Abstract
Our previous studies have found that hepatitis C virus (HCV) particles are enriched in apolipoprotein E (apoE) and that apoE is required for HCV infectivity and production. Studies by others, however, suggested that both microsomal transfer protein (MTP) and apoB are important for HCV production. To define the roles of apoB and apoE in the HCV life cycle, we developed a single-cycle HCV growth assay to determine the correlation of HCV assembly with apoB and apoE expression, as well as the influence of MTP inhibitors on the formation of HCV particles. The small interfering RNA (siRNA)-mediated knockdown of apoE expression remarkably suppressed the formation of HCV particles. However, apoE expressed ectopically could restore the defect of HCV production posed by the siRNA-mediated knockdown of endogenous apoE expression. In contrast, apoB-specific antibodies and siRNAs had no significant effect on HCV infectivity and production, respectively, suggesting that apoB does not play a significant role in the HCV life cycle. Additionally, two MTP inhibitors, CP-346086 and BMS-2101038, efficiently blocked secretion of apoB-containing lipoproteins but did not affect HCV production unless apoE expression and secretion were inhibited. At higher concentrations, however, MTP inhibitors blocked apoE expression and secretion and consequently suppressed the formation of HCV particles. Furthermore, apoE was found to be sensitive to trypsin digestion and to interact with NS5A in purified HCV particles and HCV-infected cells, as demonstrated by coimmunoprecipitation. Collectively, these findings demonstrate that apoE but not apoB is required for HCV assembly, probably via a specific interaction with NS5A.
Hepatitis C virus (HCV) is the leading cause of chronic viral hepatitis, affecting approximately 170 million people worldwide (8, 40). HCV coinfection with human immunodeficiency virus (HIV) is also common, occurring overall in 25 to 30% of HIV-positive persons (1). Individuals with chronic HCV infection are at high risk for the development of cirrhosis and hepatocellular carcinoma. A pegylated interferon and ribavirin combination is the standard therapy to treat hepatitis C but suffers from limited efficacy (<50% antiviral response among patients infected with the dominant genotype 1 HCV) and severe side effects (18, 27). More efficacious and safer antiviral drugs for effective treatment of hepatitis C are urgently needed. A thorough understanding of the HCV life cycle will likely provide novel targets for antiviral drug discovery and development to control HCV infection.
HCV is an enveloped RNA virus containing a single-stranded, positive-sense RNA genome and is classified as a Hepacivirus in the Flaviviridae family (11, 33). The viral RNA genome carries a single open reading frame flanked by untranslated regions (UTRs) at both the 5′ and 3′ ends. The 5′ and 3′ UTRs contain cis-acting RNA elements important for the initiation of HCV polyprotein translation and viral RNA replication (24). Upon translation, the HCV polyprotein precursor is proteolytically processed by cellular peptidases and viral proteases into at least 10 different viral proteins (C, E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B). Studies with subgenomic HCV RNAs demonstrated that the NS3 to NS5B proteins, in association with intracellular membranes and cellular proteins, are essential and sufficient for HCV RNA replication in the cell (5, 14, 25). The newly synthesized HCV proteins and RNA genome are assembled to form progeny HCV particles by undetermined mechanisms.
Our earlier work found that infectious HCV particles are highly enriched in apolipoprotein E (apoE), which is a major determinant of HCV infectivity and production in cell culture (10). ApoE-specific monoclonal antibodies (MAbs) effectively neutralized HCV infectivity, in a dose-dependent manner. The knockdown of apoE expression by specific small interfering RNA (siRNA) remarkably suppressed HCV production, suggesting that apoE is also important for the formation of infectious particles and/or egression (10). However, studies by others suggested that HCV assembly and production are dependent on microsomal transfer protein (MTP) and apolipoprotein B (apoB), both of which are essential components required for the assembly and secretion of very-low-density lipoproteins (VLDLs) (19, 21). In those studies, both apoB-specific siRNAs and MTP inhibitors were found to suppress HCV production (19, 21). It was speculated that HCV shares the same assembly and secretion pathway with VLDLs.
To define the roles of apoB and apoE in the formation of HCV particles and egression, we developed a single-cycle HCV growth assay. Using this assay system, we have demonstrated that apoE but not apoB is required for the infectivity and formation of infectious HCV particles. First of all, apoB-specific MAb and polyclonal antibodies did not affect HCV infection. Additionally, apoE-specific siRNA potently inhibited the formation of infectious HCV particles, whereas HCV production was unaffected by the siRNA-mediated knockdown of apoB expression. Furthermore, two MTP inhibitors, CP-346086 and BMS-2101038, efficiently blocked apoB secretion but did not significantly affect HCV production prior to the blockage of apoE expression/secretion. At higher concentrations, however, both MTP inhibitors blocked apoE secretion and consequently suppressed the formation of infectious HCV particles. To further understand the role of apoE in HCV assembly, we carried out coimmunoprecipitation (co-IP) experiments and found that apoE-specific MAb pulled down NS5A but not other HCV proteins from lysed HCV particles, suggesting a specific interaction between apoE and NS5A during the formation of infectious HCV particles. Collectively, our findings demonstrate that apoE but not apoB is required for HCV assembly, probably via a specific interaction with NS5A.
MATERIALS AND METHODS
Cell lines and cell culture.
A highly HCV-permissive human hepatoma cell line (6), Huh7.5, was generously provided by Charles M. Rice. Huh7.5 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), nonessential amino acids, penicillin, and streptomycin (Invitrogen).
Production and purification of apoB-specific MAbs.
A hybridoma cell line producing apoB (464B1B3) blocking MAb was purchased from the American Type Culture Collection (ATCC) and maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS. To make apoB-specific MAb, 50 μg of purified human apoB (Biodesign International, Saco, ME) emulsified with Freund's complete adjuvant (Sigma) was subcutaneously injected into female RBF-DnJ mice (The Jackson Laboratory). Upon three subsequent booster immunizations at 10-day intervals, splenic lymphocytes were fused with P3X myeloma cells by use of polyethylene glycol 4000 (Sigma). ApoB MAb-producing hybridomas were selected and identified as previously described (10). The MAbs were purified using a protein A antibody purification kit (Bio-Rad Laboratories). The concentration of purified MAbs was determined by spectrophotometry.
Antibody neutralization of HCV infectivity.
Huh7.5 cells in 12-well (for detection of HCV proteins) or 6-well (for quantification of HCV RNA) plates were infected with HCV of genotype 2a (JFH1) at 37°C for 2 to 3 h. The HCV-infected cells were washed twice with phosphate-buffered saline (PBS) and then incubated with 10% FBS-containing DMEM. For antibody neutralization of HCV infectivity, HCV was incubated with increasing concentrations (0, 0.4, 2.0, 10, and 50 μg/ml) of normal mouse immunoglobulin G (IgG), apoB, or apoE antibody at room temperature for 1 h and then added to Huh7.5 cells (7, 10). ApoB antibodies included polyclonal rabbit anti-apoB (Calbiochem) and goat anti-apoB (Biodesign International), MAb 464B1B3 (23), and apoB MAb3 and MAb4 (raised in our lab). ApoE MAb23 (10) was also included for comparison. At 2 h postinfection (p.i.), the antibody-HCV mixture was removed, and the HCV-infected cells were washed twice with PBS and then incubated with DMEM containing 10% FBS. At 3 days p.i., cell culture medium was collected and clarified by centrifugation to remove any cell debris. HCV-infected cells were lysed and used for detection of HCV NS3 protein by Western blotting. The level of HCV in the medium was determined by subsequent infection of Huh7.5 cells, and the levels of HCV NS3 protein and positive-strand RNA were detected by Western blotting and an RNase protection assay (RPA), respectively (7, 10). The HCV titer in the medium was determined by immunofluorescence staining for NS3-positive cell foci and is reported in focus-forming units (FFU)/ml (7, 10).
Knockdown of apoB and apoE expression by specific siRNAs.
SmartPool siRNAs targeting apoB and apoE mRNAs were purchased from Dharmacon. The most potent siRNAs identified from SmartPool siRNAs were chosen for subsequent experiments. The siRNA targeting sequences for apoB and apoE mRNAs were 5′-AGACAGAGCCGGAGCCCGA-3′ and 5′-AGACAGAGCCGGAGCCCGA-3′, respectively. A nonspecific control (NSC) siRNA was also purchased from Dharmacon. Huh7.5 cells were infected with HCV at a multiplicity of infection (MOI) of approximately 5 and then transfected with apoB, apoE, or NSC siRNA, using Lipofectamine RNAiMax (Invitrogen). The mixture of siRNA and RNAiMax was serially diluted with serum-free medium, from 50 nM to 0.4 nM. At 24 h p.i., the medium was collected and cleared by centrifugation to remove cells and cell debris. The infectivity of HCV secreted into the medium was determined by subsequent infection of Huh7.5 cells. The levels of both NS3 protein and positive-strand HCV RNA were determined by Western blotting and RPA, respectively. Infectious HCV titers were determined by immunofluorescence assay (IFA) after serial dilution (7, 10). To isolate intracellular HCV particles, the HCV-infected cells were washed twice with 1× PBS and then collected by scraping into 1× PBS. Intracellular HCV particles were prepared by freeze-thawing three times (20). Infectious titers of the intracellular HCV particles were determined by IFA and reported in FFU. HCV virion RNA (vRNA) in the medium and intracellular HCV particles were extracted with Trizol LS reagent (Invitrogen) and quantified by a real-time quantitative reverse transcription-PCR (qRT-PCR) method.
Construction of a siRNA-resistant apoE-expressing cDNA and ectopic apoE expression.
Five silent mutations (underlined in the oligonucleotide sequence of mApoE/Up) were introduced into the siRNA targeting region of the apoE3 gene, using a QuikChange Lightning site-directed mutagenesis kit (Stratagene, La Jolla, CA) and two synthetic oligonucleotides, mApoE/Up (5′-GCAAGCGGTGGAGACGGAACCCGAACCGGAGCTGCGCCAGCAG-3′) and mApoE/Bt (5′-CTGCTGGCGCAGCTCCGGTTCGGGTTCCGTCTCCACCGCTTGC-3′). The resultant apoE3-expressing cDNA was confirmed by DNA sequence analysis (Northwestern University Genomics Core, Chicago, IL) and was designated pCMV6XL5/mApoE3. This plasmid DNA was used to determine whether ectopic expression of apoE3 is able to substitute for the role of endogenous apoE in HCV assembly. Initially, Huh7.5 cells in a 12-well plate were transfected with either an NSC siRNA or apoE-specific siRNA at 50 nM. At 12 h posttransfection, the siRNA-transfected Huh7.5 cells were supertransfected with 1 μg of pCMV6XL5/mApoE3 DNA or the vector DNA as a control. Twelve hours after DNA transfection, Huh7.5 cells were infected with HCV at an MOI of approximately 5. At 24 h p.i. (single-cycle virus growth), Huh7.5 cells were lysed in RIPA buffer. The levels of HCV proteins were determined by Western blotting. The cell culture medium was collected and used to measure the levels of apoE expression (by Western blotting) and HCV infectivity. The HCV infectivity assay was carried out by infecting naïve Huh7.5 cells in a six-well plate with the collected cell culture medium. At 3 days p.i., total RNA was extracted with Trizol from the HCV-infected cells and the levels of positive-strand HCV RNA were determined by RPA.
Treatment with MTP inhibitors.
The MTP inhibitor CP-346086 (9) was synthesized by ACME Bioscience (Belmont, CA), and BMS-2101038 (21, 39) was a gift of Jin Ye (University of Texas Southwestern Medical Center). For multiple-cycle virus growth, Huh7.5 cells were infected with HCV at an MOI of about 0.3. For single-cycle HCV growth, Huh7.5 cells were infected with HCV at an MOI of 5 at 37°C for 2 h. The HCV-infected cells were washed with PBS and then incubated with DMEM containing 10% FBS and increasing concentrations of CP-346086 or BMS-2101038. At 24 h p.i. (for single-cycle HCV growth) or 3 days p.i. (for multiple-cycle HCV growth), the medium was collected and clarified by centrifugation. HCV infectivity in the medium was determined by infection of Huh7.5 cells, and the level of NS3 protein was determined by Western blotting. HCV titers in the medium were determined by IFA and reported in FFU/ml. Intracellular HCV particles were isolated from the HCV-infected cells by repeated freezing and thawing, followed by centrifugation to remove any debris, as previously described (20). Infectious titers of intracellular HCV particles were determined by IFA. The levels of HCV vRNA in the medium and in intracellular HCV particles were quantified by qRT-PCR.
Western blot analysis.
A lysate of HCV-infected cells was prepared by using standard procedures (7). Twenty-five micrograms of total protein was resolved by electrophoresis in a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel, followed by transfer onto a nitrocellulose membrane. The level of HCV proteins was determined by Western blotting, using an NS3- or NS5A-specific MAb, as previously described (7). The β-actin protein was used as an internal control. To quantify the levels of apoB and apoE, 12 μl of cell culture medium was electrophoresed in 5% and 10% SDS-polyacrylamide gels (SDS-PAGE), respectively. The apoB and apoE levels were determined by Western blotting using apoB (Biodesign)- and apoE (MAb33)-specific antibodies (10). HCV E2 in virus particles was determined by IP and Western blotting, using E2-specific MAbs provided by Michael Diamond (Washington University). Both E2-specific MAbs J6E2.1 and H77E2.7 were raised against soluble E2 proteins of the J6 and H77 strains of HCV, and both are IgG1 antibodies (Michelle Sabo and Michael Diamond, unpublished results).
HCV RNA extraction and quantification by RPA.
Total RNAs were extracted from HCV-infected cells by use of Trizol reagent (Invitrogen). The level of positive-strand HCV RNA in the cells was determined by RPA, using an [α-32P]UTP-labeled probe containing the negative-strand HCV 3′-UTR RNA, as described previously (10). After digestion with RNase A/T1, RNA products were analyzed in a 6% polyacrylamide-urea (7.7 M) gel, visualized by autoradiography, and quantified by phosphorimager analysis (7, 26).
HCV vRNA extraction and quantification by qRT-PCR.
HCV vRNA in the medium and in intracellular HCV particles were extracted using Trizol LS reagent (Invitrogen). The level of HCV vRNA was quantified by a qRT-PCR method, using a SuperScript III Platinum one-step qRT-PCR kit following the manufacturer's instructions (Invitrogen). Oligonucleotides 2aF (5′-AGCCATGGCGTTAGTATGAGTGTC-3′) and 2aR (5′-ACAAGGCCTTTCGCAACCCAA-3′), complementary to sequences within the 5′ UTR, were used as primers. The probe (5′-AAACCCACTCTATGCCCGGCCATTT-3), synthesized by Integrated DNA Technologies, contained 6-carboxyfluorescein at the 5′ end and 6-carboxytetramethylrhodamine at the 3′ end.
Determination of HCV titers by IFA.
HCV titers were determined by an IFA using an NS3-specific MAb, as previously described (10, 14). Briefly, HCV in the medium was serially diluted (10×) and then used to infect naïve Huh7.5 cells on coverslips in 24-well plates. At 3 days p.i., the number of cell FFU was determined by IFA.
Preparation and purification of HCV particles.
HCV was prepared from large-scale virus growth in 175-cm2 cell culture flasks. The HCV-containing medium was cleared by passage through a 0.22-μm filter unit (Corning). HCV particles were concentrated by filtration through Centricon Plus-70 filter devices (Millipore). The concentrated HCV was subjected to 10 to 60% sucrose gradient sedimentation analysis, as previously described (7). A total of 12 fractions with 1 ml each were collected from the bottom to the top of the sucrose gradient. The sucrose in fractions was removed by filtration through Amicon Ultra-15 filter devices (Millipore). The resulting HCV was used for subsequent analysis of apoE and NS5A interaction.
Trypsin digestion and detergent treatment of purified HCV particles.
Purified HCV particles were treated with trypsin (4 μg/ml; Sigma) in 30 μl of buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM CaCl2) in the absence or presence of 1% Triton X-100 for 1 h at 37°C. The proteolysis reactions were terminated by the addition of 1 mM phenylmethylsulfonyl fluoride and a 1/100 volume of protease inhibitor cocktail (Roche) (41). HCV virion proteins were analyzed by Western blotting, using apoE MAb33, HCV E2- and C-specific MAbs (7, 10).
Co-IP of NS5A with apoE in purified HCV particles and HCV-infected cells.
Co-IP of NS5A and apoE in purified HCV particles was performed using a co-IP kit following the manufacturer's instructions (Pierce). Briefly, purified apoE MAb23, HCV E2 MAb, and apoB MAb were coupled to AminoLink Plus coupling resin. HCV lysate was prepared by incubating purified HCV particles with M-PER protein extraction reagent (Pierce) at room temperature for 10 min. Purified HCV and HCV lysate were incubated with HCV E2-, apoB-, and apoE-specific MAb-conjugated agarose resin overnight at 4°C. Upon elution, HCV virion proteins were separated in a 10% SDS-PAGE gel and transferred to a polyvinylidene difluoride (PVDF) membrane. For co-IP of apoE and NS5A in the cells, a lysate of the HCV-infected cells was incubated with protein G-conjugated agarose beads (Invitrogen) bound with normal mouse IgG or apoB- or apoE-specific MAb. Antibody binding to protein G-conjugated beads was done by incubation with normal mouse IgG or apoB- or apoE-specific MAb at room temperature for 5 h. The unbound antibodies were removed by being washed with 1× PBS three times. The antibody-bound agarose beads were then incubated with 300 μl of cell lysate at 4°C overnight. Upon co-IP, HCV core and NSSA were detected by Western blotting using C- and NSSA-specific MAbs.
RESULTS
ApoB-specific antibodies do not neutralize HCV infectivity.
We have previously shown that infectious HCV particles are enriched in apoE and that apoE-specific MAbs efficiently neutralized HCV infectivity in a dose-dependent manner, suggesting that apoE incorporated into HCV particles is required for HCV infection (10). Several previous studies found that HCV is associated with lipoproteins in the plasma of hepatitis C patients (2, 3, 31, 32). Additionally, two recent studies suggested that apoB is important for HCV production in cell culture (19, 21). To examine the role of apoB in HCV infection, apoB-specific MAbs and polyclonal antibodies were used in HCV neutralization experiments. The commercially available apoB (46B1B3) blocking MAb was previously characterized (23). We also raised apoB-specific MAbs (apoB MAb3 and MAb4). Purified normal mouse IgG was used as a negative control. HCV neutralization experiments were carried out by mixing HCV with various amounts of antibodies prior to and during virus infection. At 3 days p.i., the levels of HCV NS3 protein and positive-strand HCV RNA in the HCV-infected cells were determined by Western blotting and RPA, respectively. Similar to normal mouse IgG, all apoB-specific MAbs and polyclonal antibodies at up to 50 μg/ml did not significantly reduce the level of HCV NS3 protein (Fig. 1A). Likewise, none of the apoB-specific antibodies affected the level of positive-strand HCV RNA (data not shown). More importantly, HCV titers in the medium of HCV-infected cells in the presence of increasing amounts of apoB-specific antibodies during infection were unaffected (Fig. 1B). In contrast, apoE MAb23 reduced the level of NS3 protein by 90% and lowered the HCV titer by 3 orders of magnitude (1,000 times) at 50 μg/ml, consistent with our previous findings (10). These results demonstrate that apoE- but not apoB-specific antibodies can neutralize HCV infectivity, suggesting that apoE but not apoB is important for HCV infection.
FIG. 1.
Effect of apoB-specific antibodies on HCV infectivity. HCV was incubated with increasing concentrations (0, 0.4, 2.0, 10, and 50 μg/ml) of normal mouse IgG, apoB polyclonal antibodies (rabbit and goat anti-apoB [α-ApoB]) or MAbs (464B1B3, apoB MAb3, and apoB MAb4), and apoE MAb23 at room temperature for 1 h, followed by infection of Huh7.5 cells. At 2 h p.i., the antibody-HCV mixture was removed, and the HCV-infected cells were washed twice with PBS and then incubated with DMEM containing 10% FBS. At 3 days p.i., the level of HCV NS3 protein in the cells was determined by Western blotting (A) and the titer of infectious HCV in the medium was determined by serial dilution and immunofluorescence staining for NS3-positive cell foci (B).
Inhibition of HCV assembly by siRNA-mediated knockdown of apoE expression.
Besides an important role in HCV infection, apoE is also required for HCV production, as demonstrated by siRNA-mediated knockdown of apoE expression and consequent suppression of HCV production (10). The question remains of whether apoE plays a role in HCV assembly and/or egression. In our previous studies, we used a multiple-cycle virus growth assay, which could not discriminate the role of apoE in infection from that in virion assembly/egression, since reduction of HCV infectivity upon the first round of virus replication will lower HCV production (10). Thus, a single-cycle HCV growth assay is necessary in order to dissect various steps of the HCV life cycle. Recently, we isolated an infectious HCV that grows to high titer (nearly 107 FFU/ml), which made it possible to determine the role of apoB and apoE in HCV virion assembly/egression. The molecular basis underlying the higher growth rate (titer) of the cell culture-adapted HCV has not been defined, although a number of amino acid mutations were identified in C, E1, NS2, NS3, NS5A, and NS5B, with most mutations clustered in NS5A (data not shown). Our ongoing work to determine the role of adaptive mutations in efficient virus growth will be reported separately. Our previous studies suggested that one round of HCV replication requires about 24 h (7). Therefore, Huh7.5 cells were infected with HCV at an MOI of approximately 5 at 37°C for 2 h and then transfected with apoB, apoE, or NSC siRNA at various concentrations up to 50 nM. At 24 h p.i., cell culture media were collected, and HCV-infected cells, with or without siRNA transfection, were lysed in RIPA buffer. The levels of apoB and apoE secreted into the medium were quantified by Western blotting (Fig. 2). The apoB-specific siRNA efficiently knocked down apoB expression, resulting in reductions of apoB-100 of 50%, 80%, and 90% at concentrations of 2, 10, and 50 nM, respectively (Fig. 2). Similarly, the apoE siRNA specifically reduced apoE, by 40%, 60%, and 80%, at 2, 10, and 50 nM, respectively (Fig. 2). In contrast, the levels of both apoB and apoE were unaffected by NSC siRNA (Fig. 2). The effects of siRNA-mediated knockdown of apoB and apoE expression on viral RNA replication were determined by measuring the NS3 protein in the HCV-infected and siRNA-transfected Huh7.5 cells. Similar to the NSC siRNA, neither apoB nor apoE siRNA had any significant effect on viral RNA replication, as the level of NS3 remained unchanged (Fig. 3A) . These results are consistent with our previous finding that siRNA-mediated knockdown of apoE expression did not affect HCV RNA replication, as determined in a subgenomic HCV RNA-harboring Huh7 cell line (10). To determine the effects of apoB and apoE knockdown on HCV production, the media of the HCV-infected and siRNA-transfected cells were used to infect naïve Huh7.5 cells. The levels of NS3 protein and positive-strand HCV RNA were then determined by Western blotting and RPA, respectively (Fig. 3B and C). Similar to the knockdown of apoE expression, the apoE-specific siRNA resulted in a remarkable reduction of infectious HCV in the medium, as shown by 80% and 95% decreases of the NS3 protein at 10 and 50 nM, respectively (Fig. 3B). The apoE siRNA lowered the level of positive-strand HCV RNA in the subsequently infected cells by 60% and 90% at 10 nM and 50 nM, respectively (Fig. 3C). In contrast, apoB and NSC siRNAs did not affect HCV production, even though the apoB-specific siRNA knocked down apoB-100 expression by 90% (Fig. 2). Additionally, infectious HCV titers were proportionally lowered by increasing concentrations of the apoE-specific siRNA, resulting in a 50-fold reduction of infectious HCV at 50 nM. However, the apoB and NSC siRNAs had no effect on the production of infectious HCV (Fig. 3D). These findings demonstrate that apoE but not apoB is required for the formation of infectious HCV.
FIG. 2.
Knockdown of apoB and apoE expression by specific siRNAs. (A) Determination of apoB and apoE secretion by Western blotting. Huh7.5 cells were infected with HCV and then transfected with apoB, apoE, or NSC siRNA at various concentrations, using Lipofectamine RNAiMax (Invitrogen) following the manufacturer's instructions. At 24 h p.i., the levels of apoB and apoE secreted into the medium were quantified by Western blotting using apoB (Biodesign) and apoE antibodies, respectively. (B) Correlation of apoB-100 secretion and siRNA concentration. The levels of apoB-100 relative to the control (without siRNA transfection) were converted to percentages of the control (100%). The relative levels of apoB-100 (y axis) were plotted against siRNA concentrations (x axis). (C) Correlation of apoE level and siRNA concentration. The relative levels of apoE were plotted (y axis) against siRNA concentrations (x axis). The relative levels of apoB and apoE shown in panels B and C are average values for three independent experiments.
FIG. 3.
Effects of siRNA-mediated knockdown of apoB and apoE expression on HCV replication and production. Huh7.5 cells were infected with HCV at an MOI of 5 and then transfected with apoB, apoE, or NSC siRNA as described in the legend to Fig. 2. At 24 h p.i., the medium was collected and cells were lysed in RIPA buffer. (A) Detection of NS3 protein in HCV-infected and siRNA-transfected cells by Western blotting using an NS3-specific MAb. (B and C) Influence of apoB and apoE siRNAs on HCV production. HCV in the medium of HCV-infected and siRNA-transfected cells was used to infect naïve Huh7.5 cells. The levels of NS3 protein (B) and positive-strand HCV RNA (C) were determined by Western blotting and RPA, respectively, as described in Materials and Methods. (D) Quantification of infectious HCV by serial dilution and IFA. HCV in the medium was serially diluted and used to infect naïve Huh7.5 cells on coverslips. The titers of infectious HCV were determined in FFU/ml as described for Fig. 1B. The titers of infectious HCV were plotted against siRNA concentrations. (E) Correlation of HCV vRNA level in the medium with siRNA concentration. HCV vRNA in the medium was extracted with Trizol reagent and quantified by real-time RT-PCR. The HCV vRNA level was calculated as a percentage of the control level (without siRNA). (F) Correlation of intracellular HCV titers with siRNA concentrations. Intracellular HCV particles were prepared from HCV-infected and siRNA-transfected Huh7.5 cells as described in Materials and Methods. Intracellular HCV titers were determined in the same way as for panel D. The titers of intracellular HCV were plotted against siRNA concentrations. The means ± standard deviations derived from three independent experiments were used for panels D to F. White bars, NSC siRNA; gray bars, apoB siRNA; black bars, apoE siRNA.
To further determine whether apoE is required for HCV assembly and/or egression, the levels of infectious HCV particles and HCV vRNA in the medium and in intracellular HCV particles were determined by IFA and a qRT-PCR method, respectively. HCV vRNA reflects all HCV particles, including infectious and noninfectious ones. Intracellular HCV particles were prepared by repeated freezing and thawing of the HCV-infected and siRNA-transfected cells, followed by centrifugation to remove any debris, as previously described (10). Like the case for infectious HCV, the level of HCV vRNA in the medium was decreased in proportion to the reduction of apoE expression, with about 60% reduction at 50 nM siRNA (Fig. 3E). However, apoB and NSC siRNAs did not influence the level of HCV vRNA in the medium, demonstrating that apoB is not required for HCV assembly/egression. Similarly, infectious titers of intracellular HCV particles were decreased >10-fold (Fig. 3F), consistent with the findings derived from the multiple-cycle HCV growth assay (10). Likewise, the level of HCV vRNA derived from intracellular HCV particles was decreased 60% at 50 nM apoE siRNA (data not shown). Again, apoB and NSC siRNAs did not affect the infectious titers and vRNA levels of intracellular HCV particles (Fig. 3F and data not shown). Taken together, these findings demonstrate that apoE but not apoB is required for HCV assembly in the cell.
To exclude a possible off-target effect of apoE siRNA on HCV assembly, we sought to determine whether ectopic expression of apoE would be able to restore HCV production in Huh7.5 cells with knockdown of endogenous apoE expression. A variant apoE-expressing cDNA (pCMV6XL5/mApoE) was constructed by introducing five silent nucleotide mutations into the siRNA targeting region. These nucleotide mutations do not change amino acids but instead disrupt the complementarity between apoE mRNA and the siRNA, therefore evading the siRNA-mediated destruction of apoE mRNA. As shown in Fig. 4A, apoE-specific siRNA efficiently reduced apoE expression and secretion. However, transfection of the apoE-expressing DNA into Huh7.5 cells with the siRNA-mediated knockdown of endogenous apoE expression resulted in similar levels of apoE in parental cells and cells transfected with NSC siRNA (Fig. 4A), indicating that apoE expressed ectopically was not affected by siRNA. Similar to the above findings, apoE siRNA and ectopic expression of apoE did not affect HCV replication, as shown by similar levels of HCV NS3 protein in the transfected cells (Fig. 4A). Strikingly, ectopic expression of apoE fully restored HCV production, as demonstrated by similar levels of both HCV NS3 protein and positive-strand RNA in Huh7.5 cells infected with HCV derived from the apoE siRNA- and cDNA-transfected cells compared to those in parental cells or cells transfected with NSC siRNA (Fig. 4B and C). These findings demonstrate that apoE is truly required for the formation of infectious HCV particles.
FIG. 4.
Ectopic expression of apoE restored HCV production, which was otherwise blocked by siRNA-mediated knockdown of endogenous apoE expression. Huh7.5 cells in 12-well plates were transfected with 50 nM of apoE or NSC siRNA. At 12 h posttransfection, pCMV6XL5/mApoE or vector DNA was transfected into cells with DMRIE-C (Invitrogen). After 12 h of incubation, Huh7.5 cells were infected with HCV at an MOI of approximately 5. At 24 h p.i., the medium was collected and cells were lysed in RIPA buffer. The medium was used for determinations of apoE levels and infectivity (HCV production). (A) Determination of levels of apoE secretion in the medium and of NS3 protein in the cells by Western blotting. Western blot analysis of apoE and HCV NS3 was performed as described in the legends to Fig. 2A and 3, respectively. (B and C) Restoration of HCV production by ectopic expression of apoE. HCV in the medium of siRNA-transfected or both siRNA- and apoE cDNA-transfected cells was used to infect naïve Huh7.5 cells. At 3 days p.i., the levels of NS3 protein (B) and positive-strand HCV RNA (C) were determined by Western blotting and RPA, respectively. The levels of positive-strand HCV RNA were quantified by phosphorimager analysis and converted into percentages of the control level, considering the level of HCV RNA without siRNA and apoE-expressing DNA as 100%. Numbers at the top of panels A and B and below panel C indicate the concentration of siRNA and the amount of DNA used in experiments.
Effects of MTP inhibitors on formation of HCV particles.
To confirm the findings derived from the above experiments with siRNAs, two MTP inhibitors, CP-346086 and BMS-2101038, were used to block the assembly and secretion of apoB-containing lipoproteins. MTP is essential for the assembly and secretion of apoB-containing lipoproteins (22). Previous studies by others suggested that the blockade of VLDLs by MTP inhibitors suppresses HCV production and maturation (19, 21). To verify the role of apoB in HCV assembly/egression, a multiple-cycle HCV growth assay was initially used to determine the effects of CP-346086 on apoB secretion and HCV production. CP-346086 is a potent MTP inhibitor, with a 50% effective concentration of 2.6 nM (9). Huh7.5 cells were infected with HCV and then incubated with CP-346086 at various concentrations for 3 days. At concentrations of up to 1 μM, CP-346086 resulted in a full blockade of apoB-100 secretion (Fig. 5A and C). However, it did not significantly affect apoE secretion, consistent with a recent report that an MTP inhibitor completely blocked apoB but did not affect apoE secretion (16). Contrary to previous reports (19, 21), CP-346086 did not affect HCV production at concentrations up to 1 μM, as shown by similar levels of NS3 protein (Fig. 5B and C) and positive-strand HCV RNA (data not shown) in the Huh7.5 cells infected with HCV derived from CP-346086-treated cells. Likewise, infectious HCV titers, which were determined by IFA, remained unchanged (Fig. 5D), even though apoB-100 secretion was completely blocked by CP-346086 at 1 μM (Fig. 5A and C). When tested at higher concentrations (3.125 to 25 μM), however, CP-346086 inhibited apoE secretion in a dose-dependent manner, reducing apoE secretion by 85% at 25 μM (Fig. 6A and C). As a consequence, HCV production was markedly suppressed, as determined by a proportional decrease of up to 90% of NS3 protein (Fig. 6B) and positive-strand RNA (data not shown) at 25 μM CP-346086 in the Huh7.5 cells that were infected with HCV derived from CP-340686-treated cells. The reduction of infectious HCV paralleled the decrease of apoE but not apoB secretion (Fig. 6C). CP-346086 reduced infectious HCV titers by up to 3 orders of magnitude (3 log) at concentrations from 3.125 to 25 μM (Fig. 6D). These findings demonstrate that suppression of HCV production by CP-346086 is due to the blockade of apoE but not apoB secretion, unlike the case in previous reports (19, 21).
FIG. 5.
Effect of CP-346086 at low concentrations on apoB and apoE secretion and HCV production in multiple-cycle HCV growth assays. Huh7.5 cells were infected with HCV at an MOI of 0.3 and then incubated with DMEM containing CP-346086 at concentrations varying from 0.008 to 1 μM. After 3 days p.i., the medium was collected for determination of apoB and apoE secretion and HCV production. (A) Determination of apoB and apoE secretion by Western blotting. (B) Determination of infectious HCV by infectivity assay. HCV in the medium was used to infect naïve Huh7.5 cells. At 3 days p.i., the level of HCV NS3 protein was determined by Western blotting. (C) Correlation of apoB-100 and apoE secretion with HCV production. The levels of apoB-100, apoE, and NS3 in panels A and B were quantified and converted to percentages of the control levels, considering the levels of apoB-100, apoE, and NS3 in the absence of CP-346086 as 100%. Relative levels of apoB-100, apoE, and NS3 were plotted against concentrations of CP-346086. (D) Determination of infectious HCV titers by IFA. Infectious HCV in the medium of HCV-infected and inhibitor-treated cells was titrated and quantified by IFA as FFU/ml.
FIG. 6.
Suppression of apoE secretion and HCV production by CP-346086 at high concentrations in multiple-cycle HCV growth assay. Huh7.5 cells were infected with HCV at an MOI of 0.3 and then treated with CP-346086 at various concentrations from 3.125 to 25 μM. After 3 days p.i., the medium was collected for determination of the levels of apoB-100 and apoE secretion and HCV production. (A) Levels of apoB-100 and apoE secretion. ApoB and apoE in the medium were detected by Western blotting. (B) Determination of infectious HCV by infectivity assay. HCV in the medium was used to infect naïve Huh7.5 cells. At 3 days p.i., NS3 was detected by Western blotting. (C) Correlation of apoB and apoE with HCV production. The data shown in panels A and B were quantified and converted to relative levels of apoB, apoE, and NS3 as percentages of the control values. Values are means ± standard deviations derived from three experiments. (D) Determination of infectious HCV titers by IFA. Infectious HCV titers (log FFU/ml) were plotted against CP-340686 concentrations (μM).
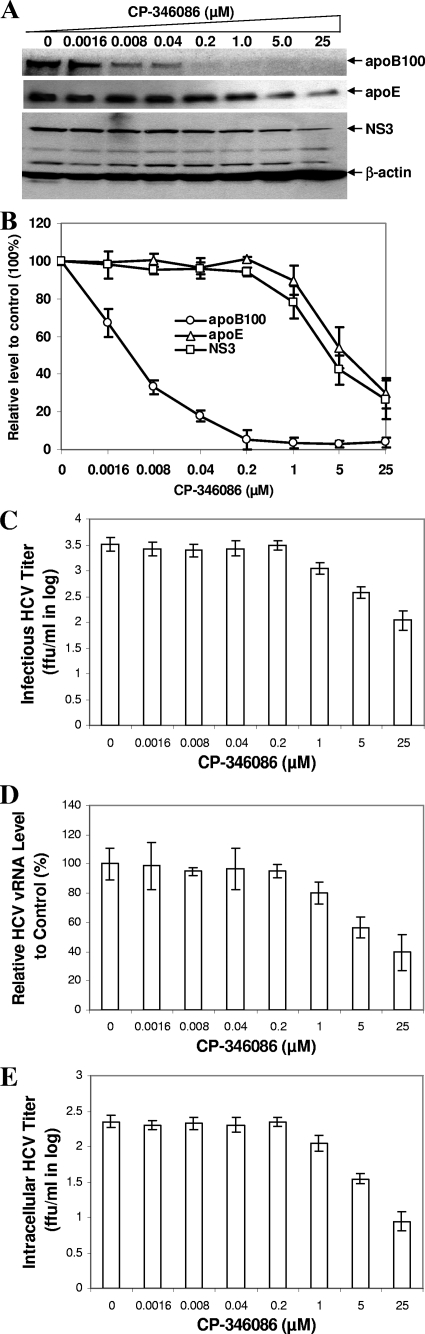
To verify that apoE but not apoB is required for the formation of infectious HCV particles, we repeated the experiments with MTP inhibitors, but using a single-cycle HCV growth assay. Huh7.5 cells were infected with HCV at a high MOI (MOI of 5) and then incubated with various concentrations (0.0016 to 25 μM) of CP-346086 for 24 h. Like the results obtained with the multiple-cycle HCV growth assay, CP-346086 remarkably reduced apoB-100 secretion, with a complete blockade of apoB-100 secretion at 0.2 μM, but did not affect HCV production (Fig. 7A). At concentrations above 0.2 μM, however, it blocked apoE secretion in a dose-dependent manner and consequently decreased HCV production by up to 70% (Fig. 7A). Infectious HCV titers were lowered by up to 1.5 log, in proportion to increasing concentrations up to 25 μM (Fig. 7C). The reduction of HCV production was in parallel with the decrease of apoE but not apoB secretion seen with CP-346086 (Fig. 7B). The level of HCV vRNA in the medium (Fig. 7D) was significantly reduced by CP-346086 only at higher (1 μM and above) concentrations. Also, the infectious titer of intracellular HCV was lowered by nearly 1.5 log (Fig. 7E). Using the single-cycle HCV growth assay, we also tested another MTP inhibitor, BMS-2101038, which was previously reported to inhibit HCV production (21). BMS-2101038 is a more potent MTP inhibitor than CP-346086, with a 50% inhibitory concentration of 0.5 nM (39). Similar to CP-346086, BMS-2101038 completely inhibited apoB secretion at 20 nM but did not influence HCV production. At higher concentrations, it blocked apoE secretion and therefore suppressed HCV production (Fig. 8A). Again, the suppression of HCV production superimposed the reduction of apoE but not apoB secretion (Fig. 8B). Infectious HCV titers and the levels of HCV vRNA in the medium and in intracellular HCV particles were reduced by BMS-2101038 only at concentrations higher than 100 nM (data not shown), consistent with the decrease of apoE secretion. All of these findings demonstrate that suppression of HCV assembly by MTP inhibitors was due to the blockage of apoE but not apoB secretion.
FIG. 7.
Effect of CP-346086 on HCV production in single-cycle HCV growth assays. Huh7.5 cells were infected with HCV at an MOI of 5 at 37°C for 2 h and then treated with CP-346086 at concentrations varying from 0.0016 to 25 μM. At 24 h p.i., the medium was collected for determination of the levels of apoB, apoE, and infectious HCV, while cells were harvested for preparation of intracellular HCV particles. (A) Effect of CP-346086 on apoB and apoE secretion and production of infectious HCV. ApoB-100 and apoE in the medium were detected by Western blotting. Infectious HCV in the medium was determined by infectivity assay, as described for Fig. 3 to 6. HCV NS3 protein in the subsequently infected Huh7.5 cells was detected by Western blotting. (B) Correlation of apoB-100 and apoE secretion with level of infectious HCV. The levels of apoB-100, apoE, and NS3 represent the means ± standard deviations derived from three different experiments, as shown in panel A. Relative levels of apoB-100, apoE, and NS3 were calculated as percentages of the control values. (C) Determination of infectious HCV titers by IFA. (D) HCV vRNA level relative to control level in the medium. The extraction and quantification of HCV vRNA in the medium were performed as described in the legend to Fig. 3. (E) Infectious titers of intracellular HCV particles determined by IFA. Intracellular HCV titers were plotted against CP-346086 concentrations (μM).
FIG. 8.
Effect of BMS-2101038 on HCV production in single-cycle HCV growth assays. Huh7.5 cells were infected with HCV at an MOI of 5 and then incubated with DMEM containing various concentrations of BMS-2101038. At 24 h p.i., the medium was collected and used to determine the levels of apoB-100 and apoE secretion and HCV production. (A) Levels of apoB and apoE determined by Western blotting. ApoB-100 and apoE in the medium were detected by Western blotting using apoB and apoE MAbs, respectively. HCV NS3 in Huh7.5 cells that were infected with medium derived from HCV-infected and BMS-2101038-treated cells was detected by Western blot analysis. (B) Correlation of apoB-100 and apoE secretion with HCV production. The levels of apoB-100, apoE, and NS3 relative to the control levels (without inhibitor treatment) were converted from the data shown in panel A and plotted against concentrations of BMS-2101038.
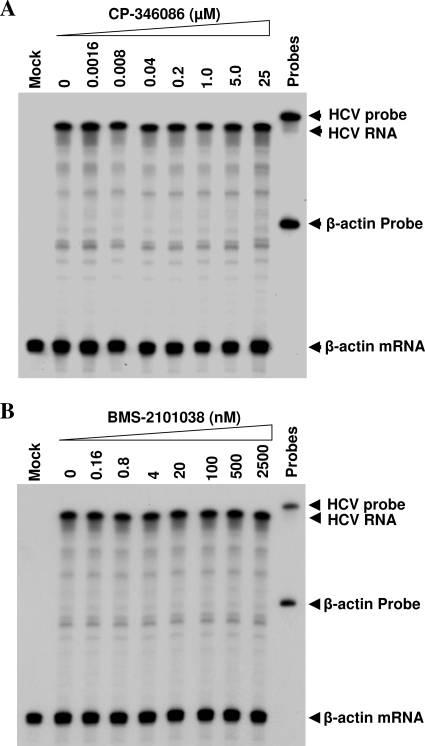
To exclude a possible inhibition of HCV RNA replication by MTP inhibitors, the levels of positive-strand HCV RNA in the HCV-infected and MTP inhibitor-treated Huh7.5 cells were determined by RPA. As shown in Fig. 9, neither CP-346086 nor BMS-2101038 had any effect on the level of HCV RNA, demonstrating that MTP inhibitors did not inhibit HCV RNA replication. Therefore, the reduction of HCV particles by MTP inhibition was due to the blockade of apoE secretion, which consequently suppressed the formation of HCV particles.
FIG. 9.
Effects of MTP inhibitors on HCV RNA replication. HCV infection and treatment with MTP inhibitors were the same as those described in the legends to Fig. 7 and 8. At 24 h p.i., total RNAs in the HCV-infected and MTP inhibitor-treated Huh7.5 cells were extracted with Trizol reagent. The levels of positive-strand HCV RNA were determined by RPA, using a radiolabeled RNA probe containing the negative-sense HCV 3′ UTR as described in Materials and Methods. The level of β-actin mRNA was used as a control for normalization of the amounts of total RNA used between samples. The concentrations of MTP inhibitors are indicated at the top. RNA probes and products are highlighted by arrows on the right. Mock, naive Huh7.5 cells without HCV infection.
Biochemical analysis of apoE in HCV particles by trypsin digestion and IP.
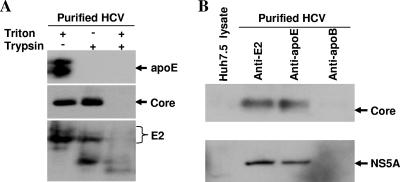
To determine the localization of apoE in HCV particles, a trypsin digestion experiment was carried out. Purified HCV particles were incubated with trypsin in the absence or presence of 1% Triton X-100 to disrupt the virus envelope. Upon trypsin digestion, apoE and HCV C (capsid) and E2 (envelope) proteins were detected by Western blotting using specific MAbs. Both apoE and HCV E2 were sensitive to trypsin digestion in the presence or absence of detergent treatment. It is not clear why E2 is less sensitive than apoE to trypsin (Fig. 10A). It is possible that protein folding and/or glycosylation may influence the sensitivity to trypsin digestion. In contrast, HCV C was resistant to trypsin digestion but became sensitive to trypsin when the viral envelope was disrupted by treatment with Triton X-100 (Fig. 10A). These results suggest that apoE is likely exposed on the viral envelope, similar to HCV E2 protein. Consistent with these observations, an apoE-specific MAb precipitated HCV particles, like HCV E2-specific MAb did (Fig. 10B), whereas apoB-specific MAb failed to do so. Interestingly, NS5A was also detected in the apoE and E2 MAb affinity-purified HCV particles, similar to the HCV C protein (Fig. 10B). Collectively, these findings suggest that apoE is likely a structural component of HCV particles.
FIG. 10.
Biochemical analysis of apoE in HCV particles. (A) Sensitivity of apoE to trypsin digestion. The preparation and purification of HCV particles were described in Materials and Methods. Purified HCV was treated with 4 μg/ml of trypsin (Sigma) in the absence or presence of 1% Triton X-100 at 37°C for 1 h. Trypsin reactions were terminated by the addition of 1 mM phenylmethylsulfonyl fluoride and a 1/100 volume of protease inhibitor cocktail (Roche). HCV C, E2, and apoE were detected by Western blotting using an ECL substrate (Pierce) and apoE-, C-, and E2-specific MAbs. (B) IP of HCV particles. HCV E2-, apoB-, and apoE-specific MAbs were individually coupled to AminoLink Plus coupling resin (Pierce). The antibody-conjugated resin was then incubated with purified HCV particles at 4°C overnight. Upon elution, proteins of HCV particles were separated by 10% SDS-PAGE, followed by transfer of proteins onto a PVDF membrane. HCV core and NS5A proteins were detected by Western blotting.
Interaction of apoE with NS5A in HCV-infected cells and in HCV particles.
To further understand the molecular basis underlying the importance of apoE in HCV assembly, co-IP experiments were performed to examine whether apoE interacts with a viral protein(s) in HCV particles and/or in HCV-infected cells. Initially, lysed HCV particles were incubated separately with apoB-, apoE-, and HCV protein-specific MAbs. As shown in Fig. 11A, an apoE-specific MAb specifically coprecipitated NS5A but not other viral proteins (data not shown), consistent with the previous finding that apoE and NS5A interact with each other, as shown by a yeast two-hybrid system (15). HCV E2- and apoB-specific MAbs were unable to coprecipitate NS5A. In HCV-infected Huh7.5 cells, NS5A was also coprecipitated by an apoE- but not apoB-specific MAb or normal mouse IgG (Fig. 11B). In contrast, apoE failed to precipitate NS5A when supernatants of subgenomic HCV replicon cells were used in the co-IP experiments (data not shown). These data suggest that the requirement of apoE for HCV production is probably through a specific interaction with NS5A during the process of HCV assembly.
FIG. 11.
Determination of apoE and NS5A interaction by co-IP. (A) Co-IP of NS5A with apoE in HCV particles. Purified HCV particles were lysed by treatment with M-PER protein extraction reagent (Pierce). The resulting HCV lysate was subjected to co-IP using apoE-, E2-, and apoB-specific MAbs as described in Materials and Methods. (B) Co-IP of NS5A with apoE in HCV-infected cells. A lysate of naïve or HCV-infected Huh7.5 cells was incubated with protein G-conjugated agarose beads (Invitrogen) bound with normal mouse IgG or apoB- or apoE-specific MAb. Upon extensive washing, precipitated proteins were separated by 10% SDS-PAGE and then transferred onto a PVDF membrane. HCV NS5A protein was subsequently detected by Western blotting using an NS5A-specific MAb.
DISCUSSION
We have previously shown that HCV grown in cell culture was enriched in apoE and that HCV infectivity was closely correlated with the level of apoE present in HCV particles (10). ApoE-specific MAbs neutralized HCV infectivity by nearly 90% and caused a reduction of infectious HCV titer of up to 4 orders of magnitude (10,000-fold). Additionally, our earlier work demonstrated that apoE is critically important for HCV production. Knockdown of apoE expression by an apoE-specific siRNA resulted in reductions of infectious HCV in the cells and in the medium by 10- and 100-fold, respectively (10). These earlier findings suggest that apoE has dual functions in cell entry (infection) and in formation and/or release of progeny HCV particles (assembly/egression). However, the previous findings were derived from multiple-cycle HCV growth assays using a low MOI of HCV for infection, in which infection and replication are coupled (10). Thus, the impairment of HCV infectivity by the lack of apoE could have resulted in a subsequent reduction of HCV production upon multiple rounds of HCV replication. In the present study, we used a single-cycle virus growth assay which measured only one round of HCV replication. In this report, we provide substantial evidence to demonstrate that apoE is likely a structural component of infectious HCV and plays an essential role in the formation of infectious HCV particles. First of all, knockdown of apoE expression by a specific siRNA resulted in a reduction of HCV by 50 times in a single round of virus replication (Fig. 2 and 3A). More importantly, HCV particles released into the medium as well as intracellular HCV particles were equally suppressed by siRNA-mediated downregulation of apoE expression (Fig. 3). However, the reduction of HCV production resulting from the siRNA-mediated knockdown of endogenous expression of apoE could be restored fully by ectopic expression of apoE (Fig. 4). Additionally, MTP inhibitors suppressed HCV production only when they blocked apoE production and/or secretion (Fig. 6, 7, and 8). Suppression of HCV production by MTP inhibitors was superimposed with the reduction of apoE but not apoB secretion (Fig. 6C, 7B, and 8B), demonstrating that HCV assembly and maturation require apoE but not VLDLs, in which apoB is a major component. Furthermore, biochemical analysis of purified HCV particles by trypsin digestion and IP suggested that apoE is a structural component of infectious HCV. Similar to our previous finding (10), apoE- but not apoB-specific MAb could precipitate HCV particles. More significantly, apoE was sensitive to protease digestion, similar to HCV E2, suggesting that apoE is likely exposed on the viral envelope. This hypothetical topology of apoE explains its dual functions in HCV entry and virion assembly. Of course, the ultimate proof for the localization of apoE in HCV particles awaits structural characterization of HCV particles.
The roles of lipoproteins in the HCV life cycle have not been defined and have remained somewhat controversial in the field. A number of previous studies suggested that HCV is associated with lipoproteins such as VLDLs in the plasma of hepatitis C patients (2, 3, 31, 32, 35). Studies with HCV pseudotype particles found that high-density lipoproteins were able to enhance the entry of HCV pseudotype particles into cells (13, 36), whereas oxidized LDLs inhibited HCV infection (37). ApoCI was also shown to associate with HCV, and an apoCI-specific antibody could inhibit HCV infection (12, 28), suggesting that lipoproteins or some apoproteins may modulate HCV infection. It is possible that lipoproteins in association with HCV found in patients may play a role in viral pathogenesis, probably by masking the host immune response and therefore facilitating persistent HCV infection. However, findings derived from our present study suggest that HCV infection, replication, and production do not depend on the assembly and secretion of VLDLs, unlike the case suggested in recent studies (19, 21). Both apoB-specific siRNA and MTP inhibitors could completely block the assembly and secretion of apoB-containing lipoproteins but did not affect HCV production. HCV production was suppressed by MTP inhibitors only at high concentrations at which apoE expression and/or secretion was blocked. Suppression of the formation of infectious HCV by MTP inhibitors was superimposed with the reduction of apoE but not apoB. In studies reported by others, MTP inhibitors were tested only at high concentrations, which are far beyond their 50% inhibitory concentrations/effective doses for inhibition of VLDL assembly and secretion. Both our studies and these previous studies had similar findings showing that high concentrations of MTP inhibitors suppressed HCV assembly, although the studies reached different conclusions. In the previous studies, the role of apoB in HCV assembly and maturation was cofounded by the reduction of apoE. In contrast to the findings derived from studies with apoB-specific siRNA, we did not detect any effect of siRNA-mediated knockdown of apoB expression on HCV production in a single-cycle HCV growth assay. However, we did observe that the apoB-specific siRNA reduced HCV production in multiple-cycle HCV growth assays. In this case, we also noticed that apoB-specific siRNA caused significant cytotoxicity 3 to 4 days after siRNA transfection. There is a close correlation between cytotoxicity and HCV reduction in multiple-cycle HCV growth assays (data not shown). This may explain the discrepancy between our findings and previous findings on the role of apoB in the HCV life cycle, as suggested by siRNA-mediated knockdown of apoB expression. Nevertheless, findings derived from single-cycle HCV growth assays clearly demonstrate that knockdown of apoB expression does not affect HCV production, suggesting that apoB does not play a significant role in HCV assembly and/or maturation.
It is not known why MTP inhibitors completely blocked the assembly and secretion of apoB-containing lipoproteins without having an influence on apoE secretion. In general, it is thought that MTP is responsible for the assembly and secretion of both apoB- and apoE-containing lipoproteins, through different steps of the assembly pathway (17). However, findings derived from our studies demonstrate a clear difference in the sensitivities of apoB and apoE to MTP inhibitors. Our findings are consistent with those recently reported by others showing that an MTP inhibitor completely suppressed apoB but had no effect on apoE secretion (16). It is possible that high concentrations of MTP inhibitors may act on a different pathway from the MTP pathway, resulting in apoE reduction. MTP inhibitors at high concentrations are toxic to cells after long incubations (data not shown). It will be interesting to determine which cellular proteins are involved in apoE assembly and secretion, which may present a novel target(s) for antiviral drug discovery and development for controlling HCV infection.
The question remains of how apoE mediates two distinct processes, cell entry and virion assembly, in the HCV life cycle. ApoE is a 34-kDa (299 amino acids) apoprotein containing a 22-kDa N-terminal domain (residues 1 to 191) that is recognized by receptors and a 10-kDa C-terminal domain (residues 222 to 299) that interacts with phospholipids (29, 30, 38). Based on its structure and roles in lipoprotein homeostasis, it is conceivable that the receptor-binding domain of apoE may mediate HCV infection, while the C-terminal domain associated with lipids is likely involved in HCV assembly. Our studies have found that apoE interacts with HCV NS5A in the cell and in HCV particles, suggesting that a specific protein-protein interaction between apoE and NS5A may determine the outcome of HCV assembly. Supportive of this concept, recent genetic studies have demonstrated that NS5A also functions in HCV assembly/production, besides its important role in viral RNA replication (4, 34). Domain III of NS5A was found to be essential for HCV production even though it is not critical to viral RNA replication (4). More interestingly, a single mutation at a phosphorylation site in domain III of NS5A ablated HCV production, although it had no effect on viral RNA replication (34). Based on these findings, we believe that the apoE-NS5A interaction is important for the formation of HCV particles and represents another unique target for antiviral drug discovery against HCV infection. Future studies are warranted to determine how apoE and NS5A interact with each other and whether mutations disrupting the apoE-NS5A interaction will result in an ablation of HCV assembly.
Acknowledgments
We thank Charles M. Rice (Rockefeller University) for providing the Huh7.5 cell line and NS5A MAb, Mike Diamond (Washington University) for HCV E2 MAbs, Jake Liang (NIDDK/NIH) for core MAb, Takaji Wakita (National Institute of Health, Japan) for the pSGR/JFH1 replicon cDNA, Lei Cai and Deneys R. van der Westhuyzen for apoB polyclonal antibodies, Jin Ye (UT Southwestern Medical Center) for the inhibitor BMS-2101038, and Wei Cun for construction of pCMV6XL5/mApoE.
This work was supported by NIH grants AI070769 and DK079293.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Alter, M. J. 2006. Epidemiology of viral hepatitis and HIV co-infection. J. Hepatol. 44:S6-S9. [DOI] [PubMed] [Google Scholar]
- 2.Andre, P., F. Komurian-Pradel, S. Deforges, M. Perret, J. L. Berland, M. Sodoyer, S. Pol, C. Brechot, G. Paranhos-Baccala, and V. Lotteau. 2002. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J. Virol. 76:6919-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre, P., G. Perlemuter, A. Budkowska, C. Brechot, and V. Lotteau. 2005. Hepatitis C virus particles and lipoprotein metabolism. Semin. Liver Dis. 25:93-104. [DOI] [PubMed] [Google Scholar]
- 4.Appel, N., M. Zayas, S. Miller, J. Krijnse-Locker, T. Schaller, P. Friebe, S. Kallis, U. Engel, and R. Bartenschlager. 2008. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 4:e1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1975. [DOI] [PubMed] [Google Scholar]
- 6.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76:13001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai, Z., C. Zhang, K. S. Chang, J. Jiang, B. C. Ahn, T. Wakita, T. J. Liang, and G. Luo. 2005. Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. J. Virol. 79:13963-13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. 1998. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Morb. Mortal. Wkly. Rep. 47:1-39. [PubMed] [Google Scholar]
- 9.Chandler, C. E., D. E. Wilder, J. L. Pettini, Y. E. Savoy, S. F. Petras, G. Chang, J. Vincent, and H. J. Harwood, Jr. 2003. CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J. Lipid Res. 44:1887-1901. [DOI] [PubMed] [Google Scholar]
- 10.Chang, K. S., J. Jiang, Z. Cai, and G. Luo. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J. Virol. 81:13783-13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 12.Dreux, M., B. Boson, S. Ricard-Blum, J. Molle, D. Lavillette, B. Bartosch, E. I. Pecheur, and F. L. Cosset. 2007. The exchangeable apolipoprotein ApoC-I promotes membrane fusion of hepatitis C virus. J. Biol. Chem. 282:32357-32369. [DOI] [PubMed] [Google Scholar]
- 13.Dreux, M., T. Pietschmann, C. Granier, C. Voisset, S. Ricard-Blum, P. E. Mangeot, Z. Keck, S. Foung, N. Vu-Dac, J. Dubuisson, R. Bartenschlager, D. Lavillette, and F. L. Cosset. 2006. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J. Biol. Chem. 281:18285-18295. [DOI] [PubMed] [Google Scholar]
- 14.El-Hage, N., and G. Luo. 2003. Replication of hepatitis C virus RNA occurs in a membrane-bound replication complex containing nonstructural viral proteins and RNA. J. Gen. Virol. 84:2761-2769. [DOI] [PubMed] [Google Scholar]
- 15.Evans, M. J., C. M. Rice, and S. P. Goff. 2004. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc. Natl. Acad. Sci. USA 101:13038-13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan, D., S. Qiu, C. D. Overton, P. G. Yancey, L. L. Swift, W. G. Jerome, M. F. Linton, and S. Fazio. 2007. Impaired secretion of apolipoprotein e2 from macrophages. J. Biol. Chem. 282:13746-13753. [DOI] [PubMed] [Google Scholar]
- 17.Fisher, E. A., and H. N. Ginsberg. 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 277:17377-17380. [DOI] [PubMed] [Google Scholar]
- 18.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 19.Gastaminza, P., G. Cheng, S. Wieland, J. Zhong, W. Liao, and F. V. Chisari. 2008. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J. Virol. 82:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gastaminza, P., S. B. Kapadia, and F. V. Chisari. 2006. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J. Virol. 80:11074-11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, H., F. Sun, D. M. Owen, W. Li, Y. Chen, M. Gale, Jr., and J. Ye. 2007. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. USA 104:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain, M. M., J. Iqbal, K. Anwar, P. Rava, and K. Dai. 2003. Microsomal triglyceride transfer protein: a multifunctional protein. Front. Biosci. 8:s500-s506. [DOI] [PubMed] [Google Scholar]
- 23.Krul, E. S., M. J. Tikkanen, T. G. Cole, J. M. Davie, and G. Schonfeld. 1985. Roles of apolipoproteins B and E in the cellular binding of very low density lipoproteins. J. Clin. Investig. 75:361-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenbach, B. D., and C. M. Rice. 2005. Unravelling hepatitis C virus replication from genome to function. Nature 436:933-938. [DOI] [PubMed] [Google Scholar]
- 25.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 26.Luo, G., S. Xin, and Z. Cai. 2003. Role of the 5′-proximal stem-loop structure of the 5′ untranslated region in replication and translation of hepatitis C virus RNA. J. Virol. 77:3312-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 28.Meunier, J. C., R. S. Russell, R. E. Engle, K. N. Faulk, R. H. Purcell, and S. U. Emerson. 2008. Apolipoprotein c1 association with hepatitis C virus. J. Virol. 82:9647-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow, J. A., K. S. Arnold, J. Dong, M. E. Balestra, T. L. Innerarity, and K. H. Weisgraber. 2000. Effect of arginine 172 on the binding of apolipoprotein E to the low density lipoprotein receptor. J. Biol. Chem. 275:2576-2580. [DOI] [PubMed] [Google Scholar]
- 30.Morrow, J. A., M. L. Segall, S. Lund-Katz, M. C. Phillips, M. Knapp, B. Rupp, and K. H. Weisgraber. 2000. Differences in stability among the human apolipoprotein E isoforms determined by the amino-terminal domain. Biochemistry 39:11657-11666. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen, S. U., M. F. Bassendine, A. D. Burt, C. Martin, W. Pumeechockchai, and G. L. Toms. 2006. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J. Virol. 80:2418-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen, S. U., M. F. Bassendine, C. Martin, D. Lowther, P. J. Purcell, B. J. King, D. Neely, and G. L. Toms. 2008. Characterization of hepatitis C RNA-containing particles from human liver by density and size. J. Gen. Virol. 89:2507-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson, B., G. Myers, C. Howard, T. Brettin, J. Bukh, B. Gaschen, T. Gojobori, G. Maertens, M. Mizokami, O. Nainan, S. Netesov, K. Nishioka, T. Shin-I, P. Simmonds, D. Smith, L. Stuyver, and A. Weiner. 1998. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. Arch. Virol. 143:2493-2503. [DOI] [PubMed] [Google Scholar]
- 34.Tellinghuisen, T. L., K. L. Foss, and J. Treadaway. 2008. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 4:e1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomssen, R., S. Bonk, C. Propfe, K. H. Heermann, H. G. Kochel, and A. Uy. 1992. Association of hepatitis C virus in human sera with beta-lipoprotein. Med. Microbiol. Immunol. 181:293-300. [DOI] [PubMed] [Google Scholar]
- 36.Voisset, C., A. Op de Beeck, P. Horellou, M. Dreux, T. Gustot, G. Duverlie, F. L. Cosset, N. Vu-Dac, and J. Dubuisson. 2006. High-density lipoproteins reduce the neutralizing effect of hepatitis C virus (HCV)-infected patient antibodies by promoting HCV entry. J. Gen. Virol. 87:2577-2581. [DOI] [PubMed] [Google Scholar]
- 37.von Hahn, T., B. D. Lindenbach, A. Boullier, O. Quehenberger, M. Paulson, C. M. Rice, and J. A. McKeating. 2006. Oxidized low-density lipoprotein inhibits hepatitis C virus cell entry in human hepatoma cells. Hepatology 43:932-942. [DOI] [PubMed] [Google Scholar]
- 38.Wetterau, J. R., L. P. Aggerbeck, S. C. Rall, Jr., and K. H. Weisgraber. 1988. Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J. Biol. Chem. 263:6240-6248. [PubMed] [Google Scholar]
- 39.Wetterau, J. R., R. E. Gregg, T. W. Harrity, C. Arbeeny, M. Cap, F. Connolly, C. H. Chu, R. J. George, D. A. Gordon, H. Jamil, K. G. Jolibois, L. K. Kunselman, S. J. Lan, T. J. Maccagnan, B. Ricci, M. Yan, D. Young, Y. Chen, O. M. Fryszman, J. V. Logan, C. L. Musial, M. A. Poss, J. A. Robl, L. M. Simpkins, W. A. Slusarchyk, R. Sulsky, P. Taunk, D. R. Magnin, J. A. Tino, R. M. Lawrence, J. K. Dickson, Jr., and S. A. Biller. 1998. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science 282:751-754. [DOI] [PubMed] [Google Scholar]
- 40.WHO. 1998. W. H. O. concerns on hepatitis C. Lancet 351:1415. [Google Scholar]
- 41.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]