Abstract
Studies in rodents have shown that male sexual function can be disrupted by fetal or neonatal administration of compounds that alter endocrine homeostasis, such as the synthetic nonsteroidal estrogen diethylstilbestrol (DES). Although the molecular basis for this effect remains unknown, estrogen receptors likely play a critical role in mediating DES-induced infertility. Recently, we showed that the orphan nuclear receptor small heterodimer partner (Nr0b2), which is both a target gene and a transcriptional repressor of estrogen receptors, controls testicular function by regulating germ cell entry into meiosis and testosterone synthesis. We therefore hypothesized that some of the harmful effects of DES on testes could be mediated through Nr0b2. Here, we present data demonstrating that Nr0b2 deficiency protected mice against the negative effects of DES on testis development and function. During postnatal development, Nr0b2-null mice were resistant to DES-mediated inhibition of germ cell differentiation, which may be the result of interference by Nr0b2 with retinoid signals that control meiosis. Adult Nr0b2-null male mice were also protected against the effects of DES; however, we suggest that this phenomenon was due to the removal of the repressive effects of Nr0b2 on steroidogenesis. Together, these data demonstrate that Nr0b2 plays a critical role in the pathophysiological changes induced by DES in the mouse testis.
Introduction
The small heterodimer partner (Shp; referred to herein as Nr0b2) is mainly known for its role in the liver, where it is involved in the feedback inhibition of bile acid synthesis (1–4). The functions of Nr0b2 have been linked to its ability to repress the transcriptional activity of other nuclear receptors (NRs) such as the liver homolog-1 (Lrh-1; referred to herein as Nr5a2; refs. 1, 3) and the estrogen receptors (Erα/β; referred to herein as Nr3a1/2; ref. 5). In addition to the liver, Nr0b2 has also been shown to be expressed in the testis (6–8). We have recently demonstrated that Nr0b2 interacts with retinoid signaling in the testis, which leads to germ cell entry in meiosis (8). Moreover, Nr0b2 controls testosterone synthesis independently of the hypothalamus/pituitary axis (8). These results suggest that in the testis, Nr0b2 interferes with several signaling pathways important for reproductive biology.
In recent years, a causal link between in utero and/or neonatal exposure to molecules that alter endocrine functions and the development of genital tract abnormalities, such as cryptorchidism, hypospadias, and impaired spermatogenesis, has emerged from studies in rodents. Most of these endocrine disrupters (EDs) exert some estrogenic and/or antiandrogenic activities (9). However, these molecules are rarely pure agonists or antagonists and can induce several signaling pathways. A good example is diethylstilbestrol (DES), known to induce reproductive disorders (10). DES has been shown to activate several members of the NR family, such as the Er and the estrogen-related receptors α/β/γ (Errα/β/γ; referred to herein as Nr3b1, Nr3b2, and Nr3b3, respectively; refs. 11–14). Even though the exact molecular basis remains unknown, the use of transgenic models suggests that Ers are involved (15–18). Moreover, some studies show similar changes in testicular gene expression induced by estradiol and DES (15). Other data, however, suggest differences in the molecular targets introduced by estradiol and DES (19).
Interestingly, Nr0b2 is a direct target gene of both the Ers (20) and the Errs (21) and inhibits their transcriptional activity (6, 22, 23). We hence hypothesized that part of the testicular effects of DES, both estrogen dependent and independent, could be mediated through Nr0b2. Using exposure to DES, to the pure estrogen agonist estradiol benzoate (EB), and to Er antagonist ICI 182,780 (referred to herein as ICI), we demonstrate here that Nr0b2 deficiency protects male mice against the harmful estrogenic and nonestrogenic effects of DES. During postnatal development, Nr0b2-null mice were more resistant to the DES-mediated inhibition of germ cell differentiation, which is explained by our finding that Nr0b2 interfered with retinoid signals that control meiosis. In adult Nr0b2-null male mice, however, protection against the negative effects of DES was caused, at least in part, by the removal of Nr0b2-repressive effects on steroidogenesis. These data demonstrate that Nr0b2 plays a critical role in the pathophysiological changes induced by EB and DES in the testis.
Results
Estrogenic compounds induce testicular expression of Nr0b2.
In order to identify a potential molecular link between Nr0b2 and the established negative effects of DES on male fertility, we monitored Nr0b2 expression at different time points during which treatment with DES is known to induce reproductive abnormalities (P1–P5). mRNA analyses demonstrated that Nr0b2 was expressed in the whole testis at these ages (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI38521DS1). Moreover, the mRNA of the NRs known to be targets of DES, such as Nr3a1, Nr3a2, and Nr3b1/2/3, were expressed in the testis (Supplemental Figure 1A). Treatment with DES caused higher mRNA accumulation of Nr0b2 in testes of P10 Nr0b2 wild-type (Nr0b2+/+) male mice (Figure 1A). In the adult testis, a dose-dependent increase in Nr0b2 mRNA was also observed upon DES administration. Interestingly, similar effects on Nr0b2 mRNA levels were observed using the pure estrogen EB (Supplemental Figure 1B), which suggests that the regulation of Nr0b2 gene was caused, at least in part, by the estrogenic effect of DES.
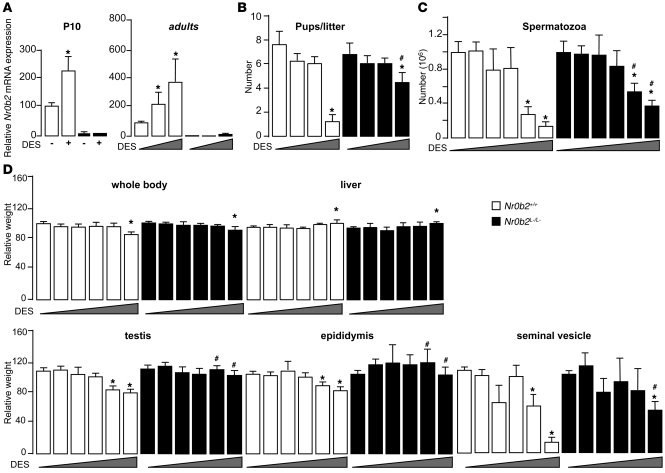
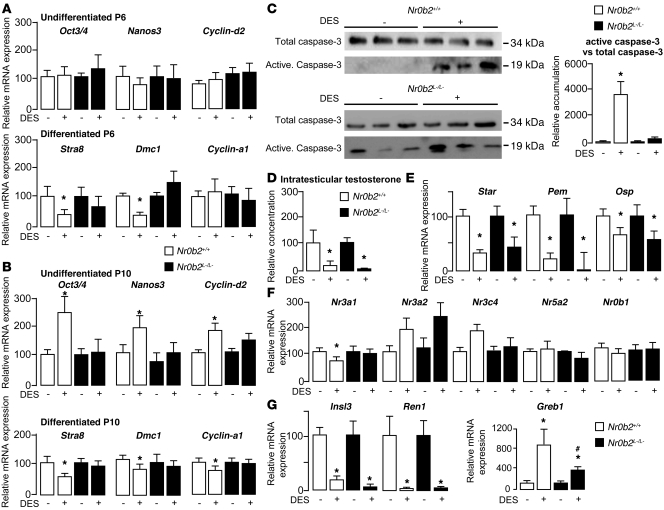
Figure 1. Nr0b2 deficiency protects male mice against DES-induced abnormalities.
(A) Nr0b2 mRNA levels in whole testes of P10 Nr0b2+/+ and Nr0b2L–/L– males exposed to 0.75 μg DES (n = 6), and in whole testes of 10-week-old males neonatally exposed to 0, 0.75, and 5 μg DES (n = 3–5). Values were normalized to β-actin. (B) Each male was bred with 5 C57BL/6J females to analyze the number of pups per litter. (C and D) Spermatozoa count in the tail of epididymis (C) and whole body weight as well as weights of testis, epididymis, seminal vehicles, and liver normalized to body weight (D) of 10-week-old Nr0b2+/+ and Nr0b2L–/L– males neonatally exposed to 0, 0.35, 0.5, 0.75, 1.5, and 5 μg DES (n = 6–14 per group). *P < 0.05 versus vehicle; #P < 0.05 versus Nr0b2+/+ given the same DES dose.
DES has a weak impact on the fertility of Nr0b2-knockout mice.
We then treated neonatal Nr0b2+/+ and knockout (Nr0b2L–/L–) male mice with increasing doses of DES (0–5 μg). Neonatal exposure of Nr0b2+/+ males to DES caused a significant 80% decrease in the number of pups per litter, whereas it had a lesser impact on litters from Nr0b2L–/L– males (Figure 1B). These data were consistent with the decreased number of spermatozoa in the epididymis tails of treated Nr0b2+/+ males, which was much less pronounced in Nr0b2L–/L– males (Figure 1C). The weights of testis, epididymis, and seminal vesicles were also more reduced in Nr0b2+/+ than in Nr0b2L–/L– male mice exposed to DES (Figure 1D). Consistent with these protective effects, the minimal dose of DES at which testis weight started to decrease was between 0.75 and 1.5 μg in Nr0b2+/+ males, whereas there was almost no effect in Nr0b2L–/L– males (Figure 1D). The protection against DES caused by Nr0b2 gene inactivation was not a general or systemic effect, as body and liver weights were similarly affected by DES in both genotypes (Figure 1D).
Even though DES belongs to the group of EDs with estrogen activity, it can also activate other signaling pathways (24). Neonatal administration of the pure estrogen, EB, to Nr0b2+/+ males decreased the adult weights of the testis, epididymis, and seminal vesicles (Supplemental Figure 1C). As for DES administration, the Nr0b2L–/L– males were significantly protected from the undesired effects of EB compared with Nr0b2+/+ males. The number of spermatozoa decreased in the epididymis tails of treated Nr0b2+/+ males, whereas no effect of EB was observed in Nr0b2L–/L– males (Supplemental Figure 1D). These effects of DES might be in part mediated by the Ers, as the use of the Er antagonist ICI on Nr0b2+/+ males was able to reverse part of the impact of DES on the decreased count of spermatozoa as well as on the decrease in organ weights (Supplemental Figure 1, E and F).
Nr0b2 deficiency protects mice from DES-induced germ cell death.
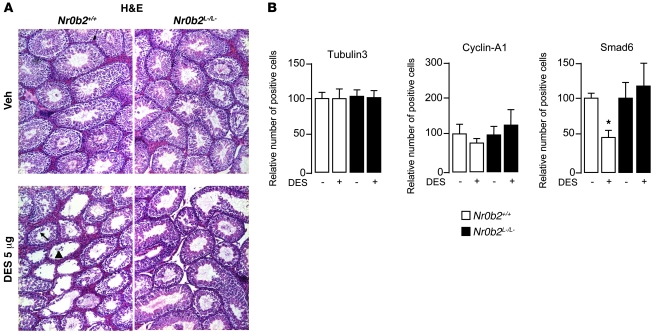
In 10-week-old adult Nr0b2+/+ mice exposed to 5 μg DES, the testes showed major morphological alterations, as reflected by the absence of germ cells in several tubules (Figure 2A). Conversely, even at this high dose, Nr0b2L–/L– males did not show apparent abnormalities. The use of cell type–specific markers (25) allowed us to determine that postmeiotic cells were the most affected cell population in DES-treated Nr0b2+/+ mice, as reflected by the decreased number of Smad6-positive cells (Figure 2B and Supplemental Figure 2A). On the contrary, no difference was observed in the number of Sertoli and premeiotic/early meiotic germinal cells (as determined by staining for Tubulin3 and Cyclin-A1, respectively; Figure 2B).
Figure 2. DES-induced histological abnormalities caused by loss of postmeiotic cells in Nr0b2+/+ mice.
(A) Representative micrographs of H&E-stained testes of 10-week-old Nr0b2+/+ and Nr0b2L–/L– mice exposed to vehicle or 5 μg DES (n = 6 per group). The arrow indicates tubules with a slight loss of germ cells; the arrowhead indicates tubes with complete loss of germ cells. Original magnification, ×100. (B) Quantification of cells per 100 seminiferous tubules (n = 4–6) positively stained for markers Smad6 (postmeiotic germ cells), Tubulin3 (Sertoli cells), and Cyclin-A1 (pre- and meiotic germ cells). Vehicle-treated mice were arbitrarily set at 100%. *P < 0.05 versus vehicle.
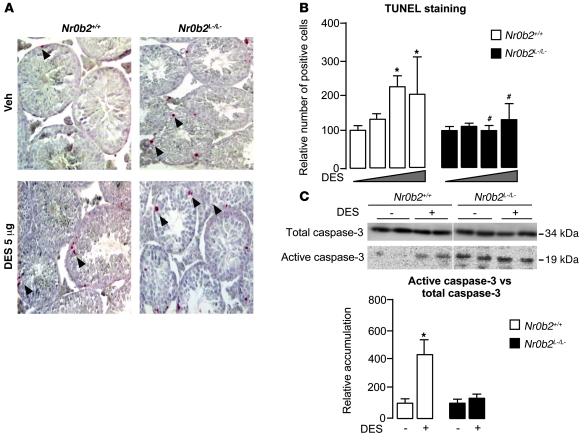
We then assessed potential changes in the rate of proliferation. No significant change in the expression of the proliferation marker Ki-67 or in the accumulation of PCNA was observed between untreated or DES-treated Nr0b2+/+ and Nr0b2L–/L– males (Supplemental Figure 2B). However, DES induced a significant increase in apoptotic cells in the testes of Nr0b2+/+ mice starting at the 0.75-μg dose, as determined by TUNEL analysis (Figure 3, A and B). The increased apoptosis in Nr0b2+/+ testis corroborated with the increased accumulation of active caspase-3 (Figure 3C). On the contrary, Nr0b2L–/L– males were protected from the increased apoptosis induced by DES (Figure 3). In a similar experiment, neonatal exposure of mice to EB resulted in germ cell loss caused by an increased number of apoptotic cells in adult Nr0b2+/+ mice, whereas Nr0b2L–/L– males were also protected from apoptosis induced by EB (Supplemental Figure 2, C and D).
Figure 3. Nr0b2 controls DES-induced adult germ cell apoptosis through regulation of testosterone synthesis.
(A) Apoptosis in 10-week-old Nr0b2+/+ and Nr0b2L–/L– mice exposed to vehicle or 5 μg DES (n = 6 per group), as analyzed by TUNEL staining. Arrowheads denote TUNEL-positive cells. Representative micrographs are shown. Original magnification, ×100. (B) Quantification of TUNEL analyses. Shown is the number of positive cells per 100 seminiferous tubules (n = 4–6). (C) Immunoblot of activate caspase-3 performed on testicular protein extracts of Nr0b2+/+ and Nr0b2L–/L– mice exposed to 0 or 5 μg DES (n = 6 per group). Quantification of activated caspase-3 protein accumulation relative to total caspase-3 is shown below; vehicle-treated mice were arbitrarily fixed at 100%. *P < 0.05 versus vehicle; #P < 0.05 versus Nr0b2+/+ given the same DES dose.
Nr0b2 reduces testosterone levels after DES exposure.
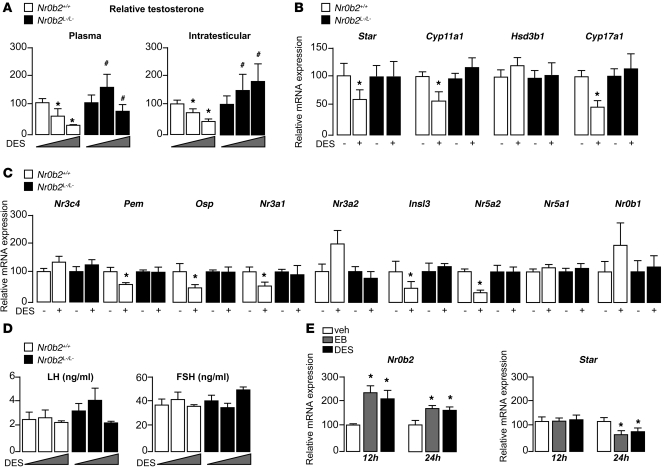
Germ cell death has previously been associated with androgen withdrawal (26, 27). Consistent with previous data (28, 29), DES induced a decrease in testosterone concentrations in Nr0b2+/+ males at as little as 0.75 μg (Figure 4A). This effect was further pronounced at 5 μg DES. The inhibition of testosterone was associated with a decrease in mRNA levels of Star, Cyp11a1, and Cyp17a1, which are critically involved in steroidogenesis (Figure 4B). Interestingly, Nr0b2L–/L– males seemed to be protected from these effects of DES, as testosterone concentrations as well as Star, Cyp11a1, and Cyp17a1 mRNA levels were not affected (Figure 4, A and B). No effect of DES was observed on mRNA accumulation of the androgen receptor Nr3c4 in Nr0b2+/+ or Nr0b2L–/L– testes (Figure 4C). The decrease in testosterone was also consistent with the reduction in mRNA abundance of the androgen-dependent genes Pem and Osp in Nr0b2+/+ males. In addition to the impact of DES on the androgen pathway, we analyzed its impact on Er target genes. The mRNA accumulation of Nr3a1 and insulin-like–3 (Insl3) decreased in Nr0b2+/+ testes, as expected, whereas this effect of DES was not observed in Nr0b2L–/L– testes (Figure 4C). Also as expected, DES had no effect on the accumulation of Nr3a2.
Figure 4. Nr0b2 controls DES-induced repression of testosterone synthesis.
(A) Plasma and intratesticular testosterone levels in 10-week-old Nr0b2+/+ and Nr0b2L–/L– mice exposed to 0, 0.75, or 5 μg DES (n = 10–15 per group). (B) Testicular mRNA expression of Star, Cyp11a1, Hsd3b1, and Cyp17a1, normalized to β-actin levels, in whole testes of 10-week-old Nr0b2+/+ and Nr0b2L–/L– mice exposed to 0 or 0.75 μg DES (n = 10–15 per group). (C) Testicular mRNA expression of Nr3c4, Pem, Osp, Nr3a1, Nr3a2, Insl3, Nr5a2, Nr5a1, and Nr0b1, normalized to β-actin levels, in whole testes of 10-week-old Nr0b2+/+ and Nr0b2L–/L– mice exposed to 0 or 0.75 μg DES (n = 10–15 per group). (D) Plasma LH and FSH concentration in 10-week-old Nr0b2+/+ and Nr0b2L–/L– mice exposed to 0, 0.75, or 5 μg DES (n = 10–15 per group). (E) mRNA expression of Nr0b2 and Star normalized to β-actin levels in MA-10 Leydig cells exposed to vehicle, EB, or DES for 12 or 24 hours (n = 6 per group). Vehicle-treated mice were arbitrarily fixed at 100%. *P < 0.05 versus vehicle; #P < 0.05 versus Nr0b2+/+ given the same DES dose.
We next analyzed the expression of known regulators of steroidogenesis to identify how Nr0b2 could control steroid synthesis. Regarding the inducers of steroidogenesis, Nr5a2 and the steroidogenic factor–1 (Sf-1, referred to herein as Nr5a1), both known targets of Nr0b2, only Nr5a2 expression decreased at 0.75 μg DES (Figure 4C). DES had no effect on the expression of dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1 (Dax-1, referred to herein as Nr0b1), a negative regulator of steroidogenesis (Figure 4C). The use of the Er agonist EB, or the antagonist ICI, suggests a significant contribution of the estrogenic effects of DES on the repression of steroidogenesis (Supplemental Figure 2, E and F). Indeed, EB exposure resulted in a decrease of the intratesticular concentration of testosterone in Nr0b2+/+ mice. Similar to what we observed after DES treatment, we observed no effect on testosterone level in Nr0b2L–/L– males after EB exposure. Moreover, ICI exposure reversed the effect of DES on testosterone in Nr0b2+/+ males.
To test whether the DES-induced effects on the testis are regulated at the level of the hypothalamus/pituitary axis, we first measured LH and FSH plasma levels. Interestingly, no impact of DES on LH or FSH level was observed, which suggests that the primary effect of DES occurs at the testicular level (Figure 4D). Several studies have demonstrated that estrogen can regulate steroidogenesis directly on the level of the Leydig cell. To confirm this hypothesis, we performed some in vitro analyses using the Ma-10 Leydig cell line. EB and DES increased Nr0b2 mRNA accumulation at 12 and 24 hours after treatment, whereas the decrease in the mRNA levels of steroidogenic genes such as Star was observed only after 24 hours (Figure 4E). Consistent with our previous report (8), these results suggest that Nr0b2 might directly repress steroid synthesis after DES and/or estrogen treatment.
DES exposure alters neonatal germ cell differentiation through Nr0b2.
DES was administered close to the beginning of germ cell differentiation (30, 31). To determine an eventual impact of DES exposure on germ cell differentiation, we analyzed the testis at P6 or P10. At P6, there was no alteration in the expression of genes specific for undifferentiated spermatogonia (Oct3/4, Nanos3, and Cyclin-d2; Figure 5A). However, in Nr0b2+/+ mice treated with DES, expression of transcripts involved in germ cell differentiation (Stra8 and Dmc1) was significantly lower. This effect was not observed in the Nr0b2L–/L– males. At P10, the accumulation of mRNAs involved in germ cell differentiation was still reduced in the testes of Nr0b2+/+ mice exposed to DES, an effect that was accompanied by the robust induction of specific transcripts of undifferentiated spermatogonia (Figure 5B), suggesting an alteration of the relative proportion of undifferentiated versus differentiating spermatogonia following DES administration in Nr0b2+/+ mice. Moreover, at P10, the meiotic marker Cyclin-a1 was found to be decreased by DES treatment, which is consistent with a decreased germ cell differentiation. Again, Nr0b2L–/L– testes were not affected. Similar studies using EB instead of DES showed that part of the effect of DES is likely mediated by Ers, as several genes, including Nanos3, Stra8, Dmc1, Cyclin-a1, Cyclin-d2, appeared to be regulated in a similar way (Supplemental Figure 3, A and B). Interestingly, some other genes, such as Oct3/4, showed a different expression pattern in mice treated with EB compared with those treated with DES (compare Figure 5B and Supplemental Figure 3B). Interestingly, this effect of DES on Oct3/4 expression was also inhibited in Nr0b2L–/L– males, which suggests that Nr0b2 is a major component of the signaling pathways activated by DES.
Figure 5. Nr0b2 inhibits neonatal germ cell differentiation in response to DES.
Nr0b2+/+ and Nr0b2L–/L– mice were exposed to 0 or 0.75 μg DES. (A and B) Testicular mRNA expression of Oct3/4, Nanos3, Cyclin-d2, Stra8, Dmc1, and Cyclin-a1, normalized to β-actin levels, in P6 (A) or P10 (B) mice (n = 5–10 per group). (C) Immunoblot of activate caspase-3 on testicular protein extracts (n = 6 per group). Quantification of activate capase-3 protein accumulation relative to total caspase-3 is also shown. (D) Intratesticular testosterone levels in P10 mice (n = 10–15 per group). (E–G) mRNA expression of Star, Pem, and Osp (E); Nr3a1, Nr3a2, Nr3c4, Nr5a2, and Nr0b1 (F); and Insl3, Ren1, and Greb1 (G) in whole testes of P10 mice (n = 10–15 per group). Values were normalized to β-actin levels. Vehicle-treated mice were set at 100%. *P < 0.05 versus vehicle; #P < 0.05 versus Nr0b2+/+ given the same DES dose.
Neonatal effect of DES exposure involves apoptosis and estrogenic signaling, but is independent of androgen status.
To understand the molecular pathways underlying the alteration in germ cell maturation, we analyzed both proliferation and apoptotic processes in P10 testis. No effect was observed on cell proliferation, as suggested by the analysis of PCNA protein accumulation (Supplemental Figure 3C). In P10 mice, DES induced apoptosis, as revealed by increased accumulation of the active caspase-3 protein, in Nr0b2+/+ mice (Figure 5C). On the contrary, the impact of DES on apoptosis was not observed in the testes of Nr0b2L–/L– males. Interestingly, the changes in the number of apoptotic cells did not seem to be linked to the androgen status, as intratesticular testosterone decreased in both genotypes (Figure 5D). In fact, DES treatment induced a drastic decrease of the intratesticular testosterone in both Nr0b2+/+ and Nr0b2L–/L– males. We also analyzed mRNA accumulation of Star and of the androgen-dependent genes Pem and Osp. The expression of these genes was decreased following DES exposure in both genotypes (Figure 5E), reflecting the decrease of intratesticular androgen levels. As for DES, EB administration resulted in a decreased expression of the steroidogenic genes Star, Cyp11a1, and Cyp17 (Supplemental Figure 3D). This result suggests that neonatal steroidogenesis, in contrast to that of adult mice, is not controlled by Nr0b2, since Nr0b2L–/L– mice showed a profile similar to that of Nr0b2+/+ mice (Figure 5, D and E).
The impact of DES on the estrogenic pathway was then analyzed in the testes of Nr0b2L–/L– mice and Nr0b2+/+ littermates. No effect of DES was observed on the expression of Nr3a2 (Figure 5F). However, consistent with the estrogenic effect of DES, the expression of known target genes of the Ers (16) — including Nr3a1, Insl3, and renin-1 (Ren1) — decreased, whereas the expression of gene regulated in breast cancer 1 protein (Greb1) increased (Figure 5, F and G). For Insl3 and Ren1, the effect of DES was similar in Nr0b2+/+ and Nr0b2L–/L– males. On the contrary, the impact of DES on the expression of Nr3a1 and Greb1 was lost or diminished in Nr0b2L–/L– versus Nr0b2+/+ mice. Similarly, the estrogenic target genes Nr3a1, Insl3, and Ren1 were affected by EB (Supplemental Figure 3E). These data demonstrated that Nr0b2 is not involved in the regulation of all the Er target genes, which suggests that there might be some compensatory mechanisms involving other cofactors of Nr3a1/2.
Nr0b2 mediates part of the neonatal effects of DES through modification of histone methylation marks.
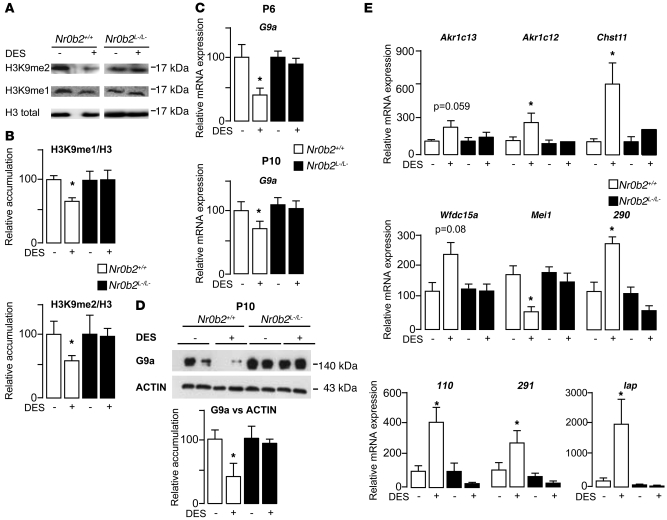
Previous studies have shown that mice lacking the H3K9 histone methyltransferase G9a are sterile, with germ cells undergoing apoptosis during the pachytene stage (32). G9a is reported to perform H3K9 mono- and dimethylation (marked by H3K9me1 and H3K9me2, respectively; refs. 33, 34). In view of the Nr0b2-dependent proapoptotic effects of DES on germ cells, we wondered whether these effects could be mediated by factors affecting histone methylation, such as G9a (31). Interestingly, in testes of DES-treated P10 Nr0b2+/+ animals, the H3K9me1/2 marks decreased, whereas they were unaffected in Nr0b2L–/L– mice (Figure 6, A and B). This suggests that DES might affect G9a expression. Consistent with this hypothesis, testicular G9a mRNA and G9a protein expression decreased in Nr0b2+/+ mice treated with DES, whereas no effect was observed in the Nr0b2L–/L– mice (Figure 6, C and D). The decrease of the transcriptional repressor G9a following DES administration in Nr0b2+/+ mice was also associated with an increase of several G9a target genes (32, 35), including Akr1c13, Akr1c12, Chst11, 290, 291, 110, Wfdc15a, and Iap, as a result of relief of repression (Figure 6E). Conversely, and in line with previous studies describing a downregulation of Mei1 in G9a-null mice (32), mRNA levels of this gene were decreased in the Nr0b2+/+ mice treated with DES (Figure 6E). In the Nr0b2L–/L– mice, neither a significant effect nor an opposite effect of DES was observed on the expression of these genes compared with Nr0b2+/+ mice. In Nr0b2+/+ males, the use of EB also induced a clear decrease in G9a mRNA levels at P6 and P10, which translated to a robust decrease in G9a protein levels (Supplemental Figure 4, A and B). The H3K9me2 mark was also decreased by EB in Nr0b2+/+ testes, whereas no effect was observed in those of Nr0b2L–/L– mice (Supplemental Figure 4C). This effect was consistent with the deregulation of G9a targets genes in the testes of Nr0b2+/+, but not Nr0b2L–/L–, mice (Supplemental Figure 4D).
Figure 6. Nr0b2 induces alterations of histone H3K9 marks through the inhibition of G9a, in response to DES.
Nr0b2+/+ and Nr0b2L–/L– mice were exposed to 0 or 0.75 μg DES. (A and B) Immunoblots of H3K9me1 and H3K9me2 performed on testis (A), and quantification of H3K9me2 and H3K9me2 accumulation, relative to total H3 (B), of P10 mice (n = 9 per group). (C) Testicular mRNA expression of G9a, normalized to β-actin levels, in whole testes of P6 and P10 mice (n = 10–15 per group). (D) Immunoblot of G9a performed on P10 mice (n = 9 per group). Quantification of G9a protein accumulation, relative to actin, is also shown. (E) Testicular mRNA expression of G9a target genes Akr1c13, Akr1c12, Chst11, Wfdc15a, Mei1, 290, 110, 291, and Iap, normalized to β-actin levels, in whole testes of P10 mice (n = 10–15 per group). Vehicle-treated mice were fixed at 100%. *P < 0.05 versus vehicle.
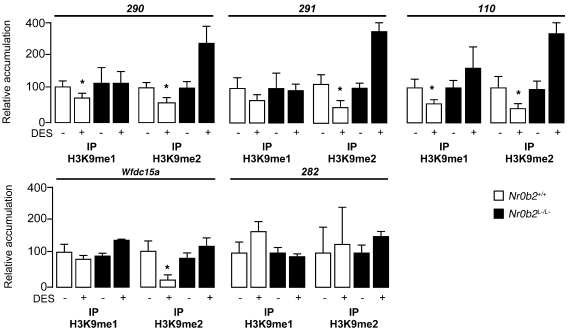
To corroborate the effect of histone modifications directly on the G9a target genes, we performed H3K9me1 or H3K9me2 IP on chromatin extracted from vehicle- or DES-treated mice at P10. DES induced a decrease in both H3K9me1 and H3K9me2 marks on the DNA sequences of G9a target genes 290, 291, 110, and Wfdc15a, whereas no effect was observed on the negative control locus 282 (Figure 7). Surprisingly, for some G9a target genes, such as 290 and 110, we noticed a significant increase of H3K9me2 in Nr0b2L–/L– mice treated with DES compared with vehicle treatment. This effect was opposite to what was observed in Nr0b2+/+ mice. In Nr0b2L–/L– mice treated with DES, the higher recruitment of H3K9me2 was consistent with the lower expression of some G9a target genes, such as 290, 291, 110, and Iap (Figures 6 and 7).
Figure 7. Nr0b2 mediates DES-induced alterations of histone H3K9 marks on the promoter of G9a target genes.
Shown are results of ChIP of crosslinked DNA from testes of P10 Nr0b2+/+ and Nr0b2L–/L– mice using H3K9me1 or H3K9me2 antibodies. The loci studied as G9a targets (290, 291, 110, Wfdc15a, and 282) were previously defined and cover at least 1 gene (35). The 282 locus was used as a negative control of H3K9 methylation. IgG was used as a negative internal control. For each condition, 6 ChIPs were performed. *P < 0.05 versus vehicle.
Nr0b2 is responsible for the cross-talk between DES and the retinoid pathway.
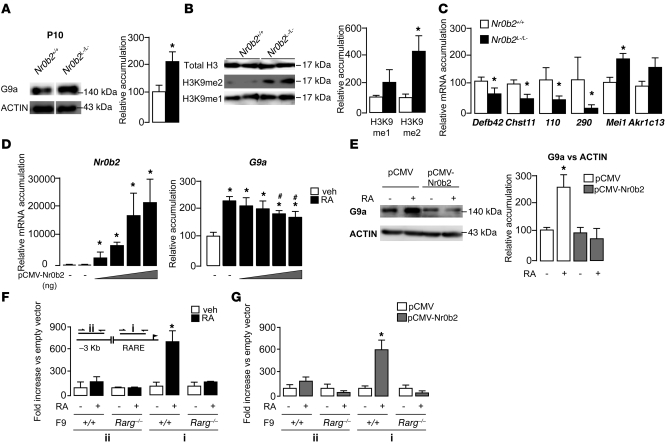
The analysis of G9a expression in the testes of untreated P10 Nr0b2+/+ and Nr0b2L–/L– mice showed higher protein accumulation in Nr0b2L–/L– mice (Figure 8A), which suggests that G9a is a target gene of Nr0b2. This was consistent with the higher levels of H3K9me1/2 marks and the deregulation of the G9a target genes observed in Nr0b2L–/L– mice (Figure 8, B and C).
Figure 8. Nr0b2 controls G9a expression.
(A) G9a immunoblot from whole testes of P10 Nr0b2+/+ and Nr0b2L–/L– untreated mice (n = 5–7 per group). Lanes were run on the same gel but were noncontiguous (white line). (B) Immunoblots of H3K9me1 or H3K9me2 performed on P10 Nr0b2+/+ and Nr0b2L–/L– mice (n = 6–7 per group). (C) mRNA levels of G9a target genes Defb42, Chst11, 110, 290, Mei1, and Akr1c13 in whole testes of P10 Nr0b2+/+ and Nr0b2L–/L– mice exposed to 0 or 0.75 μg DES (n = 10–15 per group). (A–C) Levels were normalized to actin, and normalized values of Nr0b2+/+ were set at 100%. (D) mRNA expression of Nr0b2 and G9a in F9 cells transfected with 0–800 ng pCMV-Nr0b2 (n = 3). (E) Protein accumulation of G9a in F9 cells transfected with pCMV-Nr0b2 in the absence or presence of RA (n = 3). (F and G) ChIP of crosslinked DNA from F9 wild-type or Rarg–/– cells using an anti-Rarγ antibody (F) or from F9 wild-type or Rarg–/– cells transfected with pCMV or pCMV-Nr0b2 using an anti-Flag antibody (G). Inset: A DNA sequence of ±100 bp covering either the RARE (i) or a sequence 3.0 kb upstream of the RARE (ii) was amplified. Results are expressed as fold enrichment over wild-type vehicle-treated cells (F) or over pCMV cells (G) and represent amplification variability (n = 4). *P < 0.05 versus vehicle; #P < 0.05 versus next-smallest transfected pCMV-Nr0b2 amount.
Retinoids (i.e., all-trans-retinoic acid; RA) are key components that induce the entry of germ cells into meiosis (30, 31, 36). We hence evaluated the interplay among RA signaling, Nr0b2, and G9a using the embryonic teratocarcinoma-derived F9 cell line. This cell line is a well-established cell-autonomous model for investigating retinoid signaling in vitro, as RA can induce the cells’ differentiation (37). In F9 cells, RA treatment led to an increase in G9a mRNA accumulation, which was inhibited by Nr0b2 in a dose-dependent manner (Figure 8D). These results were confirmed at the protein level (Figure 8E), which suggests that Nr0b2 could regulate the expression of G9a by inhibiting the RA receptor (Rar) pathway. Using an in silico approach based on the Genomatix program (see Methods), we mapped a putative Rar response element (RARE) in the promoter of G9a. To validate the relevance of this RARE in the regulation of G9a expression in response to retinoids, we performed chromatin IP (ChIP) experiments using either wild-type or Rarg–/– F9 cells (38). Treatment with RA led to a higher level of Rarγ IP on the RARE in wild-type cells, whereas no difference was observed in the Rarg–/– F9 cells (Figure 8F). The potential interaction of Nr0b2 with Rar on the promoter of the G9a gene was further substantiated by ChIP experiments using wild-type or Rarg–/– F9 cells transfected with pCMV or pCMV-Nr0b2 expression vectors. Consistent with our expression data, we found specific enrichment of Nr0b2 on the DNA sequences surrounding the RARE of the G9a promoter in wild-type cells, but not Rarg–/– F9 cells (Figure 8G), further underscoring the importance of the Nr0b2/Rar complex in the regulation of G9a.
Discussion
Here, we demonstrated that DES induced abnormalities in the male genital tract upon fetal and/or neonatal exposure in mice. In animal models, neonatal administration of DES decreased fertility in adult male mice as a result of altered morphology of the male genital tract, with decreased relative weights of epididymis, vesicle seminals, and testis. This was associated with a decrease in epididymis sperm count, which was caused by the increased germ cell death we observed in male mice exposed to DES. Interestingly, for all these parameters, deficiency for Nr0b2 protected the males exposed to DES, which suggests that Nr0b2 plays a critical role in the testicular pathophysiology induced by DES. However, we have demonstrated that DES acts via several signaling pathways. Through the use of estradiol and/or of the Er antagonist ICI, we showed that Nr0b2 protected the male mice against both estrogenic and nonestrogenic effects of DES. Even though it is not established in humans, these rodent data are the basis of the hypothesis of a potential link between these environmental factors (25), including the exposure to EDs (39–41), and the fast increase of the incidence of male reproductive disorders (42, 43). Our data could potentially establish a link between EDs and male reproductive disorders in humans.
High doses of EDs induce a severe decrease in testicular weight caused by germ cell death, which leads to important modifications in the relative cell type proportions. Such changes could lead to false interpretations, because the observed differences in gene expression are the result of changes in testicular cell content rather than of the real regulation of gene expression. Through a dose-effect experiment of DES, we first determined the concentration that did not dramatically affect testicular weight. This allowed us to assume that at the doses used for the molecular analyses, the modifications of gene expression were significant, not the consequences of altered cell content.
Unexpectedly, we observed higher levels of active caspase-3 in Nr0b2L–/L– mice compared with Nr0b2+/+ littermates. This increase in basal levels of active caspase-3 in Nr0b2L–/L– mice was significant in P10 animals (Figure 5C), which could reflect the earlier entry in meiosis. In adult mice, we also detected a significant difference between active caspase-3 levels in Nr0b2+/+ and Nr0b2L–/L– mice (Figure 3C). Interestingly, and in line with our previous studies (8), there was no difference in the number of TUNEL-positive cells between vehicle-treated Nr0b2+/+ and Nr0b2L–/L– males. The increase in active caspase-3 was not consistent with the unchanged results of the TUNEL assay and requires further study; this finding reflects the complexity of apoptosis, which can be regulated at both activation and inhibition levels.
Even though the present report showed that Nr0b2L–/L– mice were protected against the deleterious effect of DES and EB on the male reproductive tract, basal estrogen metabolism was similar between Nr0b2+/+ and Nr0b2L–/L– males (Supplemental Figure 5, A–C). These data suggested that the role of Nr0b2 on the estrogenic pathway is only revealed under pathophysiological conditions when exposed to estrogenic endocrine disrupters. However, the lack of difference between the genotypes under basal condition was surprising and difficult to explain; it suggests the existence of some yet-unidentified compensatory mechanisms. One could speculate that Nr0b1, which is very close to Nr0b2 at the structural level, could compensate for the lack of Nr0b2.
Nr0b1 and Nr0b2 are closely related nuclear receptors. Moreover, the impact of Nr0b1 on steroidogenesis has been well demonstrated. In our experiments, however, we have not shown a significant effect of DES treatment on Nr0b1 expression in P10 pups (Figure 5F) or in adult males (Figure 4C). These results suggest that Nr0b1 is not involved in the DES-induced phenotypes. Several studies have shown that Nr0b1 KO mice have normal levels of testosterone (44, 45). The Nr0b1 KO mouse phenotype can mostly be explained by the altered expression of aromatase, the enzyme that synthesizes estrogens, whereas other steroidogenic genes, like Star, were not affected (45). Together, these observations imply a converging role of both Nr0b members to control full testicular steroid metabolism, in which Nr0b2 would predominantly regulate steroidogenesis up to the level of testosterone synthesis, and Nr0b1 would instead control aromatization.
In adult mice, Nr0b2 was responsible for the DES-induced decrease in testosterone production. The absence of a decrease of steroidogenic genes in Nr0b2L–/L– males after DES exposure is suggestive of a major role for Nr0b2 in the DES-induced repression of steroidogenesis. This hypothesis is further corroborated by the induction of Nr0b2 mRNA in response to DES. This effect was also observed with EB, which suggests that it might be driven by Nr3a1/2 signaling. This is consistent with previous studies showing that estrogen can directly regulate steroidogenesis at the Leydig cell levels (46). The ability of estrogen to decrease steroid synthesis in the mouse Leydig cell line MA-10, highlighted by the decrease in the mRNAs of Star and Cyp11a1, has been described previously (47). To identify the possible direct effect of Nr0b2 in Leydig cells following DES administration, we also performed experiments on MA-10 cells. Similar to our in vivo results, EB and DES resulted in an increase of Nr0b2 mRNA 12 hours after treatment. This increase in Nr0b2 mRNA was followed at 24 hours by a significant decrease in Star mRNA. The kinetics of the mRNA increase of Nr0b2, followed by the decrease of Star mRNA, is consistent with Nr0b2 being a major inhibitor of steroidogenesis in Leydig cells. This is consistent with our previous work demonstrating that overexpression of Nr0b2 in a Leydig cell line represses Star and Cyp11a1 expression and that Nr0b2 binds to the promoter of these 2 genes (8). Moreover, our conclusion that Nr0b2 controls steroidogenesis directly at the testicular level after DES exposure was confirmed by the finding that plasma LH and FSH levels were both unaltered by DES as well as Nr0b2 genotype.
At the molecular level, we have previously shown that Nr0b2 regulates testicular androgen synthesis through repressed mRNA expression of 2 activators of steroidogenesis, Nr5a2 and Nr5a1, and/or through inhibited transcriptional activities of the same (8). Here, the repression of steroidogenesis by Nr0b2 at the lower 0.5-μg dose is likely caused by protein-protein interaction, as mRNA expression of both Nr5a1 and Nr5a2 was not affected, whereas Star accumulation was already reduced at this DES concentration, in Nr0b2+/+ mice (data not shown). At the dose of 0.75 μg DES, this effect was further amplified, probably by the decreased mRNA expression of Nr5a2. Even though Nr5a1 was defined as a target gene of Nr0b2, its expression was not affected by DES administration. The absence of any effect of DES was consistent with previous studies (15). These data demonstrate the complexity of the regulation of Nr5a1, which is known to be controlled by multiple factors (48); furthermore, it would be too simplistic to assume that the mRNA expression of Nr5a1 is only regulated by Nr0b2.
In contrast to our findings in adult mice (Figure 4), neonatal steroidogenesis seemed not to be controlled by Nr0b2, as Nr0b2L–/L– and Nr0b2+/+ mice showed similar profiles (Figure 5, D and E). This difference in Leydig cell response seems to occur in parallel with the cells’ transition from the fetal to the adult population and is in line with the finding that from P20 onward, Nr0b2 becomes expressed only in interstitial cells, where it controls steroidogenesis (8). Moreover, P10 testis samples showed that Nr0b2 was mainly expressed in the tubular compartment of the testis (data not shown). This suggests that, at P10, the impact of Nr0b2 following DES exposure occurs in the intratubular compartment.
In addition to its role on androgen synthesis in adult mice after neonatal DES exposure, Nr0b2 has other functions during postnatal testicular development. We administered DES during early postnatal development, close to the beginning of germ cell differentiation. Indeed, the first spermatocytes are seen at P5 (31). Neonatal administration of either DES or EB induced modified expression of genes associated with differentiated or undifferentiated cells, such as Nanos3, Stra8, and Dmc1. We demonstrated here that DES induced accumulation of specific transcripts of undifferentiated spermatogonia, specifically in treated Nr0b2+/+ testis, and reduced the expression of transcripts involved in germ cell differentiation. These results suggest a change of the relative proportion of undifferentiated versus differentiating spermatogonia after DES exposure. We thus hypothesize that Nr0b2 mediates the effect of DES on germ cell differentiation, which is in line with our previous report that Nr0b2L–/L– mice showed earlier differentiation of germ cells than did control littermates (8). To understand the alteration of these relative cell proportions, we analyzed both proliferation and apoptotic processes in P10 mouse testes. In contrast to adult mice, this differential effect observed between Nr0b2+/+ and Nr0b2L–/L– males in early postnatal development did not seem to be linked with androgenic status. The increased apoptosis observed in Nr0b2+/+ males is most likely caused by other alterations. DES treatment clearly affected the meiotic cells. In oocytes, DES induced a severe, yet reversible, deterioration of meiotic spindle microtubule organization during maturation (49). DES reduces viability of Caenorhabditis elegans and its fertility, associated with the production of aberrant gametes, as a result of nuclear abnormalities and loss of synaptonemal complexes (50). Moreover, recent studies have demonstrated the importance of germ cell–specific epigenetic processes in the initiation and early progression of meiosis (51). Interestingly, mice with a loss-of-function mutation for H3K9 histone methyltransferases are sterile, with germ cells undergoing apoptosis during the pachytene stage (32, 52). Several proteins possess H3K9 methyltransferase activity. Suv39h1, Suv39h2, and G9a are able to perform H3K9 dimethylation (33), whereas only G9a performs H3K9 monomethylation (34). In testes of DES-treated P10 Nr0b2+/+ mice, we observed decreases in the H3K9me1 and H3K9me2 marks, which suggests that DES might affect G9a expression and/or activity in the testis. Consistent with this hypothesis, G9a mRNA and G9a protein expression was decreased in Nr0b2+/+ mice treated with DES. Because of its histone methyltransferase activity, G9a acts as a repressor of transcription. The decrease of G9a after DES exposure was confirmed in Nr0b2+/+ mice by higher mRNA accumulation of known G9a target genes, such as Akr1c13 and Chst11 (32). We observed no significant effect in the Nr0b2L–/L– mice. It has been recently demonstrated that Nr0b2 physically interacts with G9a to induce repression of gene expression (53, 54). Here, we demonstrated another form of cross-talk between Nr0b2 and G9a, in which Nr0b2 inhibited the mRNA expression of G9a. It is possible that both coexist and are part of a feedback loop in which Nr0b2 inhibits G9a gene expression to attenuate the repression of their common target genes. The effect of DES on the histone methylation marks was also found when we used EB, highlighting the involvement of the estrogenic part of DES in this pathway.
If the signaling of G9a was decreased in Nr0b2+/+ mice treated with DES, as evidenced by decreased expression of G9a, decreased H3K9 methylation, and increased expression of G9a target genes, we unexpectedly observed an opposite effect of DES in the Nr0b2L–/L– males. Indeed, the expression of some target genes was found more repressed in the Nr0b2L–/L– males exposed to DES compared with vehicle. This effect, observed in Nr0b2L–/L– males, was confirmed by a higher level of methylated histones on the DNA sequences of these G9a target genes. This effect is surprising and to date remains unexplained. However, this is in line with our conclusion that Nr0b2 participates to control G9a signaling. The observed data in Nr0b2L–/L– mice treated with DES suggest that the lack of Nr0b2 induced an increase in the G9a pathway, or a compensatory pathway through other histone methyltransferases. The exact molecular mechanisms involved are not established yet and will require further studies.
The molecular mechanisms triggering the initiation of germ cell differentiation, in particular the mitotic/meiotic transition, are not completely understood. However, retinoids are key components to induce entry of germ cells in meiosis (30, 31, 36). Most intriguingly, retinoids have also been shown to induce an increase in H3K9 methylation during differentiation (55). Based on these reports and our above-described findings, we hypothesized that Nr0b2, via the control of G9a expression, is the link between RA and DES pathways in the control of the meiotic process. This was confirmed by the induction of G9a expression following RA administration in different cell lines. Moreover, we found a specific enrichment of a Rar/Nr0b2 complex on the DNA sequences surrounding the RARE of the G9a promoter. Our results therefore show, for the first time to our knowledge, a potential interaction between the retinoid signaling pathway and the expression of the histone methyltransferase G9a, which could explain, at least in part, the impact of G9a and H3K9 methylation in germ cell differentiation.
Interestingly, this effect of DES on germ cell differentiation seemed to persist in adult mice, as we observed a clear decrease in mRNA accumulation of Stra8 (Supplemental Figure 5D). We have indeed demonstrated that the deregulation of G9a was still observed at the mRNA and protein levels in Nr0b2+/+ adult males treated neonatally with DES (Supplemental Figure 5, E and F). This perpetuation of G9a deregulation in adult testis, combined with the altered testosterone synthesis, might cooperate to decrease germ cell survival and lead to subfertility after DES administration.
It has been previously demonstrated that DES can have estrogenic and nonestrogenic effects (15–18). Compared with EB, DES appeared to have a stronger effect. To determine the estrogenic part of DES activity, we used either a pure estrogenic compound, EB, or the Er antagonist ICI. Most of the macroscopic phenotypes observed with DES were also obtained using EB (e.g., organ weight, apoptotic process), and the Nr0b2L–/L– mice were also protected against the deleterious effects of EB (Supplemental Figure 1, B–D, and Supplemental Figure 2, B and E). ICI has previously been demonstrated to have deleterious effects on the male genital tract (56), with loss of germ cells and decrease of fertility. Here, we also observed a significant alteration in sperm count induced by ICI alone (Supplemental Figure 2E). In adult rats and mice, treatment with ICI induced effects similar to those previously observed in the male reproductive tract of Nr3a1 KO mice (56–59). These findings suggest that, following ICI treatment, the decrease in sperm concentration in the cauda epididymis could be explained, at least in part, by the fact that Nr3a1 is required for normal fluid reabsorption, as concluded from studies of Nr3a1 KO males (60, 61). Most interestingly, ICI partially reversed the effect of DES on all the macroscopic testicular abnormalities it induced (Supplemental Figure 1F and Supplemental Figure 2F). Notably, we used only 1 dose of ICI (50×) to compete with DES; perhaps a higher dose would produce even more pronounced competition with DES.
Most of the molecular pathways altered by DES were also found to be affected by EB (e.g., testosterone, retinoid pathway). However, some of our results highlighted differential effects of DES and EB at the dose we used: at P10, the expression of Oct3/4 was changed by DES, not by EB. However, this is an important difference, as Oct3/4 plays an important role in the determination of the pluripotency of cells, which could explain why the effect of DES was more potent compared with EB. Indeed, the altered expression of Oct3/4 by DES might more robustly inhibit germ cell differentiation than that by EB. At the molecular level, Nr5a2 has been described to induce Oct3/4 expression (62). At P10, the expression of Nr5a2 was not affected by DES treatment. This result demonstrated that the effect on Oct3/4 expression at P10 age does not seem to be related to the status of Nr5a2. On the one hand, differences in dosing and relative receptor affinity could contribute to the difference in Oct3/4 expression between DES and EB. Indeed, both compounds have a different affinity to either Nr3a1 or Nr3a2, as DES was shown to bind to Ers with a higher affinity than did estradiol (63, 64). On the other hand, it could be hypothesized that in response to DES, Oct3/4 expression might be regulated by Nr3b1/2/3. DES has previously been demonstrated to activate Nr3b1/2/3 (13), and these receptors positively regulate Oct3/4 expression (14). Even though we did not determine the underlying molecular mechanisms, the effect of DES on Oct3/4 expression was also inhibited in Nr0b2L–/L– mice; therefore, Nr0b2 deficiency might also protect against the nonestrogenic effects of DES.
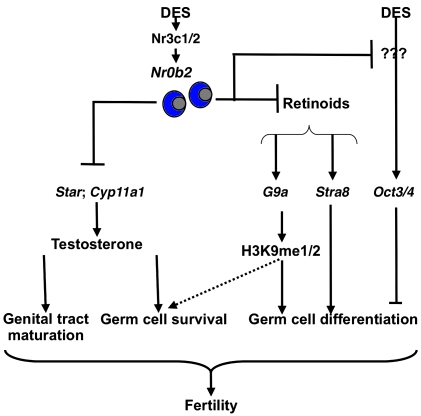
In conclusion, our results demonstrated that Nr0b2 plays a major role in the DES signaling pathway, which affects the development and function of the male reproductive system. We showed that Nr0b2L–/L– male mice were protected against the deleterious effects of DES, as they were still able to reproduce even when exposed to high doses of DES. This is caused by the multiple actions of Nr0b2 during testicular development (Figure 9). First, in neonatal animals, Nr0b2 controls germ cell differentiation through inhibition of the retinoid pathway. Nr0b2 regulates the expression of genes involved in the entry and progression of meiosis, such as Stra8 and Nanos3. It also affects meiosis through regulation of the expression of the histone methyltransferase G9a and the subsequent modification of H3K9 methylation marks. These alterations in methylation upon DES exposure induce abnormal chromosomal complexes favoring germ cell apoptosis and could affect the meiosis process. Next to these effects, which appear to be mediated through the estrogenic pathway, DES seems to inhibit germ cell differentiation at P10 through estrogen-independent pathways, as shown by Oct3/4 deregulation specifically in DES-treated mice. Second, in adult animals, the effect of Nr0b2 was dependent on the inhibition of testosterone production, leading to germ cell death. Together, our present data define Nr0b2 as one of the major actors in the molecular events leading to DES-mediated male infertility.
Figure 9. Proposed model for the role of Nr0b2 in DES-induced testicular abnormalities.
Our results indicate that Nr0b2 is a major actor in DES-induced testicular pathophysiology. Nr0b2 deficiency counteracts the negative effects of DES. In P10 mice, DES induces a blockage in meiosis entry and/or progression, which is characterized by the higher expression of genes of undifferentiated spermatogonia (Nanos3) and a decrease of meiotic genes (Stra8). The effect is stronger in DES-treated males compared with males with 1 EB treatment, which could be explained, at least in part, by the specific induction of Oct3/4 expression by DES. EB and DES treatment alters H3K9me1 and H3K9me2, which are essential for meiosis progression (29). The impact on histones is driven by the lower accumulation of G9a mRNA after DES exposure. This effect on meiosis is explained, at least in part, by the lack of the repressive activity of Nr0b2 on Rar and retinoid signaling. Finally, in adults, Nr0b2 inhibits testicular steroidogenesis, on the one hand by inhibiting the expression of Nr5a2, which controls the expression of the steroidogenic genes, and on the other hand by repressing the transcriptional activity of Nr5a2 and/or Nr5a1. All these data explain how Nr0b2 plays a major role in the subfertility induced by DES exposure.
Methods
Animals.
The Nrb02L–/L– mice used were previously described (8) and maintained on a mixed background (C57BL/6J/129sv). Mouse treatment protocols are detailed in Supplemental Methods. This study was approved by the Institut National de la Santé et de la Recherche Médicale Animal Care Committee.
Histology and immunohistochemistry.
H&E and immunohistochemistry stainings were performed as described previously (27). See Supplemental Methods for details.
Endocrine investigations.
Plasma and intratesticular concentrations were measured as described previously (27). Testosterone level is expressed as a percentage of the vehicle-treated mice for each genotype. See Supplemental Methods for details.
Plasma LH and FSH measurements.
Plasma concentrations of LH and FSH were measured as previously described (65). See Supplemental Methods for details.
Real-time PCR.
Following testis RNA extraction (TRIzol; Invitrogen) and cDNA synthesis (SuperScript II First-Strand Synthesis System; Life Technologies), real-time PCR measurement of individual cDNAs was performed using SYBR green dye to measure duplex DNA formation. Primer sequences are shown in Supplemental Tables 1 and 2. Results were analyzed using the Ct method. Quantitative PCR experiments were performed as previously described (35). See Supplemental Methods for details.
Western blot.
Proteins were extracted using SDS lysis buffer described in the ChIP protocol from Upstate (Upstate Biotechnology Inc). See Supplemental Methods for details.
Transient transfection.
F9 cells (provided by P. Chambon, Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France), were transfected with lipofectamine (Invitrogen). Rar (obtained from C. Rochette-Egly, Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) was transfected with increasing amounts of pCMV-Nr0b2 plasmid (8). The quantity of DNA was maintained constant by the addition of empty pCMV vector. After 24 hours, either 10–6 M RA or vehicle (1:1,000) was added to the cells. Cells were harvested 24 hours later, and mRNA or protein extractions were performed. See Supplemental Methods for details.
TUNEL analysis and Ki-67 staining.
TUNEL and Ki-67 experiments were performed as described previously (27) on 5 μm of testis fixed in 4% PFA. Results are expressed as the number of TUNEL-positive or Ki-67 positive cells per 100 seminiferous tubules. See Supplemental Methods for details.
ChIP.
In vitro and in vivo ChIP experiments were respectively performed from 106 cells, or P10 testis, of 3 mice. ChIP assays were carried out following the protocol provided by the manufacturer (Upstate Biotechnology Inc.). See Supplemental Methods for details.
Promoter analysis.
To analyze the promoter of G9a, we used the Genomatix MatInspector program, which identifies transcription factor binding sites in nucleotide sequences using a large library of weight matrices. The analysis of the mouse G9a promoter sequence predicted a potential binding site (599–623 nt from the transcription initiation site). Sequence identity was CAAGCCATGGGCCACAGGTGAC.
Statistics.
For statistical analysis, 2-way ANOVA was performed. When significant effects of treatment or genotype or their interactions were obtained, multiple comparisons were made with Tukey’s test. All numerical data are mean ± SEM. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, the Centre National de Recherche Scientifique, the Université Louis Pasteur, the Agence Nationale de la Recherche (ANR R06116AA, ANR R07023AA, and ANR R08008AA), the Ecole Polytechnique Fédérale de Lausanne, the Swiss National Science Foundation, the ERC, the NIH, and the Plan National de Recherche en Reproduction et Endocrinologie (to D.H. Volle). The authors also thank Pierre Chambon for the wild-type and Rarg–/– F9 cells; Cécile Rochette-Egly for the Rar expression vector; the members of the Benahmed and Auwerx laboratories for scientific discussions and support; Jean-Marc Lobaccaro, Françoise Senegalas-Balas, and Georges Pointis for critically reading the manuscript; and Geoffroy Marceau for his help on the intratesticular testosterone extractions.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 119:3752–3764 (2009). doi:10.1172/JCI38521.
References
- 1.Goodwin B., et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 2000;6:517–526. doi: 10.1016/S1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 2.Kerr T.A., et al. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev Cell. 2002;2:713–720. doi: 10.1016/S1534-5807(02)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu T.T., et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 2000;6:507–515. doi: 10.1016/S1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., et al. Redundant pathways for negative feedback regulation of bile acid production. Dev. Cell. 2002;2:721–731. doi: 10.1016/S1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 5.Brendel C., Schoonjans K., Botrugno O.A., Treuter E., Auwerx J. The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol. Endocrinol. 2002;16:2065–2076. doi: 10.1210/me.2001-0194. [DOI] [PubMed] [Google Scholar]
- 6.Johansson L., et al. The orphan nuclear receptor SHP inhibits agonist-dependent transcriptional activity of estrogen receptors ERalpha and ERbeta. J. Biol. Chem. 1999;274:345–353. doi: 10.1074/jbc.274.1.345. [DOI] [PubMed] [Google Scholar]
- 7.Lu T.T., Repa J.J., Mangelsdorf D.J. Orphan nuclear receptors as eLiXiRs and FiXeRs of sterol metabolism. J. Biol. Chem. 2001;276:37735–37738. doi: 10.1074/jbc.R100035200. [DOI] [PubMed] [Google Scholar]
- 8.Volle D.H., et al. The small heterodimer partner is a gonadal gatekeeper of sexual maturation in male mice. Genes Dev. 2007;21:303–315. doi: 10.1101/gad.409307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toppari J. Environmental endocrine disrupters. Sex Dev. 2008;2:260–267. doi: 10.1159/000152042. [DOI] [PubMed] [Google Scholar]
- 10.Bullock B.C., Newbold R.R., McLachlan J.A. Lesions of testis and epididymis associated with prenatal diethylstilbestrol exposure. Environ. Health Perspect. 1988;77:29–31. doi: 10.2307/3430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greschik H., Flaig R., Renaud J.P., Moras D. Structural basis for the deactivation of the estrogen-related receptor gamma by diethylstilbestrol or 4-hydroxytamoxifen and determinants of selectivity. J. Biol. Chem. 2004;279:33639–33646. doi: 10.1074/jbc.M402195200. [DOI] [PubMed] [Google Scholar]
- 12.Greschik H., et al. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol. Cell. 2002;9:303–313. doi: 10.1016/S1097-2765(02)00444-6. [DOI] [PubMed] [Google Scholar]
- 13.Nam K., Marshall P., Wolf R.M., Cornell W. Simulation of the different biological activities of diethylstilbestrol (DES) on estrogen receptor alpha and estrogen-related receptor gamma. Biopolymers. 2003;68:130–138. doi: 10.1002/bip.10307. [DOI] [PubMed] [Google Scholar]
- 14.Tremblay G.B., et al. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev. 2001;15:833–838. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cederroth C.R., et al. Estrogen receptor alpha is a major contributor to estrogen-mediated fetal testis dysgenesis and cryptorchidism. Endocrinology. 2007;148:5507–5519. doi: 10.1210/en.2007-0689. [DOI] [PubMed] [Google Scholar]
- 16.Prins G.S., et al. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- 17.Rivas A., et al. Neonatal coadministration of testosterone with diethylstilbestrol prevents diethylstilbestrol induction of most reproductive tract abnormalities in male rats. J. Androl. 2003;24:557–567. doi: 10.1002/j.1939-4640.2003.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 18.Singh J., Handelsman D.J. Morphometric studies of neonatal estrogen imprinting in the mature mouse prostate. J. Endocrinol. 1999;162:39–48. doi: 10.1677/joe.0.1620039. [DOI] [PubMed] [Google Scholar]
- 19.Ofner P., Bosland M.C., Vena R.L. Differential effects of diethylstilbestrol and estradiol-17 beta in combination with testosterone on rat prostate lobes. Toxicol. Appl. Pharmacol. 1992;112:300–309. doi: 10.1016/0041-008X(92)90200-C. [DOI] [PubMed] [Google Scholar]
- 20.Lai K., Harnish D.C., Evans M.J. Estrogen receptor alpha regulates expression of the orphan receptor small heterodimer partner. J. Biol. Chem. 2003;278:36418–36429. doi: 10.1074/jbc.M303913200. [DOI] [PubMed] [Google Scholar]
- 21.Sanyal S., et al. Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J. Biol. Chem. 2002;277:1739–1748. doi: 10.1074/jbc.M106140200. [DOI] [PubMed] [Google Scholar]
- 22.Johansson L., et al. The orphan nuclear receptor SHP utilizes conserved LXXLL-related motifs for interactions with ligand-activated estrogen receptors. Mol. Cell. Biol. 2000;20:1124–1133. doi: 10.1128/MCB.20.4.1124-1133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinge C.M., Jernigan S.C., Risinger K.E. The agonist activity of tamoxifen is inhibited by the short heterodimer partner orphan nuclear receptor in human endometrial cancer cells. Endocrinology. 2002;143:853–867. doi: 10.1210/en.143.3.853. [DOI] [PubMed] [Google Scholar]
- 24.Shigeta H., Zuo W., Yang N., DiAugustine R., Teng C.T. The mouse estrogen receptor-related orphan receptor alpha 1: molecular cloning and estrogen responsiveness. J. Mol. Endocrinol. 1997;19:299–309. doi: 10.1677/jme.0.0190299. [DOI] [PubMed] [Google Scholar]
- 25.Maire M., et al. Alteration of transforming growth factor-beta signaling system expression in adult rat germ cells with a chronic apoptotic cell death process after fetal androgen disruption. Endocrinology. 2005;146:5135–5143. doi: 10.1210/en.2005-0592. [DOI] [PubMed] [Google Scholar]
- 26.El Chami N., et al. Androgen-dependent apoptosis in male germ cells is regulated through the proto-oncoprotein Cbl. J. Cell Biol. 2005;171:651–661. doi: 10.1083/jcb.200507076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volle D.H., et al. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol. Endocrinol. 2007;21:1014–1027. doi: 10.1210/me.2006-0277. [DOI] [PubMed] [Google Scholar]
- 28.Goyal H.O., et al. Neonatal estrogen exposure of male rats alters reproductive functions at adulthood. Biol. Reprod. 2003;68:2081–2091. doi: 10.1095/biolreprod.102.010637. [DOI] [PubMed] [Google Scholar]
- 29.Guyot R., et al. Diethylstilbestrol inhibits the expression of the steroidogenic acute regulatory protein in mouse fetal testis. Mol. Cell. Endocrinol. 2004;220:67–75. doi: 10.1016/j.mce.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Bowles J., et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 31.Bowles J., Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007;134:3401–3411. doi: 10.1242/dev.001107. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana M., Nozaki M., Takeda N., Shinkai Y. Functional dynamics of H3K9 methylation during meiotic prophase progression. EMBO J. 2007;26:3346–3359. doi: 10.1038/sj.emboj.7601767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters A.H., et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell. 2003;12:1577–1589. doi: 10.1016/S1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 34.Tachibana M., et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikegami K., et al. Genome-wide and locus-specific DNA hypomethylation in G9a deficient mouse embryonic stem cells. Genes Cells. 2007;12:1–11. doi: 10.1111/j.1365-2443.2006.01029.x. [DOI] [PubMed] [Google Scholar]
- 36.Koubova J., et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rochette-Egly C., Chambon P. F9 embryocarcinoma cells: a cell autonomous model to study the functional selectivity of RARs and RXRs in retinoid signaling. Histol. Histopathol. 2001;16:909–922. doi: 10.14670/HH-16.909. [DOI] [PubMed] [Google Scholar]
- 38.Boylan J.F., Lohnes D., Taneja R., Chambon P., Gudas L.J. Loss of retinoic acid receptor gamma function in F9 cells by gene disruption results in aberrant Hoxa-1 expression and differentiation upon retinoic acid treatment. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9601–9605. doi: 10.1073/pnas.90.20.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delbes G., Levacher C., Habert R. Estrogen effects on fetal and neonatal testicular development. Reproduction. 2006;132:527–538. doi: 10.1530/rep.1.01231. [DOI] [PubMed] [Google Scholar]
- 40.Olesen I.A., et al. Environment, testicular dysgenesis and carcinoma in situ testis. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:462–478. doi: 10.1016/j.beem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Sikka S.C., Wang R. Endocrine disruptors and estrogenic effects on male reproductive axis. Asian J. Androl. 2008;10:134–145. doi: 10.1111/j.1745-7262.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 42.Sharpe R.M., Skakkebaek N.E. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89:e33–38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 43.Skakkebaek N.E., Rajpert-De Meyts E., Main K.M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 44.Jeffs B., et al. Blockage of the rete testis and efferent ductules by ectopic Sertoli and Leydig cells causes infertility in Dax1-deficient male mice. Endocrinology. 2001;142:4486–4495. doi: 10.1210/en.142.10.4486. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z.J., et al. Aromatase (Cyp19) expression is up-regulated by targeted disruption of Dax1. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7988–7993. doi: 10.1073/pnas.141543298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delbes G., et al. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor alpha. Endocrinology. 2005;146:2454–2461. doi: 10.1210/en.2004-1540. [DOI] [PubMed] [Google Scholar]
- 47.Houk C.P., Pearson E.J., Martinelle N., Donahoe P.K., Teixeira J. Feedback inhibition of steroidogenic acute regulatory protein expression in vitro and in vivo by androgens. Endocrinology. 2004;145:1269–1275. doi: 10.1210/en.2003-1046. [DOI] [PubMed] [Google Scholar]
- 48.Manna P.R., Wang X.J., Stocco D.M. Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids. 2003;68:1125–1134. doi: 10.1016/j.steroids.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Can A., Semiz O. Diethylstilbestrol (DES)-induced cell cycle delay and meiotic spindle disruption in mouse oocytes during in-vitro maturation. Mol. Hum. Reprod. 2000;6:154–162. doi: 10.1093/molehr/6.2.154. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein P. Nuclear aberrations and loss of synaptonemal complexes in response to diethylstilbestrol (DES) in Caenorhabditis elegans hermaphrodites. Mutat. Res. 1986;174:99–107. doi: 10.1016/0165-7992(86)90098-9. [DOI] [PubMed] [Google Scholar]
- 51.Matsui Y., Hayashi K. Epigenetic regulation for the induction of meiosis. Cell. Mol. Life Sci. 2007;64:257–262. doi: 10.1007/s00018-006-6281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters A.H., et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/S0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 53.Boulias K., Talianidis I. Functional role of G9a-induced histone methylation in small heterodimer partner-mediated transcriptional repression. Nucleic Acids Res. 2004;32:6096–6103. doi: 10.1093/nar/gkh947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang S., et al. Coordinated recruitment of histone methyltransferase G9a and other chromatin-modifying enzymes in SHP-mediated regulation of hepatic bile acid metabolism. Mol. Cell. Biol. 2007;27:1407–1424. doi: 10.1128/MCB.00944-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feldman N., et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat. Cell Biol. 2006;8:188–194. doi: 10.1038/ncb1353. [DOI] [PubMed] [Google Scholar]
- 56.Cho H.W., et al. The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy. Reprod. Biol. Endocrinol. 2003;1:57. doi: 10.1186/1477-7827-1-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K.H., et al. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol. Reprod. 2000;63:1873–1880. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira C.A., Carnes K., Franca L.R., Hess R.A. Infertility and testicular atrophy in the antiestrogen-treated adult male rat. Biol. Reprod. 2001;65:913–920. doi: 10.1095/biolreprod65.3.913. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira C.A., et al. ER function in the adult male rat: short- and long-term effects of the antiestrogen ICI 182,780 on the testis and efferent ductules, without changes in testosterone. Endocrinology. 2002;143:2399–2409. doi: 10.1210/en.143.6.2399. [DOI] [PubMed] [Google Scholar]
- 60.Hess R.A., et al. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Q., et al. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu P., et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol. Cell Biol. 2005;25:3492–3505. doi: 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuiper G.G., et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/en.138.3.863. [DOI] [PubMed] [Google Scholar]
- 64.Petersen D.N., Tkalcevic G.T., Koza-Taylor P.H., Turi T.G., Brown T.A. Identification of estrogen receptor beta2, a functional variant of estrogen receptor beta expressed in normal rat tissues. Endocrinology. 1998;139:1082–1092. doi: 10.1210/en.139.3.1082. [DOI] [PubMed] [Google Scholar]
- 65.McNeilly J.R., et al. Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology. 2000;141:4284–4294. doi: 10.1210/endo.141.11.7764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.