Abstract
Background
Erythropoiesis-stimulating agents (erythropoietin and darbepoietin) have been approved to reduce the number of blood transfusions required during chemotherapy; however, concerns about the risks of venous thromboembolism and mortality exist.
Methods
We identified patients who were aged 65 years or older in the Surveillance, Epidemiology, and End Results–Medicare database; who were diagnosed with colon, non–small cell lung, or breast cancer or with diffuse large B-cell lymphoma from January 1, 1991, through December 31, 2002; and who received chemotherapy. The main outcome measures were claims for use of an erythropoiesis-stimulating agent, blood transfusion, venous thromboembolism (ie, deep vein thrombosis or pulmonary embolism), and overall survival. We used multivariable logistic regression models to analyze the association of erythropoiesis-stimulating agent use with clinical and demographic variables. We used time-dependent Cox proportional hazards models to analyze the association of time to receipt of first erythropoiesis-stimulating agent with venous thromboembolism and overall survival. All statistical tests were two-sided.
Results
Among 56 210 patients treated with chemotherapy from 1991 through 2002, 15 346 (27%) received an erythropoiesis-stimulating agent. The proportion of patients receiving erythropoiesis-stimulating agents increased from 4.8% in 1991 to 45.9% in 2002 (P < .001). Use was associated with more recent diagnosis, younger age, urban residence, comorbidities, receipt of radiation therapy, female sex, and metastatic or recurrent cancer. The rate of blood transfusion per year during 1991–2002 remained constant at 22%. Venous thromboembolism developed in 1796 (14.3%) of the 12 522 patients who received erythropoiesis-stimulating agent and 3400 (9.8%) of the 34 820 patients who did not (hazard ratio = 1.93, 95% confidence interval = 1.79 to 2.07). Overall survival was similar in both groups.
Conclusion
Use of erythropoiesis-stimulating agent increased rapidly after its approval in 1991, but the blood transfusion rate did not change. Use of erythropoiesis-stimulating agents was associated with an increased risk of venous thromboembolism but not of mortality.
CONTEXT AND CAVEATS
Prior knowledge
Although erythropoiesis-stimulating agents have been approved to reduce the number of blood transfusions required during chemotherapy, increased risks of venous thromboembolism and mortality have been reported.
Study design
Patients who were aged 65 years or older; who were diagnosed with colon, non–small cell lung, or breast cancer or diffuse large B-cell lymphoma in 1991–2002; and who received chemotherapy were identified in the Surveillance, Epidemiology, and End Results–Medicare database. The association between erythropoiesis-stimulating agent use and venous thromboembolism and overall survival were analyzed.
Contribution
The proportion of patients receiving erythropoiesis-stimulating agents increased approximately 10-fold from 1991 through 2002. The rate of blood transfusion per year during 1991–2002 remained constant at 22%. More patients who received an erythropoiesis-stimulating agent than patients who did not developed venous thromboembolism. Overall survival was similar in both groups.
Implications
Further efforts are warranted to monitor the use and long-term toxicity of expensive oncology drugs, such as erythropoiesis-stimulating agents, to ensure that the benefits of any drug outweigh the risks in community practice.
Limitations
It is possible that venous thromboembolism would be diagnosed but not reflected in the billing claims used from the database and that not all treatments with erythropoiesis-stimulating agents or all transfusions were captured. Hemoglobin levels for individual patients were not available.
From the Editors
Two erythropoiesis-stimulating agents, erythropoietin and darbepoietin, were approved by the US Food and Drug Administration (FDA) in 1993 and 2002, respectively, for cancer patients being treated with chemotherapy (1,2). These drugs were given to reduce the need for blood transfusions. In randomized trials of anemic cancer patients (1,3), those who received erythropoiesis-stimulating agents required approximately 50% fewer transfusions, had decreased fatigue, and had an increased ability to do daily activities compared with those who did not receive erythropoiesis-stimulating agents. In these studies, unlike the trials for critically ill patients (4), there was no increased risk of venous thromboembolism (deep vein thrombosis and/or pulmonary embolism). In fact, fewer venous thromboembolism events were reported in those receiving erythropoiesis-stimulating agents than in those receiving placebo (1,3).
In 2003, a randomized, double-blind, placebo-controlled trial (5) in patients with head and neck cancer treated with radiation therapy found that overall survival of patients treated with erythropoiesis-stimulating agents was shorter than that of patients treated with placebo. In addition, the randomized trial (6) of patients with lung cancer who were randomly assigned to receive 12 injections once a week of epoetin alfa or of placebo, which was stopped early after an unplanned safety analysis, found a statistically significant higher median survival in the placebo group than in the treated group. In this study, the risk of venous thromboembolism was 9% in the placebo group and 39% in the group treated with erythropoiesis-stimulating agents. Two subsequent meta-analyses of randomized trials of the use of erythropoiesis-stimulating agents in the management of anemia (7,8) have reported a 50%–60% increase in the risk of venous thromboembolism in patients treated with erythropoiesis-stimulating agents compared with observation or placebo. The increased mortality associated with treatment with erythropoiesis-stimulating agents was uncertain until a 2008 meta-analysis that included data from newer trials (9) reported a 57% increase in the risk of venous thromboembolism and a small but statistically significant increase in mortality (hazard ratio [HR] = 1.10, 95% confidence interval [CI] = 1.01 to 1.20) (9). However, no increased risk of death has been observed among patients treated with erythropoiesis-stimulating agents who were also receiving chemotherapy (HR = 1.04, 95% CI = 0.97 to 1.11) (10).
Although erythropoiesis-stimulating agents appear to increase venous thromboembolism and possibly mortality in randomized trials, little is known about the patterns of erythropoiesis-stimulating agent use and associated outcomes in community practice. We used a retrospective cohort study to evaluate the patterns and predictors of the use of erythropoiesis-stimulating agents in elderly cancer patients with one of four common malignancies who were also receiving chemotherapy. In addition, we evaluated the impact of erythropoiesis-stimulating agents on the blood transfusion rate, long-term risk of venous thromboembolism, and overall survival in the 10 years after these agents were approved by the US FDA.
Patients and Methods
Data Source
We analyzed patient data from the Surveillance, Epidemiology, and End Results (SEER)–Medicare database (11). The SEER database contained records of patients diagnosed with cancer in regions that represented approximately 14% of the US population; in 2000, this database was expanded to include approximately 26% of the US population. SEER provides information on tumor histology, location, stage of disease, treatment, and survival, along with demographic and selected census tract–level information. The Medicare database includes Medicare A (inpatient) and B (outpatient) eligibility status, billed claims, and diagnoses. These two files are linked and provide the ability to define a population-based cancer population, to determine who has been treated with an erythropoiesis-stimulating agent or not, and then to estimate the subsequent risk of outcomes such as need for transfusions and/or venous thromboembolism.
Cohort Selection
We identified all individuals who were aged 65 years or older; who had a pathologically confirmed primary diagnosis of diffuse large B-cell lymphoma (International Classification of Disease, Ninth Revision [ICD-9] codes 9590, 9595, 9670, 9672–9675, 9677, 9680–9682, 9684, 9686, 9687, or 9690–9698), breast cancer (ICD-9 codes 800, 801, 802, 805, 814, 821, 823, 826, 848–854, 856, or 857), non–small cell lung cancer (ICD-9 codes 800–803, 804[6], 805, 807, 812, 814, 825, 826, 831, 832, 843, 848, 849, 851, 856, 857, or 898), or colon cancer (ICD-9 codes 801, 802, or 814) (12) from January 1, 1991, through December 31, 2002; and who were treated with chemotherapy. These cancers were thought to represent common cancers for which erythropoiesis-stimulating agents were frequently used. We excluded patients who were enrolled in a non-Medicare health maintenance organization because the billing claims for these patients are not submitted to Medicare for reimbursement. Patients who were enrolled in Medicare because of end-stage renal disease and dialysis rather than age were also excluded. Age at diagnosis was categorized into 5-year intervals starting at age 65 years. We recoded the SEER marital status variable as married, not married, and unknown.
Socioeconomic Status Score
We generated an aggregate socioeconomic status score from education, poverty, and income data from the 2000 census tract data, as described previously by Du et al. (13). Patients’ scores were ranked on a scale of 1–5 by use of the formula that incorporated education, poverty, and income weighted equally, with 1 being the lowest value (12). In the final analysis, patients with scores of 1 and 2 were combined into one group.
Assessment of Comorbid Disease
To assess the prevalence of comorbid disease in our cohort, we used the Klabunde adaptation of the Charlson comorbidity index (ie, the Klabunde–Charlson index) (14,15). Medicare inpatient and outpatient claims were searched for diagnostic codes of the ICD-9, Clinical Modification (12). Each condition was weighted, and patients were assigned a score that was based on the Klabunde–Charlson index (15).
Treatment Characteristics
We extracted information on chemotherapy from the date of diagnosis from the Medicare files by searching the Level II Healthcare Common Procedure Coding System: Current Procedural Terminology codes; ICD-9, Clinical Modification diagnostic codes and procedure codes; diagnostic-related group code; and the center code from physician claims files, the hospital outpatient claims files, or the Medicare provider review files. We searched for Level II Healthcare Common Procedure Coding System codes corresponding to the erythropoiesis-stimulating agents, erythropoietin and darbepoietin (Q0136-7, J0880-2, and J0885-6; Supplementary Table 1, available online). We excluded patients who received their first erythropoiesis-stimulating agent before they received chemotherapy. Use of erythropoiesis-stimulating agents was categorized on the basis of the median number of claims for erythropoiesis-stimulating agents (none, fewer than five claims, or five claims or more).
We classified patients into the following three groups: those who received chemotherapy only as nonmetastatic, those who received chemotherapy only with metastatic or recurrent cancer as metastatic, and those who received chemotherapy in both settings as both. Patients were classified as nonmetastatic if they had stage I–III breast, non–small cell lung, or colon cancer when they were treated or if they were treated within the first 12 months from diagnosis of diffuse large B-cell lymphoma. They were classified as metastatic if they had stage IV breast, non–small cell lung, or colon cancer. If chemotherapy was administered after the first 12 months, the patient was categorized as having a recurrence. Chemotherapy was classified as ever use of rituximab, platinum, 5-fluorouracil, or other.
Outcomes
The main outcomes of the study were receipt of blood transfusion and diagnostic claim for venous thromboembolism (deep vein thrombosis and/or pulmonary embolism). We identified patients who received inpatient or outpatient blood transfusions between 1991 and 2002. A venous thromboembolism event required a billing claim (the first claim). A pulmonary embolism or deep vein thrombosis event required at least two claims (16). An anemia event required at least two claims (Supplementary Table 1, available online). Patients were tracked until the end of follow-up, December 31, 2002, or death.
Statistical Analysis
Treatment with an erythropoiesis-stimulating agent and nontreatment were compared by use of χ2 tests and univariate regression, with respect to clinical and demographic variables. We used multivariable logistic regression models to analyze the association of use of erythropoiesis-stimulating agents with clinical and demographic variables (for variables, see Table 1). We used time-dependent Cox proportional hazards models from the date of diagnosis to analyze the association of erythropoiesis-stimulating agent with venous thromboembolism and with overall survival. The assumption of proportionality was confirmed visually. Patients who had a deep vein thromboembolism before receiving an erythropoiesis-stimulating agent were classified as being erythropoiesis-stimulating agent negative in this analysis. Multivariable Cox proportional hazards models were used to evaluate the relationship of erythropoiesis-stimulating agent dose (assessed as the number of claims) to a venous thromboembolism event and the relationship of erythropoiesis-stimulating agent dose to receiving blood transfusions. These analyses were also performed stratified by age category (65–69, 70–74, 75–79, or ≥80 years), extent of disease (metastatic, nonmetastatic, or both), and tumor type (colon, breast, lung, or lymphoma). A sensitivity analysis was performed by altering the number of claims for the outcome variables. Follow-up was available through December 31, 2002. We generated Kaplan–Meier curves and applied the log-rank test to compare rates of venous thromboembolism among patients who received erythropoiesis-stimulating agents within 3 months of diagnosis with those who did not, so that the time to event would be similar in both groups. In this analysis only, we excluded the 9870 patients who had had a venous thromboembolism before diagnosis to 3 months after diagnosis. We generated Kaplan–Meier curves to compare overall survival among patients who did and did not receive erythropoiesis-stimulating agents. All analyses were conducted with SAS, version 9.13 (SAS Institute, Cary, NC). All statistical tests were two-sided.
Table 1.
Univariate and multivariable analysis of associations between clinical and demographic characteristics and use of an erythropoiesis-stimulating agent (ESA) (n = 56 210; 15 346 [27%] with ESA use)*
| Characteristic | No. | ESA use, % | Univariate OR | Multivariable OR (95% CI) |
| Age at diagnosis, y | ||||
| 65–69 | 15 093 | 26.5 | 1.0 | 1.00 (referent) |
| 70–74 | 18 749 | 27.2 | 1.03 | 1.01 (0.96 to 1.07) |
| 75–79 | 13 808 | 28.2 | 1.09 | 1.02 (0.96 to 1.08) |
| ≥80 | 8560 | 27.5 | 1.05 | 0.93 (0.87 to 1.00) |
| Race | ||||
| White | 49 528 | 27.3 | 1.00 | 1.00 (referent) |
| Black | 3337 | 29.5 | 1.12 | 0.99 (0.90 to 1.08) |
| Hispanic | 617 | 28.5 | 1.06 | 0.87 (0.71 to 1.05) |
| Missing or other | 2728 | 23.8 | 0.54 | 0.86 (0.71 to 0.96) |
| Sex | ||||
| Male | 21 809 | 25.9 | 1.00 | 1.00 (referent) |
| Female | 34 401 | 28.1 | 1.11 | 1.22 (1.16 to 1.28) |
| Residence | ||||
| Metropolitan | 51 188 | 27.8 | 1.00 | 1.00 (referent) |
| Nonmetropolitan | 5021 | 22.4 | 0.75 | 0.83 (0.76 to 0.91) |
| Marital status | ||||
| Married | 32 555 | 27.5 | 1.00 | 1.00 (referent) |
| Unmarried or unknown | 21 952 | 26.9 | 0.97 | 0.91 (0.87 to 0.94) |
| Socioeconomic status | ||||
| Lowest (first) quartile | 16 575 | 25.5 | 1.00 | 1.00 (referent) |
| Second quartile | 12 803 | 26.2 | 1.04 | 1.06 (0.99 to 1.13) |
| Third quartile | 12 503 | 28.4 | 1.16 | 1.10 (1.03 to 1.17) |
| Highest (fourth) quartile | 13 466 | 30.3 | 1.27 | 1.10 (1.04 to 1.17) |
| Stage | ||||
| Nonmetastatic | 27 754 | 23.3 | 1.00 | 1.00 (referent) |
| Metastatic | 17 702 | 28.4 | 1.30 | 2.03 (1.92 to 2.13) |
| Both | 10 754 | 35.7 | 1.82 | 3.05 (2.89 to 3.23) |
| Radiation therapy | ||||
| No | 29 108 | 25.1 | 1.00 | 1.00 (referent) |
| Yes | 27 102 | 29.6 | 1.26 | 1.10 (1.05 to 1.15) |
| Comorbidity score | ||||
| 0 | 30 477 | 25.4 | 1.00 | 1.00 (referent) |
| 1 | 15 768 | 28.1 | 1.15 | 1.07 (1.02 to 1.13) |
| >1 | 9965 | 31.7 | 1.36 | 1.13 (1.07 to 1.20) |
| Period | ||||
| 1991–1995 | 14 206 | 7.8 | 1.00 | 1.00 (referent) |
| 1996–1998 | 14 896 | 19.2 | 2.82 | 3.03 (2.81 to 3.27) |
| 1999–2002 | 27 108 | 42.0 | 8.65 | 9.79 (9.11 to 10.52) |
| Tumor site | ||||
| Colon | 13 422 | 17.2 | 1.00 | 1.00 (referent) |
| Breast | 14 024 | 27.4 | 1.82 | 1.74 (1.62 to 1.87) |
| Lung | 16 939 | 32.5 | 2.32 | 1.92 (1.79 to 2.05) |
| Diffuse large B-cell lymphoma | 11 825 | 31.3 | 2.07 | 2.55 (2.38 to 2.72) |
Each variable was corrected for the other listed characteristics as well as for the Surveillance, Epidemiology, and End Results site. CI = confidence interval; OR = odds ratio.
Results
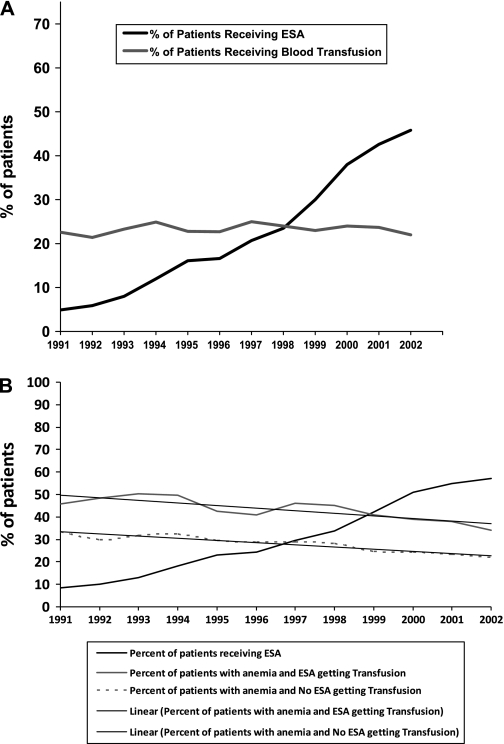
We identified 56 210 individuals who were aged 65 years or older in the SEER–Medicare database, who met our inclusion criteria, and who received chemotherapy between January 1, 1991, and December 31, 2002. Of these patients, 15 346 (27%) received erythropoiesis-stimulating agents (Table 1). The proportion of patients receiving erythropoiesis-stimulating agents increased dramatically over time, from 141 (4.8%) of a total of 2902 patients in 1991 to 2849 (45.9%) of a total of 6183 patients in 2002 (P < .001) (Figure 1). By 2002, 1451 (54%) of the 2669 patients with metastatic cancer and 2672 (45%) of the 5868 with nonmetastatic cancer received erythropoiesis-stimulating agents during chemotherapy. In a multivariable analysis, use of erythropoiesis-stimulating agents was statistically significantly decreased among patients who lived in a nonmetropolitan area, compared with a metropolitan area (odds ratio [OR] = 0.83, 95% CI = 0.76 to 0.91). Use of these agents was increased in women compared with men, in patients with higher rather than lower socioeconomic status, in patients with more rather than fewer comorbid conditions, in patients who were diagnosed in a later year rather than in an earlier year, and in patients who had metastatic disease compared with nonmetastatic disease. Patients with breast, lung, or diffuse large B-cell lymphoma were more likely to receive an erythropoiesis-stimulating agent than patients with colon cancer (Table 1). Women with diffuse large B-cell lymphoma (OR = 1.15, 95% CI = 1.05 to 1.26), colon cancer (OR = 1.38, 95% CI = 1.25 to 1.54), or lung cancer (OR = 1.23, 95% CI = 1.14 to 1.32) were statistically significantly more likely to receive erythropoiesis-stimulating agents than men with the same disease.
Figure 1.
Percentage of patients with cancer treated with chemotherapy receiving erythrocyte-stimulating agents (ESAs) and blood transfusions over time. A) Entire cohort (N = 41 161). B) Patients with anemia (n = 25 464).
The initial clinical trials (1,2) leading to FDA approval for the use of erythropoiesis-stimulating agents found a reduction in blood transfusions with use of these agents as the primary outcome. In contrast, in a population-based setting, we did not observe a change in the proportion (22%) of patients receiving chemotherapy who received at least one blood transfusion between 1991 and 2002. In fact, in the subset of patients with two claims for anemia or more, who were among the 25 464 patients in the original nine SEER sites, erythropoiesis-stimulating agent use increased from 8.5% to 60.5%. In this group of anemic patients, those who received erythropoiesis-stimulating agents had a higher transfusion rate than those who did not. Approximately 30% of patients in the erythropoiesis-stimulating agent group who received transfusions received them in the year before they received the erythropoiesis-stimulating agent, and approximately 50% received transfusions at the same time or during the year after they received the erythropoiesis-stimulating agent. Among both groups, transfusion rates decreased only slightly, but similarly, over time (Figure 1, B).
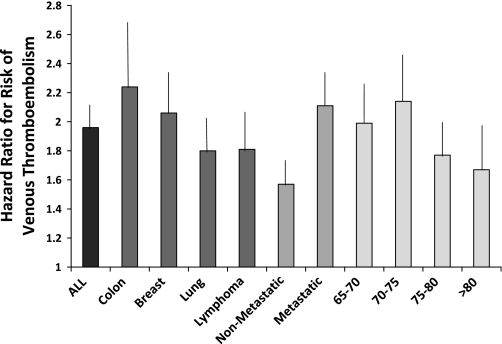
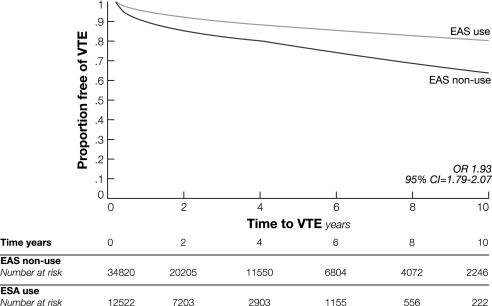
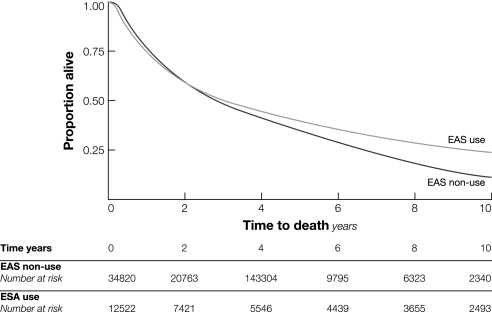
From diagnosis through the end of follow-up, 5196 (11%) of the total of 47 342 patients had a new claim for venous thromboembolism. Among the 12 522 patients who received erythropoiesis-stimulating agents, 1796 (14.3%) had a venous thromboembolism event compared with 3400 (9.8%) of the 34 820 patients who did not receive erythropoiesis-stimulating agents (P < .001). In a time-dependent Cox proportional hazards model that evaluated time to first venous thromboembolism event, use of erythropoiesis-stimulating agents (HR = 1.93, 95% CI = 1.79 to 2.07), advanced age (HR = 1.16, 95% CI = 1.07 to 1.23, for ages 70–74 years; HR = 1.21, 95% CI = 1.11 to 1.30, for ages 75–79 years; HR = 1.18, 95% CI = 1.07 to 1.28, for ages ≥80 years, all compared with ages 65–69 years), black race (HR = 1.20, 95% CI = 1.07 to 1.35), radiation therapy (HR = 1.22, 95% CI = 1.15 to 1.30), increased comorbid conditions (HR = 1.32, 95% CI = 1.22 to 1.43), and recurrent or metastatic cancer (HR = 1.53, 95% CI = 1.43 to 1.64) and diagnosis of lung cancer (HR = 1.14, 95% CI = 1.00 to 1.29) were associated with an increased risk for venous thromboembolism (Table 2). Patients with five or more claims for use of erythropoiesis-stimulating agents had a stronger association (HR = 1.55, 95% CI = 1.44 to 1.66) with venous thromboembolism than those with fewer than five claims (HR = 1.31, 95% CI = 1.19 to 1.44). A similar result was observed when the sample was limited to patients with nonmetastatic disease (for five claims or more, HR = 1.72, 95% CI = 1.51 to 1.96; for fewer than five, HR = 1.36, 95% CI = 1.17 to 1.58). The effect size for risk of venous thromboembolism was similar after altering the number of claims from two to three. The risk of a venous thromboembolism event after use of erythropoiesis-stimulating agents was slightly higher in patients with colon cancer (HR = 2.23, 95% CI = 1.88 to 2.65) (Figure 2). When we stratified the analyses by year of diagnosis and limited patient follow-up to 4 years, we found that for each time period, erythropoiesis-stimulating agent use was associated with at least a twofold increase in venous thromboembolism events. Survival free of venous thromboembolism for patients who received erythropoiesis-stimulating agents compared with those who did not was shorter (P < .001, log-rank test) (Figure 3). No association between erythropoiesis-stimulating agent use and mortality was observed, however (Figure 4).
Table 2.
Time-dependent univariate and multivariable analysis of associations between clinical and demographic characteristics and thrombosis (n = 47 342)*
| Characteristic | No. (%) | Thrombosis, No. (%) | Univariate HR | Multivariable HR (95% CI) |
| ESA use | ||||
| No | 34 820 | 3400 (9.8) | 1.00 | 1.00 (referent) |
| Yes | 12 522 | 1796 (14.3) | 1.55 | 1.93 (1.79 to 2.07) |
| Age at diagnosis, y | ||||
| 65–69 | 13 443 | 1424 (10.6) | 1.00 | 1.00 (referent) |
| 70–74 | 15 831 | 1843 (11.6) | 1.11 | 1.16 (1.07 to 1.23) |
| 75–79 | 11 235 | 1268 (11.3) | 1.07 | 1.21 (1.11 to 1.30) |
| ≥80 | 6833 | 661 (9.7) | 0.90 | 1.18 (1.07 to 1.28) |
| Race | ||||
| White | 41 535 | 4606 (11.1) | 1.00 | 1.00 (referent) |
| Black | 2801 | 385 (13.8) | 1.28 | 1.20 (1.07 to 1.35) |
| Hispanic | 532 | 48 (9.0) | 0.80 | 0.82 (0.62 to 1.10) |
| Missing or other | 2474 | 157 (6.4%) | 0.54 | 0.68 (0.57 to 0.82) |
| Sex | ||||
| Male | 18 165 | 1784 (9.8) | 1.00 | 1.00 (referent) |
| Female | 29 177 | 3412 (11.7) | 1.22 | 1.10 (1.03 to 1.18) |
| Socioeconomic status | ||||
| Lowest (first) quartile | 14 163 | 1504 (10.6) | 1.00 | 1.00 (referent) |
| Second quartile | 10 745 | 1206 (11.2) | 1.06 | 1.07 (0.99 to 1.16) |
| Third quartile | 10 408 | 1179 (11.3) | 1.07 | 1.03 (0.95 to 1.12) |
| Highest (fourth) quartile | 11 271 | 1207 (10.7) | 1.01 | 0.98 (0.90 to 1.06) |
| Treatment | ||||
| Nonmetastatic | 23 207 | 1970 (8.5) | 1.00 | 1.00 (referent) |
| Metastatic | 15 012 | 1483 (11.6) | 1.42 | 1.53 (1.43 to 1.64) |
| Both | 9123 | 1743 (16.3) | 2.09 | 1.51 (1.41 to 1.62) |
| Radiation therapy | ||||
| No | 24 281 | 2419 (10.0) | 1.00 | 1.00 (referent) |
| Yes | 23 061 | 2777 (12.0) | 1.24 | 1.22 (1.15 to 1.30) |
| Comorbidity score | ||||
| 0 | 26 943 | 2920 (10.8) | 1.00 | 1.00 (referent) |
| 1 | 13 302 | 1416 (10.6) | 0.98 | 1.06 (0.99 to 1.13) |
| >1 | 7097 | 860 (12.1) | 1.13 | 1.32 (1.22 to 1.43) |
| Period | ||||
| 1991–1995 | 12 744 | 1618 (12.7) | 1.00 | 1.00 (referent) |
| 1996–1998 | 12 495 | 1479 (11.8) | 0.92 | 0.98 (0.91 to 1.05) |
| 1999–2002 | 22 103 | 2099 (9.5) | 0.72 | 0.91 (0.84 to 0.99) |
| Tumor | ||||
| Colon | 11 434 | 1259 (11.0) | 1.00 | 1.00 (referent) |
| Breast | 12 499 | 1502 (12.0) | 1.10 | 0.83 (0.75 to 0.92) |
| Lung | 13 786 | 1350 (9.8) | 0.88 | 1.14 (1.00 to 1.29) |
| Diffuse B-cell lymphoma | 9623 | 1085 (11.3) | 1.03 | 1.11 (0.97 to 1.25) |
| Chemotherapy | ||||
| Rituximab | 3557 | 407 (11.4) | 1.15 | 0.85 (0.75 to 0.96) |
| 5-FU | 17 843 | 2074 (11.6) | 1.26 | 1.13 (1.04 to 1.22) |
| Platinum | 10 928 | 1248 (11.4) | 1.10 | 1.18 (1.07 to 1.29) |
| Other | 36 595 | 4195 (11.5) | 1.35 | 1.16 (1.07 to 1.27) |
In this analysis, 11% had had either deep vein thrombosis or pulmonary embolism. Each variable was adjusted for the other listed characteristics and for Surveillance, Epidemiology, and End Results site. 5-FU = 5-fluorouracil; CI = confidence interval; ESA = erythropoiesis-stimulating agent; HR = hazard ratio.
Figure 2.
Risk of venous thromboembolism after treatment with an erythropoiesis-stimulating agent by age group and tumor type. Data are from individual multiple logistic regression models controlling for clinical and demographic variables (as shown in Table 1). Error bars = 95% confidence intervals.
Figure 3.
Comparison of venous thromboembolism in patients with cancer who were treated with an erythropoiesis-stimulating agent (ESA) vs those who were not. Results of a Kaplan–Meier analysis are presented (odds ratio). CI = confidence interval; OR = odds ratio; VTE = venous thromboembolism.
Figure 4.
Overall survival among patients with cancer who were treated with an erythropoiesis-stimulating agent (ESA) vs those who were not. Results of a Kaplan–Meier analysis are presented.
Discussion
We found that the use of erythropoiesis-stimulating agents increased rapidly after FDA approval, with approximately 50% of patients with advanced cancer undergoing chemotherapy receiving erythropoiesis-stimulating agents by 2002. Despite erythropoiesis-stimulating agents being associated with a reduction in use of blood transfusions in clinical trials, the proportion of patients who received at least one blood transfusion remained constant during the 10-year study period, with slight decreases in the number of anemic patients who did and did not receive erythropoiesis-stimulating agents. In addition, our findings support the recent reports (7,8) that elderly patients with cancer who received erythropoiesis-stimulating agents as an adjunct to chemotherapy were at increased risk of developing venous thromboembolism. Similar to one subgroup analysis within a recent meta-analysis (17) that limited its analysis to patients who were undergoing chemotherapy, we did not observe an increase in risk of mortality that was associated with the use of erythropoiesis-stimulating agents.
Reports of adverse events associated with a number of commonly used medications that appear years after the initial FDA approval have raised concerns about the drug evaluation process (18–23). Identification of new toxic effects of FDA-approved drugs tends to increase distrust in the medical establishment. Erythropoiesis-stimulating agents were a prototypical example of the limitations of our current system. The initial approval of the erythropoiesis-stimulating agent erythropoietin for chemotherapy-related anemia was based on pooled data from two randomized trials in which patients were evaluated for 12 weeks (2,24,25). Subsequently, meta-analyses (8,9) that included thousands of patients from randomized controlled trials identified an increased risk of venous thromboembolisms that was associated with use of erythropoiesis-stimulating agents.
Medications that are approved for the supportive care of patients with cancer often have short-term outcomes, but the risks after prolonged use are not evaluated. Unfortunately, many important questions cannot be addressed in premarketing studies, which are largely focused on demonstrating short-term efficacy. This system failed to detect the risks associated with use of erythropoiesis-stimulating agents that did not come to light until years after the drugs were approved. A recent proposal in an editorial (23) calls for postmarketing studies of new drugs to address potential long-term toxic effects associated with new medications, including adequately powered safety studies, long-term studies of drugs for chronic diseases, epidemiological studies of rare adverse effects, and randomized trials that assess efficacy. After stratifying by year since marketing approval for erythropoiesis-stimulating agents, we found that the association between use of these drugs and an increased risk of venous thromboembolism could be detected as early as 4 years after FDA approval. Thus, the use of claims data for monitoring outcomes appears to be warranted as a potential way for detecting long-term toxicities.
In the spring of 2007, the FDA published an alert after results of six trials (26) of erythropoiesis-stimulating agents showed a small increased risk tumor progression and/or decreased survival associated with the use of erythropoiesis-stimulating agents. This alert culminated in a black box warning about the potential for tumor promotion, decreased survival, and venous thromboembolism in erythropoiesis-stimulating agent users (27). Their suggestions were to limit use of these drugs to specific tumor types, durations, doses, and targeted hemoglobin levels. In addition, for the first time to our knowledge, the Center for Medicare and Medicaid Services proposed the elimination or limitation of coverage for erythropoiesis-stimulating agents as treatment for some cancers. In our analysis in this article, we did not detect an increased risk of death; however, our sample was limited to elderly cancer patients who were receiving chemotherapy. A recent meta-analysis (10) of individual data from patients who were treated with chemotherapy did not find that increased mortality was associated with use of erythropoiesis-stimulating agents (HR = 1.04, 95% CI = 0.97 to 1.11).
We found greater use of erythropoiesis-stimulating agent among women with colon cancer, non–small cell lung cancer, and diffuse large B-cell lymphoma than among men with the same diseases. Among individuals older than 50 years, women have lower hemoglobin levels than men (28). It is unclear whether this observation is related to differences in hormones, diet, or other factors. Our observation that patients residing in rural areas and patients older than 80 years were less likely to receive erythropoiesis-stimulating agents may indicate difficulty in traveling to the clinic or physician’s office for injection of the erythropoiesis-stimulating agent.
Given the rapid rise in the use of erythropoiesis-stimulating agents since their approval by the FDA, we were surprised to find that rates of red blood cell transfusion did not decrease substantially during this time. The Cochrane Review (7) of more than 30 trials, including more than 6000 patients, found that the use of erythropoiesis-stimulating agents was associated with a statistically significantly reduced risk of blood transfusions (relative risk = 0.64). One possibility is that the patients in the placebo group of the clinical trial had a higher rate of transfusion than patients who are not on a clinical trial. In the original clinical trials (1–3), the blood transfusion rate was 40%–50% in patients who were randomly assigned to the placebo groups. Patients treated by physicians aware of the hypothesis may have been more inclined to proceed with transfusions than they would have been otherwise. We observed that, in the community, patients who required transfusions were those who were also treated with erythropoiesis-stimulating agents, either before or after the transfusion.
Erythropoiesis-stimulating agents are of particular interest from a public policy perspective because of the costs associated with their use. The total US sales of erythropoiesis-stimulating agents increased from $6.4 billion in 2002 to $10 billion in 2006, accounting for a greater Medicare Part B expenditure than any other drug (29). We speculate that this use was fueled by aggressive marketing to patients and physicians that focused on a promise of increased energy during chemotherapy treatment. It is estimated that direct medical care costs attributable to cancer now exceed $70 billion annually (30), with large increases in cost for elderly patients (31).
Our study had several limitations. Despite the value of the SEER–Medicare database, it has some limitations. The SEER database consists of data provided by hospital cancer registries that is obtained from patient charts. The Medicare database consists of reimbursement claims for medical care. As a result, it is possible that venous thromboembolism would be diagnosed but not reflected in the billing claims. One study (16) has shown that claims data can be used effectively in the long term to capture chronic complications especially when the complication prompts an intervention, such as urethral strictures, but may not be so useful in capturing chronic symptoms, such as impotence or incontinence. This situation is also found with deep vein thrombosis and pulmonary embolism. In addition, investigators have shown that requiring two claims decreases the likelihood of misclassification (16). The SEER–Medicare database has been used by many investigators to evaluate late effects of therapy (32–35). It is also possible that instances of patients receiving erythropoiesis-stimulating agents were not captured in Medicare claims; however, because of the expense of the medications and the exclusion of patients with secondary insurance, we do not believe that this problem occurs for a large number of patients. It was possible that not all transfusions were captured because not all blood products are reimbursed fully by Medicare; however, a claim for both the transfusion and the cost for the infusion for the transfusion does usually generate claims data. Another major limitation of our study is that we could not ascertain hemoglobin levels for individual patients; however, at least one study (36) has shown that hemoglobin level is not associated with the risk of venous thromboembolism.
All association studies are limited by lack of certainty with regard to causality. Although the association is biologically plausible and short-term randomized controlled trials have established the association, it is unclear from observational studies whether the reason for administration of an erythropoiesis-stimulating agent places the patient at higher risk for venous thromboembolism or if the agent itself increases risk. This problem of confounding by indication is particularly true for patients with more claims for erythropoiesis-stimulating agents who may be sicker as a result of the underlying cancer. Unlike the clinical trials, patients who are treated in a community practice are given erythropoiesis-stimulating agents intermittently; therefore, the length of time between dose and event varied. The increased risk of venous thromboembolism that we observed was slightly higher than that observed in the meta-analysis of randomized controlled trials in which patients were followed prospectively, but for a shorter period of time (9). The patients in this analysis had a longer follow-up and were all older than 65 years, which may have also contributed to increased risk.
Our study demonstrated that the use of erythropoiesis-stimulating agents increased rapidly in community practice after its approval in patients with both solid tumors and hematologic malignancy and among patients with either early-stage or advanced cancer who were receiving chemotherapy. Despite this increased administration to nearly half of all cancer patients, a substantial reduction in the use of blood transfusions was not observed. However, the increased risk of venous thromboembolism that was associated with use of an erythropoiesis-stimulating agent, confirming the association observed in the meta-analysis (9). Further efforts at monitoring use and long-term toxicity of expensive oncology drugs should be put in place to ensure that for any drug the benefits outweigh the risks in community practice.
Funding
National Cancer Institute (R01CA134964 to D.L.H.); American Cancer Society (RSGT-08-009-01-CPHPS to D.L.H.). R.M. was supported in part by a T32 Award (ULI RR024156) from the National Center for Research Resources of the National Institutes of Health and an R25 award from the National Cancer Institute (CA090461).
Supplementary Material
Footnotes
None of the authors have any current conflicts of interest. The authors take full and sole responsibility for the content of the manuscript, including its design, data analysis and interpretation, preparation, and submission of the manuscript.
This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; the Office of Information Services and the Office of Strategic Planning, Health Care Finance Administration; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER–Medicare database.
References
- 1.Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211–1220. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 2.Henry DH, Brooks BJ, Jr, Case DC, Jr, et al. Recombinant human erythropoietin therapy for anemic cancer patients receiving cisplatin chemotherapy. Canc J Sci Am. 1995;1(4):252–260. [PubMed] [Google Scholar]
- 3.Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19(11):2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 4.Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357(10):965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 5.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362(9392):1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 6.Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25(9):1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 7.Bohlius J, Wilson J, Seidenfeld J, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD003407.pub4. CD003407. [DOI] [PubMed] [Google Scholar]
- 8.Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299(8):914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 10.Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373(9674):1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 11.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–748. [PubMed] [Google Scholar]
- 12.American Medical Association International Classification of Disease 9th Revision. Chicago: AMA Press; 2008. [Google Scholar]
- 13.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110(3):660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG., Jr Assessing illness severity: does clinical judgment work? J Chronic Dis. 1986;39(6):439–452. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(8 suppl) doi: 10.1097/00005650-200208001-00009. IV-62-68. [DOI] [PubMed] [Google Scholar]
- 17.Brillant C, Clarke M, Kluge S, et al. Recombinant human erythropoiesis stimulating agents in cancer patients: individual patient data meta-analysis on behalf of the EPO IPD Meta-Analysis Collaborative Group. In: American Society of Hematology; 2008; San Francisco, CA. Blood. 2008 LBA-6. [Google Scholar]
- 18.Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354(13):1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 19.Couzin J. Scientific publishing. Echoing other cases, NEJM says Vioxx Safety data withheld. Science. 2005;310(5755):1755. doi: 10.1126/science.310.5755.1755. [DOI] [PubMed] [Google Scholar]
- 20.Waxman HA. The lessons of Vioxx—drug safety and sales. N Engl J Med. 2005;352(25):2576–2578. doi: 10.1056/NEJMp058136. [DOI] [PubMed] [Google Scholar]
- 21.Griffin MR, Stein CM, Ray WA. Postmarketing surveillance for drug safety: surely we can do better. Clin Pharmacol Ther. 2004;75(6):491–494. doi: 10.1016/j.clpt.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351(11):1089–1096. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 23.Ray WA, Stein CM. Reform of drug regulation—beyond an independent drug-safety board. N Engl J Med. 2006;354(2):194–201. doi: 10.1056/NEJMsb053432. [DOI] [PubMed] [Google Scholar]
- 24.Case DC, Jr, Bukowski RM, Carey RW, et al. Recombinant human erythropoietin therapy for anemic cancer patients on combination chemotherapy. J Natl Cancer Inst. 1993;85(10):801–806. doi: 10.1093/jnci/85.10.801. [DOI] [PubMed] [Google Scholar]
- 25.Henry DH, Abels RI. Recombinant human erythropoietin in the treatment of cancer and chemotherapy-induced anemia: results of double-blind and open-label follow-up studies. Semin Oncol. 1994;21(2 suppl 3):21–28. [PubMed] [Google Scholar]
- 26.Food and Drug Administration. Erythropoiesis stimulating agents (ESA) [Aranesp (darbepoetin), Epogen (epoetin alfa), and Procrit (epoetin alfa)]. Healthcare Professional Sheet text version. 2007 [Google Scholar]
- 27.Khuri FR. Weighing the hazards of erythropoiesis stimulation in patients with cancer. N Engl J Med. 2007;356(24):2445–2448. doi: 10.1056/NEJMp078101. [DOI] [PubMed] [Google Scholar]
- 28.Beregi E, Regius O, Nemeth J, Rajczy K, Gergely I, Lengyel E. Gender differences in age-related physiological changes and some diseases. Z Gerontol Geriatr. 1995;28(1):62–66. [PubMed] [Google Scholar]
- 29.Steinbrook R. Medicare and erythropoietin. N Engl J Med. 2007;356(1):4–6. doi: 10.1056/NEJMp068296. [DOI] [PubMed] [Google Scholar]
- 30.Cancer Trends Progress Report—2005 Update. National Cancer Institute, NIH. Bethesda, MD:; 2008. http://progressreport.cacer.gov/ [Google Scholar]
- 31.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100(12):888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26(31):5119–5125. doi: 10.1200/JCO.2008.16.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giordano SH, Lee A, Kuo YF, Freeman J, Goodwin JS. Late gastrointestinal toxicity after radiation for prostate cancer. Cancer. 2006;107(2):423–432. doi: 10.1002/cncr.21999. [DOI] [PubMed] [Google Scholar]
- 34.Woodward WA, Giordano SH, Duan Z, Hortobagyi GN, Buchholz TA. Supraclavicular radiation for breast cancer does not increase the 10-year risk of stroke. Cancer. 2006;106(12):2556–2562. doi: 10.1002/cncr.21943. [DOI] [PubMed] [Google Scholar]
- 35.Doyle JJ, Neugut AI, Jacobson JS, et al. Radiation therapy, cardiac risk factors, and cardiac toxicity in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;68(1):82–93. doi: 10.1016/j.ijrobp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Fullmer AC, Miller R. Retrospective review of hemoglobin and/or hematocrit levels with occurrence of thrombosis in cancer patients treated with erythropoiesis stimulating agents. J Oncol Pharm Pract. 2009 doi: 10.1177/1078155209102337. [published online ahead of print March] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.