Abstract
We created a hybrid adeno-associated virus (AAV) from two related rhesus macaque isolates, called AAVrh32.33, and evaluated it as a vaccine carrier for human immunodeficiency virus type 1 (HIV-1) and type A influenza virus antigens. The goal was to overcome the limitations of vaccines based on other AAVs, which generate dysfunctional T-cell responses and are inhibited by antibodies found in human sera. Injection of a Gag-expressing AAVrh32.33 vector into mice resulted in a high-quality CD8+ T-cell response. The resulting Gag-specific T cells express multiple cytokines at high levels, including interleukin-2, with many having memory phenotypes; a subsequent boost with an adenovirus vector yielded a brisk expansion of Gag-specific T cells. A priming dose of AAVrh32.33 led to high levels of Gag antibodies, which exceed levels found after injection of adenovirus vectors. Importantly, passive transfer of pooled human immunoglobulin into mice does not interfere with the efficacy of AAVrh32.33 expressing nucleoproteins from influenza virus, as measured by protection to a lethal dose of influenza virus, which is consistent with the very low seroprevalence to this virus in humans. Studies of macaques with vectors expressing gp140 from HIV-1 (i.e., with AAVrh32.33 as the prime and simian adenovirus type 24 as the boost) demonstrated results similar to those for mice with high-level and high-quality CD8+ T-cell responses to gp140 and high-titered neutralizing antibodies to homologous HIV-1. The biology of this novel AAV hybrid suggests that it should be a preferred genetic vaccine carrier, capable of generating robust T- and B-cell responses.
The initial interest in vectors based on adeno-associated viruses (AAV) was for applications in gene therapy. Most of the initial work was with vectors derived from AAV serotype 2 (AAV2), which is one of the six initial isolates. In the first in vivo studies, several groups showed stable expression of the transgene Escherichia coli β-galactosidase following intramuscular (i.m.) injection of AAV2-LacZ without immune responses to the transgene (23, 44). The apparent tolerance of the host to AAV-encoded antigens to a variety of transgene products has been demonstrated in mice and some large animals (1, 35, 39). Several mechanisms have been proposed to explain the lack of T-cell responses following in vivo gene transfer with AAV, including ignorance (inadequate presentation of antigen), anergy, and suppression (1, 5, 18, 37).
As applications of AAV vectors for in vivo gene transfer expanded, it became clear that the apparent immune privilege of AAV transgene products was not absolute. A number of examples emerged in which the host mounted vibrant T-cell responses to AAV-encoded transgene products. Several key parameters appeared to influence immunogenicity of the transgene. For example, Sarukhan et al. suggest that the subcellular localization of the protein influences the magnitude of the ensuing T-cell response after AAV gene transfer (37). The dose and route of administration of the AAV vector also contribute significantly to B- and T-cell responses to the transgene (3, 13). Wang et al. showed that inflammation at the site of AAV administration promotes antigen-specific immune responses to the transgene (47). A consistent observation has been that B-cell responses to AAV-encoded transgenes are much more intense and more consistently generated than CD8+ T-cell responses (8, 46, 51). A number of investigators have begun to explore AAV vectors as genetic vaccines against a variety of infectious and noninfectious diseases, based on the notion that it can be developed to stimulate transgene immune responses (14, 22, 26, 28, 48-50).
The discovery of an expanded family of AAV capsids from human and nonhuman primates has provided an opportunity to evaluate the effects of capsid structure on vector performance. Most of this work has focused on the use of novel AAV serotypes for achieving higher levels of transgene expression for applications in gene therapy (7, 12, 36). Xin et al. recently evaluated, in mice, vectors as vaccines for human immunodeficiency virus type 1 (HIV-1) based on the original AAV isolates AAV1 to AAV6 and two novel AAVs we recently discovered, AAV7 and AAV8 (48). They showed significant capsid-dependent effects on T- and B-cell responses to HIV-1 gp160. We recently confirmed these observations and more thoroughly evaluated the quality of the CD8+ T-cell responses (26). AAV vectors of multiple serotypes encoding HIV-1 Gag were injected i.m. into mice, which all showed some level of CD8+ T-cell responses based on tetramer staining and peptide-induced gamma interferon (IFN-γ) expression. However, the quality of AAV-induced, Gag-specific T cells was substantially lower than that obtained with adenoviral vectors, based on several criteria. A majority of the tetramer-positive (Tet+) T cells were nonresponsive to antigen, and those that did respond to antigen produced low levels of IFN-γ and no interleukin-2 (IL-2). Very few memory T cells were generated, and animals primed with AAV vectors were not responsive to a boost with an adenoviral vector. However, all AAV serotypes studied did generate very high levels of antibodies to the Gag transgene product.
A final issue to consider in the use of AAV as a genetic vaccine for HIV-1 is the presence of neutralizing antibodies (NAbs) to the vector due to prior AAV infections. We recently conducted an extensive screening of human populations from several continents and found high prevalence and high titers of NAbs to AAV1 and AAV2 and moderate levels of NAbs to AAV7 and -8 (4). In vivo gene transfer experiments indicate that AAV NAbs will likely impinge on vector efficacy (9, 33, 38).
This study describes the creation of a novel AAV from rhesus macaque isolates, called AAVrh32.33, and its characterization as a genetic vaccine for HIV-1. AAVrh32.33 has properties unlike those of any others we have studied. We showed that vectors based on this novel capsid elicit strong CD8+ T-cell responses to reporter transgene products that are dependent on CD4+ T-cell help and dependent on signaling through CD40L and CD28 (L. E. Mays and J. M. Wilson, submitted for publication). Important to the use of this vector in the clinic is a very low incidence of NAbs to it in human populations. This study describes the development of vectors based on AAVrh32.33 as genetic vaccines.
MATERIALS AND METHODS
Mice and immunization.
All animal procedures were performed in accordance with protocols approved by the institutional animal care and use committees of the University of Pennsylvania. BALB/c and CB6F1 hybrid mice (6 to 8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). Gag-treated mice were immunized with recombinant AAV or SAdV24 vectors diluted in 50 μl of phosphate-buffered saline (PBS) administered i.m. in the hind limb.
Vaccine vectors.
AAVrh32.33 was constructed as a hybrid of rh.32 and rh.33 (11) by cloning a BsiWI-BbvCI-restricted rh.33 fragment into AAV2/rh.32 (42). The gag transgene for all vectors is driven from a cytomegalovirus promoter and is procured on a traditional single-stranded AAV genome. All AAV and SAdV24 vectors used in this study were manufactured as described previously (20, 45) by Penn Vector at the University of Pennsylvania (Philadelphia, PA).
Peptides.
The H-2d-restricted immunodominant cytotoxic T-lymphocyte (CTL) epitope contained in the p24 portion of the Gag protein consists of amino acids 197 to 205 (AMQMLKETI). This peptide was synthesized by Mimotopes (Clayton, Victoria, Australia) and dissolved in dimethyl sulfoxide at 1 mg/ml. Dimethyl sulfoxide concentrations were kept below 0.1% (vol/vol) in all final assay mixtures. The gp140 peptide pool (NIH AIDS Research & Reference Reagent Program) used in this study spans the HIV-1 envelope gp140 protein and comprised 15 amino acid peptides overlapped by 10 amino acids. Each peptide in a pool was present at a 1-μg/ml concentration.
MHC class I tetramer staining and phenotypic analysis.
The phycoerythrin (PE)-conjugated major histocompatibility complex (MHC) class I H2-Kd-AMQMLKETI tetramer complex was obtained from Beckman Coulter (Fullerton, CA). Tetramer staining was performed as described previously (26). In brief, the isolated lymphocytes from the spleen or whole blood were costained for 30 min at room temperature with PE-conjugated tetramer and fluorescein isothiocyanate (FITC)-conjugated anti-CD8α+ (Ly-2) (BD Biosciences Pharmingen, San Diego, CA), PE-Cy5-conjugated anti-CD127, and PE-Cy7-conjugated anti-CD62L antibodies (eBioscience, CA). Red blood cells were then lysed with iTAg MHC tetramer lysing solution, and lymphocytes were fixed with fix solution (Beckman Coulter) for 10 min at room temperature. Data were acquired with an FC 500 flow cytometer and were analyzed with FlowJo software (Tree Star, San Carlos, CA). Tet+ CD8+ T cells were further used for phenotypic analysis based on the staining of anti-mouse CD127 and CD62L antibodies.
Gag p24 enzyme-linked immunosorbent assay.
Polypropylene plates (96 wells) (USA Scientific, Inc., FL) were coated with 100 μl of 0.5 μg/ml recombinant HIV-1 IIIB Gag p24 (ImmunoDiagnostics, Inc.) overnight at 4°C. The plates were blocked with PBS containing 3% fetal calf serum for 2 h at 25°C. Serum samples were twofold serially diluted with PBS containing 1% fetal calf serum and added to the plates for 2 h of incubation at 25°C. The plates were then washed three times with PBS containing 0.1% Tween 20 and incubated for 1 h with anti-mouse immunoglobulin G (IgG)-peroxidase (diluted 1:1,000 in PBS) (Sigma). Following three washes, the plates were incubated with 100 μl/well of 3,3′,5,5′-tetramethylbenzidine substrate (Sigma) for 15 min at room temperature. The reaction was stopped by using 100 μl/well of stop reagent (Sigma). The optical density was measured at a wavelength of 450 nm. The IgG titers are reported as the highest serum dilution with detectable signal over background, as measured by the optical density at 450 nm.
Intracellular cytokine staining (ICS) combined with CD107 mobilization/degranulation assay.
Measurement of cytokine production and degranulation marker CD107a in CD8+ T cells was performed by combined surface and intracellular staining with monoclonal antibodies and subsequent five-color flow cytometric analysis. Cytokine-secreting CD8+ T cells were detected using the protocol recommended by the manufacturer (Cytofix/Cytoperm Plus kit; BD Pharmingen, CA). In brief, the obtained lymphocytes (1 × 106 lymphocytes/sample) were incubated with 4 μg/ml of the HIV Gag peptide (AMQMLKETI) for 5 h at 37°C in 96-well round-bottom microtiter plates in Dulbecco modified Eagle medium supplemented in the presence of monensin (1 μg/ml), FITC-conjugated anti-CD107a (5 μg/ml) (eBioscience, CA), and GolgiPlug (1 μg/ml) (BD Pharmingen, San Diego, CA) throughout the assay period. Cells were washed and stained with PE-Texas Red-conjugated anti-mouse CD8α+ antibody (Ly-2), permeabilized in 100 μl of Cytofix/Cytoperm solution at 4°C for 20 min, and stained with anticytokine antibodies, including anti-mouse IFN-γ PE, tumor necrosis factor alpha (TNF-α) PE-Cy7, and IL-2 allophycocyanin (BD Biosciences Pharmingen, CA), at 4°C for 30 min, followed by flow cytometric analysis.
For the monkey ICS assay, 106 lymphocytes were incubated with 1 μg of gp140 peptide library in the presence of purified antibodies to CD49d and CD28 (BD Biosciences) and GolgiPlug (BD Pharmingen) for 6 h. Cells were washed and stained with phycoerythrin-Texas Red-CD8+ (Beckman Coulter) and allophycocyanin-CD4+ (BD Pharmingen) anti-human antibodies. Cells were washed, permeabilized with 250 μl of Cytofix/Cytoperm solution at 4°C for 20 min, washed with Perm/Wash solution, and stained with anticytokine antibodies, including FITC-TNF-α (BD Pharmingen), PE-IL-2 (Beckman Coulter), and PE-Cy7-IFN-γ (BD Pharmingen) at 4°C for 30 min. Cells were washed and examined by flow cytometry (FC 500; Beckman Coulter), and the data were analyzed using FlowJo software (Tree Star, OR).
Mouse model reconstituted by human Ig.
In this model, CB6F1 mice were intravenously transferred with various doses of human pooled intravenous Ig (ZLB, Switzerland) or PBS control prior to vaccination. Transfer was done twice, at 24 h and 2 h prior to immunization. The recipient mouse sera were harvested immediately prior to immunization via retro-orbital bleeding for NAb titer detection in vitro. Subsequently, serum-recipient mice were immunized with AAV2, -7, -8, or -rh32.33 vaccine expressing HIV Gag at a dose of 3 × 1010 genome copies (GC) per mouse. Gag immunoreactivity was characterized by Gag tetramer staining and Gag p24 antibody production.
IFN-γ ELISPOT assays.
MultiScreen 96-well titration plates (Millipore) were coated overnight with antibody to monkey IFN-γ (clone GZ-4; Mabtech) in PBS. Plates were washed and then blocked for 1 h with complete medium (RPMI medium containing 10% fetal bovine serum). Plates were washed with plain RPMI medium, and lymphocytes were seeded in 100 μl of complete medium at 2 × 105 cells per well. Stimulant peptide pools were added to each well to a final concentration of 0.2 μg/ml of each peptide in 100 μl of complete medium. Cells were incubated at 37°C for 20 h under 5% CO2. Plates were washed (PBS with 0.05% Tween 20) and then incubated with biotinylated antibody to monkey IFN-γ (clone B6-1; Mabtech) diluted in washing buffer containing 2% fetal bovine serum. Plates were incubated for 2 h and then washed. Avidin horseradish peroxidase (Vector Laboratories) was added to each well, and plates were incubated for 1 h. Plates were washed, and spots were developed with 3-amino-9-ethylcarbazole substrate (BD Biosciences). Spots were counted with an automated enzyme-linked immunospot (ELISPOT) reader (AID). Phytohemagglutinin, phorbol ester, and phorbol myristate acetate plus ionomycin were included as positive controls in each analysis. Only ELISPOT counts greater than 55 spot-forming units (SFU)/106 lymphocytes and with values three times over the background were considered positive.
HIV-1 neutralization assay.
Sera were also tested for HIV-1 NAbs by measuring reductions in luciferase reporter gene expression after a single round of virus infection in TZM-bl cells as described previously (24). TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu. Briefly, 200 50% tissue culture infective doses of virus was incubated with serial threefold dilutions of heat-inactivated (56°C, 1 h) test sera in duplicate in a total volume of 150 μl for 1 h at 37°C in 96-well flat-bottom culture plates. Freshly trypsinized cells (10,000 cells in 100 μl of growth medium containing 75 μg/ml DEAE dextran) were added to each well. One set of control wells received cells plus virus (virus control) and another set received only cells (background control). After incubation for 48 h, 100 μl of cells was transferred to 96-well black solid plates (Costar) for measurements of luminescence using the Britelite luminescence reporter gene assay system (PerkinElmer Life Sciences). Neutralization titers were defined as the dilution at which relative luminescence units were reduced by 50% compared to those of virus control wells after subtraction of background relative luminescence units. Assay stocks of molecularly cloned Env-pseudotyped viruses 6535.3, QH0692.42, SC422661.8, PVO.4, and TRO.11 were prepared by transfection in 293T cells and titrated in TZM-bl cells as described above; these Envs belong to a standard panel of clade B reference strains (24). An assay stock of uncloned W61D-TCLA was prepared in SupT1 cells and titrated in TZM-bl cells.
Histopathology and immunostaining.
For histopathological examination, frozen sections of gastrocnemius muscle were stained with hematoxylin and eosin. The size and number of infiltration sites together with the presence of necrotic fibers were rated by two investigators on a scale from − (normal muscle observed in naive mice) to ++++ (most extensive inflammation observed).
To analyze the cell types within infiltrates, immunostaining and confocal microscopy were performed. Frozen sections were fixed in acetone at −20°C for 5 min, blocked in PBS containing 1% donkey serum, and incubated with rat antibodies against CD8+ (1:20 dilution; BD Pharmingen) followed by tetramethyl rhodamine isocyanate-labeled donkey anti-rat antibodies (Jackson ImmunoResearch Laboratories). Sections were refixed with 4% paraformaldehyde/PBS for 15 min, permeabilized with 0.2% Triton in 1% donkey serum/PBS for 30 min, and incubated with goat antibodies against CD4+ (1:20 dilution; R&D Systems) and rabbit antibodies against FoxP3 (1:20 dilution; BioLegend). Sections were then stained with Cy5-labeled donkey anti-goat and FITC-labeled donkey anti-rabbit antibodies (all from Jackson ImmunoResearch Laboratories) and finally mounted in Vectashield with DAPI (4′,6-diamidino-2-phenylindole) (Vector Labs). All antibodies were diluted in PBS with 1% donkey serum, and slides were washed several times in PBS after each fixation and incubation step. Images were acquired with a Zeiss LSM 510 confocal microscope and pseudocolored using the Zeiss LSM image browser.
Gag mRNA real-time PCR.
RNA was extracted, according to the Trizol protocol (Invitrogen), but also included linear acrylamide (Ambion) as a coprecipitant prior to the addition of 2-propanol. Linear acrylamide (10 μg) was added to the RNA-containing aqueous phase after separation. Total RNA was quantified by a spectrophotometer and saved for quality analysis by gel electrophoresis. DNase I treatment of 10 μg total RNA was carried out in a final volume of 100 μl and included 10 units of the enzyme. The DNase I-treated total RNA was cleaned using Qiagen's RNeasy Plus minikit, eluted in 100 μl diethyl pyrocarbonate-treated water, quantified, and saved for gel electrophoresis. Reverse transcription was carried out using the high-capacity reverse transcription kit (Applied Biosystems). Briefly, 1 μg of DNase I-treated total RNA was reverse transcribed, according to the manufacturer's instructions, in a 100-μl volume. Each sample included a control without reverse transcriptase. HIV Gag target levels were estimated using both absolute and relative quantification protocols via real-time PCR. Absolute quantification was carried out against a Gag (short) insert standard. The HIV Gag real-time PCR assay had a dynamic range of 101 to 108 copies and an efficiency of amplification in the order of 94 to 96%. Relative quantification was carried out using mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as the internal reference gene, and the data were analyzed according to the threshold cycle method. The mouse GAPDH assay (Applied Biosystems) was included as a separate set of reactions in each of the absolute quantification runs. Baseline settings and threshold levels were the same for all plates analyzed.
CFSE dye dilution assay.
Freshly isolated peripheral blood mononuclear cells (PBMCs) were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) as previously described (17). Briefly, 5 million cells were resuspended in 1 ml of PBS and stained by adding an equal volume of CFSE (final concentration, 2.5 μM). Following staining, proliferation cultures were set up to be stimulated with the HIV gp140 peptide library for 6 days in minimal essential medium alpha. Control wells contained cells cultured in the absence of stimulation. Day 7 cells were harvested and stained with an anti-CD8+ antibody. The low-CFSE population was gated from the total CD8+ cells and defined as the percentage of proliferating CD8+ cells. Samples with proliferation greater than four times that observed in control wells and higher than 1% of total CD8+ cells were considered positive.
Statistical analysis.
All analyses were carried out using GraphPad Prism (version 5.00 for Windows). P values of <0.05 were considered to be significant. For comparison of the difference between two groups, a two-sided unpaired Student's t test was carried out. When comparing the means of three or more unmatched groups, one-way analysis of variance was used for analysis.
RESULTS
Identification of a novel AAV capsid capable of generating robust memory T-cell responses in mice.
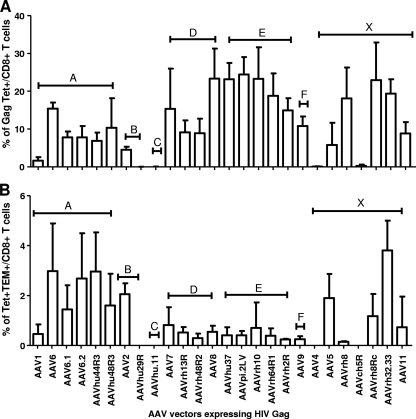
Previous studies with mice indicated that vectors based on AAV elicit dysfunctional responses to foreign transgene products following i.m. injection (27). In most cases, one can detect antigen-specific activation of T cells, although these cells are not polyfunctional, do not generate memory phenotypes, and are poorly responsive to a second exposure to antigen. In an attempt to identify an AAV vector of potential use as a genetic vaccine, we screened 26 vectors created from different capsids spanning the known phylogeny of primate AAV (42) for activation of T cells against HIV-1 Gag; the assay was based on staining PBMCs for Gag-specific T cells using a tetramer and evaluating what percentage of them had memory phenotypes based on expression of CD127 and CD62L (i.e., effector T cells, CD127−/CD62L−; effector memory T cells [TEM], CD127+/CD62L−; and central memory T cells [TCM], CD127+/CD62L+). All vectors generated some level of Tet+ cells (Fig. 1A), with variable levels of memory T cells (Fig. 1B). The one vector that produced high levels of total Tet+ T cells with a high percentage of memory phenotypes was the vector based on AAVrh32.33. It was further evaluated as a potential vector for vaccines and was compared to vectors based on AAV8, which is being considered in various clinical trials of gene therapy, and to a simian-based adenovirus, SAd24, which is being developed as a vaccine carrier for HIV-1 (16, 52).
FIG. 1.
Impact of AAV capsids on the transgene-specific T-cell response. CB6F1 mice were injected i.m. with 26 AAV isolates from clades A, B, C, D, E, and F, as well as with isolates that do not cluster in a particular clade (X). (A) Antigen-specific CD8+ T-cell responses induced by different AAV vectors expressing HIV Gag were assayed by Gag tetramer staining at 3 weeks post-vector administration. (B) HIV Gag tetramer-specific CD8+ T-cell responses were further analyzed for memory phenotype, and data are shown as percentages of tetramer HIV Gag-positive TEM (CD127+/CD62L−) in total CD8+ T cells. Data are shown as mean results with standard deviations (n = 4).
AAVrh32.33 generates robust and polyfunctional T-cell responses against HIV-1 Gag with a full spectrum of memory phenotypes.
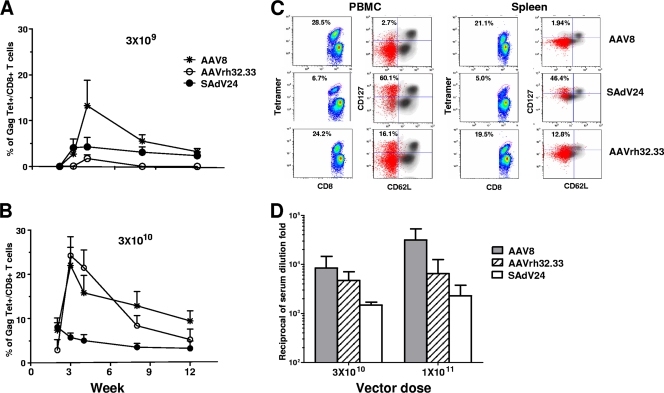
The kinetics of the CD8+ T-cell response to Gag in mice following i.m. injection of AAV8, AAVrh32.33, and SAdV24 is presented in Fig. 2A (3 × 109 GC) and B (3 × 1010 GC). Both AAV vectors and SAdV24 yielded detectable levels of Gag-specific T cells at the peak level, as measured by tetramer binding with gradual contraction over 12 weeks (Fig. 2A and B); vector dose impacted on the relative potency at the peak level. Figure 2C shows memory phenotyping of PBMCs and spleen cells from a representative animal harvested at 3 weeks old. As described previously, AAV8 yields very limited numbers of TEM or TCM compared to those of SAdV24 (26). The total number of TEM and TCM produced from AAVrh32.33 is substantial and higher than that obtained with AAV8 (Fig. 2C).
FIG. 2.
Characterization of the immune response following AAV HIV Gag vaccination. Gag tetramer responses were monitored over time in CB6F1 mice that received i.m. administration of AAV8, AAVrh32.33, or SAdV24 encoding HIV Gag at two doses, 3 × 109 (A) and 3 × 1010 (B) GC. (C) Phenotypic characterization. Three weeks following AAV vector administration, lymphocytes were isolated from whole blood and spleens of mice injected with AAV2/AAV8, SAdV24, and AAV2/AAVrh32.33 Gag-expressing vectors (3 × 1010 GC). Per lymphocyte source, on the left, a scatter plot of CD8+ T cells of a representative mouse (n = 5) illustrates the tetramer positivity (Tet+), as quantified by the percentage of Tet+ within the CD8+ population. On the right, CD62L marker expression versus that of CD127 is represented. Memory phenotype is represented with the Tet+/CD8+ population overlaid in red on the total lymphocyte population in black. The percentages of Tet+/CD8+ T cells and TEM+/Tet+ T cells are indicated within the individual panels. (D) Gag-specific antibody responses. Sera from mice immunized with AAV8, AAVrh32.33, or SAdV24 at the dose of 3 × 1010 or 1 × 1011 GC were analyzed for anti-Gag IgG, and the results are presented as means with standard deviations (n = 4).
Functional properties of Gag-specific T cells produced by AAVrh32.33 were evaluated by measuring the production of cytokines (IFN-γ, TNF-α, and IL-2) and the degranulation marker CD107a following stimulation with peptide spanning the dominant CD8+ T-cell epitope. Comparisons to results from animals injected with AAV8 and SAdV24 vectors were made (Table 1). As we noted before, the majority of Gag-specific T cells produced by AAV8 vector, as measured by tetramer staining, are not responsive to peptide, as determined by cytokine production in the ICS assay (compare Tet+/CD8+ to IFN-γ+/CD8+). The results from AAVrh32.33 and SAdV24 were indistinguishable from one another and different from those from AAV8 in that ICS and tetramer staining produced very similar estimates of Gag-specific T cells at the peak level. Characterization of the profiles and quantities of cytokines produced after peptide stimulation yielded results for AAVrh32.33 similar to those obtained with SAdV24 as opposed to those observed with AAV8 (Table 1). The proportions of Gag-specific T cells that express the two cytokines, IFN-γ and TNF-α, and CD107a (i.e., IFN-γ+ CD107a+ TNF-α+ IL-2−/total IFN-γ+) are higher with SAdV24 and AAVrh32.33 (45 and 48%, respectively) than with AAV8 (33%); a similar trend was noted for cells expressing all three cytokines and CD107a (i.e., IFN-γ+ CD107a+ TNF-α+ IL-2+/total IFN-γ+) (SAdV24, 4%; AAVrh32.22, 1%; and AAV8, 0.1%). In all subpopulations of Gag-specific T cells described in Table 1, the total quantity of IFN-γ produced per cell, as measured by mean fluorescence intensity (MFI), is higher for SAdV24 and AAVrh32.33 than for AAV8.
TABLE 1.
CD8+ T-cell phenotypes and functionality following single-vector administrationa
| Cell expression | Results from administration of animals with: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AAV8 |
SAdV24 |
AAVrh32.33 |
|||||||
| % CD8 | % IFN-γ | MFI | % CD8 | % IFN-γ | MFI | % CD8 | % IFN-γ | MFI | |
| Tet+/CD8+ | 18.66 ± 5.66 | 5.74 ± 1.43 | 19.13 ± 2.37 | ||||||
| IFN-γ+/CD8+ | 5.87 ± 1.22 | 12.81 | 6.51 ± 0.53 | 22.12 | 17.53 ± 2.18 | 18.26 | |||
| IFN-γ+ CD107a− TNF-α− IL-2−/CD8+ | 0.21 | 3.58 | 6.66 | 0.06 | 0.92 | 8.45 | 0.80 | 4.56 | 8.51 |
| IFN-γ+ CD107a+ TNF-α− IL-2−/CD8+ | 3.49 | 59.45 | 8.80 | 2.33 | 35.79 | 12.83 | 8.28 | 47.23 | 11.27 |
| IFN-γ+ CD107a+ TNF-α+ IL-2−/CD8+ | 1.95 | 33.22 | 15.90 | 2.97 | 45.62 | 28.87 | 8.56 | 48.83 | 25.2 |
| IFN-γ+ CD107a+ TNF-α+ IL-2+/CD8+ | 0.01 | 0.17 | NA | 0.26 | 3.99 | 48.5 | 0.17 | 0.97 | 38.00 |
Splenocytes from AAV8-, SAdV24-, or AAVrh32.33-immunized mice (3 × 1010 GC; n = 4) were isolated 3 weeks postimmunization and subsequently analyzed with ICS and Gag tetramer and CD8 staining for the various cytokines and CD107a. NA, not applicable.
Production of Gag antibody, as measured by enzyme-linked immunosorbent assay, was much higher following AAV8 than SAdV24, as we have shown previously (Fig. 2D) (26). Depending on the dose, AAVrh32.33 yields intermediate levels of Gag antibody or close to those obtained with AAV8.
Augmented immune responses following AAVrh32.33 prime and SAdV24 boost administration.
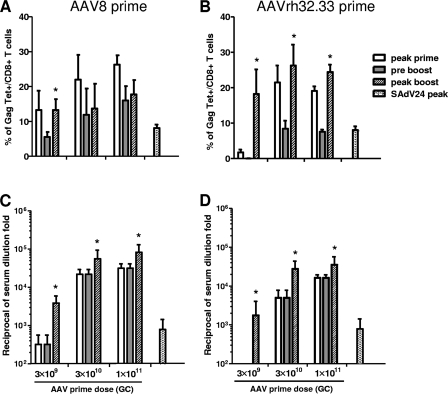
One potential application of AAV in a vaccine is its use in combination with another vaccine platform in a prime-boost regimen. Figure 3 presents levels of Tet+ cells (Fig. 3A and B) and Gag antibodies (Fig. 3C and D) in the context of use of an AAV prime (AAV8 and -rh32.33) and a SAdV24 boost using vectors expressing Gag; data are also shown for SAdV24 alone to help evaluate the potency of the boost. Studies were performed with different doses of the priming vector (3 × 109, 3 × 1010, and 3 × 1011 GC) with a fixed dose of the boosting vector (1 × 1010 particles). Both AAV8 and AAVrh32.33 yield equivalent numbers of Tet+ cells following priming at 3 × 1010 and 3 × 1011 GC, which contract to about 50% of peak values prior to the 8-week boost; similar contraction is observed at a priming dose of 3 × 109 GC, although the peak value is lower for AAVrh32.33 than for AAV8. As we showed previously, AAV8-primed CD8+ T cells are not responsive to the SAdV24 boost (26). This contrasts with results obtained with the AAVrh32.33 vector, which primed a population of T cells that were very effectively boosted with SAdV24 (the peak value following boost injection is greater than peak value following that of the prime, and the increment in Tet+ cells is at least threefold greater than that obtained with SAdV24 alone). Both AAV vectors are effectively boosted with SAdV24 in terms of Gag antibodies (Fig. 3C and D). In fact, the level of antibodies achieved with AAV8 or AAVrh32.33 prime and SAdV24 boost is 14- to 28-fold higher than that obtained with SAdV24 alone.
FIG. 3.
Dose response-effect of AAV prime on T- and B-cell responses. AAV8 or AAVrh32.33 expressing HIV Gag vectors were injected i.m. into CB6F1 mice (n = 4) at doses of 3 × 109, 3 × 1010, and 1 × 1011 GC; 8 weeks later, these mice received 1 × 1010 particles of SAdV24 Gag in parallel to a group of age-matched naïve mice. The production of CD8+ T-cell Gag tetramer responses from PBMCs (A and B) and anti-p24 Gag antibodies from sera (C and D) was monitored. The data are presented as the maximum response following the prime administration (peak prime), the measurement immediately prior to the boost administration (preboost), and peak response following the boost administration (peak boost). The value obtained when the same dose of SAdV24 was injected into naïve mice was called SAdV24 peak. Statistically significant differences (P < 0.05) between the peak boost and preboost data are marked with an asterisk. Data are shown as mean results with standard deviations.
AAVrh32.33 elicits self-limited inflammation at the site of injection associated with decline of vector genomes.
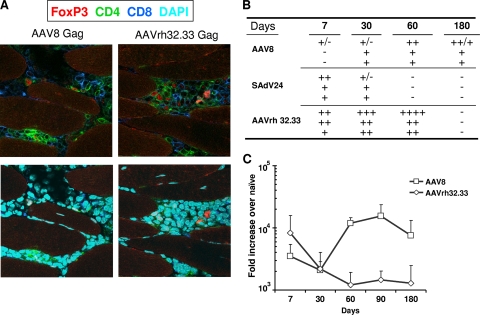
Animals injected with AAV8 and AAVrh32.33 expressing Gag were sacrificed 7, 30, 60, and 180 days later, and muscle was evaluated by histology for inflammation (Fig. 4A and B) and by TaqMan PCR for Gag mRNA (Fig. 4C); the histological consequences of i.m. injection of SAdV24 were also evaluated.
FIG. 4.
Histopathology and Gag antigen expression levels around injection site. Mice were i.m. immunized with 3 × 1010 GC of AAV8 or AAVrh32.33 expressing HIV Gag. At day 7, 30, 60, 90, and 180 postimmunization, muscle tissue from the injected areas was obtained and analyzed for inflammation, infiltration, and Gag mRNA levels. (A) At 60 days post-vector administration, infiltrated cells were immunophenotyped with antibodies against CD8+ (dark blue), CD4+ (green), and FoxP3 (red). The bottom panels show additional staining with DAPI (light blue). (B) Table scoring the degree of infiltration at different time points and compared with SAdV24 as positive control (−, no infiltrates; ++++, strongest infiltration observed). Data for individual mice in each group are shown. (C) Gag mRNA levels from muscle injected with AAV8 or AAVrh32.33 by TaqMan. Data are shown as mean results with standard deviations.
Inflammatory infiltrates localized to the injected muscle groups were observed following the injection of each vector; immunophenotyping of day 60 samples demonstrated both CD4+ and CD8+ T cells in animals receiving the AAV vectors (Fig. 4A). The severity and kinetics of inflammation differed for each vector (Fig. 4B). Injection of AAV8 resulted in a low-level but prolonged inflammatory response, while the responses elicited from both AAVrh32.33 and SAdV24 were self limited but more severe with AAVrh32.33 than with the other two (Fig. 4B). AAV8 vector genomes and its Gag mRNA persisted for at least 6 months without apparent diminution, while Gag expression from AAVrh32.33 declined by 10-fold within the first 60 days (Fig. 4C).
Human serum does not interfere with the activity of AAVrh32.33 vaccines in vivo.
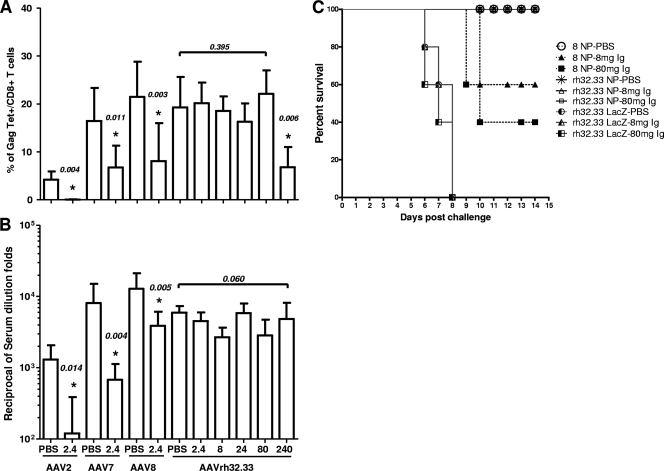
A potential impediment to the use of viral vectors as vaccines is the presence of NAbs in target human populations caused by natural infection with pathogens related to the virus used to construct the vector. In a previous study, we screened large populations of humans from Europe, Australia, the United States, and Africa for NAbs against various AAV vectors and found very high prevalence and levels to AAV1 and AAV2, intermediate levels to AAV7 and AAV8, and essentially no detectable levels to AAVrh32.22 (4). To more directly assess the impact of preexisting immunity on AAVrh32.33 vaccine efficacy, we developed an in vivo model in which mice are passively transferred with pooled human Ig for 24 and 2 h prior to vaccination with the AAV vectors. A more complete description of these studies for AAV2, -7, and -8 has been published (25). Animals received different doses of Ig in order to quantitatively assess the relative potency of human Ig in inhibiting AAV vaccine efficacy in vivo. Figure 5 presents tetramer staining (Fig. 5A) and Gag antibody titers (Fig. 5B) that result when AAV2, AAV7, and AAV8 Gag vectors are injected in mice passively transferred with 2.4 mg of pooled human Ig; for each vector, there is a substantial and statistically significant decline in both Tet+ cells and Gag antibody titers in the presence of this dose of pooled human Ig (these control data have been published previously [25]). This is in contrast to results with AAVrh32.22, in which a decline in tetramer frequencies was not realized until 100-fold-more human Ig was injected (240 mg); no dose of human Ig up to the maximal amount that we can give (i.e., 240 mg) significantly impacted Gag antibody production.
FIG. 5.
Vaccine efficacy in the presence of pooled human Ig. Mice received either PBS or the indicated doses (mg) of pooled human Ig by intravenous administration prior to immunization. Recipient mice were immunized with 3 × 1010 GC of AAV2, -7, -8, or -rh32.33 expressing HIV Gag. The Gag-specific tetramer T-cell responses (A) and Gag-specific antibody responses (B) were measured at 3 weeks postimmunization in the presence or absence of pooled human Ig. The data from 8 to 10 mice in each group are shown as the mean results with standard deviations. Statistically significant differences (P < 0.05) between the IgG-treated vector group and PBS control group are marked with an asterisk. (C) Mice passively transferred with PBS or pooled human Ig (8 or 80 mg) were immunized with 1 × 1011 GC of AAV8 or AAVrh32.33 expressing influenza virus type A NP or AAVrh32.33 expressing LacZ as the control. At day 35 postimmunization, all mice were challenged with 10 50% lethal doses of influenza virus strain PR8. The survival data analysis was plotted using GraphPad Prism (version 5.00 for Windows).
The functional consequences of NAbs on AAV vector efficacy were assessed using vectors expressing nucleoprotein (NP) from influenza virus. Animals were immunized i.m. with AAV8 and AAVrh32.33 expressing NP or an irrelevant antigen (i.e., LacZ) in the presence or absence of passively transferred human Ig, followed by challenge with a lethal dose of influenza virus PR8 (Fig. 5C); protection would be achieved if sufficient numbers of NP-specific T cells are generated. There was uniform mortality by day 8 in all groups that received vectors expressing LacZ and complete survival in animals immunized with either AAV8 or AAVrh32.33 expressing NP in the absence of human Ig. Passive transfer of human Ig at doses of 8 and 80 mg resulted in mortality in groups immunized with AAV8 NP but not in groups immunized with AAVrh32.33 NP. Passive transfer in the absence of vector had no impact on survival (data not shown).
AAVrh32.33 effectively primes a T-cell response in macaques and generates robust B-cell responses.
The biology of AAVrh32.33 as a vaccine carrier in cynomolgus macaques was evaluated in a pilot study as follows. Animals were immunized with either AAVrh32.33 (n = 4) or AAV8 (n = 3) expressing gp140 from HIV-1 and, 40 weeks later, boosted with SAdV24 expressing the same transgene; 36 weeks after the boost injection, the animals were sacrificed. An additional two animals received just SAdV24-gp140. During the in-life phase of the study, PBMCs were evaluated for gp140-specific T cells using an assortment of assays, and sera were measured for neutralizing activity against HIV-1.
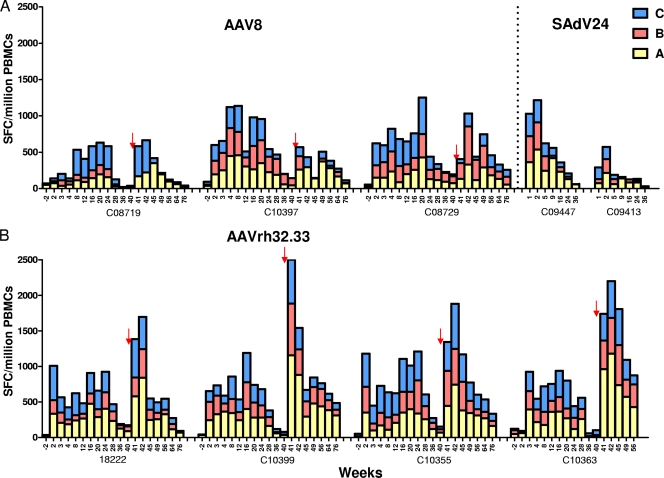
Figure 6 summarizes IFN-γ ELISPOT assays of PBMCs for T cells against gp140 using overlapping peptides collected into three distinct pools. Each animal generated substantial quantities of gp140-specific T cells following the prime injection, with little difference in the peak levels between the two AAV vectors. Kinetics of these responses did differ between the two AAV vectors. For AAV8, the peak level occurred at weeks 4 to 8 or later and slowly contracted (Fig. 6A), while AAVrh32.33 generated a peak level within 2 weeks, with a similar pattern of contraction (Fig. 6B). The major difference between the two AAV vectors was in the response following the boost injection with SAdV24. A substantial boost was observed in the AAVrh32.33-primed animals to levels substantially greater than those seen at the peak level following the prime administration; the peak level after the boost injection was also significantly higher than that achieved with SAdV24 alone (prime-boost regimen [Fig. 6B]; SAdV24 alone [Fig. 6A]). The response to the SAdV24 boost in AAV8-primed animals was remarkably blunted, with levels that never exceeded the peak level which occurred after use of the prime and that were lower than or equal to those observed with SAdV24 alone (Fig. 6A).
FIG. 6.
HIV gp140-specific IFN-γ response following AAV-Ad prime-boost vaccine regimen in cynomolgus macaques. Cynomolgus macaques were primed i.m. with 1 × 1012 GC of AAV8 (n = 3) (A) or AAVrh32.33 (n = 4) (B) expressing HIV W61D gp140. Ten months after the prime administration, animals were boosted with 2 × 1011 particles of SAdV24 HIV gp140. A control group of two naïve cynomolgus macaques received the same dose of SAdV24 HIV gp140. Freshly isolated PBMCs were assessed for IFN-γ ELISPOT responses after in vitro exposure to peptide pools A, B, and C spanning the HIV-1 gp140 proteins at different time points. The time points of boosting with SAdV24 HIV gp140 are indicated by arrows. Each bar represents the accumulative number of IFN-γ spot-forming cells (SFC) for each of the peptide pools. PBMCs without peptide stimulation showed <50 IFN-γ spot-forming cells. Animal C10363 died at week 59 of an intestinal volvulus.
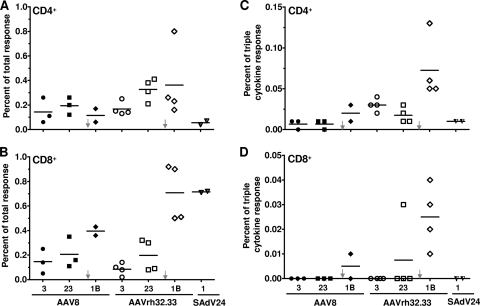
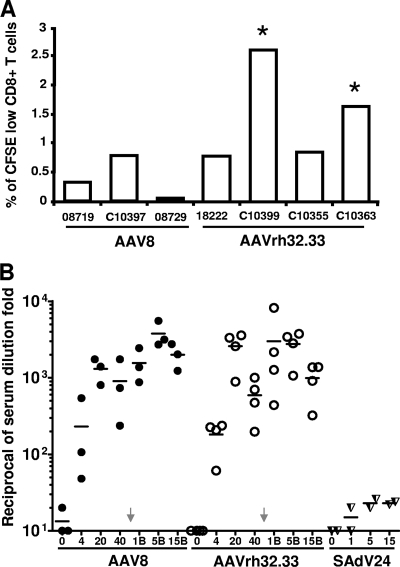
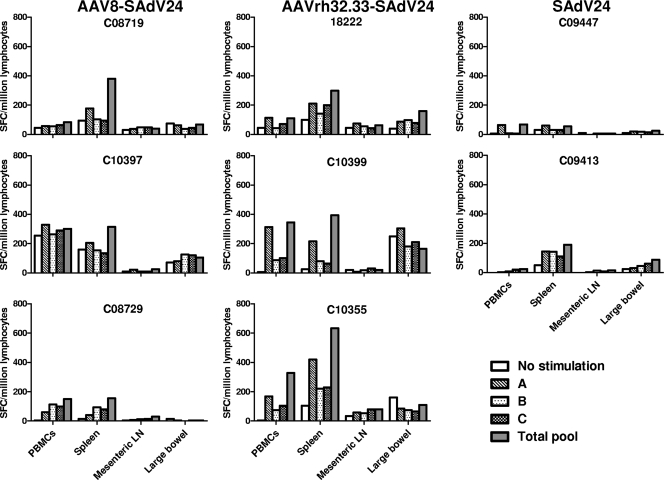
Selected PBMC samples were analyzed by ICS for function of the gp140-specific T cells. Figure 7 presents total numbers of CD4+ and CD8+ T cells to gp140 (Fig. 7A and B, respectively) and the percentage of these cells that are polyfunctional (i.e., secrete IFN-γ, TNF-α, and IL-2) (Fig. 7C and D). Data are presented from cells harvested 3 and 23 weeks after the AAV prime injection and 1 week after the SAdV24 boost injection; data are also shown from PBMCs harvested 1 week after injection of SAdV24 alone. Both AAV vectors generate detectable quantities of CD4+ and CD8+ T cells to gp140 (Fig. 7A and B, respectively) at approximately the same levels, while the numbers of polyfunctional T cells are low (Fig. 7C and D). A significant difference emerged following injection of the boost. There was little difference in the overall quantities of CD4+ T cells to gp140 following either prime injection, although significantly higher numbers of polyfunctional T cells were generated by the boost following injection of the AAVrh32.33 prime rather than the AAV8 prime. The SAdV24 boost did significantly enhance the total number of gp140-specific CD8+ T cells as well as the proportion of CD8+ T cells expressing all three cytokines that were generated following injection of the AAVrh32.33 prime, although this was not seen with animals primed with AAV8. CFSE labeling assays indicated increased proliferative capacity of the gp140-specific CD8+ T cells in at least 2/4 of the AAVrh32.33-primed animals when measured 14 weeks after vector administration (Fig. 8A). Mononuclear cells were extracted from the spleen, mesenteric lymph nodes, and large bowel at the time of necropsy 36 weeks after the boost injection and analyzed for gp140-specific T cells using the IFN-γ ELISPOT assay (Fig. 9). A signal over the no-stimulation control was detected in the spleen in 1/3 animals from the AAV8 prime group and 3/3 animals from the AAVrh32.33 prime group. T-cell frequencies were low in the mesenteric lymph nodes; interpretation of data from the large bowel was compromised due to high background.
FIG. 7.
Polyfunctional HIV gp140-specific T-cell responses following AAV-adenovirus prime-boost administration in cynomolgus macaques. Freshly isolated PBMCs were stimulated with the HIV gp140 peptide library. The secreted cytokines, including IFN-γ, IL-2, and TNF-α, as well as CD4+ and CD8+ surface markers, were stained by fluorescence-labeled antibodies and measured by flow cytometry as previously described (4). Representative plots are showing the percentages of total cytokine-secreting CD4+ (A) and CD8+ (B) T-cell responses 3 and 23 weeks post-AAV prime injection and 1 week post-SAdv24 boost injection (1B). (C and D) IFN-γ-, IL-2-, and TNF-α-cosecreting CD4+ and CD8+ T cells in response to the HIV-gp140 peptide library were plotted. The time points of boosting with SAdV24 HIV gp140 are indicated by arrows. Individual macaque data are shown. The bars represent the mean percentages of the total response.
FIG. 8.
HIV-specific CD8+ T-cell proliferative potential and NAb response following AAV-adenovirus prime-boost administration. (A) At 14 weeks postprime, the levels of CD8+ T-cell proliferation were assessed by a CFSE dye dilution assay. CFSE-labeled CD8+ T cells were stimulated ex vivo for 6 days with the HIV-1 gp140 peptide library. Proliferating cells were defined as the low-CFSE population among the total CFSE-labeled CD8+ population. Samples that had levels greater than four times the nonstimulated controls, and those higher than 1%, are indicated by an asterisk. (B) Anti-HIV NAb titers in sera were assayed at week 0, 4, 20, and 40 after AAV prime and at week 1 (1B), 5 (5B), and 15 (15B) after SAdV24 boost administrations. A control group primed with SAdV24 was also included. The bar represents the mean percentage of the total response.
FIG. 9.
HIV Env-specific IFN-γ responses from tissue-derived lymphocytes of nonhuman primates. At 36 weeks post-SAdV24 boost injection, all immunized macaques were necropsied, monocytes were isolated from the peripheral blood, spleen, mesenteric lymph nodes (LN), and large bowel, and HIV Env-specific IFN-γ responses were measured by IFN-γ ELISPOT assay. SFC, spot-forming cells.
Sera were analyzed for NAb against HIV-1 after both the prime and boost injections (Fig. 8B). Initial studies evaluated neutralizing titers against the isolate from which the gp140 was obtained. These experiments demonstrated very high titers of NAb for both AAV8 and AAVrh32.33 that persisted for the duration of the experiment. Titers of NAb increased severalfold after the boost injection. The level of NAb achieved with AAV alone was at least 2-log units higher than that generated with SAdV24 alone. There was no appreciable neutralization of heterologous laboratory and clinical isolates of HIV-1 in any groups, despite high levels of NAb against homologous virus (data not shown).
DISCUSSION
Virus-based vectors are being developed as genetic vaccines for the treatment and/or prevention of various infectious and oncologic diseases. The fact that the viral vector expresses the target antigen in cells of the recipient facilitates the production of class I-restricted CTL responses (15, 19). This has been the impetus to use recombinant adenoviruses as a vaccine carrier against HIV-1 (6, 30, 40). However, genetic vaccines have been less successful than adjuvant-protein formulations in eliciting protective and/or therapeutic antibody responses. A significant limitation of virus-based vectors is interference by antivector antibodies (21, 41, 43). This can occur de novo following a natural infection with a pathogen related to the virus used to make the vector (4). Antibodies could also arise following the first vaccine administration that could interfere with a boost or a subsequent application of a related vaccine (32).
Vaccines based on AAV have potential advantages over other virus-based systems. The vector can be rendered completely replication deficient and can be generated from a fully characterized molecular clone. In addition, AAV vectors are capable of eliciting very robust B-cell responses to the transgene (26, 50). They are serologically distinct from other virus-based vaccines and can be used in heterologous prime-boost strategies without antibody interference. However, a number of limitations of the AAV-based vaccines have emerged, based on vectors produced from the standard serotypes. These vectors do indeed generate antigen-specific T cells to the transgene, although more-detailed studies indicate that these cells are largely nonresponsive to antigen and are incapable of proliferating in the context of a heterologous boost (26). Clinical trials of AAV2 as an HIV-1 vaccine demonstrated only modest T-cell responses to the encoded HIV-1 antigens (27). This is consistent with our studies of AAV2 gag vectors in mice in which gag-specific T cells were low and nonresponsive to stimulation (24).
Seroepidemiologic studies indicate the high prevalence of NAbs to AAV1 and -2 and moderate levels to AAV7 and -8; preclinical studies indicate that these levels will interfere with vaccine efficacy (25).
Creation of the novel AAV hybrid with rh32.33 appears to overcome the apparent limitations of other AAV isolates. The CD8+ T-cell response following the prime injection is robust, persists, and leads to an attractive mix of effector T cells, TEM, and TCM with a high level of functionality based on coexpression of multiple cytokines. The ability of an adenovirus vector to substantially boost this CD8+ T-cell response is consistent with their memory phenotype and antigen-induced expression of IL-2. Most importantly, it appears that preexisting immunity to natural infections of AAV in humans will not interfere with AAVrh32.33 vaccine efficacy. We envision the use of AAVrh32.22 in an application where both antibody and CTL responses are desired. The most effective approach may be to combine an AAVrh32.33 prime with a heterologous boost using an adenovirus to increase the CTL response or protein/adjuvant to increase the antibody response.
The mechanism(s) by which AAVrh32.33 overcomes the problems of other AAV-based vectors is unclear. This vector was created as a hybrid between two very similar capsids, rh32 and rh33, isolated from the spleen of a single well-appearing rhesus macaque (11). The vectors produced with the capsid hybrid were obtained at higher yields. Phylogenetic analysis of the capsid sequence indicated that rh32 and rh33 are distinct from all previous isolates, except for having a weak relationship with AAV4. This suggests that rh32 and rh33 do not widely circulate as infectious agents in primates and that cross-reactivity between them and other more common AAVs would be minimal. The extremely low prevalence of NAbs to rh32.33 in humans is consistent with these hypotheses (4).
Several mechanisms may be considered in evaluating the vibrant and robust CD8+ T-cell responses elicited by AAVrh32.33 that do not characterize other AAVs. One mechanism to explain the absence of memory T cells following injection of vectors such as AAV8 is T-cell exhaustion due to persistent antigen expression (27); the corollary of this hypothesis is that antigen is less persistent from AAVrh32.33, diminishing the potential for exhaustion. It is interesting that expression of the commonly used reporter gene β-galactosidase is stable when delivered by any AAV vector but AAVrh32.33, where expression was diminished to very low levels and was associated with significant inflammation (31). Activation of CD8+ T cells by adenoviruses has been attributed to highly efficient transduction and activation of dendritic cells (29, 34), properties that have not been ascribed to AAVs (37). The significant differences in capsid structure noted in AAVs rh32 and rh33 compared to those noted in other known AAVs may direct unique interactions with dendritic cells that either enhance transduction or augment dendritic cell activation. Tolerance induction following administration of AAV also is associated with activation of regulatory CD4+ T cells capable of suppressing antibody and CTLs, which facilitates the use of AAV for gene therapy. Some studies provided evidence that induction of CD4 T-cell tolerance, including T-cell anergy and clonal deletion, could explain the nonresponsive state that occurs following AAV gene transfer (10). Our recent study showed that hepatic regulatory T cells together with Kupffer cells create a local suppressive microenvironment that prevents the establishment of the CTL response (2).
These studies illustrate the substantial influences that capsid structure can have on AAV vector performance. Properties conferred by the hybrid capsid of rh32.33 are indeed quite attractive as a potential component of an AAV-based vaccine.
Acknowledgments
We thank David C. Montefiori of Duke University for testing HIV-1 NAb titers. We also thank the Immunology Core personnel (University of Pennsylvania Gene Therapy Program), specifically Qiuyue Qin, Jennifer Miliaresis, and Surina Boyd, for their work on isolating lymphocytes from blood and tissues.
This research was supported by grant P30-DK-47757 (to J.M.W.), grant P01-HL-059407 (to J.M.W.), grant AI-30034 (to D. C. Montefiori), and a grant from GlaxoSmithKline Pharmaceuticals, Inc.
We declare having potential competing financial interests. L.H.V. is an inventor on pending applications and awarded patents that have been licensed and sublicensed to multiple pharmaceutical and biotechnology companies. J.M.W. is a consultant to ReGenX Holdings and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings.
Footnotes
Published ahead of print on 7 October 2009.
REFERENCES
- 1.Arruda, V. R., J. Schuettrumpf, R. W. Herzog, T. C. Nichols, N. Robinson, Y. Lotfi, F. Mingozzi, W. Xiao, L. B. Couto, and K. A. High. 2004. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood 103:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breous, E., S. Somanathan, L. H. Vandenberghe, and J. M. Wilson. 2009. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 50:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockstedt, D. G., G. M. Podsakoff, L. Fong, G. Kurtzman, W. Mueller-Ruchholtz, and E. G. Engleman. 1999. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immunol. 92:67-75. [DOI] [PubMed] [Google Scholar]
- 4.Calcedo, R., L. H. Vandenberghe, G. Gao, J. Lin, and J. M. Wilson. 2009. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 199:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, O., E. Dobrzynski, L. Wang, S. Nayak, B. Mingle, C. Terhorst, and R. W. Herzog. 2007. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood 110:1132-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, H., S. H. Wolfe, V. Valencia, K. Qian, L. Shen, M. I. Phillips, L. J. Chang, and Y. C. Zhang. 2007. Efficient and persistent transduction of exocrine and endocrine pancreas by adeno-associated virus type 8. J. Biomed. Sci. 14:585-594. [DOI] [PubMed] [Google Scholar]
- 8.Chirmule, N., W. Xiao, A. Truneh, M. A. Schnell, J. V. Hughes, P. Zoltick, and J. M. Wilson. 2000. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J. Virol. 74:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottard, V., C. Valvason, G. Falgarone, D. Lutomski, M. C. Boissier, and N. Bessis. 2004. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 24:162-169. [DOI] [PubMed] [Google Scholar]
- 10.Dobrzynski, E., and R. W. Herzog. 2005. Tolerance induction by viral in vivo gene transfer. Clin. Med. Res. 3:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, G., M. R. Alvira, S. Somanathan, Y. Lu, L. H. Vandenberghe, J. J. Rux, R. Calcedo, J. Sanmiguel, Z. Abbas, and J. M. Wilson. 2003. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. USA 100:6081-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao, G. P., Y. Lu, X. Sun, J. Johnston, R. Calcedo, R. Grant, and J. M. Wilson. 2006. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J. Virol. 80:6192-6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge, Y., S. Powell, M. Van Roey, and J. G. McArthur. 2001. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood 97:3733-3737. [DOI] [PubMed] [Google Scholar]
- 14.Hara, H., A. Monsonego, K. Yuasa, K. Adachi, X. Xiao, S. Takeda, K. Takahashi, H. L. Weiner, and T. Tabira. 2004. Development of a safe oral Abeta vaccine using recombinant adeno-associated virus vector for Alzheimer's disease. J. Alzheimers Dis. 6:483-488. [DOI] [PubMed] [Google Scholar]
- 15.Harui, A., M. D. Roth, S. M. Kiertscher, K. Mitani, and S. K. Basak. 2004. Vaccination with helper-dependent adenovirus enhances the generation of transgene-specific CTL. Gene Ther. 11:1617-1626. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto, M., J. L. Boyer, N. R. Hackett, J. M. Wilson, and R. G. Crystal. 2005. Induction of protective immunity to anthrax lethal toxin with a nonhuman primate adenovirus-based vaccine in the presence of preexisting anti-human adenovirus immunity. Infect. Immun. 73:6885-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins, E. D., M. Hommel, M. L. Turner, F. L. Battye, J. F. Markham, and P. D. Hodgkin. 2007. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat. Protoc. 2:2057-2067. [DOI] [PubMed] [Google Scholar]
- 18.Herzog, R. W., P. A. Fields, V. R. Arruda, J. O. Brubaker, E. Armstrong, D. McClintock, D. A. Bellinger, L. B. Couto, T. C. Nichols, and K. A. High. 2002. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum. Gene Ther. 13:1281-1291. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias, M. C., K. Mollier, A. S. Beignon, P. Souque, O. Adotevi, F. Lemonnier, and P. Charneau. 2007. Lentiviral vectors encoding HIV-1 polyepitopes induce broad CTL responses in vivo. Mol. Ther. 15:1203-1210. [DOI] [PubMed] [Google Scholar]
- 20.Kobinger, G. P., J. M. Figueredo, T. Rowe, Y. Zhi, G. Gao, J. C. Sanmiguel, P. Bell, N. A. Wivel, L. A. Zitzow, D. B. Flieder, R. J. Hogan, and J. M. Wilson. 2007. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine 25:5220-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostense, S., W. Koudstaal, M. Sprangers, G. J. Weverling, G. Penders, N. Helmus, R. Vogels, M. Bakker, B. Berkhout, M. Havenga, and J. Goudsmit. 2004. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 18:1213-1216. [DOI] [PubMed] [Google Scholar]
- 22.Kuck, D., T. Lau, B. Leuchs, A. Kern, M. Muller, L. Gissmann, and J. A. Kleinschmidt. 2006. Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1. J. Virol. 80:2621-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, J., D. Wang, S. Qian, Z. Chen, T. Zhu, and X. Xiao. 2003. Efficient and long-term intracardiac gene transfer in delta-sarcoglycan-deficiency hamster by adeno-associated virus-2 vectors. Gene Ther. 10:1807-1813. [DOI] [PubMed] [Google Scholar]
- 24.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, J., R. Calcedo, L. H. Vandenberghe, J. M. Figueredo, and J. M. Wilson. 2008. Impact of preexisting vector immunity on the efficacy of adeno-associated virus-based HIV-1 Gag vaccines. Hum. Gene Ther. 19:663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, J., Y. Zhi, L. Mays, and J. M. Wilson. 2007. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J. Virol. 81:11840-11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, S. W., S. E. Hensley, N. Tatsis, M. O. Lasaro, and H. C. Ertl. 2007. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J. Clin. Investig. 117:3958-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, D. W., J. L. Chang, Y. P. Tsao, C. W. Huang, S. W. Kuo, and S. L. Chen. 2005. Co-vaccination with adeno-associated virus vectors encoding human papillomavirus 16 L1 proteins and adenovirus encoding murine GM-CSF can elicit strong and prolonged neutralizing antibody. Int. J. Cancer 113:93-100. [DOI] [PubMed] [Google Scholar]
- 29.Lyakh, L. A., G. K. Koski, H. A. Young, S. E. Spence, P. A. Cohen, and N. R. Rice. 2002. Adenovirus type 5 vectors induce dendritic cell differentiation in human CD14(+) monocytes cultured under serum-free conditions. Blood 99:600-608. [DOI] [PubMed] [Google Scholar]
- 30.Malkevitch, N., L. J. Patterson, K. Aldrich, E. Richardson, W. G. Alvord, and M. Robert-Guroff. 2003. A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques. J. Immunol. 170:4281-4289. [DOI] [PubMed] [Google Scholar]
- 31.Mays, L. E., L. H. Vandenberghe, R. Xiao, P. Bell, H. J. Nam, M. Agbandje-McKenna, and J. M. Wilson. 2009. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J. Immunol. 182:6051-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papp, Z., L. A. Babiuk, and M. E. Baca-Estrada. 1999. The effect of pre-existing adenovirus-specific immunity on immune responses induced by recombinant adenovirus expressing glycoprotein D of bovine herpesvirus type 1. Vaccine 17:933-943. [DOI] [PubMed] [Google Scholar]
- 33.Peden, C. S., C. Burger, N. Muzyczka, and R. J. Mandel. 2004. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 78:6344-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rea, D., M. J. Havenga, M. van Den Assem, R. P. Sutmuller, A. Lemckert, R. C. Hoeben, A. Bout, C. J. Melief, and R. Offringa. 2001. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 166:5236-5244. [DOI] [PubMed] [Google Scholar]
- 35.Rivera, V. M., G. P. Gao, R. L. Grant, M. A. Schnell, P. W. Zoltick, L. W. Rozamus, T. Clackson, and J. M. Wilson. 2005. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 105:1424-1430. [DOI] [PubMed] [Google Scholar]
- 36.Sarkar, R., M. Mucci, S. Addya, R. Tetreault, D. A. Bellinger, T. C. Nichols, and H. H. Kazazian, Jr. 2006. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia A dogs and mice. Hum. Gene Ther. 17:427-439. [DOI] [PubMed] [Google Scholar]
- 37.Sarukhan, A., C. Soudais, O. Danos, and K. Jooss. 2001. Factors influencing cross-presentation of non-self antigens expressed from recombinant adeno-associated virus vectors. J. Gene Med. 3:260-270. [DOI] [PubMed] [Google Scholar]
- 38.Scallan, C. D., H. Jiang, T. Liu, S. Patarroyo-White, J. M. Sommer, S. Zhou, L. B. Couto, and G. F. Pierce. 2006. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 107:1810-1817. [DOI] [PubMed] [Google Scholar]
- 39.Schneider, H., C. Muhle, A. M. Douar, S. Waddington, Q. J. Jiang, K. von der Mark, C. Coutelle, and W. Rascher. 2002. Sustained delivery of therapeutic concentrations of human clotting factor IX—a comparison of adenoviral and AAV vectors administered in utero. J. Gene Med. 4:46-53. [DOI] [PubMed] [Google Scholar]
- 40.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 41.Sumida, S. M., D. M. Truitt, A. A. Lemckert, R. Vogels, J. H. Custers, M. M. Addo, S. Lockman, T. Peter, F. W. Peyerl, M. G. Kishko, S. S. Jackson, D. A. Gorgone, M. A. Lifton, M. Essex, B. D. Walker, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174:7179-7185. [DOI] [PubMed] [Google Scholar]
- 42.Vandenberghe, L. H., E. Breous, H. Nam, G. Gao, R. Xiao, A. Sandhu, J. Johnston, Z. Debyser, M. Agbandje-McKenna, and J. M. Wilson. Naturally occurring singleton residues in AAV capsid impact vector performance and illustrate structural constraints. Gene Ther., in press. [DOI] [PMC free article] [PubMed]
- 43.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, A. Y., P. D. Peng, A. Ehrhardt, T. A. Storm, and M. A. Kay. 2004. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum. Gene Ther. 15:405-413. [DOI] [PubMed] [Google Scholar]
- 45.Wang, L., R. Calcedo, T. C. Nichols, D. A. Bellinger, A. Dillow, I. M. Verma, and J. M. Wilson. 2005. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood 105:3079-3086. [DOI] [PubMed] [Google Scholar]
- 46.Wang, L., O. Cao, B. Swalm, E. Dobrzynski, F. Mingozzi, and R. W. Herzog. 2005. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 12:1453-1464. [DOI] [PubMed] [Google Scholar]
- 47.Wang, L., E. Dobrzynski, A. Schlachterman, O. Cao, and R. W. Herzog. 2005. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood 105:4226-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xin, K. Q., H. Mizukami, M. Urabe, Y. Toda, K. Shinoda, A. Yoshida, K. Oomura, Y. Kojima, M. Ichino, D. Klinman, K. Ozawa, and K. Okuda. 2006. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J. Virol. 80:11899-11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin, K. Q., T. Ooki, H. Mizukami, K. Hamajima, K. Okudela, K. Hashimoto, Y. Kojima, N. Jounai, Y. Kumamoto, S. Sasaki, D. Klinman, K. Ozawa, and K. Okuda. 2002. Oral administration of recombinant adeno-associated virus elicits human immunodeficiency virus-specific immune responses. Hum. Gene Ther. 13:1571-1581. [DOI] [PubMed] [Google Scholar]
- 50.Xin, K. Q., M. Urabe, J. Yang, K. Nomiyama, H. Mizukami, K. Hamajima, H. Nomiyama, T. Saito, M. Imai, J. Monahan, K. Okuda, K. Ozawa, and K. Okuda. 2001. A novel recombinant adeno-associated virus vaccine induces a long-term humoral immune response to human immunodeficiency virus. Hum. Gene Ther. 12:1047-1061. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, T. P., D. Y. Jin, R. M. Wardrop III, T. Gui, R. Maile, J. A. Frelinger, D. W. Stafford, and P. E. Monahan. 2007. Transgene expression levels and kinetics determine risk of humoral immune response modeled in factor IX knockout and missense mutant mice. Gene Ther. 14:429-440. [DOI] [PubMed] [Google Scholar]
- 52.Zhi, Y., J. Figueredo, G. P. Kobinger, H. Hagan, R. Calcedo, J. R. Miller, G. Gao, and J. M. Wilson. 2006. Efficacy of severe acute respiratory syndrome vaccine based on a nonhuman primate adenovirus in the presence of immunity against human adenovirus. Hum. Gene Ther. 17:500-506. [DOI] [PubMed] [Google Scholar]