Abstract
The human immunodeficiency virus type 1 (HIV-1) accessory protein Vpu enhances virus particle release by counteracting a host factor that retains virions at the surfaces of infected cells. It was recently demonstrated that cellular protein BST-2/CD317/Tetherin restricts HIV-1 release in a Vpu-dependent manner. Calcium-modulating cyclophilin ligand (CAML) was also proposed to be involved in this process. We investigated whether CAML is involved in cell surface expression of Tetherin. Here, we show that CAML overexpression in permissive Cos-7 cells or CAML depletion in restrictive HeLa cells has no effect on HIV-1 release or on Tetherin surface expression, indicating that CAML is not required for Tetherin-mediated restriction of HIV-1 release.
Human immunodeficiency virus type 1 (HIV-1) viral protein U (Vpu) is an accessory protein expressed late during the HIV-1 life cycle (2, 15). Vpu enhances virus particle release in a cell type-dependent manner (6, 11, 14, 16). Some model cell lines, referred to as permissive (including 293T, Cos-7, and HT1080), are capable of supporting HIV-1 particle release independently of Vpu expression. In contrast, in other cells, referred to as restrictive (such as HeLa cells, monocyte-derived macrophages, and primary CD4+ T lymphocytes), efficient HIV-1 release requires the expression of Vpu.
It was recently demonstrated that the interferon (IFN)-regulated cellular protein Tetherin (also called BST-2, HM1.24, and CD317) is able to restrict HIV-1 particle release by retaining virions at the surfaces of infected cells (13, 19). Evidence supporting a role of Tetherin in HIV-1 particle release restriction is well documented. Tetherin is constitutively expressed in restrictive HeLa cells but not in permissive 293T and HT1080 cells (13, 19). The expression of Tetherin in 293T or HT1080 cells is inducible by IFN-α treatment, while it is enhanced in Jurkat and primary CD4+ T lymphocytes (13). Moreover, overexpression of Tetherin in 293T and HT1080 cells inhibits the release of Vpu-defective HIV-1 particles without affecting Gag expression or processing (13). Finally, HeLa cells are able to efficiently release HIV-1 particles in the absence of Vpu if Tetherin is depleted (13, 19).
At the same time Tetherin was identified as an HIV-1 host restriction factor, another protein, calcium-modulating cyclophilin ligand (CAML), was linked to Vpu-regulated HIV-1 particle release (20). CAML is a highly conserved and ubiquitously expressed protein that is essential for cell viability (8, 17). Findings of initial functional studies demonstrated that CAML overexpression in Jurkat T cells causes a rise in intracellular calcium, followed by NFAT transcription factor activation (1, 7). Later work linked CAML with the intracellular trafficking of diverse receptors and signaling proteins (17, 18, 21, 22). Yet several properties of CAML do not fulfill the criteria expected for the putative HIV-1-tethering protein. Mainly, CAML is essential for cellular viability, is ubiquitously expressed, and is not IFN inducible (1, 8). In an attempt to reconcile these discrepancies, we sought to investigate whether CAML is involved in regulating cell surface expression of Tetherin, thus explaining its proposed role in restricting HIV-1 particle release.
Overexpression of human CAML in permissive Cos-7 cells.
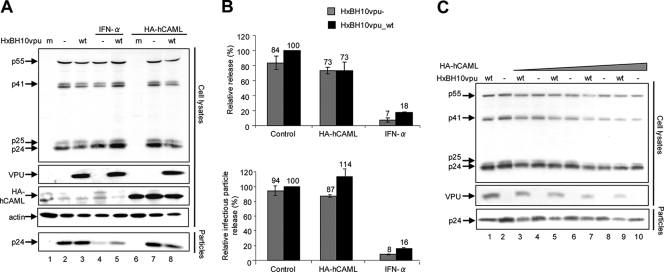
We started by testing the effect of human CAML (hCAML) on surface expression of Tetherin in Cos-7 cells. The restrictive effect of CAML on HIV-1 particle release was revealed previously by overexpressing hCAML in HIV-1- or Gag virus-like-particle-producing African green monkey Cos-7 cells in the presence or absence of Vpu (20). The hCAML open reading frame was amplified by PCR from 293T cell line DNA and cloned into the pCMV-HA plasmid (Clontech) to generate pCMV-HA-hCAML. The sequence of hemagglutinin (HA)-tagged hCAML and the integrity of the expression plasmid were confirmed by automated sequencing. A complete analysis of HIV-1 particle release and cell surface expression of Tetherin was performed using Cos-7 cells after transfection with HxBH10vpu_wt or HxBH10vpu− provirus and increasing concentrations of pCMV-HA-hCAML. HxBH10vpu_wt and HxBH10vpu− are two infectious HIV-1 molecular clones that are isogenic except for the expression of Vpu (16). As a positive control, transfected cells were treated with 10,000 U/ml of human IFN-α, since IFN-α was shown previously to induce a restriction of HIV-1 release from Cos-7 cells (12). Cells and virus-containing supernatants were collected 48 h posttransfection, as described previously (5). Protein lysates were analyzed by Western blotting to detect steady-state levels of HA-tagged CAML and viral products (processed Gag forms and Vpu) in the case of cell lysates or p24 in the case of virus lysates. HIV-1 release efficiency was evaluated by determining the ratio of the virion-associated p24 signal to the total intracellular Gag signal measured by scanning densitometry analysis of Western blots. These results were confirmed by measuring the levels of released infectious virus using HeLa-TZM indicator cells (AIDS Research and Reference Program, NIH). A second fraction of the transfected Cos-7 cells was stained to detect surface Tetherin and analyzed by flow cytometry as described elsewhere (10).
We were unable to detect an effect of hCAML on Vpu-defective HIV-1 particle release either by determining the ratio between virion- and cell-associated Gag signals by Western blotting or by evaluating infectious particle release (Fig. 1A, compare lanes 7 and 2; quantification results are shown in Fig. 1B). Indeed, the release efficiencies of the Vpu+ and Vpu-defective viruses in presence and absence of hCAML were comparable (Fig. 1A, compare lanes 7 and 8 with lanes 2 and 3; quantification results are shown in Fig. 1B). As reported previously (12), IFN-α-treated Cos-7 cells restricted HIV-1 release independently of Vpu expression (Fig. 1A, compare lanes 2 and 3 with lanes 4 and 5; quantification results are shown in Fig. 1B). Similar results were obtained when cells were transfected with increasing amounts of pCMV-HA-hCAML (Fig. 1C). Interestingly, HA-hCAML overexpression resulted in the detection of lower levels of Gag products and Vpu proteins in a dose-dependent manner (Fig. 1C).
FIG. 1.
Effect of CAML overexpression on HIV-1 particle release from permissive Cos-7 cells. (A and B) Cos-7 cells were seeded onto six-well plates and transfected using Lipofectamine 2000 according to the recommendations of the manufacturer (Invitrogen). Triplicate wells received fixed amounts of HxBH10vpu_wt (wt) or HxBH10vpu− (−) proviral DNA (2 μg) and pCMV-HA-hCAML (1 μg), along with the pCMV-HA empty vector to obtain the same final amounts of total DNA. (A) Western blot for Cos-7 cells transfected with the proviral DNA plasmid HxBH10vpu− (lanes 2, 4, and 7) or HxBH10vpu_wt (lanes 3, 5, and 8) or mock transfected (m; lanes 1 and 6). Samples from lanes 4 and 5 were treated with 10,000 U/ml of IFN-α, while samples from lanes 6 to 8 were cotransfected with the HA-tagged hCAML-expressing plasmid. Cells and supernatants containing viral particles were harvested 48 h posttransfection, and lysates were analyzed by Western blotting to detect steady-state levels of target proteins. Cell lysates were analyzed to detect HA-tagged CAML (by using anti-HA antibodies), as well as Gag products, Vpu, and cellular actin by using specific antibodies. Virus lysates were analyzed for the presence of p24 by using specific antibodies. (B) Quantification of virus particle release efficiency. (Top) Densitometric quantification of HIV-1 particle release efficiency in the presence of hCAML or upon IFN-α-treatment was performed by determining the ratio between the virion-associated Gag signal (corresponding to mature p24) and all cell-associated Gag signals (corresponding to p24/p25 and precursors p55 and p41) by Western blotting. Bands corresponding to all Gag products in cells and virus particles were scanned by laser densitometry and quantified using ImageQuant 5.0. (Bottom) Levels of released infectious virus were determined using HeLa-TZM indicator cells. HeLa-TZM indicator cells (AIDS Research and Reference Program, NIH) were inoculated with an aliquot of virus-containing supernatant. After 48 h, cells were lysed and luciferase activity was evaluated using the Promega luciferase assay system. For both top and bottom panels, the release efficiency of HxBH10vpu_wt was arbitrarily set at 100%. Error bars indicate the standard deviations of the means of results from two independent experiments. (C) Effect of increasing amounts of HA-hCAML on HIV-1 particle release. Cos-7 cells were seeded onto six-well plates and transfected as described in the legend to panel A. The cells received fixed amounts of HxBH10vpu_wt or HxBH10vpu− proviral DNA (2 μg) and increasing amounts of pCMV-HA-hCAML (from 2 to 8 μg), along with the pCMV-HA empty vector to obtain the same final amounts of total DNA. Cells and virus-containing supernatants were harvested 48 h posttransfection, and lysates were analyzed by Western blotting to detect Gag products and Vpu by using specific antibodies as indicated. Shown is a Western blot for Cos-7 cells transfected with the proviral DNA plasmid HxBH10vpu− (lanes 2, 4, 6, 8, and 10) or HxBH10vpu_wt (lanes 1, 3, 5, 7, and 9). Samples from lanes 3 to 10 were cotransfected with the HA-tagged hCAML-expressing plasmid.
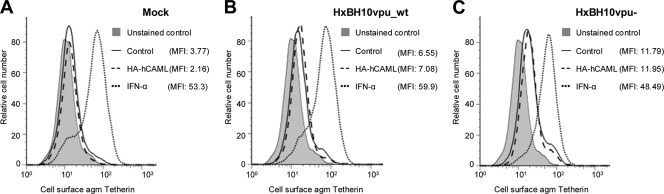
Consistent with their permissive phenotype, Cos-7 cells did not express Tetherin at the cell surface (Fig. 2A, solid line). Importantly, no significant change in the expression of the restriction factor was detected in Cos-7 cells transfected with pCMV-HA-hCAML alone or together with Vpu+ or Vpu-defective provirus (Fig. 2, compare dashed and solid lines). In contrast, IFN-α treatment significantly upregulated the surface expression of Tetherin by African green monkey Cos-7 cells (Fig. 2A, dotted line); however, this upregulation was not significantly affected by the presence of Vpu (compare Fig. 2B and C, dotted lines).
FIG. 2.
Effect of CAML overexpression on Tetherin expression at the surfaces of permissive Cos-7 cells. (A to C) Cos-7 cells were mock transfected or transfected with the HxBH10vpu_wt or HxBH10vpu− proviral construct. Forty-eight hours posttransfection, cell surface expression of Tetherin was evaluated after surface staining using anti-Tetherin antibodies, followed by flow cytometry. Tetherin antiserum was produced in rabbits by using a glutathione S-transferase-Tetherin fusion protein composed of Tetherin amino acids 40 to 181, produced in bacteria, thus generating a polyclonal antibody against the extracellular portion of Tetherin. In each panel, the gray-filled histogram represents results for mock-transfected cells stained with the preimmune rabbit serum (unstained control) while the other histograms represent results for cells stained with anti-Tetherin polyclonal rabbit serum. The histogram with a solid line represents data for mock-transfected cells, the histogram with a dotted line corresponds to mock-transfected cells treated with 10,000 U/ml of IFN-α, and the dashed-line histogram represents data for cells expressing hCAML. Mean fluorescence intensity (MFI) values after subtraction of the value for the unstained control are indicated for each sample. Stained cells were analyzed on a FACSCalibur instrument (BD Biosciences Immunocytometry Systems), and data analysis was performed by using CellQuest Pro (BD Biosciences) and FlowJo software version 7.25 (Tree Star). agm, African green monkey cells.
Depletion of CAML in restrictive HeLa cells.
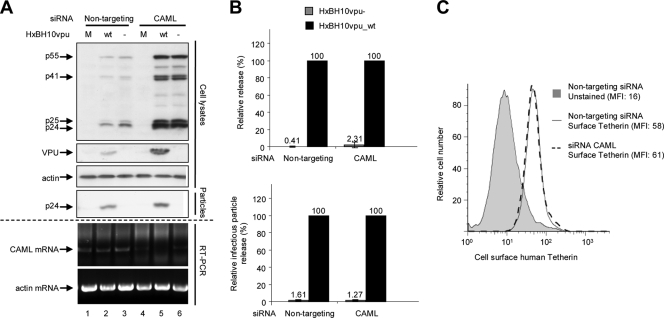
It was shown previously that small interfering RNA (siRNA)-mediated depletion of endogenous CAML relieves the Vpu-responsive block to Gag virus-like particle or Vpu-defective HIV-1 particle release from HeLa cells (20). Using RNA interference technology, we tested whether CAML downregulation affected basal cell surface Tetherin expression levels and had an impact on HIV-1 particle release. HeLa cells were transfected with siRNA specific to CAML or nontargeting siRNA by previously described methods (4). As a control, endogenous Tetherin was depleted by siRNA. Twenty-four hours posttransfection with siRNA, cells were transfected with either HxBH10vpu_wt or HxBH10vpu− provirus. Cells and virus-containing supernatants were collected and analyzed as described previously. An additional fraction of transfected cells was collected for RNA extraction. The depletion of CAML was evaluated by reverse transcription-PCR (RT-PCR) analysis of endogenous CAML mRNA. As shown in Fig. 3A, endogenous CAML mRNA levels were reduced significantly in the presence of the CAML siRNAs while control β-actin mRNA levels remained constant. Despite several attempts, we were unable to achieve complete siRNA-directed depletion of CAML mRNA. In this regard, it was recently reported that CAML-depleted cells are not viable (8, 17).
FIG. 3.
Effect of CAML depletion on HIV-1 particle release and surface expression of Tetherin in nonpermissive HeLa cells. (A) HeLa cells were transfected with nontargeting siRNA (siGENOME control siRNA [catalog no. D-001210-02-20; Dharmacon]; lanes 1 to 3) or specific siRNA against CAML (siGENOME SMART pool [catalog no. M-011601-01; Dharmacon]; lanes 4 to 6). Subsequently, cells were mock transfected (M; lanes 1 and 4) or transfected with the proviral plasmid HxBH10vpu_wt (wt; lanes 2 and 5) or HxBH10vpu− (−; lanes 3 and 6). Cells and supernatants containing viral particles were harvested 24 h posttransfection, and lysates were analyzed by Western blotting. Cell lysates were analyzed to detect Gag products, Vpu, and β-actin by using specific antibodies. Virus lysates were analyzed for the presence of p24 by using anti-p24 antibodies. Depletion of CAML mRNA was confirmed by RT-PCR using CAML mRNA-specific primers (forward primer, 5′ GGTGATTCAGTCAGTACAGG 3′; reverse primer, 5′ CTGACTCCAAGAGCAAGAAG 3′); as a control, actin mRNA levels were analyzed by RT-PCR using actin-specific primers (forward primer, 5′ACTCCTGCTTGCTGATCCAC 3′; reverse primer, 5′ TGGCTACAGCTTCACCACC 3′). (B) Quantification of virus particle release efficiency. (Top) Densitometric quantification of HIV-1 particle release efficiency after endogenous hCAML depletion. Virus release efficiency was evaluated as described in the legend to Fig. 1B. The virion-associated p24 signals were evaluated using longer blot exposure times to reveal the bands associated with HxBH10vpu−. (Bottom) The levels of released infectious virus were evaluated using HeLa-TZM indicator cells as described in the legend to Fig. 1B. For both panels, the release efficiency of HxBH10vpu_wt was arbitrarily set at 100%. Error bars indicate the standard deviations of the means of results from two independent experiments. (C) Tetherin cell surface expression was measured by flow cytometry as described in the legend to Fig. 2. The levels of surface expression of Tetherin by HeLa cells transfected with nontargeting siRNA (solid-line histogram) were compared to the levels of expression by HeLa cells transfected with specific siRNA against CAML (dashed-line histogram). As a negative control, unstained HeLa cells transfected with nontargeting siRNA were included (gray-filled histogram). Mean fluorescence intensity (MFI) values are indicated for each sample.
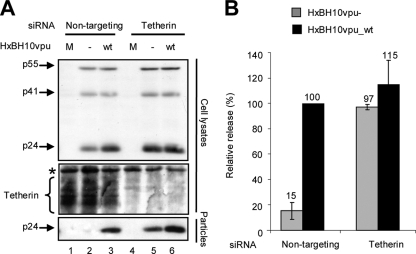
Depletion of CAML did not restore the release of HIV-1 particles from HeLa cells in the absence of Vpu, as indicated by the very low levels of mature p24 detectable only in the supernatant of cells producing HxBH10vpu− virus particles upon prolonged exposure (Fig. 3A, compare lanes 3 and 6). In contrast, the presence of Vpu overcame the block to HIV-1 particle release in the presence of CAML siRNAs or nontargeting siRNAs (Fig. 3A, lanes 2 and 5). Quantification of virus particle release efficiency by Western blot analysis or evaluation of released infectious virus (Fig. 3B) confirmed that the depletion of CAML did not restore HIV-1 particle release from HeLa cells in the absence of Vpu. Furthermore, as shown in Fig. 3C, the depletion of CAML did not affect Tetherin levels at the cell surface. In contrast, as reported previously (13, 19), the depletion of Tetherin did indeed restore HIV-1 particle release in the absence of Vpu, as indicated by the similar amounts of virion-associated p24 detected in the supernatants of HeLa cells producing HxBH10vpu_wt virus particles and those producing HxBH10vpu− virus particles (Fig. 4A, compare lanes 5 and 2 as well as lanes 5 and 6; quantification results are shown in Fig. 4B).
FIG. 4.
Effect of Tetherin depletion on HIV-1 particle release from nonpermissive HeLa cells. (A) HeLa cells were transfected with nontargeting siRNA (siGENOME control siRNA [catalog no. D-001210-02-20; Dharmacon]; lanes 1 to 3) or specific siRNA against Tetherin (ON-TARGET plus SMART pool [catalog no. L-011817-00; Dharmacon]; lanes 4 to 6). Subsequently, cells were mock-transfected (M; lanes 1 and 4) or transfected with the proviral plasmid HxBH10vpu− (−; lanes 2 and 5) or HxBH10vpu_wt (wt; lanes 3 and 6) as indicated. Cells and supernatants containing viral particles were harvested 24 h posttransfection, and lysates were analyzed by Western blotting. Cell lysates were analyzed to detect Gag products and endogenous cellular Tetherin by using anti-p24 antibodies (the asterisk denotes a nonspecific band used as an internal loading control). Virus lysates were analyzed for the presence of p24 by using specific antibodies. (B) Densitometric quantification of HIV-1 release efficiency after endogenous Tetherin depletion. HIV-1 particle release efficiency was evaluated as described in the legend to Fig. 1B. The release efficiency of HxBH10vpu_wt was arbitrarily set at 100%. Error bars indicate the standard deviations of the means of results from two independent experiments.
Discrepancies between results for nontargeting and CAML-specific siRNAs were noted. Levels of viral products were significantly increased when CAML expression was reduced (Fig. 3A, compare lanes 2 and 3 to lanes 5 and 6). It is unlikely that this effect was due to nontargeting siRNAs, since this effect was not observed when these same nontargeting siRNAs were tested against Tetherin-specific siRNAs (Fig. 4A, compare lanes 2 and 3 to lanes 5 and 6). To control for the amounts of total protein and gel loading, β-actin was probed on the same membrane used to detect Gag products in cells. No significant difference in the β-actin control was noted to account for the difference in viral product expression (Fig. 3A).
Concluding remarks.
Overexpression of hCAML in permissive Cos-7 cells did not modulate cell surface expression of Tetherin and was not sufficient to generate an HIV-1-restrictive phenotype in these cells. Furthermore, significant depletion of CAML in restrictive HeLa cells did not affect cell surface expression of Tetherin and did not overcome the requirement of Vpu for HIV-1 particle release. In contrast, depletion of Tetherin in HeLa cells restored the release of Vpu-defective HIV-1 particles to levels comparable to those observed in the presence of wild-type Vpu. Overall, we conclude that Tetherin restricts HIV-1 particle release and does not require CAML. Furthermore, these results do not support an important function of CAML in HIV-1 particle release.
Interestingly, CAML overexpression resulted in lower levels of Gag products and Vpu proteins, while in CAML-depleted cells, levels of viral proteins were found to be significantly higher. It was reported previously that CAML overexpression can affect the NFAT transcription factor pathway (1, 7), a pathway that was also reported to be important in modulating HIV-1 long terminal repeat promoter activation (3, 9). It is therefore possible that the effect observed in the previous study linking CAML to HIV-1 particle release restriction (20) is connected not to the modulation of Tetherin expression at the cell surface but rather to an earlier event that affects HIV-1 production, such as the modulation of long terminal repeat promoter activity.
Acknowledgments
We thank Eric Massicotte and Martine Dupuis for assistance in cell sorting. The HeLa-TZM cell line was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIH.
E.A.C. is the Canada Research Chair in Human Retrovirology. M.D. is the recipient of a Banting and Best studentship from the Canadian Institute of Health Research (CIHR). This work was supported by grants from the CIHR and the Fonds de Recherche en Santé du Québec (FRSQ) AIDS network to E.A.C.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Bram, R. J., and G. R. Crabtree. 1994. Calcium signalling in T cells stimulated by a cyclophilin B-binding protein. Nature 371:355-358. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, E. A., E. F. Terwilliger, J. G. Sodroski, and W. A. Haseltine. 1988. Identification of a protein encoded by the vpu gene of HIV-1. Nature 334:532-534. [DOI] [PubMed] [Google Scholar]
- 3.Cron, R. Q., S. R. Bartz, A. Clausell, S. J. Bort, S. J. Klebanoff, and D. B. Lewis. 2000. NFAT1 enhances HIV-1 gene expression in primary human CD4 T cells. Clin. Immunol. 94:179-191. [DOI] [PubMed] [Google Scholar]
- 4.Dube, M., B. B. Roy, P. Guiot-Guillain, J. Mercier, J. Binette, G. Leung, and E. A. Cohen. 2009. Suppression of Tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83:4574-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finzi, A., A. Brunet, Y. Xiao, J. Thibodeau, and E. A. Cohen. 2006. Major histocompatibility complex class II molecules promote human immunodeficiency virus type 1 assembly and budding to late endosomal/multivesicular body compartments. J. Virol. 80:9789-9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geraghty, R. J., K. J. Talbot, M. Callahan, W. Harper, and A. T. Panganiban. 1994. Cell type-dependence for Vpu function. J. Med. Primatol. 23:146-150. [DOI] [PubMed] [Google Scholar]
- 7.Holloway, M. P., and R. J. Bram. 1996. A hydrophobic domain of Ca2+-modulating cyclophilin ligand modulates calcium influx signaling in T lymphocytes. J. Biol. Chem. 271:8549-8552. [DOI] [PubMed] [Google Scholar]
- 8.Liu, Y., L. Malureanu, K. B. Jeganathan, D. D. Tran, L. D. Lindquist, J. M. van Deursen, and R. J. Bram. 2009. CAML loss causes anaphase failure and chromosome missegregation. Cell Cycle 8:940-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macian, F., and A. Rao. 1999. Reciprocal modulatory interaction between human immunodeficiency virus type 1 Tat and transcription factor NFAT1. Mol. Cell. Biol. 19:3645-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell, R. S., C. Katsura, M. A. Skasko, K. Fitzpatrick, D. Lau, A. Ruiz, E. B. Stephens, F. Margottin-Goguet, R. Benarous, and J. C. Guatelli. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 5:e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neil, S. J., S. W. Eastman, N. Jouvenet, and P. D. Bieniasz. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neil, S. J., V. Sandrin, W. I. Sundquist, and P. D. Bieniasz. 2007. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe 2:193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 14.Strebel, K., T. Klimkait, F. Maldarelli, and M. A. Martin. 1989. Molecular and biochemical analyses of human immunodeficiency virus type 1 vpu protein. J. Virol. 63:3784-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strebel, K., T. Klimkait, and M. A. Martin. 1988. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science 241:1221-1223. [DOI] [PubMed] [Google Scholar]
- 16.Terwilliger, E. F., E. A. Cohen, Y. C. Lu, J. G. Sodroski, and W. A. Haseltine. 1989. Functional role of human immunodeficiency virus type 1 vpu. Proc. Natl. Acad. Sci. USA 86:5163-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran, D. D., C. E. Edgar, K. L. Heckman, S. L. Sutor, C. J. Huntoon, J. van Deursen, D. L. McKean, and R. J. Bram. 2005. CAML is a p56Lck-interacting protein that is required for thymocyte development. Immunity 23:139-152. [DOI] [PubMed] [Google Scholar]
- 18.Tran, D. D., H. R. Russell, S. L. Sutor, J. van Deursen, and R. J. Bram. 2003. CAML is required for efficient EGF receptor recycling. Dev. Cell 5:245-256. [DOI] [PubMed] [Google Scholar]
- 19.Van Damme, N., D. Goff, C. Katsura, R. L. Jorgenson, R. Mitchell, M. C. Johnson, E. B. Stephens, and J. Guatelli. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varthakavi, V., E. Heimann-Nichols, R. M. Smith, Y. Sun, R. J. Bram, S. Ali, J. Rose, L. Ding, and P. Spearman. 2008. Identification of calcium-modulating cyclophilin ligand as a human host restriction to HIV-1 release overcome by Vpu. Nat. Med. 14:641-647. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.von Bulow, G. U., and R. J. Bram. 1997. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science 278:138-141. [DOI] [PubMed] [Google Scholar]
- 22.Yuan, X., J. Yao, D. Norris, D. D. Tran, R. J. Bram, G. Chen, and B. Luscher. 2008. Calcium-modulating cyclophilin ligand regulates membrane trafficking of postsynaptic GABA(A) receptors. Mol. Cell. Neurosci. 38:277-289. [DOI] [PMC free article] [PubMed] [Google Scholar]