Abstract
Phosphorylation of the degron of the IFNAR1 chain of the type I interferon (IFN) receptor triggers ubiquitination and degradation of this receptor and, therefore, plays a crucial role in negative regulation of IFN-α/β signaling. Besides the IFN-stimulated and Jak activity-dependent pathways, a basal ligand-independent phosphorylation of IFNAR1 has been described and implicated in downregulating IFNAR1 in response to virus-induced endoplasmic reticulum (ER) stress. Here we report purification and characterization of casein kinase 1α (CK1α) as a bona fide major IFNAR1 kinase that confers basal turnover of IFNAR1 and cooperates with ER stress stimuli to mediate phosphorylation-dependent degradation of IFNAR1. Activity of CK1α was required for phosphorylation and downregulation of IFNAR1 in response to ER stress and viral infection. While many forms of CK1 were capable of phosphorylating IFNAR1 in vitro, human CK1α and L-CK1 produced by the protozoan Leishmania major were also capable of increasing IFNAR1 degron phosphorylation in cells. Expression of leishmania CK1 in mammalian cells stimulated the phosphorylation-dependent downregulation of IFNAR1 and attenuated its signaling. Infection of mammalian cells with L. major modestly decreased IFNAR1 levels and attenuated cellular responses to IFN-α in vitro. We propose a role for mammalian and parasite CK1 enzymes in regulating IFNAR1 stability and type I IFN signaling.
Cytokines that belong to a superfamily of interferons (IFNs), including type I IFN (such as IFN-β and numerous species of IFN-α) and type II interferon (IFN-γ), are important for efficient antiviral defense (40, 51). Type I IFNs signal via interacting with the heterodimeric receptor complex composed of two chains (IFNAR1 and IFNAR2); ligand binding activates receptor-associated members of the JAK family of tyrosine kinases, Jak1 and Tyk2. These kinases phosphorylate and activate the signal transducers and activators of transcription (STAT) proteins, which increase transcription of the IFN-induced genes whose products exert antiviral, immunomodulatory, and antiproliferative effects. While some of the IFN actions might proceed in a STAT-independent manner, all biological functions of IFN-α/β reported to date rely on the function of the type I IFN receptor complex (reviewed in references 1, 54, and 55).
The IFNAR1 subunit of this receptor is essential for IFN-α/β signaling. Mice lacking IFNAR1 display a deficiency in antiviral responses (24, 36) and an altered immune activation in response to a number of microbial agents (57). Intriguingly, these mice do not display an increased susceptibility to a number of protozoans, including Leishmania spp. (57), which is lethal in animals that lack responses to IFN-γ (50). However, similar to IFN-γ, IFN-α also activates STAT1 and upregulates the inducible nitric oxide synthase, which is essential in the early defense against Leishmania (5), which by itself stimulates the production of type I IFN during the early infection stage (14).
In wild-type animals, the levels of IFNAR1 are mainly regulated by ubiquitin-dependent endocytosis and ensuing degradation of this chain and the entire type I IFN receptor (28, 30). Ubiquitination of IFNAR1 is facilitated by the βTrcp/HOS E3 ubiquitin ligase that is recruited to the destruction motif (degron) within the cytoplasmic tail of IFNAR1 upon phosphorylation of this degron on specific serines (Ser535 in humans and Ser526 in mice [29, 30]). Treatment of cells with IFN-α/β promotes this serine phosphorylation of IFNAR1 and its subsequent ubiquitination and degradation in a manner that requires catalytic activity of Tyk2 (29, 32, 34).
Intriguingly, our recent studies revealed the presence of a major JAK-independent kinase activity which is capable of phosphorylating the IFNAR1 degron in cells that are not exposed to type I IFN. Such activity confers a ligand-independent yet phosphorylation-dependent pathway by which IFNAR1 is ubiquitinated and degraded in naïve cells; this basal degradation of IFNAR1 plays an important role in limiting antiproliferative effects imposed by high levels of IFNAR1 expression (32). Remarkably, the efficacy of ligand-independent phosphorylation and turnover of IFNAR1 initially observed upon IFNAR1 overexpression (32) could be further stimulated in cells by inducers of endoplasmic reticulum (ER) stress, including thapsigargin (TG) and infection with vesicular stomatitis virus (VSV) or hepatitis C virus (31). The importance of this regulation was underscored by ensuing attenuation of IFN-α/β signaling and antiviral defenses, demonstrating that the ligand-independent pathway of IFNAR1 proteolysis plays an important role in the interaction between viruses and mammalian host. Whereas activity of the PKR-like ER kinase (PERK) has been implicated in ER stress- and virus-mediated IFNAR1 turnover, attempts to directly phosphorylate the IFNAR1 degron using this kinase were not successful (31), suggesting that another kinase mediates ligand-independent phosphorylation of IFNAR1.
Here we report identification and characterization of casein kinase 1α (CK1α) as a major bona fide kinase of IFNAR1 that mediates basal phosphorylation, ubiquitination, and turnover of IFNAR1. Experiments using genetic and pharmacological approaches further demonstrate the involvement of CK1α in ligand-independent degron phosphorylation and degradation of IFNAR1 stimulated by ER stress inducers, including VSV. Intriguingly, CK1 activity secreted by Leishmania is also capable of phosphorylating the IFNAR1 degron. Expression of leishmanial CK1 (L-CK1) in mammalian cells downregulates IFNAR1 and attenuates IFN-α/β signaling in a phosphorylation-dependent manner. Together with our previous observations with viral pathogens, these results highlight the involvement of members of the CK1 family of kinases in the ligand-independent IFNAR1 degradation pathway, which plays a role in shaping the interaction between a mammalian host and infectious agents.
MATERIALS AND METHODS
Purification of basal IFNAR1 kinase activity.
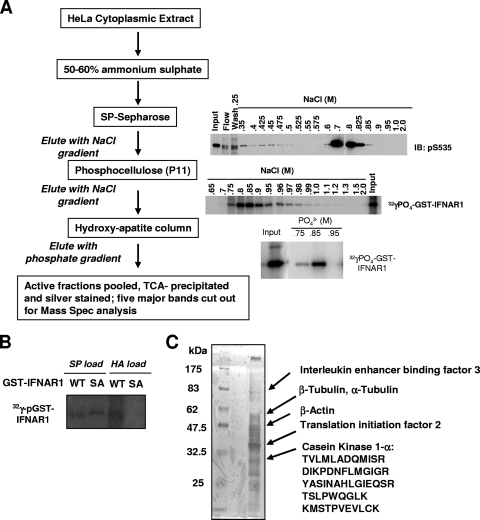
Basal IFNAR1 Ser535 kinase activity was measured in vitro using bacterially expressed glutathione S-transferase (GST)-IFNAR1 (1 μg) as a substrate, lysates from indicated cells (1 μg intact or 4 μg immunodepleted) as a source of kinase, and immunoblotting (IB) with anti-pS535 antibody as a method of detection, as described in detail elsewhere (31, 32). Untreated HeLa cells were harvested, suspended in 10 mM Tris-HCl (pH 8.0), 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol, 0.2 mM EDTA, and a cocktail of protease inhibitors (suspension buffer), and lysed by passing through a 23-gauge needle. After centrifugation, the nuclear pellet was discarded and the supernatant was ultracentrifuged at 100,000 × g for 60 min. Following centrifugation, the supernatant was kept at 4°C in buffers containing a cocktail of protease inhibitors. Approximately 90 ml of HeLa cell S100 extract (∼10 mg/ml) was precipitated with ammonium sulfate (50% to 60% saturation), and the pellet was redissolved, dialyzed, and applied onto a SP Sepharose (Amersham-Pharmacia) column and eluted with a linear gradient (100 to 2,000 mM NaCl) in buffer A containing 100 mM phosphate buffer, 50 mM KCl, 0.1 mM EDTA, and 10% glycerol. Fractions that contained Ser535 IFNAR1 kinase activity were pooled, concentrated, and further characterized by their ability to facilitate the incorporation of radioactive phosphate from 32P-labeled γ-ATP into the wild-type GST-IFNAR1 (GST-IFNAR1WT) but not the GST-IFNAR1S535A mutant. Active fractions were applied onto a phosphocellulose column (P11; Whatman) and eluted with a linear gradient (500 to 2,000 mM NaCl) in buffer B containing 20 mM Tris-HCl (pH 7.6), 100 mM KCl, 0.1 mM EDTA, and 10% glycerol. Active fractions were concentrated on a hydroxyappatite column (Bio-Rad), eluted stepwise using orthophosphate buffer, concentrated, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Five major bands (see Fig. 1B, below) were excised and subjected to in-gel tryptic digestion followed by nano-electrospray ionization (ESI) liquid chromatography-mass spectrometry (MS) by using a Waters Q-ToF mass spectrometer with a Waters nanoAcquity UPLC apparatus. The resulting tandem MS (MS/MS) spectra of the peptides derived from one of the bands contained several peptides (including DIKPDNFLMGIGR, YASINAHLGIEQSR, TSLPWQGLK, KMSTPVEVLCK, and FEEAPDYMYLR) that were identified as derivatives of human CSNK1A1 (CK1α).
FIG. 1.
Purification of cellular Ser535 kinase activity. (A) Purification scheme and results from in vitro kinase activity assays that used immunoblotting with phospho-specific antibody or [γ-32P]ATP incorporation into the GST-IFNAR1 substrate as indicated. (B) Phosphorylation of bacterium-produced GST-IFNAR1 (wild type or Ser535,539Ala mutant [SA]) by the starting fractions before loading onto either SP Sepharose (SP) or hydroxyappatite (HA) columns in the presence of radioactive [γ-32P]ATP was analyzed by SDS-PAGE and autoradiography. Mutant IFNAR1 migrates slower due to the presence of additional amino acids in the linker between GST and the cytoplasmic domain of IFNAR1 (as outlined in references 31 and 32). (C) Active fractions were pooled after the last purification step. Proteins were precipitated and separated on an SDS-PAGE gel followed by silver staining. Five indicated major bands were cut out for mass spectrometry analysis. The identities of the bands and the sequences of identified CK1α-derived peptides are shown on the right.
Constructs for mammalian expression of IFNAR1 and bacterial expression of GST-IFNAR1 were previously described (28, 30). The construct for bacterial expression of GST-CK1α (described in reference 13) was a kind gift from Jiandong Chen (H. Lee Moffitt Cancer Center, Tampa, FL). Constructs for expression of human Myc-tagged CK1 and shRNA vectors against CK1α or green fluorescent protein (GFP; a kind gift from J. Wade Harper, Harvard University, Boston, MA) were previously described (52). Human CK1α and L-CK1 cDNA (described in reference 2) were subcloned into a pEF-BOS vector with a hemagglutinin (HA) tag. A point mutation of K40R in L-CK1 was introduced via site-directed mutagenesis. Vaccinia virus B1 kinase and its kinase-dead mutant form (K149Q [KD]) expression constructs (49) were kindly provided by P. A. Lazo (Universidad de Salamaca, Salamaca, Spain). Recombinant human IFN-α2a was purchased from Roche. Thapsigargin, cycloheximide, and D4476 were from Sigma. Murine IFN-β and human IFN-γ were purchased from PBL. Small interfering RNA (siRNA) oligos against the luciferase gene (5′-CUUACGCUGAGUACUUCGAdTdT-3′) or hCK1α (5′-CCAGGCAUCCCCAGUUGCUdTdT-3′) were purchased from Dharmacon Inc. In some experiments, the siRNA oligos that contained several substitutions (underlined) of correct bases in siCK1α were used as another control (siCon#2, 5′-CCAGGCUAGGCCAGUUGCUdTdT-3′).
Cell culture, transfections, virus, and parasites.
All cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal bovine serum (FBS; HyClone) unless otherwise specified. 2fTGH human fibrosarcoma cells were a kind gift from G. Stark (Cleveland Clinic, Cleveland, OH). IFNAR1−/− mouse embryonic fibroblasts (MEFs) and their WT counterparts were kindly provided by S. Hemmi (Institute for Molecular Biology, Zurich, Switzerland). Mouse bone marrow-derived macrophages from the C57/BL6 mice were obtained by cultivating bone marrow cell isolates in RPMI medium containing 10% FBS and 30% of the L929 cell supernatant (a source of macrophage colony-stimulating factor) for 7 days according to a standard protocol. Human peripheral blood monocytes were obtained from University of Pennsylvania Human Immunology Core, and derivation of dendritic cells was done according to a standard protocol (48). A cell proliferation assay was carried out using the CellTiter 96 nonradioactive cell proliferation assay kit (catalog number G4001; Promega) according to the manufacturer's recommendations.
293T cells and HeLa cells were transfected with Lipofectamine Plus reagent and Lipofectamine 2000 reagent, respectively. VSV (Indiana serotype; a gift from R. Harty, University of Pennsylvania) was propagated in HeLa cells. L major (WHO MHOM/IL-1/80 Freidlin clone) was maintained in a log phase of growth in Schneider's growth medium containing 20% FBS.
Viral and parasite infection of cultured cells.
HeLa or 2fTGH cells were inoculated with a multiplicity (MOI) of 0.1 of VSV for 1 h, washed, and added with fresh medium. At 12.5 h later, uninfected or infected cells were treated with D4476 or vehicle (dimethyl sulfoxide [DMSO]). Total cell lysates were harvested at different ensuing time points. For Leishmania infections, the macrophages were resuspended in 106 cells/ml and were infected with a 10-fold excess of L. major (50%) metacyclic in suspension culture for 4 h. Cells were subsequently washed two times to remove free parasites and further incubated as indicated.
Measurement of L. major-secreted kinase activity.
A total of 50 × 106 confluent L. major promastigotes were washed with buffer A (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1 mM glucose, and 10 mM NaF). Cells were then resuspended in buffer A containing 50 μg/ml of GST-IFNAR1 at 30°C for 20 min as described previously (47). The supernatant was collected, supplemented with 2 mM of ATP, and further incubated at 30°C for 15 min. The substrate was captured by glutathione beads and analyzed in Western blot assay for phosphorylation at site Ser535.
Immunotechniques.
Antibodies against pSTAT1 and p-eIF2α (Cell Signaling), eIF2α (Biosources), CK1ɛ (BD Pharmingen), STAT1, Myc tag, HA tag, GST, CK1α (Santa Cruz), Flag tag, β-actin (Sigma), and ubiquitin (clone FK2; Biomol) were used for immunoprecipitation and immunoblotting. Monoclonal antibody 23H12, specific for the M protein of VSV (VSV-M), was kindly provided by D. S. Lyles (Wake Forest University School of Medicine, Winston-Salem, NC). Antibodies which recognize endogenous IFNAR1 (20) and IFNAR1 phosphorylated on Ser535 (or Ser526 in mouse IFNAR1 [29]) were described previously. Cell lysis, immunoprecipitation, and immunoblotting procedures as well as the kinase assay using cell lysates and GST-IFNAR1 as a substrate were previously described (31, 32). Quantification of IB analyses was done using Li-Cor's Odyssey infrared imaging system.
Flow cytometry.
Cell surface levels of IFNAR1 in human and mouse cells were determined by staining cells with anti-hIFNAR1 (AA3 [20]) or anti-mIFNAR1 (Leinco) in combination with anti-mouse-biotin (Jackson Laboratory) and streptavidin-phycoerythrin (e-Bioscience). Cell surface antigen levels were examined by usin a FACSCalibur flow cytometer (BD Pharmingen). The data were analyzed with the FlowJo program (Tree Star).
RESULTS
CK1α is a kinase that directly phosphorylates the IFNAR1 degron.
We previously reported detection of a major ligand- and JAK-independent Ser535 kinase activity in lysates from human cells. Such activity could be monitored by an in vitro kinase assay using the bacterially expressed cytoplasmic domain of IFNAR1 fused with GST (GST-IFNAR1) as a substrate, the cell lysates as the source of kinase, and anti-phospho-Ser535 immunoblotting as a mode of detection (32). Purification of basal IFNAR1 kinase activity was carried out as outlined in Fig. 1A. Cytoplasmic lysates from untreated HeLa cells were fractionated by ammonium sulfate precipitation followed by purification on a cation exchange SP Sepharose column (as described in detail in Materials and Methods) to identify fractions that were active in this kinase assay and that could discriminate between wild type GST-IFNAR1 and the mutant GST-IFNAR1S535A counterpart in a modified assay that used radioactive ATP for detection (Fig. 1B). This modified assay was used for further purification of enriched IFNAR1 kinase activity through additional steps (Fig. 1A). Mass spectrometry analysis of five major bands obtained from pooled active fractions resolved on SDS-PAGE (Fig. 1C) revealed the presence of several peptides derived from human CK1α (see Materials and Methods).
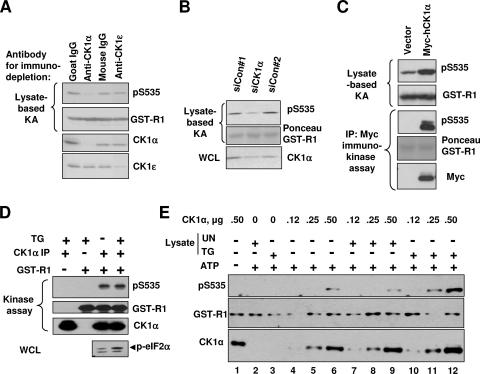
CK1α and six other members of the human CK1 family of ubiquitous pleiotropic kinases phosphorylate numerous substrates (26), some of which share the presence of a potentially phosphorylated serine or threonine residue at position n-3 to enable hierarchical mechanism of primed subsequent phosphorylation (7, 8, 16, 18, 35, 46, 56). Intriguingly, mouse and human IFNAR1 harbor similar residues (underlined), Ser529 and Ser532, in the sequence that directly precedes the degron (529SQTSQDSGNYS). Consistent with a possibility that CK1α might function as a direct basal Ser535 IFNAR1 kinase in human cells, immunodepletion of HeLa cell lysate using the antibody against CK1α (but using neither control irrelevant monoclonal or polyclonal antibodies nor antibody against CK1ɛ) indeed decreased the efficacy of GST-IFNAR1 phosphorylation in vitro by this lysate (Fig. 2A). Furthermore, while RNA interference (RNAi)-mediated knockdown of CK1α in HeLa cells decreased the ability of lysates from these cells to mediate Ser535 phosphorylation in vitro (Fig. 2B), a reverse effect was obtained upon overexpression of CK1α in 293T human embryo kidney cells (Fig. 2C). In addition, both immunopurified (Fig. 2C) and bacterially produced CK1α (Fig. 2E, lane 6) also phosphorylated GST-IFNAR1 on Ser535 in vitro. Collectively, these data validate our biochemical purification strategy and indicate that CK1α is a bona fide direct kinase of Ser535 of IFNAR1.
FIG. 2.
CK1α represents the major Ser535 kinase in the cell lysates. (A) HeLa lysates were immunoprecipitated (IP) with control immunoglobulin Gs (IgGs) or antibodies against CK1α or CK1ɛ and protein G beads. The supernatants of these reaction mixtures were analyzed for their S535 kinase activity by an in vitro kinase assay (KA) with GST-IFNAR1 as a substrate, detected by immunoblotting using anti-pS535 and anti-GST antibodies (upper panels). Efficacy of immunodepletion was verified by immunoblotting using antibodies for CK1α and CK1ɛ (lower panels). (B) Lysates from HeLa cells transfected with the indicated siRNAs were used in the kinase assay. Phosphorylation of GST-IFNAR1 and levels of CK1α in whole-cell lysates (WCL) were analyzed by IB using the indicated antibodies. Ponceau S staining of the membrane to detect GST-IFNAR1 is also depicted. (C) 293T cells were transfected with empty vector or Myc-tagged human CK1α, and lysates were prepared. These lysates were analyzed for their IFNAR1 Ser535 kinase activity (as for panels A and B). In addition, phosphorylation of GST-IFNAR1 in the immunokinase assay (as well as the levels of Myc-CK1α and GST-IFNAR1) was assessed via IP using anti-Myc antibody; results are depicted in the lower panels. (D) 293T cells were treated with DMSO or TG (1 μM) for 30 min. Lysates were subjected to CK1α IP, followed by analysis of Ser535 activity in vitro using GST-IFNAR1 as a substrate. Induction of ER stress was shown by phosphorylation of p-eIF2α as assessed by IB using phospho-specific antibody. (E) 293T cells were untreated or treated with TG (1 μM for 30 min) and harvested. Lysates from these cells were immunodepleted of CK1α as outlined for panel A. Increasing amounts (0.12 to 0.5 μg) of bacterium-produced recombinant GST-CK1α were incubated with the substrate (GST-IFNAR1) and ATP (except in lane 1) at 30°C for 30 min without any lysates (lanes 4 to 6) or in the presence of 4 μg of immunodepleted lysates from untreated (UN; lanes 7 to 9) or TG-treated (lanes 10 to 12) cells. Phosphorylation of GST-IFNAR1 on Ser535, levels of GST-IFNAR1 (using anti-GST antibody), and levels of CK1α were analyzed by IB.
A substantial body of literature indicates that members of the CK1 family are constitutively active kinases (26). However, given that ligand-independent phosphorylation of IFNAR1 can be further stimulated in cells treated with the inducers of ER stress, such as TG or viruses (31), we sought to investigate whether TG treatment activates CK1α. As expected, treatment of cells with TG caused activation of PERK as assessed via phosphorylation of its substrate, eIF2α (Fig. 2D). Remarkably, CK1α purified from the lysates from these cells (or cells treated with IFN-α [data not shown]) did not display a higher activity in an in vitro kinase reaction with GST-IFNAR1 as a substrate (Fig. 2D).
To examine whether a CK1α-independent factor may facilitate this kinase's actions in cells undergoing ER stress, we immunodepleted CK1α from the lysates of cells treated or not with TG. In line with the results shown in Fig. 2A, the supernatants of these reaction mixtures were not efficient in mediating phosphorylation of GST-IFNAR1 on Ser535 (Fig. 2E, lanes 2 and 3). However, when combined with bacterially expressed CK1α, the depleted lysates from TG-treated cells noticeably increased the efficacy of IFNAR1 phosphorylation (Fig. 2E, lanes 11 and 12). These results indicate that ER stress induces yet-to-be-identified cellular factors that cooperate with CK1α to increase the phosphorylation of the IFNAR1 degron.
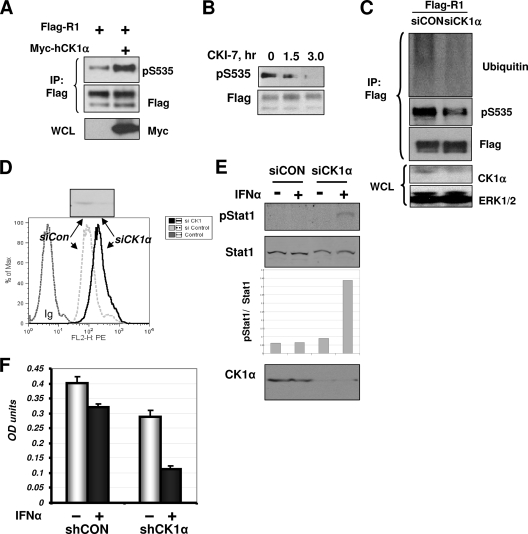
We next examined whether CK1α mediates ligand-independent IFNAR1 phosphorylation at Ser535 in the cells. Consistent with our previously published observations (32), this phosphorylation was easily detectable on Flag-tagged IFNAR1 expressed and immunopurified from human cells. Under these conditions, coexpression of human CK1α further promoted phosphorylation of the IFNAR1 degron (Fig. 3A). In addition, this phosphorylation was decreased in 293T cells treated with a CK1 inhibitor, CKI-7 (Fig. 3B). Importantly, knockdown of CK1α decreased basal Ser535 phosphorylation of coexpressed Flag-IFNAR1 (Fig. 3C).
FIG. 3.
CK1α mediates basal IFNAR1 phosphorylation, ubiquitination, and downregulation in cells. (A) 293T cells were cotransfected with Flag-IFNAR1 and Myc-hCK1α or an empty vector. Ser535 phosphorylation of Flag-IFNAR1 was analyzed by Flag immunoprecipitation (IP) followed by IB of pS535. The total levels of IFNAR1 were determined by reprobing the blot with an anti-Flag antibody. Myc-CK1α levels in the whole-cell lysates (WCL) are shown in the lower panel. (B) 293T cells expressing Flag-IFNAR1 were treated with CKI-7 (400 μM) for the indicated times. Immunopurified IFNAR1 was analyzed by IB using the indicated antibodies. (C) HeLa cells were cotransfected with Flag-IFNAR1 and siRNA against CK1α or a control siRNA. At 48 h after transfection, lysates were harvested and were subjected to IP using anti-Flag antibody followed by IB analysis using the indicated antibodies. Levels of CK1α and Erk1/2 (as a loading control) in WCL were assessed by IB using the indicated antibodies. (D) HeLa cells were transfected with RNAi against CK1α or luciferase and analyzed for the surface levels of endogenous IFNAR1 by FACS using the AA3 monoclonal antibody. Control using irrelevant immunoglobulin (Ig) is also shown. (E) HeLa cells were transfected with siRNA as for panel C and then treated with the low dose of IFN-α (5 IU/ml) for 15 min as indicated. Activation and levels of Stat1 were analyzed by immunoblotting using the indicated antibodies. The ratio between pStat1 and Stat1 signals was calculated using Li-Cor's Odyssey infrared fluorescence-based quantification system. (F) Human 2fTGH cells were cotransfected with shRNA against GFP (shCON) or against CK1α (shCK1α) and with pBABE-puro vector. After 4 days of selection in medium containing puromycin (4 μg/ml), the cells were plated into 96-well plates (5 × 104/well) and treated (+) or not treated (−) with IFN-α (250 IU/ml for 48 h) as indicated. Numbers of cells as a function of absorbance were measured using the CellTiter 96 cell proliferation assay kit (Promega) and are presented in optical density (OD) units as depicted on the graph. Averages of a total of six experiments are shown.
In line with our previous report that basal phosphorylation of IFNAR1 mediates its ubiquitination in cells not exposed to IFN (32), we also observed that knockdown of endogenous CK1α decreased the extent of IFNAR1 ubiquitination in untreated HeLa cells (Fig. 3C). Consistent with the role of IFNAR1 ubiquitination in endocytosis of this receptor (28, 30), the cell surface levels of IFNAR1 measured by fluorescence-activated cell sorting (FACS) analyses were noticeably higher in the cells transfected with siRNA against CK1α (Fig. 3D). Given that IFNAR1 levels are important for IFN-α/β signaling (24), we tested whether modulation of CK1α expression affects the extent of cellular responses to IFN-α. A brief treatment of HeLa cells that received control siRNA by a low dose of IFN-α caused a negligible level of Stat1 phosphorylation. Under these conditions, we observed a noticeably more pronounced activation of Stat1 in cells where CK1α was knocked down (Fig. 3E). Furthermore, stable downregulation of CK1α expression by shRNA constructs against CK1α augmented the antiproliferative effect of IFN-α in 2fTGH human cells (Fig. 3F). Given that CK1α is an abundant protein and its knockdown was incomplete in all these experiments, the extent of CK1α-mediated effects on IFNAR1 phosphorylation, ubiquitination, cell surface levels, and signaling are likely to be underestimated. Collectively, these data suggest that CK1α contributes to the control of IFNAR1 ubiquitination and cell surface levels of IFNAR1 as well as the sensitivity of cells to IFN-α.
CK1α is required for efficient phosphorylation and downregulation of IFNAR1 via the ligand-independent pathway.
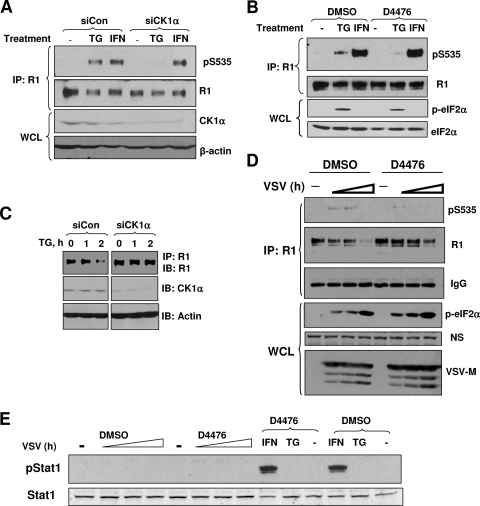
Ligand-independent phosphorylation and degradation of IFNAR1 could be further stimulated by inducers of ER stress, such as TG and infection with VSV (31). Knockdown of endogenous CK1α by RNAi noticeably decreased the extent of Ser535 phosphorylation in the cells treated with TG. Importantly, phosphorylation of IFNAR1 in response to IFN-α was not affected by siRNA against CK1α (Fig. 4A). These results indicate that CK1α is dispensable for the ligand-inducible phosphorylation of IFNAR1 but might be required for the ligand-independent pathway.
FIG. 4.
CK1α is required for efficient IFNAR1 downregulation in response to ER stress. (A) HeLa cells were transfected with control siRNA or siRNA against CK1α. After 48 h, cells were treated with vehicle control, TG (1 μM), or IFN-α (1,000 IU/ml) for 30 min, and lysates were harvested. The lysates were subjected to IFNAR1 immunoprecipitation (IP) followed by IB of pSer535 and total IFNAR1. The efficiency of CK1α knockdown is shown in the lower panel. (B) 2fTGH cells were pretreated with 15 μM of D4476 or DMSO for 1 h and then treated with vehicle control, TG (1 μM), or IFN-α (1,000 IU/ml) for 30 min. IFNAR1 Ser535 phosphorylation and total levels were determined by IP-IB. Total levels of eIF2α and its phosphorylation (as a marker of the PERK-dependent effect of TG) in whole-cell lysates (WCL) were also analyzed. (C) HeLa cells were transfected with control siRNA (siCon) or siRNA against CK1α (siCK1α). At 48 h after transfection, cells were treated with DMSO or TG (1 μM) for the indicated times. Levels of total IFNAR1 were determined by IP-IB. Levels of CK1α and actin in total cell lysate were examined by IB. (D) 2fTGH cells were infected with VSV (MOI, 0.1) for 13 h. The infected cells were then treated with DMSO or 20 μM of D4476, and cells were further incubated for 0.5, 1.0, or 2.0 h. At these time points, cells were harvested. Endogenous IFNAR1 from these cells was analyzed by IP-IB using the indicated antibodies. Levels of viral protein VSV-M and phosphorylation of eIF2α (indicative of ER stress) were also assessed by IB in WCL. The nonspecific band (NS) is indicative of the loading of the gel. (E) Lysates from experiments shown panels B and D were analyzed for Stat1 phosphorylation and Stat1 levels by IB using the indicated antibodies.
The latter possibility was further tested by a pharmacologic approach using a cell-permeable and selective CK1 inhibitor, D4476 (3, 45). Although TG caused a comparable induction of phosphorylation of eIF2α (a canonical substrate of TG-inducible PERK [21, 59]) regardless of pretreatment with D4476, this inhibitor noticeably attenuated the Ser535 phosphorylation of IFNAR1 in response to TG but not to IFN-α in 2fTGH cells (Fig. 4B). These data together suggest that CK1 activity is required for ligand-independent phosphorylation of the degron of IFNAR1.
ER stress induces S535 phosphorylation of IFNAR1 and accelerates its phosphorylation-dependent endocytosis and subsequent degradation (31). Consistently, in cells transfected with siRNA against CK1α, thapsigargin-induced downregulation of IFNAR1 was noticeably attenuated (Fig. 4C). Collectively, these results demonstrate that CK1α phosphorylates S535 to accelerate subsequent downregulation of IFNAR1, therefore controlling the levels of IFNAR1 in cells that undergo ER stress.
To further test this possibility we investigated the role of CK1 in phosphorylation and downregulation of IFNAR1 in 2fTGH cells infected with VSV, which was previously shown to induce IFNAR1 phosphorylation and degradation in a ligand- and JAK-independent manner (31). We were limited in our approach and chose not to use RNAi because of the potential pleiotropic effects of loss of CK1α on viral replication and expression of viral proteins reported in literature (4, 6, 12, 17, 23, 33, 42, 43). Instead, we used a pharmacological approach to acutely inhibit CK1 activity by treatment with D4476. Previous reports demonstrated that VSV infection promoted ER stress (21) and phosphorylation-dependent ubiquitination and degradation of IFNAR1 (31). When D4476 was added to the VSV-infected cells shortly before a point where significant accumulation of a viral protein (VSV-M) can be seen, this inhibitor markedly attenuated virus-induced S535 phosphorylation of IFNAR1 and downregulation of IFNAR1 without affecting eIF2α phosphorylation (Fig. 4D). Under these conditions, it is unlikely that IFNAR1 downregulation is driven by signaling initiated by endogenous IFN-α/β because of the lack of basal Stat1 phosphorylation in these lysates (Fig. 4E), although a possibility that type I IFN might be produced and act at other time points of infection cannot be ruled out. In all, these results indicate the involvement of CK1α in VSV-induced S535 phosphorylation and ensuing degradation of IFNAR1.
Leishmanial casein kinase regulates IFNAR1 levels and IFN-α/β signaling.
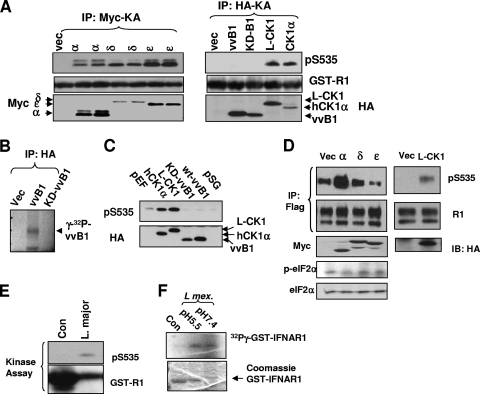
Casein kinase 1 comprises a large family of evolutionarily conserved kinases that include numerous isoforms in mammalian cells as well as CK1 orthologs and CK1-like proteins expressed in some lower organisms. We next examined whether different members in the CK1 superfamily are capable of phosphorylating S535 of IFNAR1 in vitro and in the cells. Vaccinia virus is known to express a CK1-like kinase B1 (vvB1) that plays an important role in its replication (44). When expressed and immunopurified from 293T cells, this kinase was not capable of direct phosphorylation of IFNAR1 on Ser535 (Fig. 5A, right panel) despite being active in autophosphorylation (Fig. 5B) and against other substrates, including casein (49). On the contrary, immunopurified human CK1δ, CK1ɛ, and protozoan parasite L-CK1 were active against IFNAR1 S535 in the immunokinase assay in vitro (Fig. 5A). Accordingly, lysates from cells overexpressing hCK1α and L-CK1, but not vvB1, exhibited elevated levels of S535 kinase activity (Fig. 5C). Interestingly, although all tested human CK1 isoforms were capable of phosphorylating GST-IFNAR1 in vitro, only expression of hCK1α increased the phosphorylation of Flag-IFNAR1 in the cells (Fig. 5D, left panel). Such an effect of hCK1α was unlikely to represent an artifact of specific induction of ER stress, since levels of phosphorylated eIF2α were similar in cells overexpressing all tested human CK1 forms. Similar to hCK1α, expression of L-CK1 also sufficed to promote phosphorylation of the IFNAR1 degron in the cells (Fig. 5D, right panel). These results together suggest that there is a specificity in the ability of diverse CK1 species to phosphorylate Ser535 of IFNAR1 and that there are certain structural determinants present in hCK1α and L-CK1 that enable this function in cells.
FIG. 5.
Characterization of the S535 kinase activity of several human CK1 isoforms and CK1-like proteins from other organisms. (A) 293T cells were transfected with an empty vector (Vec) or Myc-tagged CK1α (α), CK1δ (δ), or CK1ɛ (ɛ) or, as shown in the right panel, with HA-tagged vaccinia virus B1 kinase (vvB1), the kinase-dead vvB1 (KD-B1), L. major CK1 (L-CK1), or human CK1α. These transfected kinases were IPed with Myc or HA and were subjected to in vitro immunokinase assay (KA) to determine Ser535 phosphorylation of GST-IFNAR1. Levels of the substrate as well as kinases expression were also analyzed. (B) Autophosphorylation of HA-tagged vvB1 (expressed in and immunopurified from 293T cells) was carried out in the presence of labeled [γ-32P]ATP and detected by SDS-PAGE and autoradiography. Immunoprecipitation (IP) reactions from the lysates of the vector-transfected cells or cells expressing catalytically inactive KD-vvB1 mutant were used as a negative control. (C) In vitro phosphorylation of GST-IFNAR1 using lysates from cells transfected with indicated kinases as a source of the kinase activity was measured by immunoblotting using phospho-specific anti-pS535 antibody (upper panel). Expression of CK1 species was analyzed by IB using anti-HA antibody (lower panel). pEF and pSG indicate cells transfected with the indicated empty vectors. (D) 293T cells were cotransfected with Flag-IFNAR1 together with an empty vector (Vec) or Myc-tagged CK1α (α), CK1δ (δ), or CK1ɛ (ɛ) or, as shown in the right panel, with HA-tagged L-CK1. Phospho-S535 and total IFNAR1 signals were analyzed by IP-IB. Ectopic expression levels of the kinases were determined by Myc or HA IB. In the left panel, phosphorylation and total eIF2α levels are indicative of comparable levels of ER stress in cells transfected with different CK1 isoforms. (E) In vitro phosphorylation of GST-IFNAR1 with supernatant from L. major promastigote culture. Buffer lacking Leishmania was used as a control (Con). These fractions were incubated with ATP and GST-IFNAR1 (5 μg) at 30°C for 30 min. The products of this kinase reaction were analyzed by IB for pS535 and GST. (F) In vitro phosphorylation of GST-IFNAR1 by concentrated supernatant of cultured amastigotes of L. mexicana (obtained upon treatment with buffers with indicated pHs that mimicked the phagosomal or cytosolic environments) was measured by incorporation of radioactive phosphate as described in Materials and Methods.
It is plausible that mammalian IFNAR1 encounters L-CK1 when the cells are infected with Leishmania parasites that shuffle between sandflies and mammalian hosts during the infectious life cycle. Within this cycle, Leishmania promastigotes are released from the insect gut to invade macrophages and dendritic cells in the mammalian hosts via phagocytosis to become mammal-parasitizing amastigotes (reviewed in reference 41). Intriguingly, there are reports that various species of Leishmania are capable of secreting the CK1-like kinase that is active against several host mammalian substrates, including membrane proteins (47, 58). We have used the reported experimental conditions to test whether such activity is capable of phosphorylating IFNAR1. Incubation of concentrated medium obtained from L. major promastigotes with ATP and GST-IFNAR1 led to a noticeable phosphorylation of this substrate on Ser535 (Fig. 5E). In addition, kinase activity secreted by amastigotes from another Leishmania species (L. mexicana) under two different acidity conditions resulted in phosphorylation of IFNAR1 detected via incorporation of radiolabeled ATP into this substrate (Fig. 5F). These results suggest that different forms of Leishmania secrete a kinase activity that is capable of directly phosphorylating IFNAR1 within its degron.
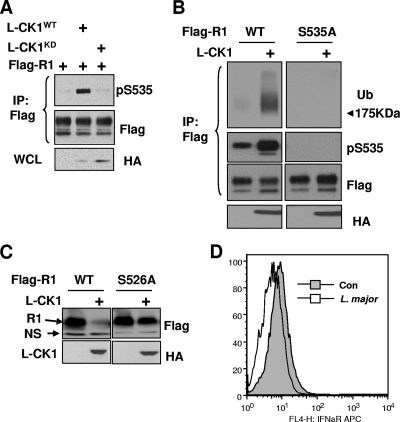
L-CK1 has been cloned and, based on studies that used inhibitors of this kinase, is implicated in controlling the growth of Leishmania (2, 15, 27). We further sought to investigate whether this kinase might regulate phosphorylation-dependent ubiquitination and degradation of IFNAR1. Expression of wild-type L-CK1 but not of its catalytically inactive mutant promoted phosphorylation of coexpressed Flag-tagged IFNAR1 on Ser535 (Fig. 6A). Furthermore, expression of L-CK1 increased ubiquitination of wild-type Flag-IFNAR1 but not of its S535A mutant, which was insensitive to the phosphorylating effects of L-CK1 (Fig. 6B). In some of these experiments, we observed a slight decrease in the levels of wild-type Flag-IFNAR1 in the cells where L-CK1 was coexpressed; however, these changes were difficult to interpret because of the presence of endogenous IFNAR1. To test whether the presence of leishmanial kinase might affect the levels of IFNAR1, we used mouse embryo fibroblasts obtained from IFNAR1 knockout animals. These fibroblasts were reconstituted with either wild-type mouse Flag-IFNAR1 or its mutant that harbors the S526A mutation (analogous to the human S535A substitution). Given that coexpression of L-CK1 decreased the levels of wild-type Flag-IFNAR1 much more dramatically than that of the phosphorylation-insensitive receptor mutant (Fig. 6C and 7B, lower panel), it is likely that L-CK1 downregulates IFNAR1 at least in part through a phosphorylation-dependent mechanism.
FIG. 6.
Expression of L-CK1 promotes phosphorylation-dependent IFNAR1 ubiquitination, and degradation. (A) 293T cells were cotransfected with Flag-IFNAR1 and HA-tagged L-CK1 (WT or kinase-dead K40R mutant) or an empty vector. The levels of pS535 and total IFNAR1 were determined by IP-IB. The levels of L-CK1 in whole-cell lysates (WCL) were determined by IB using anti-HA antibody. (B) 293T cells were cotransfected with IFNAR1 (WT or S535A mutant) and L-CK1 or an empty vector. The levels of ubiquitinated, S535-phosphorylated, and total IFNAR1 were analyzed by IP-IB. The levels of L-CK1 were assessed by HA IB. (C) MEFs derived from IFNAR1−/− mice reconstituted with WT or S526A mouse IFNAR1 were transfected with L-CK1 or an empty vector. The levels of IFNAR1 and L-CK1 were determined by Flag and HA IB, respectively. N.S., nonspecific band. (D) Human blood monocyte-derived dendritic cells were infected with L. major promastigote culture (containing ∼50% metacyclics at an MOI of 10) or left uninfected as a control (Con). After overnight incubation, cells were subjected to FACS analysis of cell surface IFNAR1 using AA3 monoclonal antibody.
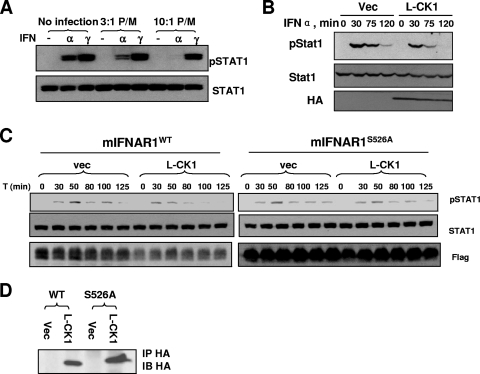
FIG. 7.
L. major infection or L-CK1 expression suppresses type I IFN signaling. (A) Mouse bone marrow-derived macrophages were infected or not with L. major parasites (as outlined for Fig. 6D) at the indicated ratios. After overnight incubation, cells were treated with mouse IFN-α (200 IU/ml) or IFN-γ (10 ng/ml) for 30 min. Levels of phosphorylated and total Stat1 were determined by IB. (B) 293T cells transfected with the indicated plasmids were subjected to pulse treatment with human IFN-α (500 IU for 15 min). Cells were harvested at the indicated time points after beginning of treatment and analyzed for Stat1 activation using the indicated antibodies. Levels of L-CK1 were analyzed by IB. (C) MEFs from IFNAR1−/− mice reconstituted as described for Fig. 6C were transfected with empty vector or L-CK1 as indicated. After 24 h, cells were trypsinized and equal numbers of cells were plated into 12-well plates. After overnight incubation, cells were pulsed with murine IFN-α (50 IU/ml) for 30 min and then chased with fresh medium for the indicated times (relative to the initial addition of IFN). Lysates were harvested, and the levels of pStat1, total Stat1, and Flag-IFNAR1 were determined by IB. (D) Lysates from untreated cells from the experiment shown in panel C were analyzed for expression of HA-tagged L-CK1 using IP-IB with anti-HA antibody.
Furthermore, infection of human dendritic cells with L. major led to a modest but reproducible decrease in the cell surface levels of endogenous IFNAR1 assessed by FACS (Fig. 6D). Similar results were obtained when mouse bone marrow macrophages were used for infection (data not shown). Collectively these data suggest that the presence of L-CK1 in mammalian cells leads to phosphorylation of the IFNAR1 degron and ensuing phosphorylation-dependent downregulation of IFNAR1.
Maintenance of IFNAR1 levels plays an important role in regulation of the duration and magnitude of type I IFN signaling (22, 24, 30). The results that L. major secretes an S535 kinase activity and that L-CK1 is sufficient to cause S535-dependent IFNAR1 loss suggested that Leishmania may attenuate the extent of IFN signaling. Infection of mouse bone marrow macrophages with L. major indeed led to a dose-dependent inhibition of Stat1 phosphorylation in response to IFN-α (Fig. 7A). Remarkably, this suppression was specific, as Leishmania infection did not affect Stat1 phosphorylation induced by type II IFN (IFN-γ). Since type I and II IFNs utilize different receptors, yet similar intracellular kinases, to activate Stat1, the latter data suggest that L. major inhibits cellular responses to type I IFN via targeting its receptor.
To directly test the role of L-CK1 in the inhibition of type I IFN signaling we transfected plasmid for expression of L-CK1 or empty vector in human 293T cells and followed up activation of Stat1 after pulse treatment with human IFN-α. Cellular responses to this cytokine were noticeably attenuated in cells that received L-CK1 (Fig. 7B). A similar experiment was performed on IFNAR1-null mouse embryo fibroblasts that were reconstituted with either wild-type IFNAR1 or its L-CK1-insensitive IFNAR1S526A mutant. A pulse treatment of cells with mouse IFN-α led to a temporal induction of Stat1 phosphorylation, the extent of which was reduced over time (Fig. 7C). Expression of L-CK1 in cells that harbor wild-type IFNAR1 led to a noticeable signaling inhibition that manifested itself in both a lesser magnitude and a shorter course of Stat1 phosphorylation. Importantly, these changes were much less prominent when L-CK1 was expressed in cells that harbor the IFNAR1S526A mutant (Fig. 7C), despite similar levels of L-CK1 achieved in these cells (Fig. 7D). These results collectively indicate that the presence of the leishmanial CK1 in the host cells suppresses the cellular responses to IFN-α in a manner that at least in part depends on phosphorylation of the IFNAR1 degron.
DISCUSSION
We have previously reported that a ligand- and Jak-independent signaling pathway leads to Ser535 phosphorylation-dependent ubiquitination and degradation of IFNAR1. This pathway plays an important role in regulating the levels of IFNAR1 in naïve cells and in determining the sensitivity of cells to future exposures to type I IFN. A major basal kinase activity in cell lysates that phosphorylates IFNAR1 within its degron has been described (32). In the present study, we purified CK1α as a kinase capable of phosphorylating IFNAR1 in vitro. We further characterized CK1α as the direct kinase responsible for basal IFNAR1 kinase activity and basal phosphorylation of IFNAR1 in unstimulated cells. These conclusions are based on the facts that kinase activity in cell lysates and basal IFNAR1 phosphorylation are decreased when CK1α is removed from cells (by knockdown) or lysates (by immunodepletion). Furthermore, recombinant CK1α was capable of directly phosphorylating IFNAR1 within its degron (Fig. 1 and 2).
Recent studies from our laboratory also revealed that phosphorylation, ubiquitination, and degradation of IFNAR1 via the ligand-independent pathway can be accelerated by ER stress stimuli such as treatment with TG or infection with VSV. These stimuli initiated a PERK-dependent pathway and, given that PERK itself did not directly phosphorylate IFNAR1, were proposed to act upon IFNAR1 via another protein kinase that was to be identified (31). Here the data of experiments using pharmacological (CK1 inhibitors) and genetic (RNAi) approaches demonstrated that CK1α is required for phosphorylation and augmented downregulation of IFNAR1 in cells that were treated with TG or infected with VSV. Given that modulations of CK1 activity did not affect IFNAR1 phosphorylation in response to IFN (Fig. 4), we conclude that CK1α is a bona fide IFNAR1 degron kinase that functions within the ligand-independent pathway.
While human cells express several members of the CK1 family that share highly conserved kinase domains (26) and are capable of phosphorylating IFNAR1 in vitro, specific knockdown of CK1α sufficed to effectively reduce the ligand-independent Ser535 phosphorylation of IFNAR1 in human cells. Furthermore, expression of CK1α and L-CK1 but not other tested members of the CK1 family induced IFNAR1 phosphorylation in the cells. These data suggest that CK1α and L-CK1 might be unique in their ability to efficiently target S535 of IFNAR1 in cells. The structural basis and the mechanisms underlying this specificity are to be delineated in future studies.
Further studies are also needed to understand how CK1α, which is known as a constitutively active kinase (26), can cooperate with ER stress stimuli to increase IFNAR1 phosphorylation and promote the degradation of this receptor. In cells that undergo ER stress, levels of CK1α and its Ser535 kinase activity are not affected (Fig. 2D). This suggests that additional regulatory events occur to prompt increased Ser535 phosphorylation in response to ER stress stimuli. Indeed, the lysates from TG-treated cells stimulated the activity of CK1α toward Ser535 phosphorylation of IFNAR1 in vitro (Fig. 2E). One likely mode of regulation may involve a posttranslational modification of IFNAR1. It has been widely reported that ability of CK1 to phosphorylate many of its substrates is often augmented by a “priming” phosphorylation event at an S/T residue at the n-3 position (7, 8, 16, 18, 26, 35, 46, 56). Interestingly, residues 529/532 in IFNAR1 is serine, suggesting a possible involvement of priming phosphorylation in triggering CK1α targeting Ser535. Given that ER stress requires PERK for promoting IFNAR1 degron phosphorylation but PERK cannot directly phosphorylate IFNAR1 (31), it is possible that another kinase downstream of PERK provides such priming and increases the efficacy of CK1α actions. In addition, subcellular localization of CK1 may also determine the efficiency of IFNAR1 targeting. Studies aimed to test these hypotheses are currently under way.
In addition to human CK1α, an ortholog kinase from Leishmania, L-CK1, was also capable of mediating IFNAR1 phosphorylation. Either expression of L-CK1 or infection of cells with Leishmania led to downregulation of IFNAR1 and inhibition of cellular responses to type I IFN (Fig. 6 and 7). It remains to be seen exactly how L-CK1 gets to the vicinity of the type I IFN receptor. The parasite molecules involved in host cell regulation are poorly defined; however, activation of SHP-1 appears to depend on the presence of a parasite molecule, Leishmania EF-1, which binds to and activates SHP-1 (37, 38). Studies with Leishmania EF-1 indicate that it gains access to the cytosol in order to mediate its function, although the mechanism involved remains undefined. Similarly, cysteine proteases from L. mexicana are implicated in altering the NF-κB signaling in the cytosol (11). It is plausible that L-CK1 is also capable of being transported to the cytoplasm in order to mediate its effect on IFNAR1. The mechanisms of this transport remain to be investigated. Studies of these mechanisms might lead to identification of novel targets for interfering with Leishmania-mediated IFNAR1 degradation and suppression of IFN-α signaling.
Numerous parasites, including Toxoplasma spp. (15), Leishmania spp. (47, 58), Trypanosoma spp. (9, 10, 15, 19, 53), Plasmodium spp. (25), and others, express CK1 orthologs. These kinases and their substrates (among both parasite and host proteins) as well as a potential role in regulating IFNAR1 are yet to be sufficiently characterized. These studies are of interest given that targeting parasite protein kinases might be useful for developing novel antiparasitic agents (39). Our results provide a rationale for future testing of the efficacy of a combination of the L-CK1 inhibitors, such as purvalanol B (27) and imidazopyridine (2), with type I IFNs as a means of antileishmanial treatment.
Acknowledgments
We thank J. W. Harper, S. Pellegrini, J. Chen, P. A. Lazo, D. S. Lyles, S. Hemmi, G. Stark, and R. Harty for reagents.
This work was supported by NIH grants CA092900 (to S.Y.F.), AI073347 (to S.Y.F. and P.S.), and AI076257 (to P.S.).
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who know JAK-STAT. Science 296:1653-1655. [DOI] [PubMed] [Google Scholar]
- 2.Allocco, J. J., R. Donald, T. Zhong, A. Lee, Y. S. Tang, R. C. Hendrickson, P. Liberator, and B. Nare. 2006. Inhibitors of casein kinase 1 block the growth of Leishmania major promastigotes in vitro. Int. J. Parasitol. 36:1249-1259. [DOI] [PubMed] [Google Scholar]
- 3.Bain, J., L. Plater, M. Elliott, N. Shpiro, C. J. Hastie, H. McLauchlan, I. Klevernic, J. S. Arthur, D. R. Alessi, and P. Cohen. 2007. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408:297-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya, D., I. H. Ansari, and R. Striker. 2009. The flaviviral methyltransferase is a substrate of casein kinase 1. Virus Res. 141:101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdan, C., J. Mattner, and U. Schleicher. 2004. The role of type I interferons in non-viral infections. Immunol. Rev. 202:33-48. [DOI] [PubMed] [Google Scholar]
- 6.Boyle, K. A., and P. Traktman. 2004. Members of a novel family of mammalian protein kinases complement the DNA-negative phenotype of a vaccinia virus ts mutant defective in the B1 kinase. J. Virol. 78:1992-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustos, V. H., A. Ferrarese, A. Venerando, O. Marin, J. E. Allende, and L. A. Pinna. 2006. The first armadillo repeat is involved in the recognition and regulation of beta-catenin phosphorylation by protein kinase CK1. Proc. Natl. Acad. Sci. USA 103:19725-19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustos, V. H., O. Marin, F. Meggio, L. Cesaro, C. C. Allende, J. E. Allende, and L. A. Pinna. 2005. Generation of protein kinase Ck1α mutants which discriminate between canonical and non-canonical substrates. Biochem. J. 391:417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabokis, M., L. Kurz, M. I. Gonzatti, and J. Bubis. 2003. Protein kinase CK1 from Trypanosoma cruzi. J. Protein Chem. 22:591-599. [DOI] [PubMed] [Google Scholar]
- 10.Calabokis, M., L. Kurz, J. Wilkesman, J. M. Galan-Caridad, C. Moller, M. I. Gonzatti, and J. Bubis. 2002. Biochemical and enzymatic characterization of a partially purified casein kinase-1 like activity from Trypanosoma cruzi. Parasitol. Int. 51:25-39. [DOI] [PubMed] [Google Scholar]
- 11.Cameron, P., A. McGachy, M. Anderson, A. Paul, G. H. Coombs, J. C. Mottram, J. Alexander, and R. Plevin. 2004. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-κB signaling pathway. J. Immunol. 173:3297-3304. [DOI] [PubMed] [Google Scholar]
- 12.Campagna, M., M. Budini, F. Arnoldi, U. Desselberger, J. E. Allende, and O. R. Burrone. 2007. Impaired hyperphosphorylation of rotavirus NSP5 in cells depleted of casein kinase 1α is associated with the formation of viroplasms with altered morphology and a moderate decrease in virus replication. J. Gen. Virol. 88:2800-2810. [DOI] [PubMed] [Google Scholar]
- 13.Chen, L., C. Li, Y. Pan, and J. Chen. 2005. Regulation of p53-MDMX interaction by casein kinase 1α. Mol. Cell. Biol. 25:6509-6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diefenbach, A., H. Schindler, N. Donhauser, E. Lorenz, T. Laskay, J. MacMicking, M. Rollinghoff, I. Gresser, and C. Bogdan. 1998. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 8:77-87. [DOI] [PubMed] [Google Scholar]
- 15.Donald, R. G., T. Zhong, L. Meijer, and P. A. Liberator. 2005. Characterization of two T. gondii CK1 isoforms. Mol. Biochem. Parasitol. 141:15-27. [DOI] [PubMed] [Google Scholar]
- 16.Donella-Deana, A., N. Grankowski, W. Kudlicki, R. Szyszka, E. Gasior, and L. A. Pinna. 1985. A type-1 casein kinase from yeast phosphorylates both serine and threonine residues of casein. Identification of the phosphorylation sites. Biochim. Biophys. Acta 829:180-187. [DOI] [PubMed] [Google Scholar]
- 17.Eichwald, C., G. Jacob, B. Muszynski, J. E. Allende, and O. R. Burrone. 2004. Uncoupling substrate and activation functions of rotavirus NSP5: phosphorylation of Ser-67 by casein kinase 1 is essential for hyperphosphorylation. Proc. Natl. Acad. Sci. USA 101:16304-16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flotow, H., P. R. Graves, A. Q. Wang, C. J. Fiol, R. W. Roeske, and P. J. Roach. 1990. Phosphate groups as substrate determinants for casein kinase I action. J. Biol. Chem. 265:14264-14269. [PubMed] [Google Scholar]
- 19.Galan-Caridad, J. M., M. Calabokis, G. Uzcanga, F. Aponte, and J. Bubis. 2004. Identification of casein kinase 1, casein kinase 2, and cAMP-dependent protein kinase-like activities in Trypanosoma evansi. Mem. Inst. Oswaldo Cruz 99:845-854. [DOI] [PubMed] [Google Scholar]
- 20.Goldman, L. A., M. Zafari, E. C. Cutrone, A. Dang, M. Brickelmeier, L. Runkel, C. D. Benjamin, L. E. Ling, and J. A. Langer. 1999. Characterization of antihuman IFNAR-1 monoclonal antibodies: epitope localization and functional analysis. J. Interferon Cytokine Res. 19:15-26. [DOI] [PubMed] [Google Scholar]
- 21.He, B. 2006. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 13:393-403. [DOI] [PubMed] [Google Scholar]
- 22.Huang-Fu, W. C., J. Liu, R. N. Harty, and S. Y. Fuchs. 2008. Cigarette smoking products suppress anti-viral effects of type I interferon via phosphorylation-dependent downregulation of its receptor. FEBS Lett. 582:3206-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber, E., D. Vlasny, S. Jeckel, F. Stubenrauch, and T. Iftner. 2004. Gene profiling of cottontail rabbit papillomavirus-induced carcinomas identifies upregulated genes directly involved in stroma invasion as shown by small interfering RNA-mediated gene silencing. J. Virol. 78:7478-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang, S. Y., P. J. Hertzog, K. A. Holland, S. H. Sumarsono, M. J. Tymms, J. A. Hamilton, G. Whitty, I. Bertoncello, and I. Kola. 1995. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. USA 92:11284-11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappes, B., C. D. Doerig, and R. Graeser. 1999. An overview of Plasmodium protein kinases. Parasitol. Today 15:449-454. [DOI] [PubMed] [Google Scholar]
- 26.Knippschild, U., A. Gocht, S. Wolff, N. Huber, J. Lohler, and M. Stoter. 2005. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 17:675-689. [DOI] [PubMed] [Google Scholar]
- 27.Knockaert, M., N. Gray, E. Damiens, Y. T. Chang, P. Grellier, K. Grant, D. Fergusson, J. Mottram, M. Soete, J. F. Dubremetz, K. Le Roch, C. Doerig, P. Schultz, and L. Meijer. 2000. Intracellular targets of cyclin-dependent kinase inhibitors: identification by affinity chromatography using immobilised inhibitors. Chem. Biol. 7:411-422. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, K. G., H. Barriere, C. J. Carbone, J. Liu, G. Swaminathan, P. Xu, Y. Li, D. P. Baker, J. Peng, G. L. Lukacs, and S. Y. Fuchs. 2007. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J. Cell Biol. 179:935-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar, K. G., J. J. Krolewski, and S. Y. Fuchs. 2004. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J. Biol. Chem. 279:46614-46620. [DOI] [PubMed] [Google Scholar]
- 30.Kumar, K. G., W. Tang, A. K. Ravindranath, W. A. Clark, E. Croze, and S. Y. Fuchs. 2003. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 22:5480-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, J., W. C. Huang-Fu, K. G. Kumar, J. Qian, J. P. Casey, R. B. Hamanaka, C. Grigoriadou, R. Aldabe, J. A. Diehl, and S. Y. Fuchs. 2009. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe 5:72-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, J., A. Plotnikov, A. Banerjee, K. G. Suresh Kumar, J. Ragimbeau, Z. Marijanovic, D. P. Baker, S. Pellegrini, and S. Y. Fuchs. 2008. Ligand-independent pathway that controls stability of interferon alpha receptor. Biochem. Biophys. Res. Commun. 367:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacLaine, N. J., B. Oster, B. Bundgaard, J. A. Fraser, C. Buckner, P. A. Lazo, D. W. Meek, P. Hollsberg, and T. R. Hupp. 2008. A central role for CK1 in catalyzing phosphorylation of the p53 transactivation domain at serine 20 after HHV-6B viral infection. J. Biol. Chem. 283:28563-28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marijanovic, Z., J. Ragimbeau, K. G. Kumar, S. Y. Fuchs, and S. Pellegrini. 2006. TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochem. J. 397:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meggio, F., A. Donella-Deana, and L. A. Pinna. 1979. Studies on the structural requirements of a microsomal cAMP-independent protein kinase. FEBS Lett. 106:76-80. [DOI] [PubMed] [Google Scholar]
- 36.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 37.Nandan, D., and N. E. Reiner. 2005. Leishmania donovani engages in regulatory interference by targeting macrophage protein tyrosine phosphatase SHP-1. Clin. Immunol. 114:266-277. [DOI] [PubMed] [Google Scholar]
- 38.Nandan, D., T. Yi, M. Lopez, C. Lai, and N. E. Reiner. 2002. Leishmania EF-1α activates the Src homology 2 domain containing tyrosine phosphatase SHP-1 leading to macrophage deactivation. J. Biol. Chem. 277:50190-50197. [DOI] [PubMed] [Google Scholar]
- 39.Naula, C., M. Parsons, and J. C. Mottram. 2005. Protein kinases as drug targets in trypanosomes and Leishmania. Biochim. Biophys. Acta 1754:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pestka, S. 2000. The human interferon alpha species and receptors. Biopolymers 55:254-287. [DOI] [PubMed] [Google Scholar]
- 41.Polonio, T., and T. Efferth. 2008. Leishmaniasis: drug resistance and natural products (review). Int. J. Mol. Med. 22:277-286. [PubMed] [Google Scholar]
- 42.Quintavalle, M., S. Sambucini, C. Di Pietro, R. De Francesco, and P. Neddermann. 2006. The alpha isoform of protein kinase CKI is responsible for hepatitis C virus NS5A hyperphosphorylation. J. Virol. 80:11305-11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quintavalle, M., S. Sambucini, V. Summa, L. Orsatti, F. Talamo, R. De Francesco, and P. Neddermann. 2007. Hepatitis C virus NS5A is a direct substrate of casein kinase I-alpha, a cellular kinase identified by inhibitor affinity chromatography using specific NS5A hyperphosphorylation inhibitors. J. Biol. Chem. 282:5536-5544. [DOI] [PubMed] [Google Scholar]
- 44.Rempel, R. E., and P. Traktman. 1992. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J. Virol. 66:4413-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rena, G., J. Bain, M. Elliott, and P. Cohen. 2004. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 5:60-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roach, P. J. 1991. Multisite and hierarchal protein phosphorylation. J. Biol. Chem. 266:14139-14142. [PubMed] [Google Scholar]
- 47.Sacerdoti-Sierra, N., and C. L. Jaffe. 1997. Release of ecto-protein kinases by the protozoan parasite Leishmania major. J. Biol. Chem. 272:30760-30765. [DOI] [PubMed] [Google Scholar]
- 48.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos, C. R., F. M. Vega, S. Blanco, R. Barcia, and P. A. Lazo. 2004. The vaccinia virus B1R kinase induces p53 downregulation by an Mdm2-dependent mechanism. Virology 328:254-265. [DOI] [PubMed] [Google Scholar]
- 50.Scharton, T. M., and P. Scott. 1993. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schreiber, R. D., and M. A. Farrar. 1993. The biology and biochemistry of interferon-gamma and its receptor. Gastroenterol. Jpn. 28(Suppl. 40):88-94. [DOI] [PubMed] [Google Scholar]
- 52.Shirogane, T., J. Jin, X. L. Ang, and J. W. Harper. 2005. SCFbeta-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J. Biol. Chem. 280:26863-26872. [DOI] [PubMed] [Google Scholar]
- 53.Spadafora, C., Y. Repetto, C. Torres, L. Pino, C. Robello, A. Morello, F. Gamarro, and S. Castanys. 2002. Two casein kinase 1 isoforms are differentially expressed in Trypanosoma cruzi. Mol. Biochem. Parasitol. 124:23-36. [DOI] [PubMed] [Google Scholar]
- 54.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 55.Taniguchi, T., and A. Takaoka. 2001. A weak signal for strong responses: interferon-alpha/beta revisited. Nat. Rev. Mol. Cell Biol. 2:378-386. [DOI] [PubMed] [Google Scholar]
- 56.Umphress, J. L., P. T. Tuazon, C. J. Chen, and J. A. Traugh. 1992. Determinants on simian virus 40 large T antigen are important for recognition and phosphorylation by casein kinase I. Eur. J. Biochem. 203:239-243. [DOI] [PubMed] [Google Scholar]
- 57.van den Broek, M. F., U. Muller, S. Huang, R. M. Zinkernagel, and M. Aguet. 1995. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148:5-18. [DOI] [PubMed] [Google Scholar]
- 58.Vieira, L. L., N. Sacerdoti-Sierra, and C. L. Jaffe. 2002. Effect of pH and temperature on protein kinase release by Leishmania donovani. Int. J. Parasitol. 32:1085-1093. [DOI] [PubMed] [Google Scholar]
- 59.Welihinda, A. A., W. Tirasophon, and R. J. Kaufman. 1999. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expr. 7:293-300. [PMC free article] [PubMed] [Google Scholar]