The acronym CARS stands for the compensatory anti-inflammatory response syndrome and was coined in a 1996 paper by Bone to help describe an immunologic phenomenon that increasingly was noticed to occur in sepsis.1 Like its precursor, the systemic inflammatory response syndrome (SIRS), CARS is a complex and incompletely defined pattern of immunologic responses to severe infection. The difference was that while SIRS was a proinflammatory syndrome that seemed tasked with killing infectious organisms through activation of the immune system, CARS was a systemic deactivation of the immune system tasked with restoring homeostasis from an inflammatory state. Moreover, it has become apparent that CARS is not simply the cessation of SIRS, it can exist separately from SIRS. Additionally, it has a distinct set of cytokines and cellular responses and may have a powerful influence on clinical outcomes in sepsis.
BACKGROUND/HISTORY
The studies that led to this concept came from two different streams of medical research, one that was new at the time of Bone's article and one quite old. The new information to which Bone referred in his paper was the large set of data that recently had emerged from numerous studies in which agents that blocked inflammation were used in human sepsis patients.2 In stark contrast to the animal data, the human studies showed poor efficacy of these agents and even suggested that harm could be done in some cases. Bone hypothesized that a powerful anti-inflammatory response already existed to balance the destructive killing of the proinflammatory response and that agents that upset the balance too far in either direction could lead to death, either through uncontrolled inflammation, or failure to defend against infectious organisms.
This concept of autoimmunosuppression was not new, however. It had existed in the medical literature for many decades, largely in the surgical and burn literature. Doctors in these fields long had observed that massive tissue injury such as that caused by burns or trauma made patients more susceptible to infections. It soon was recognized that many of these patients were anergic, indicating impaired lymphocyte function. Much research followed in the 1970s and 1980s trying to characterize the cause and nature of this impaired immunity.3–7 In the decades that have followed, much has been learned about the mechanisms of the body's anti-inflammatory response, and it is clear that the immunosuppression of sepsis described by Bone is likely another form of that previously described. The term CARS, as used today, usually reflects all autoimmunosuppression caused by a major insult such as sepsis, burns, or tissue injury. This article reviews the literature on CARS, focusing on the major lines of inquiry in human and animal, research and discusses some of the possible therapeutic benefits this research may generate in the future.
WHAT DEFINES THE COMPENSATORY ANTI-INFLAMMATORY RESPONSE SYNDROME?
To better understand CARS, it is helpful to understand what responses characterize the proinflammatory state that precedes it. It now is known that inflammation can be triggered in two main ways, either by infections with pathogens like bacteria, or by the products of tissue destruction. The innate immune system describes a network of immune cells and their surface receptors designed to recognize and react to either dead tissue or pathogens. When elements of either of these encounter certain lymphocytes or monocytes, they bind to pre-existing receptors and cause activation (lymphocytes) or are ingested and then presented on cell surface receptors to activate other cells (monocytes). What follows is an expansion and activation of several immune cell lines such as polymorphonucleocytes (PMNs) and B lymphocytes stimulated by the proinflammatory cytokines interleukin (IL)-1 and tumor necrosis factor (TNF). The presence of these cytokines also leads to other clinical manifestations of infection such as fever, capillary leak, vasodilation, and the expression of heat shock proteins from the liver.8
The CARS response essentially reverses many of these processes and has been characterized over the last several decades to include:
Cutaneous anergy
Reduction of lymphocytes by means of apoptosis
Decreased cytokine response of monocytes to stimulation
Decreased numbers of human leukocyte antigen (HLA) antigen-presenting receptors on monocytes
Expression of cytokines such as IL-10 that suppress TNF expression (Box 1)
Much research suggests that the clinical effect of this has a profound impact on patient outcomes. Indeed clinicians long have noted that many people who succumb from sepsis die after the initial proinflammatory insult has ceased, often from a second infection. Additionally, many patients who have sepsis, especially those who have poor pre-existing health, seem never to mount the inflammatory response that should characterize infection, instead presenting with low leukocyte counts and hypothermia (Fig. 1). This article discusses some of the major studies that led to current knowledge of CARS and what may be coming in the future.
Fig.1.
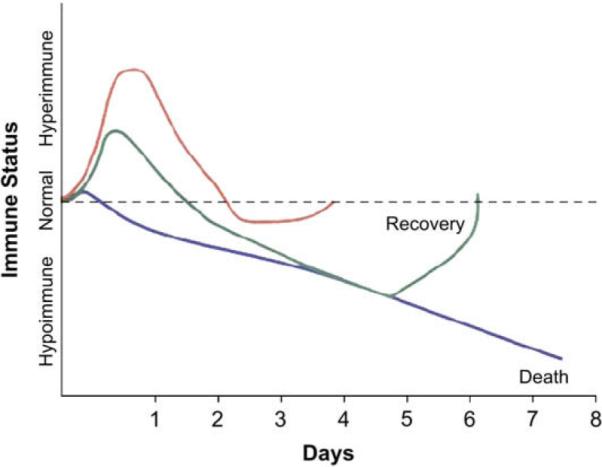
Three different immunologic responses to sepsis in three hypothetical patients of varying pre-existing health status. The relative magnitude of the anti-inflammatory (CARS) response in relation to the proinflammatory (SIRS) response is what is important in determining death in many sepsis patients. (From Hotchkiss RS, Karl IE. Medical progress: the patho-physiology and treatment of sepsis. N Engl J Med 2003;348(2):47; with permission.)
Lymphocytes in Compensatory Anti-inflammatory Response Syndrome
Lymphocytes play a central role in modulating the sepsis response. This is highlighted by altered proinflammatory immune response and increased mortality, after polymicrobial septic challenge in mice lacking both T- and B-cells.9 Their importance relates to their capacity to interact with the innate and adaptive immune responses and their ability to coordinate, amplify, and attenuate the inflammatory response. Lymphocyte anergy (the inability to respond to recall antigens in vivo, [eg, tetanus toxin]) or decreased responsiveness to mitogenic stimulus long has been demonstrated in patients following major surgery, blunt trauma, and thermal injury.10–12 Further studies using both animal and human in vitro models helped better characterize these lymphocyte alterations.
In 1985, Abraham and Chang13 demonstrated reduced ability of T lymphocytes to respond to the mitogens concanavalin A and phytohemagglutinin following traumatic injury. Later, it was discovered that the period of immunoparalysis after trauma was characterized by increased expression of inhibitory coreceptors (PD-1, CD47, CTLA4) on T lymphocytes and decreased expression of coactivator receptors such as CD28 on lymphocytes.14 This altered phenotype of lymphocytes correlated with diminished proliferation response contributing to anergy. Most significantly, a relationship between the loss of cell-mediated immunity in patients following traumatic injury, and the development of sepsis and late death had been established.12,15
As investigators began to look at lymphocyte function in sepsis (as opposed to trauma), it was clear that similar, if not identical patterns of dys-function were occurring. For example, it was shown that patients who have sepsis exhibit defects in their T lymphocytes, because the cells fail to proliferate in response to mitogenic stimuli and also fail to produce IL-2 or -12.16–18 Activated CD4 T-cells can be subdivided into two functionally distinct, highly polarized subsets, termed type 1 helper T-cell (Th1) and type 2 helper T-cell (Th2), depending on their pattern of lymphokine secretion and related functional activities. They secrete either cytokines with inflammatory Th1 properties, including TNF-α, interferon-γ, and IL-2, or cytokines with more anti-inflammatory Th2 properties (eg, IL-4 and -10). Lymphocytes from patients with burns or sepsis have reduced levels of Th1 cytokines but increased levels of the Th2 cytokines IL-4 and -10, and reversal of the Th2 response improves survival among patients who have sepsis.17,18
Some newer studies, however, show a somewhat different picture. For example, global down-regulation of Th1 and Th2 responses in patients after sepsis or severe trauma also has been observed.19,20 This suggests that there may be complete down-regulation of T-cell effector response rather than a shift to an anti-inflammatory response. Similarly, Heidecke and colleagues16 examined T-cell function in patients who had peritonitis and found that they had decreased Th1 function without increased Th2 cytokine production. In this study, effective T-cell proliferation and cytokine secretion correlated with mortality.
More recent findings have been identified a role for other regulatory T-cell populations suppressing T-cell immunity, including natural killer T-cells (NKT) and Gamma delta T-cells (γδ). Blocking the activation of NKT cells by means of an anti-CD1d antibody prevented this immune suppression.21 Using a model of burn injury on γδ T-cell deficient mice, Schwacha and colleagues22 demonstrated a reduced production of proinflammatory cytokines, suggesting that γδ T-cells played an important role in postburn survival. The exact mechanisms by which these regulatory lymphocyte subsets affect the immune response remain subjects of controversy but may represent future therapeutic targets in the management of sepsis.
ANTIGEN-PRESENTING CELLS
Monocytes
Critical to the inflammatory response are the recognition and killing of invading organisms by monocytes. Equally important, monocytes present antigens by means of expression of HLA receptors and secrete proinflammatory cytokines to amplify the immune response.23 Multiple studies have demonstrated clearly that following either trauma or sepsis, monocytes have diminished capacity for both these responses. Specifically, they secrete fewer cytokines when stimulated and down-regulate expression of HLA receptors.24–30 Monocytes from septic patients who had decreased mHLA-DR produced low amounts of TNF-α and IL-1 in response to bacterial challenges.31 This down-regulation of monocyte function generally predicts increased risk of secondary infection and poor prognosis. The role of this phenomenon in critically ill human patients is discussed in more detail in a later section.
DENDRITIC CELLS
Dendritic cells (DC) function as an important mediator in immune responses. Several investigators have found that their numbers decrease following the cecal ligation and puncture (CLP) model of sepsis in rodents.32,33
In people, Guisset and colleagues34 have observed that septic patients who survived exhibited significantly higher circulating blood DC counts than those who died. In a postmortem study of spleen from 26 septic patients and 20 trauma patients, Hotchkiss and colleagues35 observed that sepsis caused a dramatic reduction in the percentage area of spleen occupied by follicular DCs. The importance of DCs in mediating the immune response is emphasized further by murine studies showing improved outcome in sepsis by replenishing the DC population.36 Also, Fujita and colleagues37 have demonstrated improved survival in septic patients using the adoptive transfer of bone marrow-derived regulatory DCs (DCregs) in mice. These approaches may represent therapeutic modalities of the future.
Cytokines in Compensatory Anti-inflammatory Response Syndrome
Just as the SIRS response is characterized by many different and sometimes redundant cytokines, the CARS response also seems to involve many cytokines. The most important however is clearly IL10. IL10 first was characterized around 1990 and was shown to regulate T-cell populations.38,39 It now has been established that IL-10 has multiple immunosuppressive roles,40 with its most important being the down-regulation of TNF. In animal models of sepsis, the administration of IL10 has been shown to have both positive41–43 and negative44,45 effects on outcome, which likely depend on the time of administration and the severity of the infection. In one carefully done animal model, Ashare and colleagues46 followed levels of proinflammatory and anti-inflammatory cytokines throughout the whole course of sepsis in mice. They found that bacterial levels in tissue correlated with IL-10 levels and that if the complementary proinflammatory response was blocked by pretreatment with IL-1 receptor antagonist, bacterial levels were higher, as was mortality. Similarly, Song and colleagues47 showed that blocking IL-10 activity early had no effect on mortality; blocking it late (12 hours) after sepsis, however, improved mortality. These studies help illustrate how IL-10 helps maintain a careful balance of the immune system in inflammation; thus manipulation of it is so dangerous.
APOPTOSIS
Regulation of apoptosis of immune cell populations during sepsis and other traumatic states may play a crucial role balancing the hyperactive inflammatory state with excessive injury to the host. Several studies suggest this balance is critical to outcome of experimental animals and possibly septic patients.48,49 The immunoparalysis that has been shown to be a hallmark of CARS response in sepsis may be a pathologic result of increased immune effector cell apoptosis. Additionally, it has been proposed that the clearance of increased numbers of apoptotic cells may drive immune suppression through the cells that handle them. Uptake of apoptotic cells by macrophages and DCs stimulates immune tolerance by inducing the release of anti-inflammatory cytokines, including IL-10 and transforming growth factor-β (TGF), and suppressing the release of proinflammatory cytokines.50,51
A key role of apoptosis in patients with sepsis was illustrated by Hotchkiss and colleagues49,52 in two studies that compared patients who died of sepsis with patients who died of nonseptic etiologies. Autopsies of patients who had sepsis revealed extensive apoptosis of lymphocytes and gastrointestinal (GI) epithelial cells. These findings were similar to animal studies showing comparable widespread lymphocyte and GI epithelial cell death in sepsis.53,54 Le Tulzo and colleagues55demonstrated exaggerated lymphocyte apoptosis is present in peripheral blood of patients with septic shock, contrary to those with simple sepsis or critically ill nonseptic patients. Additionally, lymphocyte apoptosis occurs rapidly, leads to a profound and persistent lymphocyte loss, and is associated with poor patient outcome. Evidence that apoptosis is a direct mediator of the immune dysfunction rather than just a marker of immune dysfunction comes from murine studies, where prevention of apoptosis improves survival in sepsis.56,57
CLINICAL SIGNIFICANCE OF UNDERSTANDING COMPENSATORYANTI-INFLAMMATORY RESPONSE SYNDROME
Biomarkers
In addition to the studies mentioned previously, there have been many efforts to study the magnitude of the CARS response in relation to patient outcomes. This has led some to see CARS bio-markers as a possible tool for prognosis and therapy.58 In an early study, Keane and colleagues59 looked at lymphocytes removed and cultured from 31 patients who had severe trauma. They found that, overall, lymphocyte response to stimulation with mitogens was reduced markedly from controls. Furthermore, responses were lower, and the duration of suppression was longer in those patients who became infected. Additionally, the suppression of response preceded the onset of infection. Extremely low responses were found in three patients who later died.
A larger number of studies, however, have focused on monocytes and their apparent down-regulation of HLA receptors as a biomarker.24,25,27,28,60–65 In 1995, Asadulla and colleagues24 studied 57 neurosurgical patients and found that HLA-DR expression was lower in 14 patients who developed infection, compared with patients who had an uncomplicated postoperative course (P < .0001). Out of 10 patients who had less than 30% HLA-DR positive monocytes, nine developed infection. They hypothesized that the mechanism of this down-regulation was high levels of endogenous cortisol, as the effect coincided with high corticotropin (ACTH) and cortisol concentrations. Additionally, similar down-regulation was seen in other patients who received high doses of exogenous corticosteroids. Subsequent studies supported the theory that the magnitude of HLADR receptor down-regulation predicted various other poor outcomes such as sepsis in liver transplant patients.28 That story, however, was confounded by exogenous steroids in some patients.27 Allen and colleagues66 found HLA levels predicted sepsis in pediatric cardiac surgery patients. In a small study of septic adults, Su and colleagues64 found that levels of HLA-DR positive monocytes less than 30% were more predictive of mortality than Acute Physiology and Chronic Health Evaluation (APACHE) II scores.
More recent studies looking at the predicative power of HLA receptor have yielded different results. In 2003, three papers were published that yielded similar findings. Hynninen and colleagues61 evaluated the HLA-DR expression of 61 patients who had sepsis at admission and showed no predictive power of HLA expression for survival. Another study of 70 septic patients also found no correlation between HLA expression and infectious or mortality outcomes.63 Interestingly, this study showed that if patients' monocytes were stimulated with G-CSF ex-vivo, their HLA expression increased. The third study looked at 85 cardiac surgery patients. HLA expression was measured at presurgery, immediately after, and 1 day later. Their data showed that although all patients' HLA levels declined after surgery, the magnitude of the response did not correlate with sepsis/SIRS or other infectious complications.62 Reasons for the different results still are being investigated but may be the result of small sample sizes, timing, or well-described variation caused by the different laboratory techniques used. In one study, the same samples were analyzed in two different laboratories and differed by as much as 20%.62
Other studies have looked at anti-inflammatory cytokine levels as predictors of poor outcomes; most of these studies have been on human patients and have borne mixed results. These data likely reflect the varied magnitudes and time courses of both pro- and anti-inflammatory cytokine expression in real patients. In 1998, Doughty and colleagues67 sampled 53 pediatric ICU patients and found that high IL-10 levels correlated with three or more organ dysfunctions and mortality. Ahlstrom and colleagues60 found no predictive value in IL-10 levels in patients who had SIRS, but Simmons and colleagues68 found that IL-10 levels did correlate with mortality in a sample of 93 critically ill patients who had acute renal failure. Perhaps the most interesting data come from two studies that looked at the ratio of IL-10 to TNF. In a large study of over 400 patients admitted to the hospital for fever, van Dissel and colleagues69 showed that a higher IL-10 to TNF ratio was predictive of mortality. A similar study by Gogos and colleagues58 in a population of patients who had mixed sepsis showed the same results.
Potential Therapeutics
Given the still incomplete understanding of CARS response, it is not surprising that little has been done to use this knowledge as a point of therapeutic intervention in sepsis. Nevertheless, there have been several studies that have addressed therapeutics, and they can be grouped into two main categories: hormonal and cytokine therapies.
The hormonal therapy came from earlier studies showing that testosterone seemed to have a negative impact on sepsis and trauma outcomes and is believed to act through augmenting post-injury immunosuppression.70 Two subsequent studies by the same investigators showed that administration of the estrogen-like drug dehydroepiandrosterone reduced the immunosuppression and improved mortality in septic mice.71,72
More research has focused on manipulation of cytokines to reverse the CARS immunosuppression. At least five studies have examined the use of gamma interferon, which has been shown in vivo to reverse monocyte deactivation.73,74 Two very similar small trials were done on human subjects who had sepsis.75,76 In both studies, subjects who had sepsis and monocyte HLA-DR expression of 30% or less were given interferon-γ. Both groups reported increases in HLA-DR expression, usually after just one dose. One of the studies also examined the monocytes ex vivo and showed that interferon improved monocyte cytokine production also.75 A third human trial was different, in that it sought study the effects of interferon-γ regionally.77 In this study, the authors selected 21 patients who had severe trauma and alveolar macrophage dysfunction as determined by a bronchoalveolar lavage sample showing macrophage HLA-DR expression of 30% or less. Interferon-γ was the administered via inhalation. They found about 50% of the subjects had an increase in their alveolar macrophage HLA-DR expression. These patients had a lower incidence of pneumonia but no other differences in outcomes. The small numbers and lack of a control population in all three of these studies limit the conclusions that can be drawn, especially because HLA-DR expression is known to increase as patients recover.
SUMMARY
It has become clear that during sepsis or other major inflammatory stresses, there is a carefully orchestrated balance within the host organism. The proinflammatory forces rise to eliminate pathogens and dead tissue, and in doing so, often cause injury to the host. The timely arrival of anti-inflammatory responses such as the CARS response seeks to limit the damage while not interfering with the pathogen elimination. Just like its mirror image SIRS, however, the CARS response can be dangerous when its effects are unchecked or poorly timed, leaving the host too vulnerable to the next set of pathogens. Hopefully, further work in this field will allow one to manipulate this response favorably to improve outcomes in sepsis and related injuries.
Box 1 Characterization of compensatory anti-inflammatory response syndrome.
Cellular/molecular elements
Lymphocyte dysfunction (ie, reduced proliferative and/or type 1 helper T-cell [Th1] cytokine production in response-defined antigens or specific T-cell stimuli)
Lymphocyte Apoptosis
Down-regulation of monocyte HLA receptors Monocyte deactivation (ie, reduced Th1/proinflammatory cytokine production in response stimuli)
IL-10 production
Transforming growth factor-beta production Prostaglandin E2 production
Clinical elements
Cutaneous anergy
Hypothermia
Leukopenia
Susceptibility to infection
Failure to clear infection
REFERENCES
- 1.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24(7):1125–8. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Freeman BD, Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs. 2000;9(7):1651–63. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- 3.MacLean LD, Meakins JL, Taguchi K, et al. Host resistance in sepsis and trauma. Ann Surg. 1975;182(3):207–17. doi: 10.1097/00000658-197509000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munster AM. Post-traumatic immunosuppression is due to activation of suppressor T cells. Lancet. 1976;1(7973):1329–30. doi: 10.1016/s0140-6736(76)92658-1. [DOI] [PubMed] [Google Scholar]
- 5.Meakins JL, Pietsch JB, Bubenick O, et al. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186(3):241–50. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller CL, Baker CC. Changes in lymphocyte activity after thermal injury. The role of suppressor cells. J Clin Invest. 1979;63(2):202–10. doi: 10.1172/JCI109290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe JH, Wu AV, O'Connor NE, et al. Anergy, immunosuppressive serum, and impaired lymphocyte blastogenesis in burn patients. Arch Surg. 1982;117(10):1266–71. doi: 10.1001/archsurg.1982.01380340002002. [DOI] [PubMed] [Google Scholar]
- 8.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16(2):83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 9.Shelley O, Murphy T, Paterson H, et al. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20(2):123–9. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 10.Daniels JC, Sakai H, Cobb EK, et al. Evaluation of lymphocyte reactivity studies in patients with thermal burns. J Trauma. 1971;11(7):595–601. doi: 10.1097/00005373-197107000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Hensler T, Hecker H, Heeg K, et al. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65(6):2283–91. doi: 10.1128/iai.65.6.2283-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Mahony JB, Wood JJ, Rodrick ML, et al. Changes in T lymphocyte subsets following injury. Assessment by flow cytometry and relationship to sepsis. Ann Surg. 1985;202(5):580–6. doi: 10.1097/00000658-198511000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham E, Chang YH. The effects of hemorrhage on mitogen-induced lymphocyte proliferation. Circ Shock. 1985;15(2):141–9. [PubMed] [Google Scholar]
- 14.Bandyopadhyay G, De A, Laudanski K, et al. Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med. 2007;35(3):794–801. doi: 10.1097/01.CCM.0000256847.61085.A5. [DOI] [PubMed] [Google Scholar]
- 15.Stephan RN, Kupper TS, Geha AS, et al. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg. 1987;122(1):62–8. doi: 10.1001/archsurg.1987.01400130068010. [DOI] [PubMed] [Google Scholar]
- 16.Heidecke CD, Hensler T, Weighardt H, et al. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178(4):288–92. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 17.O'Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222(4):482–90. doi: 10.1097/00000658-199522240-00006. discussion 490–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrick ML, Wood JJ, Grbic JT, et al. Defective IL-2 production in patients with severe burns and sepsis. Lymphokine Res. 1986;5(Suppl 1):S75–80. [PubMed] [Google Scholar]
- 19.Puyana JC, Pellegrini JD, De AK, et al. Both T-helper-1- and T-helper-2-type lymphokines are depressed in posttrauma anergy. J Trauma. 1998;44(6):1037–45. doi: 10.1097/00005373-199806000-00017. discussion 1045–1036. [DOI] [PubMed] [Google Scholar]
- 20.Wick M, Kollig E, Muhr G, et al. The potential pattern of circulating lymphocytes TH1/TH2 is not altered after multiple injuries. Arch Surg. 2000;135(11):1309–14. doi: 10.1001/archsurg.135.11.1309. [DOI] [PubMed] [Google Scholar]
- 21.Palmer JL, Tulley JM, Kovacs EJ, et al. Injury-induced suppression of effector T cell immunity requires CD1d-positive APCs and CD1d-restricted NKT cells. J Immunol. 2006;177(1):92–9. doi: 10.4049/jimmunol.177.1.92. [DOI] [PubMed] [Google Scholar]
- 22.Schwacha MG, Ayala A, Chaudry IH. Insights into the role of gammadelta T lymphocytes in the immunopathogenic response to thermal injury. J Leukoc Biol. 2000;67(5):644–50. [PubMed] [Google Scholar]
- 23.Krakauer T, Oppenheim JJ. IL-1 and tumor necrosis factor-alpha each up-regulate both the expression of IFN-gamma receptors and enhance IFN-gamma-induced HLA-DR expression on human monocytes and a human monocytic cell line (THP-1) J Immunol. 1993;150(4):1205–11. [PubMed] [Google Scholar]
- 24.Fumeaux T, Pugin J. Is the measurement of monocytes HLA-DR expression useful in patients with sepsis? Intensive Care Med. 2006;32(8):1106–8. doi: 10.1007/s00134-006-0205-7. [DOI] [PubMed] [Google Scholar]
- 25.Volk HD, Reinke P, Docke WD. Immunological monitoring of the inflammatory process: Which variables? When to assess? Eur J Surg Suppl. 1999;(584):70–2. doi: 10.1080/11024159950188600. [DOI] [PubMed] [Google Scholar]
- 26.Volk HD, Reinke P, Krausch D, et al. Monocyte deactivation-rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22(Suppl 4):S474–81. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 27.Asadullah K, Woiciechowsky C, Docke WD, et al. Very low monocytic HLA-DR expression indicates high risk of infection–immunomonitoring for patients after neurosurgery and patients during high dose steroid therapy. Eur J Emerg Med. 1995;2(4):184–90. doi: 10.1097/00063110-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 28.van den Berk JM, Oldenburger RH, van den Berg AP, et al. Low HLA-DR expression on monocytes as a prognostic marker for bacterial sepsis after liver transplantation. Transplantation. 1997;63(12):1846–8. doi: 10.1097/00007890-199706270-00026. [DOI] [PubMed] [Google Scholar]
- 29.Denzel C, Riese J, Hohenberger W, et al. Monitoring of immunotherapy by measuring monocyte HLA-DR expression and stimulated TNFalpha production during sepsis after liver transplantation. Intensive Care Med. 1998;24(12):1343–4. doi: 10.1007/s001340050775. [DOI] [PubMed] [Google Scholar]
- 30.Haveman JW, van den Berg AP, van den Berk JM, et al. Low HLA-DR expression on peripheral blood monocytes predicts bacterial sepsis after liver transplantation: relation with prednisolone intake. Transpl Infect Dis. 1999;1(3):146–52. doi: 10.1034/j.1399-3062.1999.010302.x. [DOI] [PubMed] [Google Scholar]
- 31.Astiz M, Saha D, Lustbader D, et al. Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med. 1996;128(6):594–600. doi: 10.1016/s0022-2143(96)90132-8. [DOI] [PubMed] [Google Scholar]
- 32.Ding Y, Chung CS, Newton S, et al. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004;22(2):137–44. doi: 10.1097/01.shk.0000131194.80038.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flohe SB, Agrawal H, Schmitz D, et al. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol. 2006;79(3):473–81. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- 34.Guisset O, Dilhuydy MS, Thiebaut R, et al. Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Med. 2007;33(1):148–52. doi: 10.1007/s00134-006-0436-7. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168(5):2493–500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 36.Toliver-Kinsky TE, Cui W, Murphey ED, et al. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J Immunol. 2005;174(1):404–10. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 37.Fujita S, Seino K, Sato K, et al. Regulatory dendritic cells act as regulators of acute lethal systemic inflammatory response. Blood. 2006;107(9):3656–64. doi: 10.1182/blood-2005-10-4190. [DOI] [PubMed] [Google Scholar]
- 38.MacNeil IA, Suda T, Moore KW, et al. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145(12):4167–73. [PubMed] [Google Scholar]
- 39.O'Garra A, Stapleton G, Dhar V, et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–32. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- 40.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: A complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30(1 Supp):S58–63. [PubMed] [Google Scholar]
- 41.Berg DJ, Kuhn R, Rajewsky K, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96(5):2339–47. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howard M, Muchamuel T, Andrade S, et al. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177(4):1205–8. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Poll T, Jansen PM, Montegut WJ, et al. Effects of IL-10 on systemic inflammatory responses during sublethal primate endotoxemia. J Immunol. 1997;158(4):1971–5. [PubMed] [Google Scholar]
- 44.Remick DG, Garg SJ, Newcomb DE, et al. Exogenous interleukin-10 fails to decrease the mortality or morbidity of sepsis. Crit Care Med. 1998;26(5):895–904. doi: 10.1097/00003246-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 45.Steinhauser ML, Hogaboam CM, Kunkel SL, et al. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162(1):392–9. [PubMed] [Google Scholar]
- 46.Ashare A, Powers LS, Butler NS, et al. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L633–40. doi: 10.1152/ajplung.00231.2004. [DOI] [PubMed] [Google Scholar]
- 47.Song GY, Chung CS, Chaudry IH, et al. What is the role of interleukin 10 in polymicrobial sepsis: anti-inflammatory agent or immunosuppressant? Surgery. 1999;126(2):378–83. [PubMed] [Google Scholar]
- 48.Ayala A, Xin Xu Y, Ayala CA, et al. Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood. 1998;91(4):1362–72. [PubMed] [Google Scholar]
- 49.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27(7):1230–51. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voll RE, Herrmann M, Roth EA, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658):350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 52.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166(11):6952–63. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 53.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7(4):247–53. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Hotchkiss RS, Swanson PE, Cobb JP, et al. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25(8):1298–307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Le Tulzo Y, Pangault C, Gacouin A, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18(6):487–94. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Hotchkiss RS, Swanson PE, Knudson CM, et al. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162(7):4148–56. [PubMed] [Google Scholar]
- 57.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96(25):14541–6. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro-versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181(1):176–80. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 59.Keane RM, Birmingham W, Shatney CM, et al. Prediction of sepsis in the multitraumatic patient by assays of lymphocyte responsiveness. Surg Gynecol Obstet. 1983;156(2):163–7. [PubMed] [Google Scholar]
- 60.Hynninen M, Pettila V, Takkunen O, et al. Predictive value of monocyte histocompatibility leukocyte antigen-DR expression and plasma interleukin-4 and -10 levels in critically ill patients with sepsis. Shock. 2003;20(1):1–4. doi: 10.1097/01.shk.0000068322.08268.b4. [DOI] [PubMed] [Google Scholar]
- 61.Oczenski W, Krenn H, Jilch R, et al. HLA-DR as a marker for increased risk for systemic inflammation and septic complications after cardiac surgery. Intensive Care Med. 2003;29(8):1253–7. doi: 10.1007/s00134-003-1826-8. [DOI] [PubMed] [Google Scholar]
- 62.Perry SE, Mostafa SM, Wenstone R, et al. Is low monocyte HLA-DR expression helpful to predict outcome in severe sepsis? Intensive Care Med. 2003;29(8):1245–52. doi: 10.1007/s00134-003-1686-2. [DOI] [PubMed] [Google Scholar]
- 63.Ahlstrom A, Hynninen M, Tallgren M, et al. Predictive value of interleukins 6, 8 and 10, and low HLA-DR expression in acute renal failure. Clin Nephrol. 2004;61(2):103–10. doi: 10.5414/cnp61103. [DOI] [PubMed] [Google Scholar]
- 64.Su L, Zhou DY, Tang YQ, et al. [Clinical value of monitoring CD14+ monocyte human leukocyte antigen (locus) DR levels in the early stage of sepsis] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18(11):677–9. [PubMed] [Google Scholar]
- 65.Zhang YT, Fang Q. [Study on monocyte HLA-DR expression in critically ill patients after surgery] Zhonghua Wai Ke Za Zhi. 2006;44(21):1480–2. [PubMed] [Google Scholar]
- 66.Allen ML, Peters MJ, Goldman A, et al. Early postoperative monocyte deactivation predicts systemic inflammation and prolonged stay in pediatric cardiac intensive care. Crit Care Med. 2002;30(5):1140–5. doi: 10.1097/00003246-200205000-00031. [DOI] [PubMed] [Google Scholar]
- 67.Doughty L, Carcillo JA, Kaplan S, et al. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113(6):1625–31. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]
- 68.Simmons EM, Himmelfarb J, Sezer MT, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65(4):1357–65. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 69.van Dissel JT, van Langevelde P, Westendorp RG, et al. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351(9107):950–3. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 70.Angele MK, Wichmann MW, Ayala A, et al. Testosterone receptor blockade after hemorrhage in males. Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997;132(11):1207–14. doi: 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- 71.Angele MK, Catania RA, Ayala A, et al. Dehydroepiandrosterone: an inexpensive steroid hormone that decreases the mortality due to sepsis following trauma-induced hemorrhage. Arch Surg. 1998;133(12):1281–8. doi: 10.1001/archsurg.133.12.1281. [DOI] [PubMed] [Google Scholar]
- 72.Catania RA, Angele MK, Ayala A, et al. Dehydroepiandrosterone restores immune function following trauma-haemorrhage by a direct effect on T lymphocytes. Cytokine. 1999;11(6):443–50. doi: 10.1006/cyto.1998.0458. [DOI] [PubMed] [Google Scholar]
- 73.Hershman MJ, Appel SH, Wellhausen SR. Interferon-gamma treatment increases HLA-DR expression on monocytes in severely injured patients. Clin Exp Immunol. 1989;77(1):67–70. [PMC free article] [PubMed] [Google Scholar]
- 74.Bundschuh DS, Barsig J, Hartung T, et al. Granulocyte-macrophage colony-stimulating factor and IFN-gamma restore the systemic TNF-alpha response to endotoxin in lipopolysaccharide-desensitized mice. J Immunol. 1997;158(6):2862–71. [PubMed] [Google Scholar]
- 75.Docke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3(6):678–81. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 76.Kox WJ, Bone RC, Krausch D, et al. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: proof of principle. Arch Intern Med. 1997;157(4):389–93. [PubMed] [Google Scholar]
- 77.Nakos G, Malamou-Mitsi VD, Lachana A, et al. Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med. 2002;30(7):1488–94. doi: 10.1097/00003246-200207000-00015. [DOI] [PubMed] [Google Scholar]


