Synopsis
Pulmonary arterial hypertension remains a vexing clinical disease with no cure. Despite advances and the discovery of a gene (BMPR2) associated with many of the hereditary forms of the disease, and some cases not previously known to be inherited, the reasons for mutations in this gene as a cause remain somewhat elusive. Clearly, a complex interplay exists between genetic alterations, environmental exposures (including infections) and disease development. This article addresses the advances in the genetics of PAH, including the identification of genetic etiologies and modulators, and the role of genetics in predicting disease progression and targeting therapeutics.
Keywords: Pulmonary Hypertension, Genetics, Genomics, Gene expression, Microarray
Introduction
The first descriptions of pulmonary arterial hypertension have fascinated clinicians and scientists since these brief initial descriptions. Despite significant advances in the genetic determinations for disease risk, PAH remains an elusive disease in terms of defined pathogenesis and targeted therapies. This article focuses on historical perspectives of the pathophysiology and the vasodilatory components, leading to the three approved classes of medication for treatment. Furthermore, insight into the genetic ideology of selected cases and the pathobiology surrounding more mechanistic investigations are offered. The influences of environment and environmental exposures are considered in the context of the potential for genetic by environmental interactions in terms of etiology. Future considerations involve ongoing approaches to utilize targeted therapies in the treatment of this disease. Moreover, the utilization of genomic technologies for the discovery of meaningful biomarkers is underway.
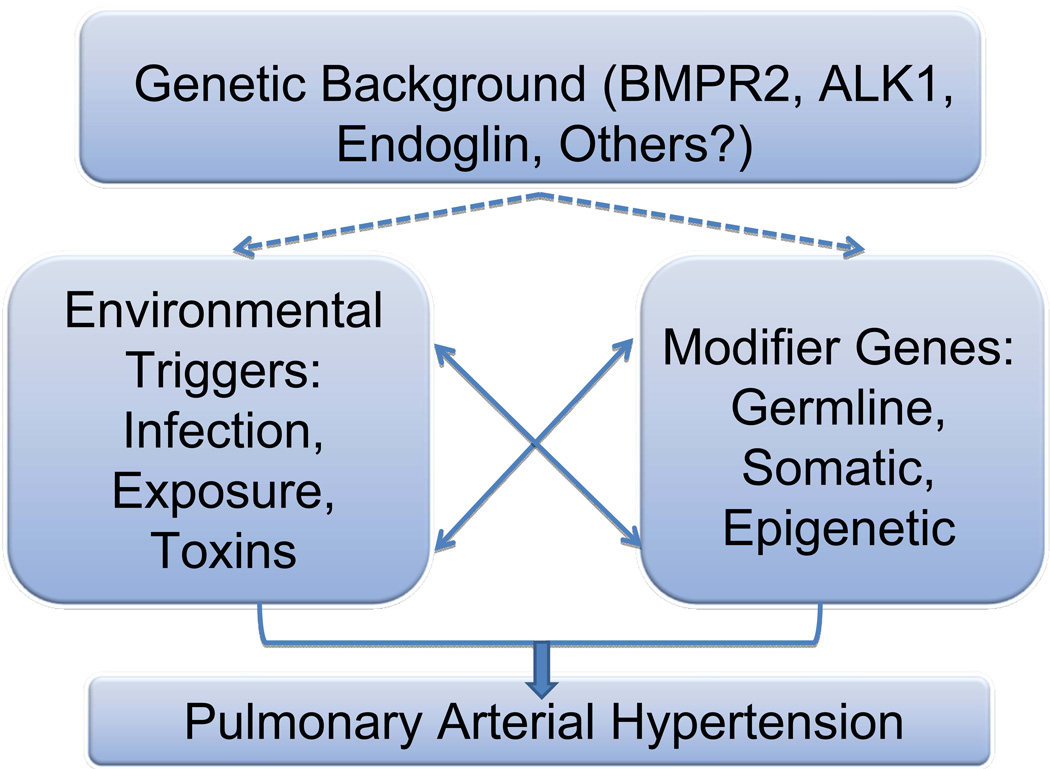
Many investigators now subscribe to a “multiple hit” hypothesis for the causes of pulmonary arterial hypertension (PAH). This theory subscribes that, in a given genetic background, selected patients may be more prone to the development of disease. Other distinct influences on disease acquisition, such as exposure to certain drugs or infections, may alter the incidence of disease. Furthermore, modifier genes may alter the disease incidence / development. These gene modifiers could be either germline, somatic, or epigenetic. Yhe general outline for this hypothesis is illustrated in Figure 1.
Figure 1. “Multiple Hit” Hypothesis for the Development of Pulmonary Hypertension.
In this model, individuals may possess a genetic background that could predispose to the development of pulmonary hypertension. Since there is incomplete penetrance, even for heritable cases of PAH, other modifier events are highly likely to be causal, and can include environmental triggers such as exposures or infections, or the co-existence of modifier genes, either germline, somatic or epigenetic modifications. The interplay between genes and environment will undoubtedly prove extremely important in the definition of the etiology of the disease.
Vasoactive Pathways
Insight into the pathogenesis and, importantly, initial treatments of pulmonary artery hypertension come from the sentinel works of Vane and Moncada by elucidating the vasodilatory potency of prostacyclin and its analogs, initial work into the potential for treatment was begun (1). One of the first utilization for the use of prostacyclin occurred in the persistent fetal circulation syndrome (2). Utilization in the adult was reported in the Lancet in 1980 (3). Investigations into the acute hemodynamic of infused prostacyclin were reported in 1982 (4). Of note, from an early point in time, selected vasodilation of the pulmonary vascular bed was not a universal finding in a minority of patients exhibited vasodilation. Dr. Higenbottam and others were the first to report on the long term success treatment of primary pulmonary hypertension with continuous intravenous epoprostenol (prostacyclin) in a landmark article in Lancet in 1984 and subsequently the neonatal forms of this disease were treated in a similar fashion (5). Deficiencies in prostacyclin began to be reported first in animal models of the disease. Badesch et al, described deficiencies in prostacyclin production in the neonatal calf model of pulmonary hypertension (6). It was not until a decade that deficiencies of the enzyme prostacyclin synthase were determined to be present in the human condition of pulmonary arterial hypertension (7). Therefore, a common thread surrounding the initial treatment of pulmonary arterial hypertension were to engage pharmacological interventions directed primarily at vasodilatory responses, albeit further analysis revealed the importance of antiproliferative properties. By describing alterations in physiologic levels, supplementation with pharmacologic compounds became the mainstay of treatment approaches and currently provides the three major classifications of treatment options for this disorder: prostaglandins, nitric oxide agonists, and endothelin receptor blockers.
Endothelin
Unlike prostacyclin, targeting the endothelin-1 pathway proceeded historically from basic science discovery, preclinical animal models, human translational studies, and finally human clinical trials. Endothelin-1 (ET-1) is a peptide which is produced and endogenously by predominantly vascular endothelial cells. This peptide represents one of the most potent vasoconstrictors and smooth muscle cell mitogens described (8, 9). Human studies demonstrated that Endothelin-1 expression was elevated in plasma and lung tissue of patients with PAH (10). Furthermore, the magnitude of elevation of ET-1 was shown to correlate with disease severity (11). Pre-clinical animal modeling demonstrated that blocking, in a non-selective fashion, both Endothelin-A and Endothelin-B receptors successfully prevented disease in a rat model (12). A separate model of rat pulmonary hypertension using monocrotaline demonstrates profound vascular remodeling in rats. The effectiveness of Endothelin receptor blockade was demonstrated in this pre-clinical model as well (12). Utility for the utilization of Endothelin receptor blockade in neonatal diseases was demonstrated by the effectiveness of Endothelin receptor blockade in ovine fetal disease (13). Once safety and efficacy were defined in human dosing, effectiveness in the treatment of the disease was demonstrated (14). Indeed, a randomized double-blind placebo-controlled trial was performed demonstrating the effectiveness of non-selective ET-A and ET-B receptors with the drug Bosentan (15). Novel selective ET-A inhibitors are currently in clinical trials and utilization and the potential for combinatorial therapy is great and will be discussed later in the review.
Nitric Oxide
Nitric Oxide (NO) is a free radical gas that can diffuse away from the site of production in endothelial cells to various targets. The nitric oxide system represents one of the potent vasodilator compounds for the pulmonary vascular endothelium. The NO system has been an indirect target for molecular pharmacology in the treatment of pulmonary hypertension. Investigative work into the role of the NO system in pulmonary vascular disease parallels, in many ways, investigations for the other vasoactive pathways including prostacyclins and endothelins. The supply of NO is tightly regulated by the interplay of three synthetic enzymes and the synthesis of NO is through the oxidation of L-Argenine by the NO synthase family. This family represents three isozymes with overlapping patterns of expressions. Germaine to the pulmonary vascular bed NOS-3 Nitric Oxide Synthase-3 (Endothelial NOS) is expressed in vascular endothelial cells throughout the body. Through a series of animal modeling investigations, the role of each NOS isozyme has been examined regarding the pulmonary vascular bed by Fagan et al (16). Through this examination of targeted disruption of each of the NOS enzymes, a conclusion reached is that NOS-3 may be the major NO synthetic enzyme relevant to the pulmonary vascular bed. NO synthase has been found to be deficient in patients with pulmonary hypertension in a landmark article (17). The ability to modulate the NO system can occur either by inhaled nitric oxide gas directly, as is prevalently used for diagnostic catheterizations, and in the treatment of patients with acute lung injury and ARDS. In 1999, the FDA approved inhaled NO therapy for term and near-term infants with hypoxic respiratory failure and pulmonary hypertension. Long-term treatment with inhaled NO is not a viable option, however. The NO pathway and downstream levels of NO are impacted by signaling through the cyclic GMP classical pathway. Reaction of NO with the SGC-heme (in the ferrous state) induces a conformational change at the catalytic site leading to several hundred-fold increased production of cyclic GMP from GTP.
The cyclic GMP signal is limited primarily by degradation of this cyclic nucleotide by phosphodiesterases. Several phosphodiesterases (PDEs) degrade both cyclic AMP and cyclic GMP. In particular, phosphodiesterase-5 is a specific phosphodiesterase for cyclic GMP. Inhibition of PDE-5 can occur through selective pharmacologic inhibition and in particular the drug Sildenafil. Inhibitors of PDE-5 such as Sildenafil augment NO/cyclic GMP mediated vascular relaxation by preventing the breakdown of cyclic GMP to GMP. Sildenafil has been shown to cause dose-dependent reductions in pulmonary vascular resistance in animal models of acute pulmonary hypertension without significant changes in systemic vascular resistance (18). This effect has also been translated into human clinical trials (19). A large randomized controlled trial of patients with idiopathic PAH demonstrated the effectiveness of Sildenafil in the treatment of pulmonary arterial hypertension (20).
Insights from Genetics and Immunogenetics
A number of lines of investigation have been performed leading to significant insight into the pathogenesis of PAH. Historically, some of the earliest immunogenetic association studies have resulted from the autoantibody correlations with the Human Leukocyte Antigen (HLA) class II alleles. HLA class II alleles encoded within the Major Histocompatibility Complex (MHC) appear to play important roles in selected autoimmune diseases. Indeed, many autoimmune diseases are associated with increased frequencies of HLA DR, DQ, or DP. HLA genotyping can be performed by the utilization of either direct DNA sequencing or the use of sequence specific probes. These HLA susceptibility genes, in many cases, are permissive for the disease state and are subject to additional genetic and environmental modifying factors. HLA DR-II is found more frequently in patients with scleroderma and pulmonary hypertension (21). In a similar fashion, HLA DR3 and HLA-DR 52 are found more frequently in children with PAH (22).
For patients with Idiopathic Arterial Hypertension, both in children and adults, HLA-DRQ7 was shown to be more frequently occurring allele (23). While these associations prove to be intriguing, they represent susceptibility loci and are not sufficient to cause disease. Indeed, a separate genetic or environmental insult appears to be required for the disease to become manifest when these alleles are present within individuals. The relative risk of disease for an individual harboring these alleles has yet to be defined.
The historical observations of heritability in some families with pulmonary hypertension ultimately led researchers to the discovery of a single gene locus, mutated in nearly all familial cases of PAH. Initially, vertical transmission of PAH within some families over many generations led to the speculation of a single dominant gene as the cause in these families. The transmission patterns were compatible with an autosomal dominant disorder, but the analysis was complicated by the finding of incomplete penetrance (not all those inheriting the mutated allele are stricken with the disease) and an increased prevalence in women with a variable age of onset (24). Researchers at Vanderbilt University described the finding that, very often, subsequent generations of patients with familial PAH develop the disease at younger ages in each succeeding generation – a phenomenon known as genetic anticipation (25). The initial attempts to map the disease causing genetic locus were undertaken using microsatellite markers spread across defined intervals of the human genome. Using this methodology, two independent groups were able to localize the familial PAH locus with a high and definitive logarithm of odds (LOD) scores mapping to a rather broad region of chromosome 2q31 (26, 27). In 2000, researchers from both Columbia University and Vanderbilt University reported that a mutation in the Transforming Growth Factor β Receptor (TGF-β) Superfamily member was strongly associated with familial PAH (28, 29). Heterozygous germline mutations of bone morphogenetic protein receptor 2 (BMPR2) (a component of the TGF-β-related family) is found to underlie up to 60% of cases of familial IPAH (30, 31).
More recently, the investigation of other signaling molecules within the BMPR2 pathway have led investigators to discover other mutations associated with PAH. Patients with hereditary hemorrhagic telangiectasia (HHT, previously known as Osler-Weber-Rendu), can exhibit pulmonary hypertension. In these cases, coding changes in activin-receptor-like kinase 1 (ALK1) are demonstrated to be associated with disease (32). Further functional analysis of ALK1 in these kindreds demonstrated that these mutations are the cause of pulmonary hypertension in patients with HHT (33). Endoglin, an accessory TGF-β receptor, highly expressed during angiogenesis, is essential for ALK1 signaling. Mutations in endoglin have also been reported in patients with PAH (34).
The Proliferative / Neoplastic Paradigm
The prevailing concept is the one which dysfunctional endothelial cells plays the key pathobiological role in PH (35). However, to the present day, the pathogenesis of intimal vascular lesions remains mostly undetermined. Plexiform lesions, typically located in branching pointes of muscular arteries(36), consists of a network of vascular channels lined up by endothelial cells (37) and a core of myofibroblastic or less well-differentiated cells (38). In our experience, these lesions are characteristically found in cases of severe PH, including IPAH, and PH associated with HIV infection, liver cirrhosis, CREST, congenital heart malformations, and schistosomiasis.
We have proposed that plexiform lesions represent a process of misguided angiogenesis based on the findings of expression of vascular endothelial growth factor (VEGF), its receptors 1 (flt) and 2(kdr), and hypoxia inducible factor (HIF)-1α and β (39). The finding of monoclonality of endothelial cells in plexiform lesions in IPAH, but not in similar lesions in PH associated with congenital heart malformation, suggests that these lesions might arise from mutations in tumor suppressor genes (40). Somatic loss of expression of transforming growth factor β receptor 2 ((TGFβ-R2) and the propapoptotic Bax, potentially due to microsatellite instability, is also documented in IPAH plexiform lesions (41). BMPR2 expression is documented in plexiform lesions (42) and in remodeled pulmonary arteries in IPAH lungs, predominantly in endothelial cells. However, our studies reveal that the cells in the central core of plexiform lesions lack the expression of TGF-β receptor 2, TGF-β receptor 1 and their signaling Smad(s) 2,1 (which shares common epitopes with Smads 5 and 8), 3 and 4, including the phosphorylated Smad 1/5/8 and 2. The absence of expression of these phosphorylated Smads is the best indication that there is no TGF-β (via Smad 2 or Smads 1/5/8) or BMP (via Smads 1/5/8) signaling in these cells (43). Loss of cytostatic signaling from TGF-β would allow the plexiform cells to abnormally proliferate. Notwithstanding the evidence of preserved BMPR expression and signaling in IPAH endothelial cells, recent studies indicate that BMP signaling protects against endothelial cell apoptosis in vitro (44); the loss of this protection by germline mutations in BMPR2 would thus favor enhanced susceptibility to apoptosis of lung endothelial cells.
VEGF (39), endothelin-1 (45), and survivin (46, 47) are among the factors present in plexiform lesions that may enhance endothelial cell and smooth muscle cell proliferation or decrease vascular cell apoptosis. These lesions have decreased expression of anti-remodeling mediators such as nitric oxide synthase (48) and prostacyclin synthase (49), and tumor suppressors, such as caveolin-1(50). These phenotypic characteristics lead us to propose that the plexiform lesions have characteristics in common with neoplasms (51). As discussed below, there is emerging evidence that viral factors may play a role in endothelial cell proliferative lesions in severe PH.
Medial smooth muscle cell hypertrophy is a characteristic pathological feature of PH that involves muscularized arteries (ranging between 70 and 500 µm in diameter), and precapillary vessels (below 70 µm in diameter). The medial smooth muscle cell layer represents approximately 10–15% of the outside diameter of normal muscularized pulmonary arteries, while it approaches 30–60% of the outside diameter in vessels of IPAH lungs (52–55). Although careful morphometric assessments of medial remodeling have not been carried out in non-IPAH PH, it is apparent that medial thickening occurs in mild/moderate or severe PH and in cases of normal individuals exposed to cigarette smoke with no evidence of PH (56). This diagnostic limitation of the finding of medial hypertrophy has led us to propose that the identification of pulmonary medial remodeling warrants additional clinical evidence of the presence of potential PH (57).
The mechanisms underlying the thickening of the pulmonary vascular medial layer have been linked mostly to cell proliferation and, more recently, to inhibition of cell apoptosis. The identification of mutations in BMPR2 led to several in vitro studies aimed at relating smooth muscle cell proliferation to abnormal BMPR2 signaling. IPAH smooth muscle cells isolated from proximal arteries (i.e., elastic vessels, >500 µm in diameter) exhibit decreased inhibitory effect on cell proliferation mediated by TGF-β1 or BMP-4 when compared with smooth muscle cells isolated from normal human pulmonary arteries (58). Of note, these altered responses are not due to abnormal expression of Smad signaling or ligand binding to its receptors in IPAH smooth muscle cells. Similar studies were extended to more peripheral smooth muscle cells (i.e., from arteries less than 2 mm in diameter-(that are still elastic arteries) obtained from normal pulmonary arteries, which undergo cell proliferation and protection against apoptosis when exposed to BMP-4, a ligand for BMPR2. Therefore, loss of function mutations in MPR2 would be predicted to cause smooth muscle cell arrest and increased cell death (59), i.e., paradoxically opposite to that predicted to occur in familial IPAH in which mutations would facilitate smooth muscle cell growth and remodeling. Given these somewhat discrepant results (59), the evidence of reduced expression of BMPR2 found in IPAH alveolar septa (42), and the finding of decreased levels of the activated form of Smad 1 (the signaling Smad for BMPR2) in smooth muscle cells of muscular pulmonary arteries in IPAH, it remains unclear how BMPR2 mutations either can cause or trigger the disease. Indeed, when compared with wild type mice, heterozygous mice lacking a single copy of BMPR2 have no pulmonary vascular phenotype at baseline or under chronic hypoxia (60), but show moderately increased pulmonary artery pressures and minimal remodeling when stressed with intratracheal delivery of a 5-lipoxygenase expressing vector (60).
The role of serotonin (5-hydroxytryptamine) in the growth stimulation of pulmonary artery smooth muscle cells has been intensively studied as a mechanism of medial remodeling in PH and a potential modifier gene to the familial IPAH. Serotonin is internalized in smooth muscle cells after binding to serotonin transporter (5-HTT) or to its receptors 5-HT1B-R, 5-HT-2A R, 5-HT7-R, and 5-HT2B -R, when it promotes vasoconstriction, cell growth, and enhancement of hypoxia-induced remodeling and PH (61). Vascular smooth muscle cells in IPAH lungs express higher levels of 5-HTT (62) or 5-HT2B R (61) by immunohistochemistry, and undergo enhanced cell proliferation when treated in vitro with serotonin compared with normal smooth muscle cells (62). These findings are supported by the report that mice deficient in 5HTT or 5-HT-2B are protected against PH caused by hypoxia alone (61) or hypoxia combined with the anorexigen dexfenfluramine (63), respectively. Of interest is the observation that increased serotonin levels affect signaling pathways downstream of mutated BMPR2, as serotonin infusion enhances normoxic and hypoxic pulmonary pressures and vascular remodeling in BMPR2 heterozygous mice as compared with wild type mice (59).
Despite evidence supporting a loss of function for the TGF-β family signaling, particularly in endothelial cells of IPAH, TGF-β may produce gain-of-function alterations underlying medial smooth muscle cell growth and adventitial fibroblast activation. TGF-β isoforms 1, -2, and -3 are expressed in hypertensive pulmonary arteries (64), and could signal via activation of Smad 2 or 3 by serine phosphorylation, whose expression was documented in pulmonary vascular smooth muscle cells (43). Recent evidence implicates PDGF in the pathogenesis of both monocrotaline- and chronic hypoxia-induced PH (65). Monocrotaline treatment or chronic hypoxia exposure leads to increased PDGF receptor (PDGFR) -β expression and phosphorylation and activation of ERK Map kinase in rats. The findings of positive response of hypertensive animals to Gleevac™, also an inhibitor of PDGFR signaling, and the evidence of increased expression of PDGFR-β and phosphorylated PDGFR-β in IPAH lungs, led the authors to translate their finding by treating an IPAH patient with Gleevac™, with promising early results (66).
It is becoming clear that the ultimate fate of vascular smooth muscle cells in PH is determined by their resistance to apoptosis. In fact, “apoptosis-resistance” might play a central role in both the endothelial- and smooth muscle cell-based pulmonary vascular lesions (67), since IPAH lungs have lower number of apoptotic cells than normal or emphysematous lungs (68). As growth signals originated by PDGF, TGF-β, EGF, serotonin, and extracellular matrix proteins are interrupted in animal models of PH, pulmonary arteries undergo de-remodelling associated with apoptosis of pulmonary artery smooth muscle cells (69). Targeting apoptosis of the hypertrophic smooth muscle cells might represent a more viable approach towards treatment.
Recent studies of K+ channel activity have provided a novel insight of the lack of proapoptotic signals in IPAH smooth muscle cells. An exciting and complementary paradigm based on the interplay between K+ channels and apoptosis in pulmonary artery smooth muscle cells has emerged based on the demonstration of activation of K+ channels causes cytochrome C release from the mitochondria and water efflux from the dying cells (70). Conversely, inhibition of K+ channels causes cell depolarization, enhances contractility, and decreases apoptosis of pulmonary artery smooth muscle cells. One potential mechanism linking this paradigm to BMPR2 mutations is the finding that BMPR2 activation upregulates K+ channels (71) and causes apoptosis of normal pulmonary artery smooth muscle cells, but not of cells from patients with IPAH (72). McMurtry et al provide recent additional evidence that mechanisms akin to cancer operate in pulmonary vascular remodelling (47). Survivin protects cancer cell against apoptosis by inhibiting caspase activation and apoptosis inducing factor (73). Not only do pulmonary artery smooth muscle cells in IPAH lungs express higher levels of the antiapoptotic survivin, but monocrotaline-induced pulmonary vascular remodelling requires survivin expression. Indeed, transduction of a functionally deficient survivin in monocrotaline-treated lungs prevented pulmonary vascular remodelling and, when administered after monocrotaline treatment, it enhances apoptosis of pulmonary artery smooth muscle cells and reduces pulmonary artery pressures. This mutant survivin increases levels of K+ channel activity and leads to depolarization of mitochondria with enhanced cytochrome C release.
Influence of Exposures and Infections on PAH
The development of PAH is associated with exposure to several classes of compounds. However, a common mechanism of action for each compound is the property of central nervous system stimulation. Reports of PAH are associated with the use of cocaine (74). Importantly, in the 1960’s there were reports of an epidemic of primary pulmonary hypertension associated with the use of a particular anorexigen, aminorex fumarate (75). In the early 1990s, a group of French investigators reported a clustering of PAH in patients using fenfluramine (76). Dexfenfluramine is the main ingredient in this drug, used to treat obesity. Subsequently, a 23-fold increased risk of the development of PAH was reported in persons using this anorexigen for more than 3 months duration (77).
Viral infection may disrupt normal immunoregulatory and homeostatic cellular pathways, which result in endothelial or smooth muscle cell injury and activate inflammation. Most of the pathways involved in virus pathogenesis converge on either pro-survival or pro-angiogenic signals, the same signals associated with severe PH.
The important role of inflammation is further highlighted in cases of PH where a viral etiology can be identified. For HIV-related pulmonary hypertension, the first clinical report of an association between infection and the development of lesions appeared in 1987 (78), followed by other reports (79–81) but with no evidence for the presence of the virus in PH vascular lesions. As PH is frequently diagnosed when it is advanced, the incidence of PH in HIV-infected patients is likely underestimated, although a recent report demonstrates a high prevalence of PH in HIV-infected children (82). BMPR2 mutations are not required for severe PH to occur in HIV-infected patients, yet the vascular lesions in the lungs from HIV-infected (80) patients are identical to those with familial PH and sporadic IPAH with BMPR2 mutations. Some studies showed no correlation between viral load and right heart changes (83, 84). However, a case report (85) and a recent unpublished observation showed that viral load control with HARRT therapy can be associated with an improved clinical outcome. Furthermore, Bosentan, an endothelin receptor antagonist has been successfully used in some HIV-PH patients (86), suggesting that shear stress contributes to the disease independently of the viral load.
In the lung, HIV-1 primarily infects macrophages providing a potential reservoir not only for the transmission of the virus to circulating T-cells, but also a source for localized viral proteins such as Nef, Tat, and gp120, all of which may have direct effects on innocent by-stander cells. The chronic exposure to viral products in the lung, a deficiency in regulatory T cells, and an altered production of chemokines/cytokines, may all contribute to pulmonary vascular dysfunction, with endothelial cells being particularly sensitive target.
The HIV Nef (for negative factor) protein is found in plexiform lesions of macaques infected with a chimeric virus containing the simian immunodeficiency virus (SIV) backbone with the human immunodeficiency virus Nef (in place of SIV Nef) (87). Nef is also present in endothelial cells of HIV infected patients with PH. These recent studies suggest that the viral protein may exert direct effects on cells not necessarily permissive for viral replication. Foci of mononuclear cells and ectopic lymphoid tissues characteristically found in regions adjacent to the lesions may be sources of this viral protein.
The Nef protein appears to be dispensable for viral replication in vitro, but is a critical virulence factor for pathogenesis and maintenance of high viral loads in vivo (88, 89). Nef is an N-terminus-myristoylated protein with a relative molecular mass of 27 kDa is found associated with cellular membranes and the cytoskeleton (90). Myristoylation is essential for almost all the functions ascribed to Nef, including membrane localization within lipid raft microdomains. The localization and adaptor functions recruit signaling proteins to discrete regions in the membrane and affect T cell signaling pathways (91). Proteins associated with a survival and pro-angiogenic phenotype in severe PH such as PI-3 kinase, MAP kinases, and a p21 kinase-2 are all recruited to the rafts by Nef (92–95). In human monocyte-derived macrophages (MDMs), Nef activates the STAT1 pathway and the secretion of MIP-1, IL-1-α, IL-6, and TNF-α (96). Extracellular Nef found in HIV patients (approximately 10 ng/ml) enters the vascular endothelium in vivo via CXCR4 (97). Finally, Nef can be proapoptotic or pro-survival, depending on the context of expression and the particular cell type (98). Thus, localization of Nef to the lipid rafts may be sufficient to trigger the changes associated with the endothelial cell expansion characteristic of plexiform lesions. On the other hand, a second hit such as infection with other viruses (e.g. gammaherpesviruses such as HHV8) or a genetic susceptibility may be necessary as well. Human herpesvirus 8 (HHV8) is a gammaherpesvirus, also known as Kaposi's sarcoma-associated herpes virus (KSHV) (99, 100). Evidence of HHV8 is found in a large percentage of plexiform lesions of PH patients examined in Denver, USA. suggesting for the first time that this virus was a contributing factor (101). There are several pro-angiogenic or oncogenic genes present in its genome, including a viral IL-8 and a viral IL-6 both shown to play a role in IPAH. In addition, the genome encodes a seven-transmembrane-spanning G protein-coupled receptor (GPCR) with extensive sequence similarity to cellular chemokine receptors (102). When expressed in NIH 3T3 fibroblasts, this gene increases their ability to grow in soft agar and to induce tumor formation in nude mice (103). GPCR increases secretion of vascular endothelial growth factor and activation of the ERK1/2 (p44/42) mitogen-activated protein kinase signaling pathway (104). Endothelial cells that express this gene become immortalized with constitutive activation of the VEGF-receptor 2 (KDR) (105). In addition, this gene can cause KS-like lesions in nude mice (103), and over-expression within hematopoietic cells results in angioproliferative lesions, resembling those found in KS (106). These are yet more examples of viral factors with the potential of altering cellular phenotype in the absence of viral replication. Nevertheless, in spite of their recognized angioproliferative potential and the initial association with plexiform lesions, several groups do not reproduce these results with HHV8 (107). Studies of patients from a San Francisco clinic along with Japanese and German cohorts find no evidence of latent virus in the lesions or serum antibodies against viral antigens (108–111). The discrepancy between groups may be a reflection of the methodology used to detect the virus or of regional (genetic/environmental) differences in the study population. Of note, latency-associated transcripts may be undetectable if the virus is going through a lytic replication cycle. In addition, serological tests for viral antibodies are notoriously difficult and in many cases, hard to interpret. Thus, further studies are necessary to address these questions. Recent sentinel studies suggest that, indeed, HHV8 can infect pulmonary vascular endothelial cells in vitro and that cells infected with HHV8 downregulate the BMPR2 pathway and acquire an apoptosis-resistant phenotype upon infection, lending credence to the possible causative role for this infection (112).
PH represents one of the extrahepatic complications of Hepatitis C virus infection, with a prevalence of 1–5% (113). In the majority of patients, portal hypertension precedes pulmonary hypertension (113, 114). The pathogenesis is also poorly understood, but the histological hallmarks are similar to IPAH. Whether these lesions are secondary to increased inflammatory cytokine production or to direct viral replication or to presence of viral products in the lung remain to be determined. As in HIV-mediated pulmonary hypertension, an associated immune dysregulation may trigger uncontrolled intrapulmonary angiogenesis.
Expression Profiling in PAH
In the investigation of PH, gene microarrays have been employed in a variety of study designs performed on a diverse array of cell types and animal species. Human studies have examined the gene expression profile of whole lung homogenates as well as individual cell types, such as smooth muscle cells isolated from the pulmonary arteries of patients and mononuclear cells isolated from peripheral blood.(115, 116) Animal microarray studies have been performed on both whole lung tissue and micro-dissected pulmonary vasculature of hypoxic and monocrotaline induced PAH.(117, 118) In aggregate, these studies have employed “hypothesis building” strategies, helping to focus attention on potentially novel pathologic pathways. However, gene expression has also been utilized as a biomarker, potentially useful in classification or identification of an individual’s risk of disease.(119)
Geraci et al analyzed the gene expression profile of lung tissue obtained from 6 patients with IPAH (2 with familial PAH) vs. 6 “normal” controls.(120) The normal lung tissue was obtained during surgery and was free from pathology on histologic review. All the patients included in the IPAH cohort had severe PAH (PA mean > 50 mmHg). Two of the IPAH patients had been diagnosed with familial PAH (FPAH). The study from Geraci et al provided a wealth of data that requires further investigation. For example, this study indicated increased expression of the gene encoding the anti-apoptotic protein BCL-2. Indeed, IHC again confirmed over expression of BCL-2 protein in the plexiform lesions from patients with IPAH (unpublished data, personal communication with Norbert Voelkel). Zhang et al have demonstrated that BMP-2 and 7 induce apoptosis in cultured pulmonary artery smooth muscle cells (PASMCs) associated with a marked down regulation of BCL-2.(121) When exposed to BMP-2 and BMP-7, PASMCs isolated from patients with IPAH had decreased apoptosis as compared to PASMCs from patients with other secondary causes of PAH.
Inflammation and autoimmunity are possible contributing factors in the development of PAH.(122–125) We hypothesized that the gene expression of peripheral blood mononuclear cells (PBMCs) would be altered in patients with PAH as compared to normal controls. Furthermore, we hypothesized that PBMCs could serve as a readily available surrogate tissue in patients with PAH, and that the gene expression profile of these cells could act as a biomarker of disease. The gene expression of PBMCs from 15 patients with PAH (including IPAH and PAH associated with a variety of other conditions such as CREST, portal hypertension and thromboembolic disease) and compared this to the PBMC gene expression of 6 normal controls. We identified a signature set of 106 genes which discriminated with high certainty (p≤0.002) between patients with PAH and normal controls. A subset of these genes was then validated by q-PCR both retrospectively on the initial group, and prospectively on a novel cohort of patients. The 106 gene signature identified genes previously recognized to be associated with PAH (i.e. adrenomedullin) as well as genes, such as endothelial cell growth factor-1 (ECGF-1), which may play a currently unrecognized role in the disease.(126) Notably, we were unable to identify a gene expression signature which discriminated between IPAH and PAH associated with other conditions such as scleroderma or thromboembolic disease, by unsupervised analysis in this study. This inability may have been due to inadequate sample size or to the diverse nature of diseases included in the non-IPAH cohort.(127) A third possibility is that a unique expression profile for IPAH does not exist in peripheral blood cells, however a supervised cluster analysis performed on these samples identified genes that may be differentially expressed between the groups. This strategy identified a list of 28 genes differentially expressed between IPAH and PAH associated with secondary conditions such as CREST syndrome, thromboembolic disease and portal hypertension.(116) One of these genes, Herpesvirus entry mediator (HVEM), was retrospectively and prospectively confirmed by q-PCR. This study again represents two potential uses of microarray gene expression in PAH. These include: 1) a hypothesis generating tool to identify previously unsuspected genes or pathways that contribute to disease and 2) gene expression as a biomarker of disease. If a gene expression pattern predictive of PAH could be identified, then “at risk” populations could be screened. Patients who are identified as being at high risk for disease could initiate therapy early in hopes of impacting the natural history of PAH. The strategy of gene expression as a biomarker has been successfully demonstrated in AML.(128, 129)
While many studies to date have used microarray analysis as a “hypothesis generating” tool, this technology also has vast potential as means of identifying and quantifying biomarkers of disease. The oncology literature has demonstrated the utility of gene microarray analysis to predict important outcomes such as response to therapy and survival.(130, 131) While many of these studies have examined the gene expression of tissue biopsies, others have examined less invasive options such as expression analysis of cells obtained from peripheral blood.(132, 133) It is likely that in the near future, gene microarrays will also be employed in a pharmacogenomics approach in PAH, helping to identify the most appropriate therapies for individual patients.(134, 135) These goals are ambitious, but certainly accomplishable. Such approaches will significantly increase our understanding of the pathobiology of PAH and aid in our struggle against this disabling and deadly disease.
Future Challenges in Pulmonary Hypertension
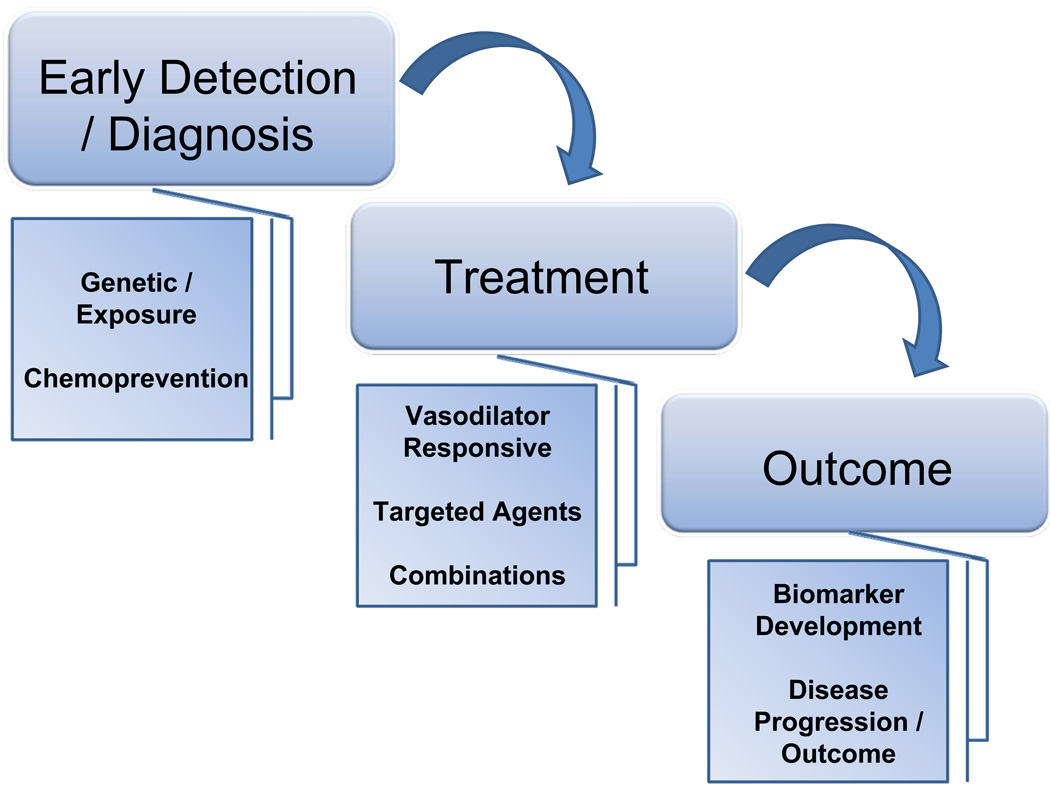
There are many future challenges in the field of pulmonary hypertension. However, the focus can be placed on diagnosis, treatment options and outcomes. There are several potential novel ares of discovery within this continuum, as outlined in Figure 2. Within that framework, one can investigate the recent advances in treatment, including the exciting promise of combinatorial treatment. No current treatment of PAH achieves a cure for the condition (136). Until very recently, treatment of PAH was reserved for the most severely affected individuals with NYHA functional class III and IV. However, the EARLY trial demonstrated that a proportion of patients with NYHA class II early disease benefited from treatment with a dual endothelin receptor antagonist (137). Newer compounds within this class include selective endothelin A receptor blockers, which show promise (138). Combination trials using compounds with different mechanisms of action demonstrate significant improvement in exercise capacity, hemodynamics, and time to clinical worsening (139). In this case, the PACES trial demonstrated the addition of sildenafil to epoprostenol was shown to be effective (139). So the future is clearly brighter, with more treatment options. However, a cure is not currently present. Moreover, treatment very early in the disease, or indeed chemoprevention, remains to be tested.
Figure 2. Future Challenges for Research in Pulmonary Hypertension.
There are a number of exciting avenues for research. These follow along the continuum of diagnosis, treatment and outcome. Early diagnosis may lead to earlier interventions of treatment. Ultimately, with individuals at particularly high risk for disease development, chemoprevention strategies could be employed. Treatment will focus on new, targeted pathways based on novel pathway investigations. The hope of combinatorial treatments are emerging. The field is in dire need of biomarker development to follow, more accurately, disease progression.
The evolution of biomarker development in PAH continues to demonstrate promise. Ideally, biomarkers would involve the serial measurement of circulating biomarkers, which would have sensitivity, specificity, and reliability. Biomarkers should be developed to not only accurately diagnose PAH with potential for early intervention trials, but should also be able to accurately map disease progression. With these goals in mind, array-based applications and proteomic analysis may have great promise. The most widely studied (and hence utilized) biomarker for disease severity in PAH reflects neurohumoral activation by the measurement of brain natriuretic peptide and N-terminal probrain natriuretic peptide (140). Demonstration of circulating cell microparticles might reflect PAH severity (141, 142). The stress-responsive, TGF-β-related cytokine growth-differentiation factor (GDF-15) has recently demonstrated promise as a serum biomarker (143, 144). The examination of autoimmunity and its role in PAH has been a long-time source of investigation. Using a clever strategy of immune sera from patients with PAH, a French group was able to undertake a large proteomic analysis to define the potential targets of fibroblast antibodies in some patients with IPAH (145).
Acknowledgments
This work was supported by Grant No. HL 089508 from the NHLBI (MWG), the CMREF grants to (MWG, RMT)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moncada S, Vane JR. Prostacyclin: its biosynthesis, actions and clinical potential. Philos Trans R Soc Lond B Biol Sci. 1981;294:305–329. doi: 10.1098/rstb.1981.0108. [DOI] [PubMed] [Google Scholar]
- 2.Cassin S, Tod ML, Frisinger JE, Jordan JA, Philips JB. Use of prostacyclin in persistent fetal circulation. Lancet. 1979;2:638. doi: 10.1016/s0140-6736(79)91698-2. [DOI] [PubMed] [Google Scholar]
- 3.Watkins WD, Peterson MB, Crone RK, Shannon DC, Levine L. Prostacyclin and prostaglandin E1 for severe idiopathic pulmonary artery hypertension. Lancet. 1980;1:1083. doi: 10.1016/s0140-6736(80)91522-6. [DOI] [PubMed] [Google Scholar]
- 4.Rubin LJ, Groves BM, Reeves JT, Frosolono M, Handel F, Cato AE. Prostacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertension. Circulation. 1982;66:334–338. doi: 10.1161/01.cir.66.2.334. [DOI] [PubMed] [Google Scholar]
- 5.Higenbottam T, Wheeldon D, Wells F, Wallwork J. Long-term treatment of primary pulmonary hypertension with continuous intravenous epoprostenol (prostacyclin) Lancet. 1984;1:1046–1047. doi: 10.1016/s0140-6736(84)91452-1. [DOI] [PubMed] [Google Scholar]
- 6.Badesch DB, Orton EC, Zapp LM, et al. Decreased arterial wall prostaglandin production in neonatal calves with severe chronic pulmonary hypertension. Am J Respir Cell Mol Biol. 1989;1:489–498. doi: 10.1165/ajrcmb/1.6.489. [DOI] [PubMed] [Google Scholar]
- 7.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 8.Stelzner TJ, O'Brien RF, Yanagisawa M, et al. Increased lung endothelin-1 production in rats with idiopathic pulmonary hypertension. Am J Physiol. 1992;262:L614–L620. doi: 10.1152/ajplung.1992.262.5.L614. [DOI] [PubMed] [Google Scholar]
- 9.Hassoun PM, Thappa V, Landman MJ, Fanburg BL. Endothelin 1: mitogenic activity on pulmonary artery smooth muscle cells and release from hypoxic endothelial cells. Proc Soc Exp Biol Med. 1992;199:165–170. doi: 10.3181/00379727-199-43342. [DOI] [PubMed] [Google Scholar]
- 10.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004;61:227–237. doi: 10.1016/j.cardiores.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Bonvallet ST, Zamora MR, Hasunuma K, et al. BQ123, an ETA-receptor antagonist, attenuates hypoxic pulmonary hypertension in rats. Am J Physiol. 1994;266:H1327–H1331. doi: 10.1152/ajpheart.1994.266.4.H1327. [DOI] [PubMed] [Google Scholar]
- 13.Ivy DD, Parker TA, Ziegler JW, et al. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Invest. 1997;99:1179–1186. doi: 10.1172/JCI119274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson DJ, Wallman LL, Jones R, et al. Hemodynamic effects of Bosentan, an endothelin receptor antagonist, in patients with pulmonary hypertension. Circulation. 2000;102:411–418. doi: 10.1161/01.cir.102.4.411. [DOI] [PubMed] [Google Scholar]
- 15.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 16.Fagan KA, Fouty BW, Tyler RC, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest. 1999;103:291–299. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 18.Weimann J, Ullrich R, Hromi J, et al. Sildenafil is a pulmonary vasodilator in awake lambs with acute pulmonary hypertension. Anesthesiology. 2000;92:1702–1712. doi: 10.1097/00000542-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Wilkens H, Guth A, Konig J, et al. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation. 2001;104:1218–1222. doi: 10.1161/hc3601.096826. [DOI] [PubMed] [Google Scholar]
- 20.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 21.Steen VD, Powell DL, Medsger TA., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 22.Barst RJ, Flaster ER, Menon A, Fotinox M, Morse JH. Evidence for the association of unexplained pulmonary hypertension in children with the major histocompatibility complex. Circulation. 1992;85:249–258. doi: 10.1161/01.cir.85.1.249. [DOI] [PubMed] [Google Scholar]
- 23.Morse JH, Barst RJ, Fotino M, et al. Primary pulmonary hypertension, tissue plasminogen activator antibodies, and HLA-DQ7. Am J Respir Crit Care Med. 1997;155:274–278. doi: 10.1164/ajrccm.155.1.9001324. [DOI] [PubMed] [Google Scholar]
- 24.Loyd JE, Primm RK, Newman JH. Familial primary pulmonary hypertension: clinical patterns. Am Rev Respir Dis. 1984;129:194–197. doi: 10.1164/arrd.1984.129.1.194. [DOI] [PubMed] [Google Scholar]
- 25.Loyd JE, Butler MG, Foroud TM, Conneally PM, Phillips JA, III, Newman JH. Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;152:93–97. doi: 10.1164/ajrccm.152.1.7599869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols WC, Koller DL, Slovis B, et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31–32. Nat Genet. 1997;15:277–280. doi: 10.1038/ng0397-277. [DOI] [PubMed] [Google Scholar]
- 27.Morse JH, Jones AC, Barst RJ, Hodge SE, Wilhelmsen KC, Nygaard TG. Mapping of familial primary pulmonary hypertension locus (PPH1) to chromosome 2q31–q32. Circulation. 1997;95:2603–2606. doi: 10.1161/01.cir.95.12.2603. [DOI] [PubMed] [Google Scholar]
- 28.Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension.The International PPH Consortium. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 30.The International PPH Consortium. Lane KB, Machado RD, et al. Heterozygous germline mutations in BMPR2 encoding a TGF-B receptor cause familiar pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 31.Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (gene PPH1) Is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trembath RC, Thomson JR, Machado RD, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 33.Harrison RE, Flanagan JA, Sankelo M, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaouat A, Coulet F, Favre C, et al. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax. 2004;59:446–448. doi: 10.1136/thx.2003.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 36.Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol. 1997;28:434–442. doi: 10.1016/s0046-8177(97)90032-0. [DOI] [PubMed] [Google Scholar]
- 37.Tuder RM, Groves BM, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 38.Heath D, Smith P, Gosney J. Ultrastructure of early plexogenic pulmonary arteriopathy. Histopathology. 1988;12:41–52. doi: 10.1111/j.1365-2559.1988.tb01915.x. [DOI] [PubMed] [Google Scholar]
- 39.Tuder RM, Chacon M, Alger LA, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 40.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest. 1998;101:927–934. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res. 2001;88:e8–e11. doi: 10.1161/01.res.88.1.e2. [DOI] [PubMed] [Google Scholar]
- 42.Atkinson C, Stewart S, Upton PD, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 43.Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth Factor β signaling in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170:1340–1348. doi: 10.1164/rccm.200311-1602OC. [DOI] [PubMed] [Google Scholar]
- 44.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, et al. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res. 2006;98:209–217. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 45.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet S, Michelakis ED, Porter CJ, et al. An abnormal mitochondrial-hypoxia inducible factor-1 alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats - Similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 47.McMurtry MS, Archer SL, Altieri DC, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 49.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 50.Achcar RO, Demura Y, Rai PR, et al. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest. 2006;129:696–705. doi: 10.1378/chest.129.3.696. [DOI] [PubMed] [Google Scholar]
- 51.Voelkel NF, Cool CD, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest. 1999;114:225S–230S. doi: 10.1378/chest.114.3_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 52.Yamaki S, Wagenvoort CA. Comparison of primary plexogenic arteriopathy in adults and children.A morphometric study in 40 patients. Br Heart J. 1985;54:428–434. doi: 10.1136/hrt.54.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chazova I, Loyd JE, Newman JH, Belenkov Y, Meyrick B. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol 199. 146:389–397. [PMC free article] [PubMed] [Google Scholar]
- 54.Yi ES, Kim H, Ahn H, et al. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am J Respir Crit Care Med. 2000;162:1577–1586. doi: 10.1164/ajrccm.162.4.9912131. [DOI] [PubMed] [Google Scholar]
- 55.Palevsky HI, Schloo BL, Pietra GG, et al. Primary pulmonary hypertension. Vascular structure, morphometry, and responsiveness to vasodilator agents. Circulation. 1989;80:1221. doi: 10.1161/01.cir.80.5.1207. [DOI] [PubMed] [Google Scholar]
- 56.Santos S, Peinado VI, Ramirez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Resp J. 2002;19:632–638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 57.Tuder RM, Zaiman AL. Pathology of pulmonary vascular disease. In: Peacock A, Rubin LJ, editors. Pulmonary circulation. London: Hodder Arnold, Health Sciences; 2003. [Google Scholar]
- 58.Morrell NW, Yang X, Upton PD, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 59.Yang X, Long L, Southwood M, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–1063. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 60.Song YL, Jones JE, Beppu H, Keaney JF, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Launay JM, Herve P, Peoc'h K, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- 62.Eddahibi S, Humbert M, Fadel E, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eddahibi S, Hanoun N, Lanfumey L, et al. Attenuated hypoxic pulmonary hypertension in mice lacking the 5- hydroxytryptamine transporter gene. J Clin Invest. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Botney MD, Bahadori L, Gold LI. Vascular remodeling in primary pulmonary hypertension. Potential role for transforming growth factor-beta. Am J Pathol. 1994;144:286–295. [PMC free article] [PubMed] [Google Scholar]
- 65.Schermuly RT, Dony E, Ghofrani HA, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. The New England Journal of Medicine. 2005;353:1412–1413. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 67.Tuder RM, Yeager ME, Geraci M, Golpon HA, Voelkel NF. Severe pulmonary hypertension after the discovery of the familial primary pulmonary hypertension gene. Eur Respir J. 2001;17:1065–1069. doi: 10.1183/09031936.01.00202701. [DOI] [PubMed] [Google Scholar]
- 68.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163:737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 69.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med. 2000;6:698–702. doi: 10.1038/76282. [DOI] [PubMed] [Google Scholar]
- 70.Remillard CV, Yuan JXJ. Activation of K+ channels: an essential pathway in programmed cell death. Amer J Physiol -Lung Cell M PH. 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- 71.Fantozzi I, Platoshyn O, Wong AH, et al. Bone morphogenetic protein-2 upregulates expression and function of voltage-gated K+ channels in human pulmonary artery smooth muscle cells. AJP - Lung Cellular and Molecular Physiology. 2006:00191. doi: 10.1152/ajplung.00191.2005. [DOI] [PubMed] [Google Scholar]
- 72.Dawson CA. Hypoxic pulmonary vasocontriction: Heterogeneity. In: Yuan JX, editor. Hypoxic pulmonary vasoconstriction: Cellular and Molecule Mechanisms. Norwell: Massachussets; 2002. pp. 15–32. [Google Scholar]
- 73.Altieri DC. Validating survivin as a cancer therapeutic target. Nature Reviews Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 74.Collins E, Hardwick RJ, Jeffery H. Perinatal cocaine intoxication. Med J Aust. 1989;150:331–332. 334. doi: 10.5694/j.1326-5377.1989.tb136497.x. [DOI] [PubMed] [Google Scholar]
- 75.Gurtner HP. Aminorex and pulmonary hypertension. A review. Cor Vasa. 1985;27:160–171. [PubMed] [Google Scholar]
- 76.Brenot F, Herve P, Petitpretz P, Parent F, Duroux P, Simonneau G. Primary pulmonary hypertension and fenfluramine use. Br Heart J. 1993;70:537–541. doi: 10.1136/hrt.70.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abenhaim L, Moride Y, Brenot F, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 78.Kim KK, Factor SM. Membranoproliferative glomerulonephritis and plexogenic pulmonary arteriopathy in a homosexual man with acquired immunodeficiency syndrome. Hum Pathol. 1987;18:1293–1296. doi: 10.1016/s0046-8177(87)80417-3. [DOI] [PubMed] [Google Scholar]
- 79.Kanmogne GD, Kennedy RC, Grammas P. Is HIV involved in the pathogenesis of noninfectious pulmonary complications in infected patients? Curr HIV Res. 2003;1:385–393. doi: 10.2174/1570162033485177. [DOI] [PubMed] [Google Scholar]
- 80.Mette SA, Palevsky HI, Pietra GG, et al. Primary pulmonary hypertension in association with human immunodeficiency virus infection. A possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis. 1992;145:1196–1200. doi: 10.1164/ajrccm/145.5.1196. [DOI] [PubMed] [Google Scholar]
- 81.Pellicelli AM, Barbaro G, Palmieri F, et al. Primary pulmonary hypertension in HIV patients: a systematic review. Angiology. 2001;52:31–41. doi: 10.1177/000331970105200105. [DOI] [PubMed] [Google Scholar]
- 82.Pongprot Y, Sittiwangkul R, Silvilairat S, Sirisanthana V. Cardiac manifestations in HIV-infected Thai children. Ann Trop Paediatr. 2004;24:153–159. doi: 10.1179/027249304225013439. [DOI] [PubMed] [Google Scholar]
- 83.Recusani F, Di M, Gambarin F, D'Armini A, Klersy C, Campana C. Clinical and therapeutical follow-up of HIV-associated pulmonary hypertension: prospective study of 10 patients. AIDS. 2003;17 Suppl 1:S88–S95. doi: 10.1097/00002030-200304001-00013. [DOI] [PubMed] [Google Scholar]
- 84.Barbaro G, Lucchini A, Pellicelli AM, Grisorio B, Giancaspro G, Barbarini G. Highly active antiretroviral therapy compared with HAART and bosentan in combination in patients with HIV-associated pulmonary hypertension. Heart. 2006;92:1164–1166. doi: 10.1136/hrt.2005.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Speich R, Jenni R, Opravil M, Jaccard R. Regression of HIV-associated pulmonary arterial hypertension and long-term survival during antiretroviral therapy. Swiss Med Wkly. 2001;131:663–665. doi: 10.4414/smw.2001.09842. [DOI] [PubMed] [Google Scholar]
- 86.Sitbon O, Gressin V, Speich R, et al. Bosentan for the treatment of human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2001;170:1212–1217. doi: 10.1164/rccm.200404-445OC. [DOI] [PubMed] [Google Scholar]
- 87.Marecki JC, Cool CD, Parr JE, et al. HIV-1 Nef is Associated with Complex Pulmonary Vascular Lesions in SHIV-nef-infected Macaques. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200601-005OC. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 89.Kestler HW, Ringler DJ, Mori K, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 90.Harris M. The role of myristoylation in the interactions between human immunodeficiency virus type I Nef and cellular proteins. Biochem Soc Trans. 1995;23:557–561. doi: 10.1042/bst0230557. [DOI] [PubMed] [Google Scholar]
- 91.Wang JK, Kiyokawa E, Verdin E, Trono D. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc Natl Acad Sci U S A. 2000;97:394–399. doi: 10.1073/pnas.97.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Graziani A, Galimi F, Medico E, et al. The HIV-1 nef protein interferes with phosphatidylinositol 3-kinase activation 1. J Biol Chem. 1996;271:6590–6593. doi: 10.1074/jbc.271.12.6590. [DOI] [PubMed] [Google Scholar]
- 93.Linnemann T, Zheng YH, Mandic R, Peterlin BM. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology. 1915;294:246–255. doi: 10.1006/viro.2002.1365. [DOI] [PubMed] [Google Scholar]
- 94.He JC, Husain M, Sunamoto M, et al. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest. 2004;114:643–651. doi: 10.1172/JCI21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krautkramer E, Giese SI, Gasteier JE, Muranyi W, Fackler OT. Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts. J Virol. 2004;78:4085–4097. doi: 10.1128/JVI.78.8.4085-4097.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olivetta E, Percario Z, Fiorucci G, et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: involvement of Nef endocytotic signals and NF-kappa B activation. J Immunol. 1915;170:1716–1727. doi: 10.4049/jimmunol.170.4.1716. [DOI] [PubMed] [Google Scholar]
- 97.James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J Virol. 2004;78:3099–3109. doi: 10.1128/JVI.78.6.3099-3109.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi HJ, Smithgall TE. HIV-1 Nef promotes survival of TF-1 macrophages by inducing Bcl-XL expression in an extracellular signal-regulated kinase-dependent manner. J Biol Chem. 2003;279:51688–51696. doi: 10.1074/jbc.M410068200. [DOI] [PubMed] [Google Scholar]
- 99.Damania B, Desrosiers RC. Simian homologues of human herpesvirus 8. Philos Trans R Soc Lond B Biol Sci. 1929;356:535–543. doi: 10.1098/rstb.2000.0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Desrosiers RC, Sasseville VG, Czajak SC, et al. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J Virol. 1997;71:9764–9769. doi: 10.1128/jvi.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cool CD, Rai MD, Yeager ME, et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med. 2003;349:1113–1122. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 102.Boshoff C, Endo Y, Collins PD, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 103.Guo HG, Sadowska M, Reid W, Tschachler E, Hayward G, Reitz M. Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J Virol. 2003;77:2631–2639. doi: 10.1128/JVI.77.4.2631-2639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Estep RD, Axthelm MK, Wong SW. A G protein-coupled receptor encoded by rhesus rhadinovirus is similar to ORF74 of Kaposi's sarcoma-associated herpesvirus. J Virol. 2003;77:1738–1746. doi: 10.1128/JVI.77.3.1738-1746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bais C, Van G, Eroles P, et al. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell. 2003;3:131–143. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 106.Yang TY, Chen SC, Leach MW, et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J Exp Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galambos C, Montgomery J, Jenkins FJ. No role for kaposi sarcoma-associated herpesvirus in pediatric idiopathic pulmonary hypertension. Pediatr Pulmonol. 2006;41:122–125. doi: 10.1002/ppul.20349. [DOI] [PubMed] [Google Scholar]
- 108.Laney AS, De M, Peters JS, et al. Kaposi sarcoma-associated herpesvirus and primary and secondary pulmonary hypertension. Chest. 2005;127:762–767. doi: 10.1378/chest.127.3.762. [DOI] [PubMed] [Google Scholar]
- 109.Katano H, Ito K, Shibuya K, Saji T, Sato Y, Sata T. Lack of human herpesvirus 8 infection in lungs of Japanese patients with primary pulmonary hypertension. J Infect Dis. 2001;191:743–745. doi: 10.1086/427824. [DOI] [PubMed] [Google Scholar]
- 110.Nicastri E, Vizza CD, Carletti F, et al. Human herpesvirus 8 and pulmonary hypertension. Emerg Infect Dis. 2005;11:1480–1482. doi: 10.3201/eid1109.040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Henke-Gendo C, Mengel M, Hoeper MM, Alkharsah K, Schulz TF. Absence of Kaposi's sarcoma-associated herpesvirus in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 1915;172:1581–1585. doi: 10.1164/rccm.200504-546OC. [DOI] [PubMed] [Google Scholar]
- 112.Bull TM, Meadows CA, Coldren CD, et al. Human herpesvirus-8 infection of primary pulmonary microvascular endothelial cells. Am J Respir Cell Mol Biol. 2008;39:706–716. doi: 10.1165/rcmb.2007-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moorman J, Saad M, Kosseifi S, Krishnaswamy G. Hepatitis C virus and the lung: implications for therapy. Chest. 2005;128:2882–2892. doi: 10.1378/chest.128.4.2882. [DOI] [PubMed] [Google Scholar]
- 114.Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492–498. doi: 10.1016/s0735-1097(10)80121-4. [DOI] [PubMed] [Google Scholar]
- 115.Fantozzi I, Huang W, Zhang J, et al. Divergent effects of BMP-2 on gene expression in pulmonary artery smooth muscle cells from normal subjects and patients with idiopathic pulmonary arterial hypertension. Exp Lung Res. 2005;31:783–806. doi: 10.1080/01902140500461026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bull TM, Coldren CD, Moore M, et al. Gene microarray analysis of peripheral blood cells in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170:911–919. doi: 10.1164/rccm.200312-1686OC. [DOI] [PubMed] [Google Scholar]
- 117.Hoshikawa Y, Nana-Sinkam P, Moore MD, et al. Hypoxia induces different genes in the lungs of rats compared with mice. Physiol Genomics. 2003;12:209–219. doi: 10.1152/physiolgenomics.00081.2001. [DOI] [PubMed] [Google Scholar]
- 118.Kwapiszewska G, Wilhelm J, Wolff S, et al. Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension. Respir Res. 2005;6:109. doi: 10.1186/1465-9921-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Buermans HP, Redout EM, Schiel AE, et al. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics. 2005;21:314–323. doi: 10.1152/physiolgenomics.00185.2004. [DOI] [PubMed] [Google Scholar]
- 120.Geraci MW, Moore M, Gesell T, et al. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88:555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 121.Zhang S, Fantozzi I, Tigno DD, et al. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L740–L754. doi: 10.1152/ajplung.00284.2002. [DOI] [PubMed] [Google Scholar]
- 122.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 123.Isern RA, Yaneva M, Weiner E, et al. Autoantibodies in patients with primary pulmonary hypertension: association with anti-Ku. Am J Med. 1992;93:307–312. doi: 10.1016/0002-9343(92)90238-7. [DOI] [PubMed] [Google Scholar]
- 124.Voelkel NF, Tuder R. Interleukin-1 receptor antagonist inhibits pulmonary hypertension induced by inflammation. Ann N Y Acad Sci. 1994;725:104–109. doi: 10.1111/j.1749-6632.1994.tb39794.x. [DOI] [PubMed] [Google Scholar]
- 125.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest. 1998;114:225S–230S. doi: 10.1378/chest.114.3_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 126.Vizza CD, Letizia C, Sciomer S, et al. Increased plasma levels of adrenomedullin, a vasoactive peptide, in patients with end-stage pulmonary disease. Regul Pept. 2005;124:187–193. doi: 10.1016/j.regpep.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 127.Sheppard D. Fishing in the bloodstream: insights into the mechanisms of pulmonary hypertension? Am J Respir Crit Care Med. 2004;170:827–828. doi: 10.1164/rccm.2406008. [DOI] [PubMed] [Google Scholar]
- 128.Bullinger L, Dohner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350:1605–1616. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 129.Valk PJ, Verhaak RG, Beijen MA, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350:1617–1628. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- 130.Chatterjee SK, Zetter BR. Cancer biomarkers: knowing the present and predicting the future. Future Oncol. 2005;1:37–50. doi: 10.1517/14796694.1.1.37. [DOI] [PubMed] [Google Scholar]
- 131.Reis-Filho JS, Westbury C, Pierga JY. The impact of expression profiling on prognostic and predictive testing in breast cancer. J Clin Pathol. 2006;59:225–231. doi: 10.1136/jcp.2005.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y, Elashoff D, Oh M, et al. Serum circulating human mRNA profiling and its utility for oral cancer detection. J Clin Oncol. 2006;24:1754–1760. doi: 10.1200/JCO.2005.03.7598. [DOI] [PubMed] [Google Scholar]
- 133.Lacayo NJ, Meshinchi S, Kinnunen P, et al. Gene expression profiles at diagnosis in de novo childhood AML patients identify FLT3 mutations with good clinical outcomes. Blood. 2004;104:2646–2654. doi: 10.1182/blood-2003-12-4449. [DOI] [PubMed] [Google Scholar]
- 134.Murali S. Pulmonary arterial hypertension. Curr Opin Crit Care. 2006;12:228–234. doi: 10.1097/01.ccx.0000224867.78581.0c. [DOI] [PubMed] [Google Scholar]
- 135.Woodcock J. Pharmacogenetics: on the road to 'personalized medicine'. FDA Consum. 2005;39:44. [PubMed] [Google Scholar]
- 136.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 137.Galie N, Rubin L, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 138.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 139.Simonneau G, Rubin LJ, Galie N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 140.Fijalkowska A, Kurzyna M, Torbicki A, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 141.Bakouboula B, Morel O, Faure A, et al. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:536–543. doi: 10.1164/rccm.200706-840OC. [DOI] [PubMed] [Google Scholar]
- 142.Amabile N, Heiss C, Real WM, et al. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1268–1275. doi: 10.1164/rccm.200710-1458OC. [DOI] [PubMed] [Google Scholar]
- 143.Lankeit M, Kempf T, Dellas C, et al. Growth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med. 2008;177:1018–1025. doi: 10.1164/rccm.200712-1786OC. [DOI] [PubMed] [Google Scholar]
- 144.Nickel N, Kempf T, Tapken H, et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:534–541. doi: 10.1164/rccm.200802-235OC. [DOI] [PubMed] [Google Scholar]
- 145.Terrier B, Tamby MC, Camoin L, et al. Identification of target antigens of antifibroblast antibodies in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1128–1134. doi: 10.1164/rccm.200707-1015OC. [DOI] [PubMed] [Google Scholar]