Abstract
The adrenal cortex is a critical steroidogenic endocrine tissue, generated at least in part from the coelomic epithelium of the urogenital ridge. Neither the intercellular signals that regulate cortical development and maintenance nor the lineage relationships within the adrenal are well defined. We have explored adrenal Shh activity and found that Shh is expressed in relatively undifferentiated steroidogenic cells, which signal to the overlying capsule and subjacent nonsteroidogenic mesenchyme cells that we also find are progenitors of steroidogenic lineages. Shh-expressing cells also generate all steroidogenic cell types, but not nonsteroidogenic ones. Shh mutant adrenals have a thin capsule and small cortex. Our findings both support a novel dual lineage, Shh-independent and Shh-dependent, model of adrenocortical development, and identify distinct populations of adrenocortical progenitor and candidate stem cells.
Keywords: adrenal cortex, Gli1, stem cell, differentiation
The adrenal gland contains a neuroendocrine medulla within a steroidogenic cortex. The medulla produces catecholamines in response to sympathetic inputs, while the cortex produces essential steroids in response to hormonal inputs. Cortical production of aldosterone regulates sodium retention and blood volume (1), and is controlled by the renin–angiotensin system. Glucocorticoid output, which mediates the stress response (2), is modulated by the hypothalamic–pituitary–adrenal (HPA) axis. Although adrenocortical function requires precise control of the steroidogenic cell lineages, the developmental and postnatal mechanisms that regulate their production and maintenance are poorly defined (3).
The adrenal cortex is organized in concentric layers, with steroidogenic cells arrayed in radial columns surrounded by a mesenchymal capsule. Murine steroidogenic cortical cells are organized into three zones. The outermost zone is the aldosterone-producing zona glomerulosa (ZG); the next zone is the zona fasciculata (ZF), which produces the glucocorticoid corticosterone; and the innermost zone is the transient fetal/X-zone, a potentially catabolic region that regresses in adults (4). The ZG and ZF express a shared cohort of enzymes that catalyze the initial steps in the synthesis of steroids from cholesterol, as well as zone-specific terminal enzymes that catalyze the final steps: aldosterone synthase (Cyp11B2) in the ZG and 11β-hydroxylase (Cyp11B1) in the ZF (3, 5).
During embryogenesis, the cortex develops from intermediate mesoderm (Fig. S1). At approximately embryonic day (e)9.5 in the mouse, cells in the coelomic epithelium initiate expression of the transcription factor SF1, a marker that defines steroidogenic cells (6, 7) and delaminate into the mesenchyme, forming the nascent adrenogonadal primordium (AGP) (8, 9). At approximately e10.5, a subset of SF1-positive (SF1+) cells, at least some of which derive from the coelomic epithelium (9), segregate dorsomedially from the AGP and coalesce into the adrenal anlagen. Although the inductive and migratory cues are not understood, adrenocortical cells are specified by interactions between SF1 and other transcription factors (10, 11). At e12.5–e13.0, neural crest medullary precursors invade the anlagen (10), as mesenchymal cells encapsulate the gland. As the adrenal grows, SF1-dependent steroidogenic enzyme expression begins, and steroidogenic zones segregate (12). Steroidogenic cell proliferation depends on β-catenin activity (13). By e14.5, the first cells of the definitive adult cortex are forming the ZF, while inner cortical cells form the fetal adrenal (14, 15). In mice the ZG forms perinatally. The mechanisms that control these differentiation processes are also not understood.
Adrenocortical progenitor and/or stem populations are thought to contribute to homeostatic maintenance and remodeling of steroidogenic lineages. Lineage analyses, BrdU/3H thymidine pulse–chase experiments, and tissue remodeling and regeneration experiments all support the idea that most proliferation occurs near the cortical periphery and that cells transit inward by centripetal displacement to eventually die near the medullary border (reviewed in refs. 3 and 16). It is believed that adrenal stem cells reside in the outer cortex, either in an “undifferentiated zone” at the ZG/ZF boundary (17, 18) or in the capsule or subcapsule mesenchyme, from which they are recruited into the cortex to become steroidogenic (3, 19). However, the molecular identity, specific location, and mechanisms that regulate such adrenocortical stem/progenitor cells are undefined.
Sonic hedgehog (Shh) encodes a secreted signal implicated in adrenal development. Shh mRNA is expressed in the outer murine adrenal cortex, and several human and murine hedgehog pathway mutant phenotypes include adrenal aplasia or dysgenesis (20, 21). Despite these data, neither the specific roles of Shh signaling in adrenal ontogeny nor the signaling and responding cell populations have been rigorously defined. Here we perform expression, mutant, and lineage analyses of adrenal Shh activity to identify Shh signaling and responsive cells and their roles in adrenal development and differentiation.
Results
Shh Is Expressed in Steroidogenic Adrenocortical Outer Zone Cells.
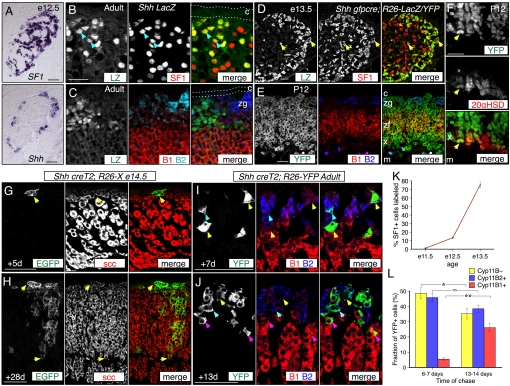
We identified Shh and the Shh pathway components Ptch1, Ptch2, Smo, Gli1, Gli2, and Gli3 as mRNAs enriched in the capsule and ZG, in a differential screen comparing gene expression in rat adrenal ZG plus capsule with inner zone plus medulla cells (Fig. S1). We examined Shh expression in the mouse adrenal gland by in situ hybridization or via the ShhnLacZ (Shh-LacZ) reporter allele (22), from e10.5 to adult ages. Shh expression was detected in cells clustered at the cortical periphery, in SF1+ regions, from e12.5 into adulthood (Fig. 1 A–C and Fig. S1) but not in intermediate mesoderm, gonad or dorsal aorta tissue (Fig. S1 and Tables S1–S5).
Fig. 1.
Sonic hedgehog expression marks adrenocortical progenitors. (A–C) Shh expression in mouse adrenal cortex. (A) SF1 and Shh mRNA expression in adjacent e12.5 transverse sections. Shh is expressed by SF1+ outer adrenal cortex, whereas SF1 is throughout adrenal cortex. Up, dorsal; left, medial. (B) Coimmunofluorescence images showing colocalization of nuclear LacZ and SF1 in Shh-LacZ adrenal (blue arrows). (C) Coimmunofluorescence images showing that most LacZ+ Shh LacZ cells do not coexpress Cyp11B2 or Cyp11B1. Note that weak nuclear B2 signal is nonspecific. (D–L) Adrenocortical Shh lineages defined using constitutive and inducible Shh Cre drivers and R26-YFP, R26-LacZ, or R26-X reporter alleles. Recombined R26-X cells express membrane EGFP. (D–F) Coimmunofluorescence images showing cells labeled by constitutive Shhgfpcre Cre activity. (D) At e13.5, the adrenal contains numerous SF1+ LacZ+ cells (yellow arrows). (E and F) At P12, labeled YFP+ cells coexpress Cyp11B2, Cyp11B1 (E), or 20αHSD (yellow arrow, F). (G–J) Representative coimmunofluorescence images showing adrenocortical cells labeled by tamoxifen-inducible Shh creT2 Cre activity. (G) After a 5-day chase from e14.5, EGFP+ scc+ cells are clustered at the cortical periphery. (H) By 28 days, the clusters have grown into radial columns. (I) After a 7-day chase in adults, 95% of YFP+ cells express either no Cyp11B2 or Cyp11B1 (Cyp11B–, yellow arrows) or Cyp11B2 (B2, blue arrow) and are in the zona glomerulosa. (J) After a 13-day chase, in adults 26% of YFP+ cells are Cyp11B1+ and in the zona fasciculata (B1, magenta arrows). (K) Quantification of lineage data from e11.5 to e13.5. n = 8 Adrenals at e11.5, 4 at e12.5, and 6 at e13.5 (see Table S6). Graphs, mean ± SEM. (L) Quantification of data from (H and I). n = 3 Adrenals at 6–7 days; n = 4 at 13–14 days. Error bars, SEM (see Table S6). *, P < 0.05; **, P < 0.01. c, capsule; m, medulla; x, X-zone; zf, zona fasciculata; zg: zona glomerulosa. (Scale bars, 50 μm.)
By comparing the distribution of LacZ+ Shh-LacZ nuclei with the steroidogenic cell marker SF1, we found that all LacZ+ cells also express SF1 (616 LZ+ SF1+/616 total LZ+ cells; Fig. 1B). Most LacZ+ cells expressed neither Cyp11B1 nor Cyp11B2 (Cyp11B–; Fig. 1C). Using the Shhgfpcre (Shh-gfpcre) allele, in which a GFP-cre gene fusion replaces the Shh coding sequence (23), GFP expression was detected only in peripheral cortical cells at e17.5 and P20 (Fig. S1). Shh is thus expressed in relatively undifferentiated steroidogenic cell clusters at the periphery of the adrenal cortex from early organogenesis to adulthood.
Shh Expression Marks Progenitors of Functional Steroidogenic Cells.
We defined the Shh lineage using the cre driver alleles Shh-gfpcre or Shh-creT2 (23) and several reporter alleles [R26-YFP; R26-LacZ or R26-mR/mG (R26-X)] (24–26). Reporter expression using the Shh-gfpcre driver was first detected at e11.5, when 1.1% of SF1+ adrenal cells were labeled (Fig. 1K and Fig. S2), which increased to 75% at e13.5 (Fig. 1 D and K). We did not detect labeled cells in the gonads or mesenchyme near the adrenal. We also examined differentiation marker expression in the postnatal Shh-gfpcre; R26-YFP lineage. At P12, YFP was expressed throughout the cortex but not in the capsule or medulla (Fig. 1E). YFP expression colocalized with Cyp11B2 in the ZG, Cyp11B1 in the ZF, 20-alpha hydroxysteroid dehydrogenase (20αHSD) in the X zone (4), and with the pan-steroidogenic cell marker, cholesterol side chain cleavage enzyme (scc), in the ZG, ZF, and X zones of both male and female adrenals (Fig. 1 E and F and Fig. S2). The mRNA expression data plus these lineage results reveal adrenocortical cells are thus the sole source of Shh in or near the developing adrenal. Furthermore, Shh expression marks progenitors of all adrenocortical steroidogenic cell types.
We next asked whether Shh expression marks progenitors after anlagen segregation or postnatally. Shh-creT2; R26-X embryos were exposed to tamoxifen at e14.5. After 5 days, small clusters of EGFP+, scc+ cells were located at or near the cortical periphery (Fig. 1G), which had grown into columns that extended from the periphery almost to the medulla by 28 days (Fig. 1H). We also examined adrenals of Shh-creT2; R26-YFP adult males treated with tamoxifen. After 6–7 days, single YFP+ cells were scattered around the cortical periphery, and all expressed scc (n = 126/126 YFP+ cells, Fig. S2). Of the YFP+ cells, 95% were in the ZG, and 49% expressed neither Cyp11B2 nor B1, whereas the remaining 5% were near the ZF/ZG boundary and expressed Cyp11B1 (Fig. 1 I and L). At 13–14 days, clusters were larger and contained significantly more Cyp11B1+ cells (26%; P = 0.004 vs. 6–7 days; Fig. 1 J and L). These data demonstrate centripetal displacement of Shh descendants, both embryonically and postnatally, into multiple functional adrenocortical zones.
Adrenocortical Shh Signals Are Received by Nonsteroidogenic Cells.
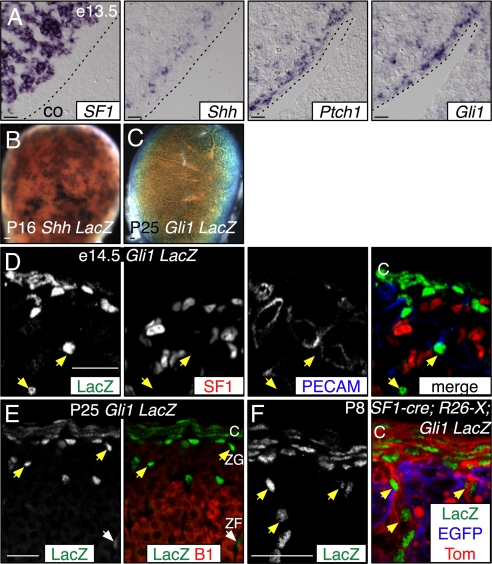
Expression of the transcription factor Gli1 and the Shh receptor Ptch1 is induced by Shh signaling, and their elevated expression marks cells transducing Shh signals (27, 28). We examined Gli1 and Ptch1 mRNA expression and expression of the Gli1LacZ (Gli1-LacZ) reporter allele (29) in and around the adrenal cortex (Fig. 2). At e12.5–14.5, both genes were expressed in mesenchyme surrounding the SF1+ cortex and in scattered cells within the adrenal (Fig. 2 A and D and Fig. S3). At later ages, expression was consolidated into the inner capsule, and cells subjacent to the capsule with some deeper in the cortex (Fig. 2E). Histochemically stained Gli1-LacZ cells were uniformly scattered over the surface of the adrenal, in contrast to clustered Shh-LacZ cells, consistent with Gli1 expression reflecting dispersion of the Shh ligand (Fig. 2 B and C and Fig. S3). At embryonic ages, the noncapsule cells were distributed throughout the cortex, whereas postnatally they were primarily in the ZG (Fig. 2). The LacZ+ Gli1-LacZ cells do not coexpress SF1 (n = 0/551 LacZ+ cells at e16.5, n = 4 adrenals) or PECAM, an endothelial marker (Fig. 2D).
Fig. 2.
Adrenocortical Shh signals are received by nonsteroidogenic cells. Expression of the Shh targets Ptch1 and Gli1 in the adrenal gland. (A) Adjacent sections showing SF1, Shh, Ptch1, and Gli1 expression in e13.5 adrenal tissue. Ptch1 and Gli1 are expressed within and surrounding the SF1+ cortex. Up, dorsal; left, medial; dotted line, coelom border. (B and C) X-gal-stained Shh LacZ and Gli1 LacZ postnatal adrenals. Shh expression is patchy, whereas Gli1 is uniform. (D and E) Gli1 LacZ coimmunofluorescence comparison of (D) e14.5 LacZ, SF1 and endothelial PECAM or (E) P25 LacZ and Cyp11B1 expression. Gli1 is expressed in capsule cells, and in SF1–PECAM–Cyp11B1– subcapsule cells (yellow arrows). Some LacZ+ cells are in the ZF (white arrows; E). (F) Coimmunofluorescence comparison of SF1 lineage (EGFP+) with Gli1 LacZ expression (LacZ) reveals Gli1 is expressed in capsule (Tom+) and nonsteroidogenic subcapsule (EGFP – Tom+) cells. Unrecombined R26-X cells express membrane tdTomato (Tom). Scale bars, 50 μm. Abbreviations: B1, Cyp11B1; C, capsule; co, coelom; ZF, zona fasciculata; ZG, zona glomerulosa.
We next asked whether cells descended from SF1+ cells might express Gli1, using Gli1-LacZ; SF1-cre; R26-X mice (13). SF1-cre induced recombination in all steroidogenic cortical cells but not capsule or medulla (Fig. 2F and Fig. S4). In Gli1-LacZ; SF1-cre; R26-X adrenals, LacZ, and EGFP were not coexpressed (Fig. 2F). These data indicate that Gli1 marks the inner capsule and a novel noncapsule population that is neither endothelial nor steroidogenic. The data further reveal that the adrenal response to Shh produced by steroidogenic cells is restricted to nonsteroidogenic populations.
Cells That Express Gli1 Are Progenitors of Steroidogenic Lineages.
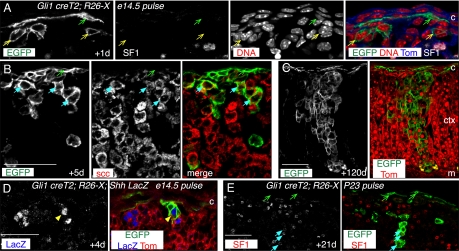
The discovery of Gli1+ nonsteroidogenic cells primarily at the cortical periphery raised the novel possibility that some Gli1+ SF1− cells are progenitors of SF1+ steroidogenic cells. To test this we used a Gli1-creT2 allele (28). We exposed Gli1-creT2; R26-X embryos to tamoxifen at e14.5 and analyzed adrenal tissue thereafter (Fig. 3 and Fig. S5).
Fig. 3.
Nonsteroidogenic cells that express Gli1 are steroidogenic progenitors. Gli1 lineage analyses were performed using tamoxifen-inducible Gli1-creT2 and the R26-X reporter (R26-mR/mG). (A–C) e14.5 Embryos were tamoxifen treated and adrenals harvested after up to 4 months. (A) After a 1-day chase, there are labeled SF1− capsule cells (green arrow) and small clusters of SF1− subcapsule cells (yellow arrows). (B) After a 5-day chase, there are larger subcapsule clusters of labeled scc+ cells (blue arrows). (C) After a 4-month chase, labeled clusters extend from the capsule to the medulla. There are also labeled capsule cells. (D) Gli1 creT2;R26-X;Shh-LacZ e14.5 embryos were tamoxifen treated and harvested after 4 days. EGFP+LacZ+ cell (yellow arrow) reveals Shh expression in Gli1 descendants. Note that the Shh-nLacZ allele is also floxed. Thus, LacZ labeling of EGFP+ cells is not quantitative, as it is restricted to cells with an unrecombined Shh-LacZ allele. (E) P23 Gli1 creT2;R26-X male was tamoxifen-treated and adrenals harvested after 21 days. Labeled clusters of SF1+ cells extend from the subcapsule into the cortex. Scale bars, 50 μm. Abbreviations: c, capsule; ctx, cortex; m, medulla.
After 1 day (Fig. 3 A and Fig. S5), EGFP+ cells were present near the cortical periphery primarily in the inner capsule, with single cells or small cell clusters present underneath the capsule. The majority of these were SF1−. By 5 days (Fig. 3B and Fig. S5), there were SF1+, scc+, EGFP+ clusters subjacent to labeled capsule cells, and by 21 days clusters extended as far as 20 cell diameters into the cortex (Fig. S5). After 4 months (Fig. 3C and Fig. S5), EGFP+ cells were still present throughout the capsule and in scc+ clusters that could extend to the medullary border. To determine whether Gli1 descendants might initiate Shh expression, we labeled cells in Gli-creT2; R26-X; Shh-nLacZ mice at e14.5 or P2 and chased for 4 or 7 days (Fig. 3D and Fig. S5). Some cortical EGFP+ cells were also LacZ+, indicating that steroidogenic Gli1 descendants can express Shh.
We also labeled cells at P23 and examined adrenals 21 days later (Fig. 3E and Fig. S5). We detected both EGFP+ capsule cells and EGFP+ subjacent clusters that were SF1+ and scc+, demonstrating that similar Gli1 lineages can be generated postnatally. Together these data provide compelling evidence for the existence of long-lived nonsteroidogenic progenitors of steroidogenic adrenocortical cells, as well as additional evidence for centripetal displacement of cells through the cortex.
Shh Modulates the Capsule and Regulates Steroidogenic Cells Indirectly.
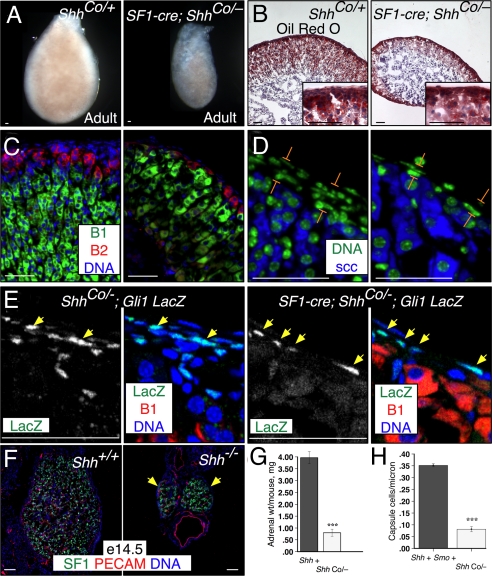
The Gli1 and Ptch1 expression data imply that steroidogenic cells do not transduce the Shh signal. Thus, removing the obligate Shh signal transducer Smo from the steroidogenic cells should not affect adrenocortical development. We tested this using a conditional Smo allele, SmoCo (30). SF1-cre; SmoCo/Co mice were viable, and the weight (mutant, 1.8 mg/adrenal; control, 1.9 mg; P = 0.6; Fig. S6), cortical size, capsule cell density, and differentiation of SF1-cre; SmoCo/Co adrenals were similar to those of controls (mutant, 0.37 cells/μm; control, 0.35 cells/μm; P = 0.3; Fig. S6). We confirmed recombination at the SmoCo locus in the adrenal cortex by genomic PCR (Fig. S6). These genetic data provide additional evidence that although steroidogenic cells produce the Shh signal, they do not significantly respond to it, and that Shh affects steroidogenic cortical cells indirectly.
To determine whether Shh is required in the steroidogenic cortex, the apparent Shh source, we used a conditional Shh allele, ShhCo (31). SF1-cre; ShhCo/− mice were viable. The adrenal glands were small (mutant, 0.8 mg/mouse; control, 4.0 mg/mouse; P = 0.001; Fig. 4 A and G), with the right visibly smaller than the left (Fig. S6). They also had a dramatically thinned and asymmetric cortex covered by a thin capsule (mutant, 0.08 capsule cells/μm; control, 0.35 cells/μm; P = 9 × 10−5; Fig. 4 D and H). Cortical features and zonal steroidogenic enzyme expression, as well as medulla size, appeared normal (Fig. 4 B–D), although ZF cells were hypertrophic, presumably to compensate for the smaller number of steroidogenic cells (Fig. 4 C and E). We observed similar phenotypes at e18.5 (Fig. S6). This excludes the possibility that these phenotypes are secondary to HPA axis activity defects, because although SF1-cre is expressed in the hypothalamus and pituitary, pituitary POMC derivatives are not required for prenatal adrenocortical development (32).
Fig. 4.
Shh signaling is required for cortical growth and capsule maintenance. Adrenal phenotypes in steroidogenic cell conditional SF1-cre;ShhCo/− and Shh−/− mutants. (A) Adult (3-month) adrenals are small after conditional deletion of Shh (ShhCo). (B) Oil red O staining of neutral lipids, hematoxylin counterstain. (Insets) High magnification. (C and D) Cyp11B2, Cyp11B1 (C) and scc (D) are expressed in appropriate zones in SF1-cre; ShhCo/− adrenal sections. (D) Adult SF1-cre; ShhCo/− mutant adrenals have a thin capsule (brackets). (E) Coimmunofluorescence images showing Gli1 LacZ expression in control (left) and SF1-cre; ShhCo/− (right) adrenal sections. LacZ+ cells (yellow arrows) are more sparse but present in mutant adrenal capsule. (F) An SF1+ adrenal cortex forms in Shh−/− mutants, but at e14.5 is much smaller than in controls. (G) Quantification of adrenal weights. Shh+, n = 3 mice; SF1-cre; ShhCo/−, n = 3. Error bars, SEM (see Table S6). (H) Quantification of capsule cell density. Shh+, n = 4 adrenals; SF1-cre; ShhCo/−, n = 3. Error bars, SEM (see Table S6). Scale bars, 50 μm. B1, Cyp11B1; B2, Cyp11B2.
To address whether the hedgehog signaling was completely removed from the conditional mutant, we examined SF1-cre; Shhco/−; Gli1-LacZ adrenals. We detected LacZ expression in scattered capsule cells in the mutant, which were substantially fewer than in controls (Fig. 4E). Thus, residual hedgehog activity might partially ameliorate the conditional null phenotype. We therefore examined adrenals in Shh−/− embryos and found that at e14.5 and e16.5 they were also much smaller than those in control Shh+/+ embryos (Fig. 4F and Fig. S6). LacZ expression was not detected in the e16.5 Shh−/−; Gli1-LacZ adrenal capsule (Fig. S6). Vascularization appeared normal, consistent with healthy mutant tissue. These results demonstrate that an adrenal cortex forms even in the complete absence of Shh.
Discussion
We have identified Gli1 and Shh expression as markers of distinct adrenocortical progenitor and candidate stem populations, and Shh signaling as a major regulator of adrenal development and, probably, postnatal maintenance. Together these data lead us to propose a novel dual lineage model of adrenocortical development that also has implications for postnatal regulation of the gland.
Gli1 and Shh Lineages.
Our lineage data identify Gli1+ and Shh+ cells as two populations that contain adrenocortical progenitors. Gli1+ cells convert from a nonsteroidogenic, SF1− identity to a steroidogenic SF1+ phenotype and subsequently contribute to all steroidogenic lineages. Gli1 descendants also persist and generate steroidogenic cells for months. The relationship between capsule and subcapsule mesenchyme, both of which express Gli1, is unclear, and thus either might house founder cells of steroidogenic lineages. Salmon and Zwemer (19) found that trypan blue staining of the capsule progressively labeled more central adrenal regions, which suggested that adrenocortical cells are descended from the capsule; however, these observations could not be repeated (33, 34). Our results provide direct experimental evidence in support of this idea.
Shh is expressed by clusters of predominantly undifferentiated cells localized in the ZG. Descendants of Shh+ cells can differentiate into all steroidogenic cell types and grow into radial centripetal columns, although they do not contribute to the capsule or medulla. Thus Shh expression marks exclusively steroidogenic progenitors. One Shh lineage is established at the earliest stages of adrenal anlagen formation and is independent of Shh signaling. A second class of Shh+ cells is descended from Gli1+ cells, which are responding to Shh signals. Although the relative contribution of each type of Shh+ cell to the mature adrenal remains to be determined, their descendants are probably functionally equivalent, as there is no evidence for functionally distinct lineages distributed radially around the gland. Thus our data argue strongly for dual adrenocortical origins: a primary lineage derived from SF1+ coelomic epithelium, and a secondary lineage derived from SF1− mesenchyme, either of which might contain adrenocortical stem cells.
The adrenal cortex appears as the adrenal anlagen segregates from the rostral adrenogonadal primordium (3, 10, 35). Prior lineage experiments revealed a coelomic epithelial contribution to the cortex (9). Our Gli1 lineage data define a mesenchymal SF1− contribution that likely begins after Shh expression is initiated in the adrenal anlagen. However, recent SF1 lineage data appear to conflict with the idea that these cells are initially SF1− (15). Fate maps generated using a small SF1/Ad4BP fetal adrenal enhancer element to drive inducible Cre expression labeled the entire cortex into adulthood after Cre induction at e11.5. This suggests that progenitors of all cortical cells transiently express SF1. If so, our Gli1 lineage results suggest that these progenitors generate mesenchyme that forms the capsule or subcapsule. However, a much larger SF1-cre transgene (13) does not label the capsule, and thus the smaller transgene might display ectopic mesenchymal expression at early developmental stages.
Functions of Shh.
Our data reveal multiple adrenocortical requirements for Shh signaling. These include effects on capsule thickness and growth of the cortical SF1 population. Our Gli1 and Ptch1 expression data argue that the effects on the capsule are probably direct, whereas, in combination with the Smo mutant data, the effects on the SF1+ cells are indirect.
Removing Shh signaling reduces the capsule to a single cell layer over much of the cortex. This might result if Shh is a capsule cell mitogen or trophic factor or a chemoattractant for noncapsule mesenchyme. Alternatively, Shh might maintain capsule progenitors, which otherwise could be rapidly depleted as the nascent adrenal grows. Although mechanisms controlling capsule growth and maintenance are poorly understood, the capsule is thickened in adrenals mutant for MC2R, the ACTH receptor (36). This might reflect feedback signaling between the MC2R+ cortex and the overlying capsule, perhaps via MC2R regulation of Shh expression.
The second major Shh phenotype is the failure of the SF1+ population to expand normally, resulting in a smaller cortex. One cause of this defect might be failed recruitment of nonsteroidogenic mesenchymal cells into the cortex. Shh signaling might regulate the transition of responsive progenitors into steroidogenic lineages, with reduced signaling causing a smaller cortex. Alternatively, Shh might maintain progenitor potency and/or competence to respond to non-Shh cues. In support of these ideas, hedgehog signaling is known to regulate stem cell niches in multiple organs (37, 38). Shh might independently also regulate mitogenic secondary signals from capsule mesenchyme to the steroidogenic cells. Wnt family growth factors are candidates, as β-catenin is required for SF1+ cell proliferation and survival (13), and hedgehog and Wnt signals frequently are regulated reciprocally (38).
Ching and Vilain (21) recently reported adrenal Shh and Gli gene expression patterns and described SF1-cre; ShhCo/Co adrenal phenotypes. They detect Shh mRNA expression in subcapsular cortical cells, and Gli1, Gli2 and Gli3 expression at the margin of the cortex. Similar to our results, they find that conditional Shh mutant adrenal glands are small, with an eccentric medulla but normal zonation. They also detect reduced proliferation in the mutant cortex, which we have not examined. In contrast to our results, they describe loss of the right adrenal gland in adult mutant animals. We always found a right adrenal gland, although, unlike the left adrenal, it was located deep within adipose tissue. Whether this difference is due to genetic variation or to other causes is unclear.
Two-Lineage Model of Adrenocortical Development.
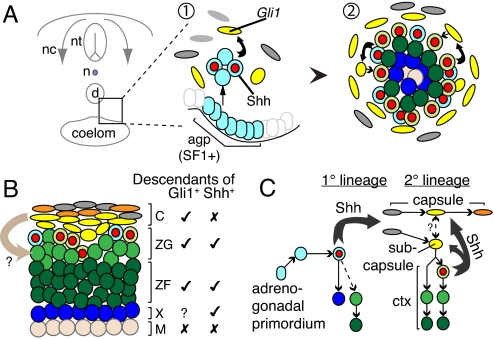
Our data lead us to the following model of adrenocortical development (Fig. 5). Adrenal anlagen formation, in which SF1+ cells delaminate from the adrenogonadal primordium, occurs independent of Shh signaling and establishes a primary adrenal lineage. These cells then initiate Shh expression, and nearby mesenchymal cells convert to a steroidogenic identity, perhaps dependent on Shh signaling, thus forming a secondary adrenal lineage. Surrounding mesenchymal cells encapsulate the gland, concurrent with investment by medullary sympathoadrenal neural crest cells. Although rudimentary capsule formation is Shh independent, capsule maturation and maintenance are not. Newly converted secondary adrenal cells at the cortical periphery rapidly up-regulate Shh expression. Some of their descendants and those of the primary lineage down-regulate Shh expression and continue to differentiate, while centrally displacing older cells. A bootstrapping circuit is thus established between cortical cells that signal via Shh to the overlying mesenchyme to modulate further recruitment of cells into the cortical lineage, and this system functions into adulthood to maintain the cortex and capsule.
Fig. 5.
Two-lineage model of adrenocortical development. (A) Diagram of transverse section of embryo. 1, Primary adrenal lineage delaminates from the coelomic epithelium, initiates Shh expression (blue “olives”) and induces Gli1 expression (yellow) in surrounding mesenchyme. Gli1+ cells convert into secondary adrenal lineage and initiate Shh expression (green “olives”). 2, Shh+ cells continually induce Gli1 in overlying mesenchyme, as differentiating cells down-regulate Shh. (B) Mature gland, with potential capsule-derived signal (arrow) and cell types descended from Gli1+ or Shh+ cells. (C) Signaling between primary 1° and secondary 2° adrenal lineages and mesenchyme. Thick arrows, signals; thin arrows, lineages. Abbreviations: d, dorsal aorta; n, notochord; nc, neural crest; nt, neural tube.
The adrenal cortex remodels in response to physiological changes in the salt/water balance or stress levels, via either the renin–angiotensin system or the HPA axis. Each system is thought to mobilize or to repress progenitor and/or stem populations as needed to modulate steroidogenic capacity (18, 39). Shh expression is likely to identify outer cortical cells that are targeted during remodeling. It will be interesting to learn how these cells and Shh signaling contribute to these processes.
Experimental Procedures
Rats and Mice.
All live animal procedures were performed according to Columbia University Institutional Animal Care and Use Committee or UK Home Office guidelines in the Animal (Scientific Procedures) Act 1986. Male Sprague–Dawley rats (Charles River Laboratories) and mice (Table S1) were fed standard laboratory chow. Noon on the day of the vaginal plug was considered embryonic day 0.5. CreT2 activity was induced by IP injection of 0.5 mg to 6 mg of tamoxifen (50 mg/ml in corn oil).
Immunostaining, in Situ mRNA Hybridization, and LacZ Histochemistry.
Serial cryosections (5 μm) were cut from tissue fixed in 4% paraformaldehyde in PBS at 4 °C for less than 2 hours for immunostaining or overnight for in situ hybridization. Immunostaining was performed at 4 °C overnight with primary antibodies (Table S4), followed by secondary antibodies (Table S5) for 1–2 h at RT and counterstaining with H33342 or Toto3. Nonradioactive section in situ hybridization probes are detailed in SI Experimental Procedures. LacZ activity was detected by X-gal staining for 4 hours to overnight (24).
Quantification and Statistical Analyses.
All analyses are based on examination of three or more adrenal glands. For lineage and capsule density analyses, cells were counted in at least three sections per adrenal gland. Wet adrenal glands were weighed after removal of adherent fat. P values were calculated using unpaired heteroscedastic two-tailed t tests, and values of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments.
We thank J. Kirkland and M. Mendelsohn for mouse husbandry; F. Costantini, A. Joyner, A. Kottmann, K. Parker, C. Gomez-Sanchez, Y. Weinstein, K. Morohashi, and A. Swain for reagents; and R. Axel, B. Capel, F. Doetsch, O. Hobert, T. Jessell, L. Guasti, and G. Vinson for discussions. This work was supported by the Medical Research Council and Bart's Foundation for Research (P.K), and by the American Heart Association, the Medical Research Council, and the National Institute of Diabetes and Digestive and Kidney Diseases (E.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909471106/DCSupplemental.
References
- 1.Lumbers ER. Angiotensin and aldosterone. Regul Pept. 1999;80:91–100. doi: 10.1016/s0167-0115(99)00026-9. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 3.Kim AC, Hammer GD. Adrenocortical cells with stem/progenitor cell properties: Recent advances. Mol Cell Endocrinol. 2007;265:10–16. doi: 10.1016/j.mce.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148:976–988. doi: 10.1210/en.2006-1100. [DOI] [PubMed] [Google Scholar]
- 5.Heikkila M, et al. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 7.Honda S, et al. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993;268:7494–7502. [PubMed] [Google Scholar]
- 8.Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- 9.Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- 10.Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- 11.Else T, Hammer GD. Genetic analysis of adrenal absence: Agenesis and aplasia. Trends Endocrinol Metab. 2005;16:458–468. doi: 10.1016/j.tem.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 13.Kim AC, et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- 14.Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28:7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: Initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26:4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinson GP. Adrenocortical zonation and ACTH. Microsc Res Tech. 2003;61:227–239. doi: 10.1002/jemt.10331. [DOI] [PubMed] [Google Scholar]
- 17.Wright N, Voncina D. Studies on the postnatal growth of the rat adrenal cortex. J Anat. 1977;123:147–156. [PMC free article] [PubMed] [Google Scholar]
- 18.Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim Biophys Acta. 2003;1619:317–324. doi: 10.1016/s0304-4165(02)00490-7. [DOI] [PubMed] [Google Scholar]
- 19.Salmon T, Zwemer R. A study of the life history of corticoadrenal gland cells of the rat by means of trypan blue injections. Anat Rec. 1941;80:421–429. [Google Scholar]
- 20.King PJ, Guasti L, Laufer E. Hedgehog signalling in endocrine development and disease. J Endocrinol. 2008;198:439–450. doi: 10.1677/JOE-08-0161. [DOI] [PubMed] [Google Scholar]
- 21.Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. genesis. 2009;47:628–637. doi: 10.1002/dvg.20532. [DOI] [PubMed] [Google Scholar]
- 22.Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 25.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 27.Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 28.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 30.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 31.Lewis PM, et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 32.Karpac J, et al. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology. 2005;146:2555–2562. doi: 10.1210/en.2004-1290. [DOI] [PubMed] [Google Scholar]
- 33.Calma I, Foster C. Trypan Blue and cell migration in the adrenal cortex of rats. Nature. 1943;152:536. [Google Scholar]
- 34.McPhail M. Trypan Blue and growth of the adrenal cortex in mice. Nature. 1944;153:460–461. [Google Scholar]
- 35.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 36.Chida D, et al. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci USA. 2007;104:18205–18210. doi: 10.1073/pnas.0706953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watt FM. Unexpected Hedgehog-Wnt interactions in epithelial differentiation. Trends Mol Med. 2004;10:577–580. doi: 10.1016/j.molmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Engeland WC, Ennen WB, Elayaperumal A, Durand DA, Levay-Young BK. Zone-specific cell proliferation during compensatory adrenal growth in rats. Am J Physiol Endocrinol Metab. 2005;288:E298–E306. doi: 10.1152/ajpendo.00307.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.