemicals and reagents were obtained from Sigma.
Data analysis
Data obtained from coronary hemodynamic studies were expressed in CR units (RU = mmHg X min x g /mL). Vasoconstrictor responses to THX were expressed as the ratio between stimulated- and the basal CR, and vasodilator responses to BKN as the percent relaxation induced by bradykinin in THX-precontracted coronary arteries (THX-BKN/THX-basal). Data were analyzed using ANOVA with Scheffe’s post-hoc test when multiple comparisons were made on CT and CM at different age. In addition, paired or unpaired t-test (StatView, SAS Institute, Inc.) was used to compare differences between two groups. Differences were considered significant when P≤ 0.05.
Results
General characteristics of animals
Table 1 shows the general characteristics of hamsters of 1, 2, and 6 months of age. Body (BW) and heart weight (HW) were slightly lower for CM than for CT in all age groups. HW to BW ratios, however, were 15% lower for CM at 6 months of age (P<0.05) when compared with 1- month-old CM. No difference in HW/BW ratios were observed for CT animals at the different ages evaluated. The reduced HW/BW ratio in CM could be related to cardiac dilation at 6 months of age.45 Intrinsic heart rates (HR) of the isolated heart were significantly lower than values reported in the literature for the retroperfused heart.39–40 The reason for this observation is not known. It is possible, however, that such differences arise from the fact that we worked in zero-load conditions while in previous studies39–40 the heart was loaded to determine dp/dt. Heart rate was significantly higher (31%) for CM than for CT in all age groups (P<0.05). Previous echocardiographic studies in these animal groups failed to reveal differences in heart function or structure at 2 months of age33. However, alterations in these parameters were observed in CM at 6 months of age. Indeed, cardiac output index and ejection fraction decreased, whereas left-ventricular end-diastolic- and end-systolic volume increased significantly in 6-month-old CM.42
Table 1.
Characteristics of Experimental Animals
| Age (months) | 1 | 2 | 6 |
|---|---|---|---|
| Control (CT) | |||
| Body Weight (g) | 65.7±1 | 99.2±2 | 130.2±1 |
| Heart Rate (beats/min) | 61±1.2 | 61±1.9 | 67±2.2 |
| Heart Weight (mg) | 61.8±3 | 91.8±3 | 118.4±8 |
| HW/BW (mg dw/g) | 0.9406 | 0.9254 | 0.9094 |
| Cardiomyopathic (CM) | |||
| Body Weight (g) | 58.9±2 | 92.7±2 | 126.0±2 |
| Heart Rate (beats/min) | 82±2* | 81±2* | 80±2* |
| Heart Weight (mg) | 57.5±3 | 83.4±2 | 103.9±4 |
| HW/BW (mg dw/g) | 0.9762 | 0.9004 | 0.8246** |
The values shown are the means ± SEM. Eight hamsters per group.
: P< 0.05 when compared with age-matched CT.
: P<0.05 vs. CM of 1-month of age. All other comparisons did not reach statistical significance. HW = heart weight, BW= body weight, dw = dry weight
Adequacy of the preparation used
Figure 1 depicts a representative experiment in 2-month-old CT in which we evaluated the effects of sequential additions of 0.1 μmol/L THX, 10 μmol/L BKN, and 10 μmol/L SNP on CR as a function of time. As it can be observed, CR was markedly increased by THX (about 3-fold). Infusion of BKN into the perfusion line decreased 42% the THX-induced contraction. Subsequent infusion of SNP, fully relaxed the coronary vasculature reducing CR to basal values. These results indicate the usefulness of our preparation to evaluate the reactivity of the coronary vasculature to THX, the endothelium-dependent relaxation by BKN, and the endothelium-independent relaxation by SNP.
Figure 1.
Sequential Effects of Thomboxane (THX), Bradykinin (BKN), and Sodium Nitroprussiate (SNP) on Coronary Resistance in Syrian Hamsters. The figure is a representative experiment conducted in an isolated, beating heart preparation from a 2-month-old control hamster. The agonists were infused into the perfusion line at the indicated times (arrows). The concentrations used were: THX 0.1 μmol/L, BKN 10 μmol/L, and SNP 10 μmol/L.
Increased THX-dependent CR and decreased BKN-mediated relaxation in 2 month-old CM
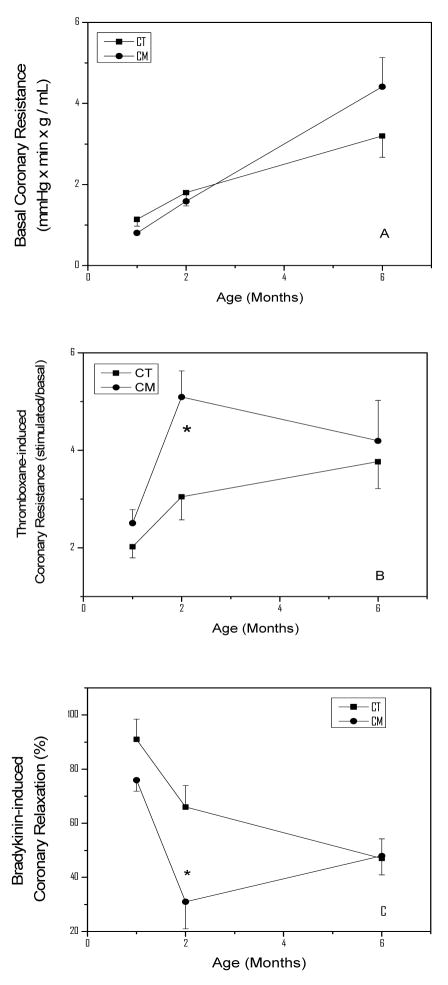
Figure 2 (panel A) illustrates the basal CR as a function of age in CT and CM animals. CR increased linearly in CT from 1.1 ± 0.1 RU in 1-month-old hamster to 3.20 ± 0.52 RU at 6 months of age (P<0.05). Similar changes in CR were observed in CM hamsters at these ages. Panel B depicts the THX-induced CR in these animal groups as a function of age. The THX-induced CR was 2.02 ± 0.23 RU in 1-month-old CT hamsters, and increased 50% and 86% (P<0.05) at 2 and 6 months of age, respectively. In CM, the THX-induced CR increased markedly (2-fold higher, P<0.05) at 2 months of age when compared with its basal value (2.58 ± 0.28), and was 69% (P<0.05) higher than the CR of CT hamsters of similar age. Thereafter, the THX-induced CR of CM decreased to 4.24 ± 0.83 RU at 6 months of age, a value that was not statistically different from that of similarly aged CT. Panel C illustrates the BKN-induced relaxation in THX-precontracted coronaries. The parameter decreased with age in CT hamsters, being 91±8% at 1 month, 68±8 at 2 months, and 48±7% at 6 months of age. In CM, the relaxation induced by BKN was reduced to 76±4% at 1 month of age, and to 32±12% (P<0.05) at 2 months of age. Thereafter, the BKN-induced relaxation in CM increased to values not different from those of CT at 6 months.
Figure 2.
Coronary Hemodynamic in Control (CT) and Cardiomyopathic (CM) hamsters as a function of age. The results shown represent the mean ±SEM of 8 hearts per group. Panel A illustrates the coronary resistance (CR), panel B the THX-induced increase in CR, and panel C the BKN-induced CR reduction in THX-precontracted coronaries. Statistical analysis: Panel B = *P<0.05 for 2-month-old CM vs. age-matched CT. Panel C = * P<0.05 for CM vs. age-matched CT.
L-NAME does not affect basal CR, but markedly stimulate THX-dependent CR in 1-month-old animals
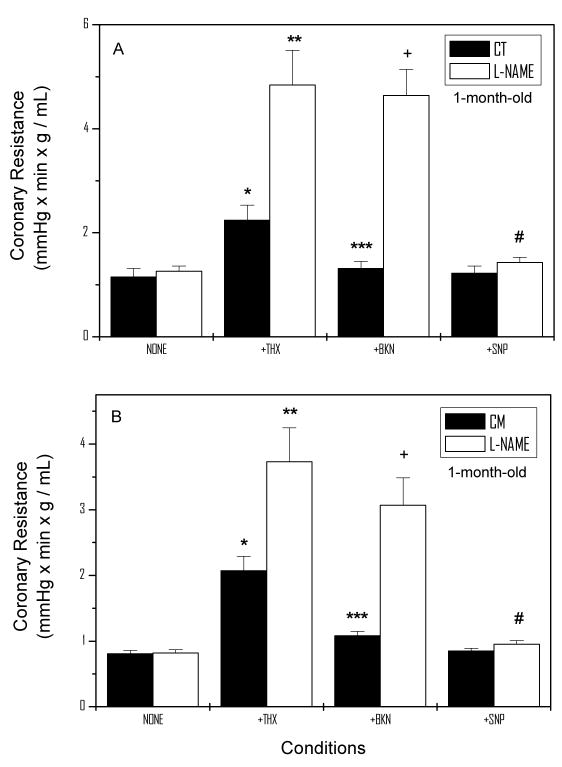
The relaxation induced by BKN in both CT and CM suggests that the NO-cGMP-PKG cascade is involved in the modulation of CR. This is also supported by the strong relaxing effect of SNP in this preparation. To investigate further whether NO is a modulator of basal and stimulated CR, similar experiments to those shown in Figure 1 were conducted after a 20 min L-NAME infusion. Figure 3 illustrates the basal and stimulated CR in the absence and presence of 10 μmol/L L-NAME at 1 month of age in CT (panel A) and CM (panel B). Basal CR (1.14 ± 0.16 RU) in CT was not affected by L-NAME. However, when THX was infused into the coronary circulation, CR increased 96% (P<0.05) in the absence and 3.84-fold (P<0.05) in the presence of L-NAME. Furthermore, BKN-abolished the THX-induced CR in CT, but failed to do so in L-NAME treated hearts. Under these conditions, SNP abolished the BKN-resistant, THX-induced contraction. Similar overall responses in basal CR, THX, BKN, and SNP were observed in 1 month-old CM.
Figure 3.
Basal and Stimulated CR in the Presence and Absence of L-NAME in 1-month-old CT (Panel A) and CM (Panel B) hamsters. The figure depicts the mean ±SEM of 8 determinations in each group. Statistical analysis: Panel A = *P<0.05 vs. None, ** P<0.05 vs. None-L-NAME, *** P<0.05 vs. THX, +P<0.05 vs. BKN, # P<0.05 vs. BKN-L-NAME. Panel B = * P<0.05 vs. None, **P<0.05 vs. None-L-NAME, *** P<0.05 vs. THX, +P<0.05 vs. BKN, and # P<0.05 vs. BKN-L-NAME.
L-NAME stimulates both basal CR and THX-dependent CR in 2-month-old hamsters
In 2 month-old CT (Figure 4), L-NAME increased basal CR (1.79 ± 0.31 RU) 79% (P<0.05). Following infusion of THX, CR increased 2.87-fold (P<0.05) in the absence and 2.31-fold (P<0.05) in the presence of L-NAME. L-NAME abolished the BKN-mediated relaxation in THX-precontracted coronaries. The BKN-resistant relaxation was reduced to basal values by SNP in the absence or presence of L-NAME. Similar overall responses to L-NAME were observed in 2-month-old CM (Figure 4B). However, THX was more effective in CM than in CT in enhancing CR in the absence (4.87-fold, P<0.05) and presence of L-NAME (3.45-fold, P<0.05). The effect of L-NAME in the presence of BKN and that of SNP on the BKN-resistant CR were similar to that observed in CT at this stage.
Figure 4.
Basal and Stimulated CR in the Presence and Absence of L-NAME in 2-month-old CT (Panel A) and CM (Panel B) hamsters. The figure depicts the mean ±SEM of determinations in 8 CT and 8 CM hamsters. Statistical analysis: Panel A = * P<0.05 vs. None, ** P<0.05 vs. None, *** P<0.05 vs. THX, +P<0.05 vs. BKN, # P<0.05 vs. BKN-L-NAME. Panel B = ** P<0.05 vs. None, +P<0.05 vs. BKN and # P<0.05 vs. BKN-L-NAME.
L-NAME stimulation of CR is lost in 6-month-old CM
Experiments conducted in 6-month-old CT (not shown) yielded a response profile similar to that of 2-month-old animals. However, in CM of similar age L-NAME failed to significantly increase the basal CR (4.38 ± 0.54 in None to 6.04 ± 1.3 RU in L-NAME, NS), nor the THX-induced CR (20.64 ± 4.0 in THX vs. 13.17 ± 2.15 RU in THX + L-NAME). The response of coronary smooth muscle to SNP in the presence of BKN + L-NAME was preserved in these animals (from 13.54 ± 2.4 in the absence to 5.36 ± 0.95 RU in the presence of SNP, P<0.05).
Increased THX-dependent CR and reduced BKN-mediated relaxation is reversed by the antioxidant NAC
To investigate whether the reduced BKN-induced relaxation of THX-precontracted coronaries was due to ROS-mediated endothelial dysfunction, experiments were conducted in 2-month-old animals treated for 30 days with the antioxidant NAC. Figure 5 illustrates the effect of NAC on CT (panel A) and CM (panel B) in 2-month-old animals. Panel A shows that NAC treatment did not affect the BKN-induced relaxation in CT in the absence or presence of L-NAME. By contrast in CM, NAC-treatment improved the impaired BKN-induced relaxation (from 31.3 ±10% to 53.1 ± 7%, P<0.05), and decreased the L-NAME-resistant, BKN-induced relaxation (from 21 ± 9% to 5.6 ± 2%, P<0.05). These effects of NAC were associated with inhibition of superoxide generation in aortas from 2-month-old CM. Panel C shows that superoxide generation increased 73% in CM over CT values (from 13,029 ± 2482 in CT to 25,645 ± 5,387 cpm/mg dry weight in CM, P<0.05). This effect was abolished by NAC treatment (from 25,645 ± 5,387 to 10,550 ± 2,786 cpm/mg dry weight, P<0.05).
Figure 5.
Effect of N-acetylcysteine (NAC) on Bradykinin-induced Coronary Relaxation and Superoxide Generation in CT and CM hamsters of 2 months of age. The results shown are the means ±SEM of 8 determinations per group. Panel A illustrates the BKN-induced relaxation in the presence and absence of L-NAME, in NAC-treated and non-treated CT hamsters. Panel B depicts similar studies for CM hamsters. Panel C shows aortic superoxide generation determined by lucigenin chemiluminescence in CT and CM in NAC-treated and non-treated animals. Statistical analysis, Panel A: * P<0.05 when compared to its respective –None- control. Panel B: * P<0.05 when compared with CM, and # P<0.05 when compared with CM-L-NAME. Panel C: * P<0.05 when compared with CT, and ** P<0.05 when compared with None-CM.
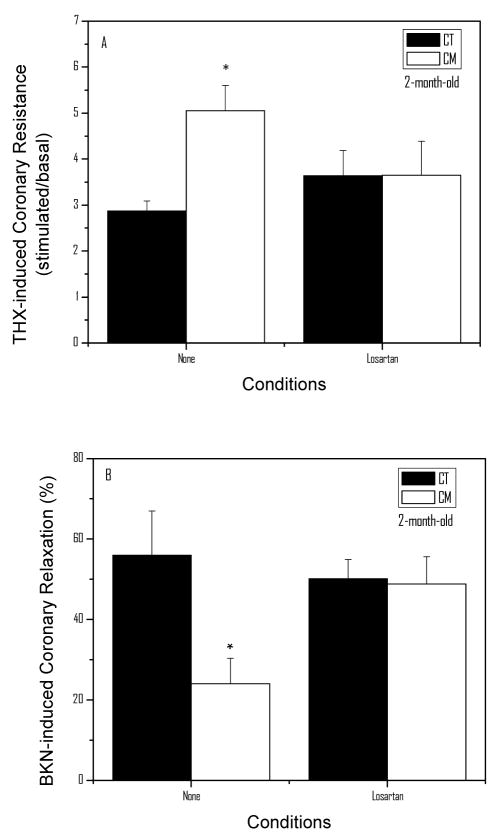
Losartan reversed the increased THX-dependent CR and improved the BKN-mediated relaxation in 2-month-old CM
Our findings in the aorta suggest that Ang II-mediated ROS generation33 induces endothelial dysfunction and increased vascular reactivity. To evaluate whether a similar mechanism causes ED and the hyper-reactive coronary circulation in CM, we treated CT and CM with losartan from 4 to 8 weeks of age. Figure 6A shows the THX-induced coronary resistance in CT and CM treated with losartan. As illustrated in the figure, CM shows an enhanced THX response (76%, P<0.05) when compared with CT. In CM animals treated with the AT-1 receptor blocker, the THX-induced CR was not observed. Figure 6B depicts the BKN-induced coronary relaxation following pretreatment with THX. As shown in the figure, the BKN-induced relaxation was markedly reduced (57%, P<0.05) in CM when compared with CT. In CM pre-treated with losartan, however, the BKN-induced relaxation was normalized.
Figure 6.
Effect of losartan on Coronary Resistance and Relaxation in CT and CM of 2 months of age. The results shown are the means ±SEM of 8 determinations per group. Panel A illustrates the THX-induced coronary resistance in CT and CM with and without treatment with losartan (10 mg/kg/day) for 30 days. Panel B shows the effect of losartan on the BKN-induced coronary relaxation in THX-precontracted coronaries. Statistical analysis: *P<0.05 when compared with None-CT.
Discussion
The evidence presented in this study indicates that the coronary vasculature in CM is hyper-reactive in the necrotic phase. Similar results have been reported in cremaster muscle arterioles30, and in the aorta of young CM hamsters.27–29,33 The increased reactivity of the coronary vasculature is evidenced by the fact that following THX infusion, CR increased markedly in 2-month-old CM when compared with age-matched CT although basal CR was normal. Conway et al.26 also reported increased CR by arginine vasopressin in 2–3 month-old CM (CHF 148 strain). In the present work, we extended these observations by characterizing the nature of the increased CR in BIO-T02 hamsters. We found that the increased CR is associated with reduced BKN-induced relaxation (Figure 2C) at 2 months of age. Indeed, the percentage relaxation induced by BKN in THX-precontracted coronaries was about 50% lower in CM than CT at this age. This observation does not appear to result from the increased CR induced by THX because at 6 months of age the BKN-induced relaxation (%) was similar in CT and CM (Figure 2C), although the difference in CR generated by THX (stimulated - basal) was greater in CM than in CT (16.25 ± 3.6 vs. 9.60 ± 2.5 RU, respectively). Therefore, the reduction in BKN-induced relaxation at 2 months of age in CM must result from the presence of ED at this stage. The observation that L-NAME abolished the BKN-induced relaxation strongly suggests that ED is secondary to reduced NO bioavailability at this stage. In addition, the BKN-resistant, THX-induced increase in CR in the presence or absence of L-NAME was abolished by SNP, suggesting that the mechanisms distal to the coronary smooth muscle NO cascade (cGMP-PkC-dependent pathways) are not affected in CM. Finally, NAC prevented the increased THX-induced CR and normalized the BKN-mediated relaxation in CM supporting the idea that oxidative stress is an important factor in coronary dysfunction in 2-month-old CM. Indeed, the antioxidant also reduced superoxide generation in the aorta of similarly aged CM. Therefore, it is likely that the increased CR in CM is secondary to ED caused by ROS-dependent, NO levels reduction.
The state of coronary hyperreactivity in young CM could reflect a vascular state predisposing to focal vasospasms during the necrotic phase as originally proposed by Factor and colleagues.12–13 Coronary vasospasms could arise in hyperreactive vascular areas where contractile agonists are released secondary to hemodynamic, metabolic, or cardiomyocyte alterations.19 It is of interest that the increased coronary reactivity correlate positively with findings of oxidative stress,46 increased release of cardiac troponin-T and plasma α-hydroxybutyrate dehydrogenase in young CM.4 Therefore, coronary dysfunction could contribute to ischemic tissue damage and myolysis of cardiomyocytes through the generation of vasospasms.19 However, even in the presence of a hyperreactive circulation at 2 months of age, we did not find differences in basal CR between CT and CM as reported previously by Conway et al.26 This indicates that the presence of vasospasms does not affect the basal coronary resistance at this stage.
An important finding of the present study is that of age-dependent alterations in NO-mediated modulation of basal- and stimulated-CR. Our studies with L-NAME in CT of 1, 2, and 6 months of age indicate that the NO-dependent modulation of basal coronary vasculature appears at 2 months of age and is maintained in 6-month-old hamsters. By contrast, NO appears to exert a braking effect on the THX-induced CR in CT starting at 1 month of age, because this parameter increased significantly in the presence of L-NAME. The same behavior concerning the THX-induced NO-mediated regulation operates in 2-month-old CM. Therefore, while 1-month-old hamsters only show the NO-dependent modulation of stimulated CR, both components of NO control (basal and stimulated) are present in 2 and 6-month-old hamsters. These findings could reflect differences between an immature signal transduction system for basal endothelial NO release (EDRF) and a fully operational stimulated-EDRF release47 at 1 month of age. To our knowledge, this is the first time that such differential development of NO-dependent pathways is described in the coronary circulation of the hamster.
Our findings also indicate that the endothelial NO-vasodilator system is lost in 6-month-old CM. This conclusion is based on the observation that infusion of L-NAME in these animals did not stimulate the basal or the THX-induced CR. These findings agree with a previous study by Gutierrez et al.48 who reported that the N-Nitro-L-arginine- (LNA) dependent increase in mean arterial pressure was reduced in young CM and abolished in old CM. Gutierrez et al.48 also reported that the loss of LNA -dependent increase in mean arterial pressure in old CM was prevented by the antioxidant tiron. Subsequently, Clark and Fuchs49 reported marked reductions in NOS-dependent relaxations in coronary arteries isolated from 10-month-old CM (Bio 14.6). However, despite the loss of endothelium-dependent relaxation (EDR) in 6 months-old CM, coronary relaxation was maintained at the same level of age-matched CT (Figure 2C). This finding agrees with previous observations by other research groups49 and suggests that in adult or old CM, other vasodilator substances released by BKN compensate for the loss of NO-dependent vasodilation. The vasodilator released by BKN appears to be ROS-dependent, because the L-NAME resistant, BKN-induced relaxation in 2-month-old CM was inhibited by NAC treatment (Figure 5B). In transgenic mice with dilated cardiomyopathy (Tgαq*44 model), the decrease in NO-dependent coronary vasodilation with age is associated with an increase in PGI2, both of which are promoted by superoxide production.50 These results are in line with a study in 200-day-old CM (UM-X7.1) showing inhibition of coronary flow by indomethacin.40 However, a recent report20 suggests that H2O2 could be the vasodilator released by BKN because it elicits catalase-sensitive relaxation, endothelium-dependent superoxide generation, and H2O2 production in isolated coronary artery segments from human patients. Therefore, studies are necessary to establish the identity of the EDR factor in adult CM.
The similarities of the present findings (i.e. NAC-sensitive ED and increased reactivity to contractile agonists), with those reported earlier in the aorta of 2-month-old CM by our group,27–29,33 support the idea that similar RAS-dependent mechanisms are operational in coronary arteries. Indeed, pretreatment of CM with losartan from 4 to 8 weeks of age, prevented these alterations in the coronary circulation of 2-month-old animals. These findings, together with the improvement of BKN-induced coronary relaxation by NAC in 2-month-old animals, indicate for the first time that the coronary alterations observed in the early stages of cardiomyopathy in the hamster are secondary to Ang II-dependent, ROS mediated pathways. In adult CM, chronic blockade of RAS with enalapril and losartan (25 and 10 mg/Kg/day for 5 months, respectively), increases plasma nitrite/nitrate levels to values not different from those of CT (from 18.07 ± 0.99 to 68.30 ± 18.8 μM, n=3, P<0.05), suggesting that RAS upregulation decreases plasma NO levels. It is noteworthy, however, that in rat coronary arteries, upregulation of vascular RAS by L-NAME leads to coronary oxidative stress, inflammation, and atherosclerotic changes that are prevented by ACE inhibitors and angiotensin receptor blockers.36–37,51 Therefore, low NO bioavailability secondary to oxidative stress in CM could promote RAS upregulation, inducing in turn, a positive feedback cycle by the stimulation of Ang II-dependent NAD(P)H oxidase activity.33,43
The relevance of RAS in the etiology of cardiomyopathy in the hamster is underlined by recent findings that treatment of CM with enalapril (ACEI) and losartan (ARB) from 1 to 6 months of age, significantly reduces the development of dilated cardiomyopathy.42 This finding together with those of the present study, indicate that prevention of early coronary dysfunction preserves cardiac function in CM. This suggests that RAS inhibition using ACE inhibitors and/or losartan could be useful for the treatment of patients with sarcoglycanopathies that are prone to develop early vascular (systemic and coronary) disease and cardiomyopathy.52–54 Interestingly, recent studies in muscular dystrophy patients suggest that early diagnosis and treatment with ACE inhibitors could improve the dilated cardiomyopathy and reduce the premature death frequently associated with this condition.55–56 Although the precise mechanism for the effects of ACE inhibitors in these patients has not been established, it is likely that suppression of early RAS-dependent, coronary alterations could be instrumental in this effect.
Due to limitations of the isolated heart preparation used, we could not establish the location within the vascular tree of the coronary alterations reported here. Important functional differences exist between epicardial arteries (capacitative vessels) and arterioles (resistance elements).57–58 Another potential limitation of our heart preparation, is that we used of zero-load conditions as opposed to an isovolumetric contracting heart. Although hearts from CT and CM were compared under identical conditions, it is possible that lower oxygen demands in the unloaded heart could make data comparison with other studies difficult. However, our results are consistent with those reported in the literature concerning the effect of age on CR, the increase in CR by THX, the BKN-induced relaxation, the effect of L-NAME on CR and the vasorelaxant effect of SNP.26, 38–40, 49 Therefore, we consider the results of the present work valid notwithstanding these limitations.
In summary, the results presented in this study indicate that the coronary vasculature of the cardiomyopathic hamster is hyperreactive during the necrotic phase of cardiomyopathy. The hyperreactivity of coronary vasculature is associated with ED, and both of these alterations are Ang II-dependent. It is likely that these alterations predispose to focal vasospasms with ischemia and necrosis during this critical stage of development. The similarities of these findings with those reported by us in the aorta of young CM, suggest that coronary alterations are an intrinsic vascular event- independent of cardiomyocyte dysfunction. Altogether, these results implicate the vascular RAS in the etiology of cardiac disease in patients with sarcoglycanopathies.
Acknowledgments
This work was supported by a grant from NIH-SCORE S06-RR 08224. The authors are grateful for the contribution of Ms. N. Cruz to lucigenin chemiluminiscence studies, and Dr. A.M. Preston from the Biochemistry Department for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Factor SM, Sonnenblick EH. The pathogenesis of clinical and experimental congestive cardiomyopathies: recent concepts. Prog Cardiovasc Dis. 1985;27:395–20. doi: 10.1016/0033-0620(85)90002-7. [DOI] [PubMed] [Google Scholar]
- 2.Forman R, Parmley WW, Sonneblick EH. Myocardial contractility in relation to hypertrophy and failure in myopathic Syrian hamster. J Mol Cell Cardiol. 1972;4:203–11. doi: 10.1016/0022-2828(72)90058-2. [DOI] [PubMed] [Google Scholar]
- 3.Gertz EW. Animal model of human disease: myocardial failure, muscular Dystrophy. Amer J Pathol. 1973;70:151–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Kato Y, Iwase M, Takagi K, Nishizawa T, Kanazawa H, Matsushita A, et al. Differential myolysis of myocardium and skeletal muscle in hamsters with dilated cardiomyopathy: beneficial protective effect of diltiazem. Circ J. 2006;70(11):1497–02. doi: 10.1253/circj.70.1497. [DOI] [PubMed] [Google Scholar]
- 5.Kawada T, Nakatsuru Y, Sakamoto A, Koizumi T, Shin WS, Okai-Matsuo Y, et al. Strain- and age-dependent loss of sarcoglycan complex in Cardiomyopathic hamster hearts and its re-expression by delta-sarcoglycan gene transfer in vivo. FEBS Lett. 1999;458(3):405–8. doi: 10.1016/s0014-5793(99)01164-3. [DOI] [PubMed] [Google Scholar]
- 6.Kawada T, Nakazawa M, Nakauchi S, Yamazaki K, Shimamoto R, Urabe M, et al. Rescue of hereditary form of dilated cardiomyopathy by rAAV-mediated somatic gene therapy: amelioration of morphological findings, sarcolemmal permeability, cardiac performances, and the prognosis of TO-2 hamsters. Proc Natl Acad Sci U S A. 2002;99 (2 ):901–6. doi: 10.1073/pnas.022641799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lossnitzer K, Janke J, Hein B, Stauch M, Fleckenstein A. Disturbed myocardial calcium metabolism: a possible pathogenic factor in the hereditary cardiomyopathy of the Syrian hamster. Recent Adv Stud Cardiac Struct Metab. 1975;6:207–17. [PubMed] [Google Scholar]
- 8.Sonnenblick EH, Fein F, Capasso JM, Factor SM. Microvascular spasm as a cause of cardiomyopathies and the calcium blocking-agent verapamil as potential primary therapy. Am J Cardiol. 1985;55:179B–84B. doi: 10.1016/0002-9149(85)90629-0. [DOI] [PubMed] [Google Scholar]
- 9.Strobeck JE, Factor SM, Bhan A, Sole M, Liew CC, Fein F, et al. Hereditary and acquired cardiomyopathies in experimental animals; mechanical, biochemical and structural features. Ann NY Acad Sci. 1979;317:58–88. doi: 10.1111/j.1749-6632.1979.tb56511.x. [DOI] [PubMed] [Google Scholar]
- 10.Ueyama T, Ohkusa T, Hisamatsu Y, Nakamura Y, Yamamoto T, Yano M, et al. Alterations in cardiac SR Ca2+ -release channels during development of heart failure in cardiomyopathic hamsters. Am J Physiol. 1998;274(43):H1–7. doi: 10.1152/ajpheart.1998.274.1.H1. [DOI] [PubMed] [Google Scholar]
- 11.Galle J, Mohr W, Lossnitzer K, Haferkamp O. Development of necrosis and its sequelae in the myocardium of polymyopathic hamsters (BIO 8262). An electron microscopic study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;36(1):87–100. doi: 10.1007/BF02912058. [DOI] [PubMed] [Google Scholar]
- 12.Factor SM, Sonnenblick EH. Hypothesis: is congestive cardiomyopathy caused by a hyperreactive myocardial microcirculation (microvascular spasm)? Am J Cardiol. 1982;50(5):1149–52. doi: 10.1016/0002-9149(82)90435-0. [DOI] [PubMed] [Google Scholar]
- 13.Factor SM, Minase T, Cho S, Dominitz R, Sonnenblick EH. Microvascular spasm in the cardiomyopathic Syrian hamster: a preventable cause of focal myocardial necrosis. Circulation. 1982;66(2):342–54. doi: 10.1161/01.cir.66.2.342. [DOI] [PubMed] [Google Scholar]
- 14.Kawano H, Ogawa H. Endothelial function and coronary spastic angina. Intern Med. 2005;44(2):91–9. doi: 10.2169/internalmedicine.44.91. [DOI] [PubMed] [Google Scholar]
- 15.Cohn RD, Durbeej M, Moore SA, Coral-Vazquez R, Prouty S, Campbell KP. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J Clin Invest. 2001;107(2):R1–7. doi: 10.1172/JCI11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coral-Vazquez R, Cohn R, Moore SA, Hill JA, Weiss RM, Davisson RL, et al. Disruption of sarcoglycan-sarcospan complex in vascular smooth muscle: A novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–74. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- 17.Rouleau JL, Chuck LHS, Hollosi G, Kidd P, Sievers RE, Wikman-Coffelt J, et al. Verapamil preserves myocardial contractility in the hereditary cardiomyopathy of the hamster. Circ Res. 1982;50:405–12. doi: 10.1161/01.res.50.3.405. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler MT, Allikian MJ, Heydemann A, McNally EM. The sarcoglycan complex in striated and vascular smooth muscle. Cold Spring Harb Symp Quant Biol. 2002;67:389–97. doi: 10.1101/sqb.2002.67.389. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler MT, Korcarz CE, Collins KA, Lapidos KA, Hack AA, Lyons MR, et al. Secondary coronary artery vasospasm promotes cardiomyopathy progression. Am J Pathol. 2004;164(3):1063–71. doi: 10.1016/S0002-9440(10)63193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, et al. Hydrogen peroxide inhibits cytochrome P450 epoxygenases. Interaction between two endothelium-dervied hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen L, Smith TW. T-type Ca2+ channels are abnormal in genetically determined cardiomyopathic hamster hearts. Circ Res. 1994;75(1):149–55. doi: 10.1161/01.res.75.1.149. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Ohkusa T, Ono K, Sato T, Yoshida M, Yano M, et al. Beneficial effect of the L- and T-type Ca2+-channel blocker efonidipine on cardiomyopathic hamsters. Circ J. 2007;71:1970–76. doi: 10.1253/circj.71.1970. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y-W, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: Identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151(6):1321–36. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNally E, Allikian M, Wheeler MT, Mislow JM, Heydemann A. Cystoskeletal defects in cardiomyopathy. J Mol Cell Cardiol. 2003;35:231–41. doi: 10.1016/s0022-2828(03)00018-x. [DOI] [PubMed] [Google Scholar]
- 25.Chang Q, Natelson BH, Golstein CD, Ottenweller JE. The role of stressor intensity and underlying vasculopathy in altering coronary reactivity in cardiomyopathic hamsters. Psychosom Med. 1997;59:51–7. doi: 10.1097/00006842-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Conway RS, Natelson BH, Chen WH, Ting W. Enhanced coronary vasoconstriction in the Syrian myopathic hamster supports the microvascular spasm hypothesis. Cardiovasc Res. 1994;28:320–4. doi: 10.1093/cvr/28.3.320. [DOI] [PubMed] [Google Scholar]
- 27.Crespo MJ, Altieri PI, Escobales N. Enhanced Contractility of Ang II in the Aorta of Cardiomyopathic Hamsters is Mediated by an Increased Ang II-Binding Capacity and Release of ET-1. Vasc Pharmacol. 2006;44(4):247–52. doi: 10.1016/j.vph.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Crespo MJ, Escobales N, Altieri PI. Altered Vascular Function in Early Stages of Heart Failure in Hamsters. J Card Fail. 1997;3(4):311–18. doi: 10.1016/s1071-9164(97)90031-2. [DOI] [PubMed] [Google Scholar]
- 29.Crespo MJ. Vascular Alterations During the Development and Progression of Experimental Heart Failure. J Card Fail. 1999;5(1):55–63. doi: 10.1016/s1071-9164(99)90025-8. [DOI] [PubMed] [Google Scholar]
- 30.Conway RS, Factor SM, Sonnenblick EH, Baez S. Microvascular reactivity of the myopathic Syrian hamster cremaster muscle. Cardiovasc Res. 1987;21:796–03. doi: 10.1093/cvr/21.11.796. [DOI] [PubMed] [Google Scholar]
- 31.Chang Q, Natelson BH, Ottenweller JE, Conway RS. Stress triggers different pathophysiological mechanisms in younger and older cardiomyopathic hamsters. Cardiovasc Res. 1995;30(6):985–91. [PubMed] [Google Scholar]
- 32.Munzel T, Shinning C, Post F, Warnoholtz A, Schulz E. Pathophysiology, diagnosis, and prognostic implications of endothelial dysfunction. Ann Med. 2008;40(3):180–96. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 33.Escobales N, Crespo MJ. Angiotensin II-dependent Vascular Alterations in Young Cardiomyopathic Hamsters: Role of Oxidative Stress. Vasc Pharmacol. 2006;44(1):22–8. doi: 10.1016/j.vph.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Mollnau H, Oelze M, August M, Wendt M, Daiber A, Schulz E, et al. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler Thromb Vasc Biol. 2005;25(12):2554–9. doi: 10.1161/01.ATV.0000190673.41925.9B. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92(3):322–9. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
- 36.Katoh M, Egashira K, Kataoka C, Usui M, Koyanagi M, Kitamoto S, et al. Regression by ACE inhibition of arteriosclerotic changes induced by chronic blockade of NO synthesis in rats. Am J Physiol. 2001;280(5):H2306–12. doi: 10.1152/ajpheart.2001.280.5.H2306. [DOI] [PubMed] [Google Scholar]
- 37.Usui M, Egashira K, Tomita H, Koyanagi M, Katoh M, Shimokawa H, et al. Important role of local angiotensin II activity mediated via type 1 receptor in the pathogenesis of cardiovascular inflammatory changes induced by chronic blockade of nitric oxide synthesis in rats. Circulation. 2000;101(3):305–10. doi: 10.1161/01.cir.101.3.305. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs LC. Superoxide anions contribute to impaired endothelium-dependent relaxation in coronary arteries of young cardiomyopathic hamsters. Endothelium. 1996;4:141–9. [Google Scholar]
- 39.Mokuno S, Ito T, Numaguchi Y, Matsui H, Toki Y, Okumura K, Hayakawa T. Impaired nitric oxide production and enhanced autoregulation of coronary circulation in young spontaneously hypertensive rats at prehypertensive stage. Hypertens Res. 2001;24(4):395–01. doi: 10.1291/hypres.24.395. [DOI] [PubMed] [Google Scholar]
- 40.Véronneau M, Tanguay M, Fontaine E, Jasmin G, Dumont L. Reactivity to endothelium-dependent and -independent vasoactive substances is maintained in coronary resistance vessels of the failing hamster heart. Cardiovasc Res. 1997;33(3):623–30. doi: 10.1016/s0008-6363(96)00256-8. [DOI] [PubMed] [Google Scholar]
- 41.Matsuhisa S, Otani H, Okazaki T, Yamashita K, Akita Y, Sato D, et al. N-acetylcysteine abolishes the protective effect of losartan against left ventricular remodeling in cardiomyopathy hamster. Antioxid Redox Signal. 2008;10(12):1999–08. doi: 10.1089/ars.2008.2069. [DOI] [PubMed] [Google Scholar]
- 42.Crespo MJ, Cruz N, Altieri PI, Escobales N. Enalapril and losartan are more effective than carvedilol in preventing dilated cardiomyopathy in the Syrian cardiomyopathic hamster. J Cardiovasc Pharmacol Ther. 2008;13(3):199–06. doi: 10.1177/1074248408320006. [DOI] [PubMed] [Google Scholar]
- 43.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97(8):1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skatchkov MP, Sperling D, Hink U, Mulsch A, Harrison DG, Sindermann I, et al. Validation of lucigenin as a chemiluminiscent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun. 1999;254(2):319–24. doi: 10.1006/bbrc.1998.9942. [DOI] [PubMed] [Google Scholar]
- 45.Cruz N, Arocho L, Rosario L, Crespo MJ. Chronic administration of carvedilol improves cardiac function in 6-month-old Syrian cardiomyopathic hamsters. Phamacology. 2007;80:144–50. doi: 10.1159/000103254. [DOI] [PubMed] [Google Scholar]
- 46.Nishizawa T, Iwase M, Kanazawa H, Ichihara S, Ichihara G, et al. Serial alterations of –adrenergic signaling in dilated cardiomyopathic hamsters- possible role of myocardial oxidative stress. Circ J. 2004;68:1051–60. doi: 10.1253/circj.68.1051. [DOI] [PubMed] [Google Scholar]
- 47.Bassenge E. In: Endothelial regulation of vascular tone. Ryan US, Rubanyi GM, editors. New York: Marcel Dekker Inc; 1992. pp. 225–64. [Google Scholar]
- 48.Gutierrez JA, Clark SG, Giulumian AD, Fuchs LC. Superoxide anions contribute to impaired regulation of blood pressure by nitric oxide during the development of cardiomyopathy. J Pharmacol Exp Ther. 1997;282(3):1643–9. [PubMed] [Google Scholar]
- 49.Clark SG, Fuchs LC. BKCa channels compensate for loss of NO-dependent coronary artery relaxation in cardiomyopathy. Am J Physiol Heart Circ Physiol. 2000;279:H2598–03. doi: 10.1152/ajpheart.2000.279.6.H2598. [DOI] [PubMed] [Google Scholar]
- 50.Drelicharz L, Kozlovki V, Skorka T, Heinze-Paluchowska S, Jasinski A, Gebska A, et al. NO and PGI2 in coronary endothelial dysfunction in transgenic mice with dilated cardiomyopathy. Basic Res Cardiol. 2008;103:417–30. doi: 10.1007/s00395-008-0723-2. [DOI] [PubMed] [Google Scholar]
- 51.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39(2):245–50. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 52.Politano L, Nigro V, Passamano L, Petretta V, Comi LI, Papparella S, et al. Evaluation of cardiac and respiratory involvement in sarcoglycanopathies. Neuromuscul Disord. 2001;11(2):178–85. doi: 10.1016/s0960-8966(00)00174-7. [DOI] [PubMed] [Google Scholar]
- 53.Barresi R, Di Blasi C, Negri T, Brugnoni R, Vitali A, Felisari G, et al. Disruption of heart sarcoglycan complex and severe cardiomyopathy caused by beta sarcoglycan mutations. J Med Genet. 2000;37(2):102–07. doi: 10.1136/jmg.37.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsubata S, Bowles KR, Vatta M, Zintz C, Titus J, Muhonen L, et al. Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. J Clin Invest. 2000;106(5):655–62. doi: 10.1172/JCI9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jefferies JL, Eidem BW, Belmont JW, Craigen WJ, Ware SM, Fernbach SD, et al. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation. 2005;112(18):655–62. doi: 10.1161/CIRCULATIONAHA.104.528281. [DOI] [PubMed] [Google Scholar]
- 56.Duboc D, Meune C, Lerebours G, Devaux JY, Waksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45(6):855–57. doi: 10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 57.Camisi PG, Crea F. Coronary Microvascular Dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 58.Van de Voorde J, Brochez V, Vanheel B. Heterogeneous effects of histamine on isolated rat coronary arteries. Eur J Pharmacol. 1994;271(1):17–23. doi: 10.1016/0014-2999(94)90259-3. [DOI] [PubMed] [Google Scholar]