Abstract
Almost 40 y ago the scientific community was taking actions to control environmental factors that contribute to variation in the responses of laboratory animals to scientific manipulation. Laboratory animal diet was recognized as an important variable. During the 1970s, the American Institute of Nutrition, National Academy of Science, Institute of Laboratory Animal Resources, and Laboratory Animals Centre Diets Advisory Committee supported the use of ‘standard reference diets’ in biomedical research as a means to improve the ability to replicate research. As a result the AIN76 purified diet was formulated. During this same time, the laboratory animal nutritionist at the NIH was formulating open-formula, natural-ingredient diets to meet the need for standardized laboratory animal diets. Since the development of open-formula diets, fixed-formula and constant-nutrient–concentration closed-formula laboratory animal natural ingredient diets have been introduced to help reduce the potential variation diet can cause in research.
The ability to replicate research is fundamental for good science. One key to replicating research is to control all variables except those being studied. To meet this requirement, the microbiologic and genetic characterization of laboratory animals has become increasingly well-defined over the years. Health status and environmental factors (for example, diet, bedding, light cycles, noise, humidity, temperature, and personnel interactions with animals) are all factors that can influence research outcomes and should be controlled to the extent possible.11 Diet is an important environmental factor that affects reproduction, growth, disease, and response to experimental manipulation in laboratory animals. Furthermore, diet is a substantial cost to an animal care program. In 2005, 25% ($700 million) of the intramural NIH budget supported research projects that used laboratory animals.28 That year, the NIH used more than 2.6 million pounds of feed at a cost of over US$1,000,000 to maintain its laboratory animals.2
Background
During the past 35 y, various individuals and scientific institutions have developed guidelines intended to promote standardization of the selection, use, and reporting of diets used for research animals. The purpose of these dietary guidelines was to improve the quality of research. In the early 1970s, Dr Joseph Knapka, laboratory animal nutritionist at the NIH Veterinary Resources Program, initiated a program to standardize laboratory animal diets by formulating the first “open-formula laboratory animal diets.”15 The NIH open-formula diets were intended to improve research by reducing experimental variability and to reduce the cost of laboratory animal diets by allowing open competition for the purchase of those diets. A 1977 publication from the American Institute of Nutrition recommended that the NIH07 open-formula, natural-ingredient rodent diet and the AIN76 purified diet be used as “standard reference diets.”2 This report acknowledged that although a single standard diet that met the requirements of every research program could not be formulated, the use of a standard reference diet, which could be modified to meet specific research needs, would reduce 1 source of variability.
The Laboratory Animals Centre Diets Advisory Committee and The National Academy of Sciences also supported the use of standard reference diets in biomedical research.8,13 The National Academy of Sciences stated that the detailed composition of laboratory animal diets used for experimental purposes was important knowledge for scientists and strongly recommended feeding open-formula diets.13 Other authors recommended feeding open-formula diets as a means to eliminate the potential for drug-nutrient interactions confounding toxicology studies.10 In 1993, to improve the AIN76 diet, the AIN93 Growth (G) and the AIN93 Maintenance (M) diets were formulated (Table 1).26 At this time, the AIN reasserted the need to standardize experimental laboratory animal diets to reduce the variation intrinsic in natural ingredient diets and to facilitate interpretation of results among experiments and laboratories.26
Table 1.
Composition of AIN93 growth (G) and maintenance (M) diets
| Ingredient concentration (g/ kg) |
||
| AIN93G | AIN93M | |
| Cornstarch | 397.486 | 465.692 |
| Casein (≥85% protein) | 200.000 | 140.000 |
| Dextrinized cornstarch | 132.000 | 155.000 |
| Sucrose | 100.000 | 100.000 |
| Soybean oil | 70.000 | 40.000 |
| Fiber (Solka–Floc) | 50.000 | 50.000 |
| AIN93G Mineral mix | 35.000 | 35.000 |
| AIN93VX Vitamin mix | 10.000 | 10.000 |
| L-Cystine | 3.000 | 1.800 |
| Choline bitartrate | 2.500 | 2.500 |
| Tert-butylhydroquinone | 0.014 | 0.008 |
A new comprehensive approach toward biologic research, systems biology, is being developed. It is defined as, “the understanding of how all relevant components of a biologic system interact functionally over time and under varying conditions.”28 New technologies including genomics, proteomics, metabolomics, and nutrigenomics are evolving and being used to advance the systems biology approach.14 These technologies are affected by both intrinsic and environmental factors, such as diet.4,14 Therefore, the importance of controlling dietary variables in research will not diminish and probably will increase in the future.
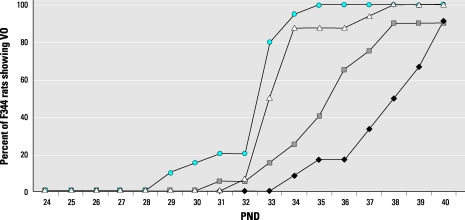
Recently, adverse experimental outcomes related to plant phytoestrogens in the diet emphasize the effect of diet on research and the importance of standardized open-formula diets.6,12,36 For example, 1 study showed that rodent diets significantly differ in phytoestrogen content.31 In addition, the phytoestrogen content of the same laboratory animal diet varied 3-fold depending on mill date, producing significant effects on reproductive, toxicology, and comparative estrogenic or hormonal endpoints such as uterotrophic bioassays and vaginal opening assays.30,33 These assays are used to determine the estrogenic or antiestrogenic activity of endocrine disruptor compounds. Figure 1 shows the effect of variable levels of the phytoestrogens daidzein and genistein in different mill dates of the same diet on the results of VO endpoints.32 The time of VO was significantly (P < 0.05) advanced for the F44 rats fed different mill dates of PMI 5002 rodent diet varying in phytoestrogen content. This batch-to-batch variation in the phytoestrogen content of different mill dates of the same diet makes it difficult for investigators to reproduce estrogenic results within or between laboratories over time. Based on these findings, the authors of these studies recommended that a standardized open-formula diet, ideally free of phytoestrogens, should be used for studies that could be confounded by dietary estrogens or for studies evaluating the estrogenic activity of endocrine disruptor compounds.32,33 For research that can be affected by dietary estrogens, the results of the cited studies show how important it is to identify the diet being fed and its phytoestrogen content in all publications.
Figure 1.
The effect of batch-to-batch variation in the total daidzein + genistein (D + G) content in batches of the same diet (PMI 5002) with different mill dates on the timing of vaginal opening in F344 rats. The total daidzein + genistein content can vary 3-fold depending on mill date, producing significant (P < 0.05) differences in the time of vaginal opening between different mill dates at postnatal days (PND) 34 and 36. The low-phytoestrogen diet in the chart is PMI 5K96. Diamond, PMI 5K96 control (D + G = 7 µg/g); square, PMI 5002 with mill date of 2003 Aug 20 (D + G = 98 µg/g); triangle, PMI 5002 with mill date of 2004 Nov 07 (D + G = 223 µg/g); circle, PMI 5002 with mill date of 1997 Jul 23 (D + G = 431 µg/g). This figure is based on that in reference 32 and appears with permission.
Types of Diets
Open-Formula Diets.
In open-formula diets, the concentrations of all ingredients (that is, the quantitative ingredient formulations) are publicly available, thus allowing researchers to control for this important environmental variable. Open-formula diets also enable retrospective analysis of possible diet composition effects on research results, an option unavailable with a closed-formula (proprietary) diet. The AIN93 diet formulations (Table 1) are examples of semipurified open-formula diets providing the quantitative ingredient formulations. Information regarding the 11 NIH open-formula laboratory animal diets currently available is at http://dvrnet.ors.od.nih.gov/diets_info.asp. These diets have been in use at the NIH since their development and are used at other scientific institutions and commercial breeders. New open formula diets are formulated and made available as needed. For example, the NIH31 open-formula extruded autoclavable rodent diet was formulated in response to requests from the NIH animal research community. This extruded diet is an alternative to both the NIH31 open-formula pelleted rodent diet and closed-formula extruded rodent diets. The formulation and nutrient content of the extruded NIH31 diet are comparable to those of the NIH31 pelleted diet. However, when extruded diets are autoclaved, minimal starch gelatinization occurs, thus eliminating the problems of pellet clumping and increased hardness associated with autoclaved pelleted diets.9 A feeding trial showed that the extruded NIH31 diet is palatable.3 All laboratory animal diet manufacturers can produce open-formula diets in addition to their proprietary brands.
Closed-Formula Diets.
The commercial diets produced and marketed under vendor trade names typically are known as ‘closed formula diets’ and are proprietary. Although the ingredients are listed, the quantitative ingredient formulation is not publicly available. Therefore, ingredient composition can vary without public disclosure. Variations in formulation can occur if feed manufacturers use ‘least cost’ strategies to formulate closed-formula diets. A least-cost strategy refers to formulating diets to maximize profit by using the least-expensive ingredients. Terminology such as ‘fixed formula’ and ‘Constant Nutrition’ (LabDiets, PMI Nutrition International, St Louis, MO) has been used by manufacturers to describe closed-formula diets.
Fixed-Formula Diets.
In a fixed-formula diet, the quantitative ingredient formulation does not change. Because the quantitative formulations for open-formula diets do not change, the terms fixed formula and open formula have mistakenly been thought of as synonymous. Both fixed- and open-formula diets occasionally may require changes in formulation to maintain nutrient composition or meet changing nutrient requirements. However, changes in quantitative ingredient formulation are made public when open-formula diets are modified, whereas information regarding modifications to fixed-closed formula diets is proprietary and therefore not disclosed publically.
Constant Nutrition Diets.
Constant Nutrition is the trademarked phrase of PMI Nutrition International and describes their laboratory animal diets (LabDiets) for which the concentrations of known nutrients and the group of ingredients used remain constant. However, the quantitative ingredient formulations of Constant Nutrition diets can be varied without public disclosure. Alteration in the diet formulation can change undefined nutrient or dietary components, such as fatty acids,27 phytoestrogens,6,32,33 phytosterols,5 nitrosamines,14,25 mycotoxins,20,35 and methylmercury,37 potentially affecting research outcomes. For example, Table 2 shows the ingredients from 3 diets that provide a total crude protein concentration of 12%. As shown, the 12% protein concentration is achieved in the 3 diets by using different amounts of the same ingredients: corn, wheat, fish meal, and soybean meal. Although the protein concentration remains at 12%, other nutrients and dietary components, such as amino acids, fatty acids, phytoestrogens, phytosterols, nitrosamines, and mycotoxins, which are found in 1 or more of these ingredients, could differ among the 3 diets. These dietary components potentially can cause physiologic effects in animals and affect research results.7,1,17,18,27 For example, the nutritional composition of commercial laboratory rodent diets altered exocrine pancreatic function in rats;27 the investigators concluded that differences in the fatty acid composition of the diets affected pancreatic secretion and cholecystokinin release. In another report, changes in diet due to moving to a different research institute resulted in unexpected, significant variation in what were well-established results on uterine gene expression and reproductive functions.36 It took 3 y to confirm that differences in dietary phytoestrogen concentrations between the 2 diets—from the same manufacturer—fed at the different institutions were responsible for the variation.
Table 2.
How 3 diets achieve 12% protein content the same set of ingredients
| Diet 1 |
Diet 2 |
Diet 3 |
|||||
| Ingredient | % protein in ingredient | % ingredient in diet | % dietary protein due to ingredient | % ingredient in diet | % dietary protein due to ingredient | % ingredient in diet | % dietary protein due to ingredient |
| Corn | 8.9 | 15 | 1.34 | 12 | 1.07 | 14 | 1.25 |
| Oats | 11 | 12 | 1.32 | 12 | 1.32 | 12 | 1.32 |
| Fish meal | 61 | 9 | 5.49 | 11.5 | 7.02 | 7 | 4.27 |
| Wheat | 12.7 | 8 | 1.02 | 6 | 0.76 | 9 | 1.14 |
| Soybean meal | 48.5 | 6 | 2.91 | 4 | 1.94 | 8.5 | 4.12 |
| Total dietary protein | 12.08 | 12.11 | 12.10 | ||||
The same issue applies for other nutrients. The fat level can be constant but the fatty acid composition can vary, depending on the fat or protein sources. For example, although fish meal, a common protein source, contains approximately 8% to12% fat that is rich in omega-3 fatty acids, it is seldom considered a fat source in a diet formulation.19, 24 In the example shown in Table 2, the amounts of fish meal varied from 7% to 11.5% of the diet, thereby as much as doubling the omega-3 fatty acid concentration.24 Similarly, corn has high amounts of omega-6 fatty acids. The importance of a change in diet composition is not always known, but the potential to affect the animal—and thus the research—remains.
Other Considerations
Five other considerations merit discussion. First is the potential for significant nutrient and contaminant variation in laboratory animal diets due to seasonal variation in the ingredients and variation among batches of diet within season. These are concerns for both open- and closed-formula diets. If these problems could not be controlled, then producing open- and closed-, fixed-formula diets would be impossible. However, both seasonal as well as batch-to-batch nutrient variation can be minimized by rigorous quality control by the diet manufacturers.34
A unique concern for open-formula diets is that nutrient and contaminant concentrations in the ingredients from different geographic sources could be so vastly different that the nutrient or contaminant compositions for any open-formula diet could not be reproduced among different manufacturers without significant variation. However, diet manufacturers have produced open-formula diets for more than 30 y with only occasional minimal formulation modifications due to ingredient nutrient differences. If and when problems were discovered, they were quickly resolved, and any changes to the formulations of the NIH open formula diets are made available to the public.2 Therefore, with effective quality-assurance programs at the feed manufacturers, variation in nutrient and contaminant concentrations can be made insignificant. The quality-assurance program at the NIH ensures that all laboratory animal diets meet their nutrient and contaminant specifications.
Second is the ability to evaluate diet quality. Whether using open- or closed-formula diets, assessing the quality of the diet is important. The quality of ingredients, for instance, may vary for a number of reasons such as drought, flood, mold, disease contamination, or insect infestation. For this reason, testing for both nutrient composition and contaminants is important.21,22 Ideally, ingredients should be tested by the diet manufacturer prior to being accepted for use in formulating a diet. Purchasers of laboratory animal diet should request nutrient and contaminant data from manufacturers or develop institutional quality-assurance programs.
A third issue is the ability to obtain products on the competitive market. Soliciting contracts to provide open-formula diets by competitive bidding has been shown to result in a 30% savings to the NIH.23 However, unless quantitative ingredient formulations of diets are known, competitive bidding of equivalent diets is not possible, and closed-formula proprietary diets do not accommodate direct comparisons of diets. When closed-formula diets are used institutionally, it is common to contract the purchase as ‘sole source,’ where the proprietary vendor is the only provider. A sole-source contract is used when only one vendor can provide the product, such as a closed formula diet. This practice would complicate matters if the cost of a diet becomes noncompetitive, the manufacturer does not maintain expected production or storage standards, or diet quality deteriorates. Contracts for open-formula diets minimize these problems because the user defines the diet formulations, allowing solicitation through competitive bidding and facilitating a change in diet manufacturers, if warranted.
The fourth consideration is emergency planning to ensure that research can continue with minimal interruption during a disaster or some other event. If the vendor cannot fulfill its contract obligations, the use of open-formula diets would allow the buyer to purchase the same open-formula diets from another manufacturer without changing diets and possibly interfering with research.
A final consideration is the ability to maintain diet continuity should a researcher transfer his or her research to a different scientific institution. Open-formula diets are globally available and can be manufactured with little change in quality by diet manufacturers. This situation would serve to minimize research variability due to changes in diet between research institutions.
Conclusions
The proprietary nature of closed-formula diets may create less flexibility within a research program. These diets should be selected with the understanding that changes to diet formulation may occur without notice. Therefore, institutions are advised to closely monitor the specifications made available by the manufacturer.
Open-formula diets offer several advantages to the research community: (1) they meet the scientific requirements for standard diets as established by the American Institute of Nutrition and National Academy of Sciences; (2) their quantitative formulations are available to the user; (3) they facilitate the control of potential research variables; (4) any changes made to formulations are available to the user; (5) new formulas can be developed as needs change; (6) diets can be purchased from multiple vendors, prompting competitive and quality incentives; (7) they allow alternative vendors to make the product in the event of an emergency; and (8) researchers globally can evaluate results that are affected by diet on a comparative basis. These advantages strengthen the argument for the use of open-formula diets.
References
- 1.Ait-Yahia D, Madani S, Savelli JL, Prost J, Bouchenak M, Belleville J. 2003. Dietary fish protein lowers blood pressure and alters tissue polyunsaturated fatty acid composition in spontaneously hypertensive rats. Nutrition 19:342–346 [DOI] [PubMed] [Google Scholar]
- 2.American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies 1977. Report of the Committee. J Nutr 107: 1340–1348 [DOI] [PubMed] [Google Scholar]
- 3.Barnard D.2005. Personal communication.
- 4.Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. 2005. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed 18:143–162 [DOI] [PubMed] [Google Scholar]
- 5.Bouic PJD. 1999. Plant sterols and sterolins: a review of their immune-modulating properties. Altern Med Rev 4:170–177 [PubMed] [Google Scholar]
- 6.Brown NM, Setchell DR. 2001. Animal models impacted by phytoestrogens in commercial chow: Implications for pathways influenced by hormones. Lab Invest 81:735–747 [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain WJ, Voss KA, Norred WP. 1993. Analysis of commercial laboratory rat rations for fumonisin B1, a mycotoxin produced on corn by Fusarium moniliforme. Contemp Top Lab Anim Sci 33:26–28 [PubMed] [Google Scholar]
- 8.Clarke HE, Coates ME, Eva JK, Ford DJ, Milner CK, O'Donoghue PN, Scott PP, Ward RJ. 1977. Dietary standards for laboratory animals: report of the Laboratory Animals Centre Diets Advisory Committee. Lab Anim 11:1. [DOI] [PubMed] [Google Scholar]
- 9.Cole J.2006. Personal communication.
- 10.Conner MW, Newberne PM. 1984. Drug-nutrient interactions and their implications for safety evaluations. Fundam Appl Toxicol 4:S341–S356 [DOI] [PubMed] [Google Scholar]
- 11.Faith R, Hessler JR. 2006. Housing and environment, p 303–337. Suckow MA, Weisbroth SH, Franklin CL. The laboratory rat. San Diego (CA): Academic Press [Google Scholar]
- 12.Hartley DE, Edwards JE, Spiller CE, Alon N, Tucci S, Seth P, Forsling ML, File S. 2003. The soya isoflavone content of rat diet increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 167:46–53 [DOI] [PubMed] [Google Scholar]
- 13.Institute of Laboratory Animal Resources Committee on Laboratory Animal Diets 1978. Control of diets in laboratory animal experimentation. Washington (DC): National Academies of Sciences [Google Scholar]
- 14.Kaput J, Rodriguez RL. 2004. Nutritional genomics: the next frontier in the postgenomic era. Physiol Genomics 16:166–177 [DOI] [PubMed] [Google Scholar]
- 15.Knapka JJ. 1983. Nutrition, p 52–67. Foster HL, Small JD, Fox JG. The mouse in biomedical research, vol III: normative biology, immunology, and husbandry. New York (NY): Academic Press [Google Scholar]
- 16.Koppang N. 1974. Dimethylnitrosamine—formation in fish meal and toxic effects in pigs. Am J Pathol 74:95–108 [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis SM, Ullrey DE, Barnard DE, Knapka JJ. 2006Nutrition in the laboratory rat, p 219–301. Suckow MA, Weisbroth SH, Franklin CL. The laboratory rat. San Diego (CA): Academic Press [Google Scholar]
- 18.Marcus A. 2005. Muddled studies? Blame the chow. Scientist 19:12 [Google Scholar]
- 19.National Research Council 2001. Nutrient requirements of dairy cattle. Washington (DC): National Academies Press [Google Scholar]
- 20.Newberne PM. 1974. Mycotoxins: toxicity, carcinogenicity, and the influence of various nutritional conditions. Environ Health Perspect 9:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newberne PM, Fox JG. 1980. Nutritional adequacy and quality control of rodent diets. Lab Anim Sci 30:352–365 [PubMed] [Google Scholar]
- 22.Newberne PM, McConnell RG. 1980. Dietary nutrients and contaminants in laboratory animal experimentation. J Environ Pathol Toxicol 4:105–122 [PubMed] [Google Scholar]
- 23.Nichols C. 1990. Animal nutritionist cooks up healthy menu. Animal care matters. 01 Sep 1990. Bethesda (MD): NIH Office of Animal Care and Use [Google Scholar]
- 24.Omega Protein. [Internet]. Menhaden meal specifications. 2009. [Cited Oct 2009]. Available at http://www.omegaproteininc.com/products-natural-nautic.html.
- 25.Rao GN, Knapka JJ. 1987. Contaminant and nutrient concentrations of natural ingredient rat and mouse diet used in chemical toxicology studies. Fundam Appl Toxicol 9:329–338 [DOI] [PubMed] [Google Scholar]
- 26.Reeves PG, Nielsen FH, Fahey GC. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN76A Rodent Diet. J Nutr 123:1939–1951 [DOI] [PubMed] [Google Scholar]
- 27.Sabbatini ME, Pellegrino N, Rios M, Bianciotti LG, Vatta MS. 2006. Variation in exocrine pancreatic secretion in rats due to different commercial diets. Lab Anim (NY) 35:41–49 [DOI] [PubMed] [Google Scholar]
- 28.Schmelz EM, Wang MD, Merrill AH. 2006. Genomics, proteomics, metabolomics, and systems biology approaches to nutrition, p 3–19. Bowman BA, Russell RM. Present knowledge in nutrition. Washington (DC): ILSI [Google Scholar]
- 29.Taylor J. 2005. NIH Office of Animal Care and Use. Personal communication
- 30.Thigpen JE, Haseman JK, Saunders HE, Setchell KDR, Grant MG, Forsythe DB. 2003. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med 53:607–615 [PubMed] [Google Scholar]
- 31.Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. 1999. Phytoestrogen content of purified, open-, and closed-formula laboratory animal diets. Lab Anim Sci 49:530–536 [PubMed] [Google Scholar]
- 32.Thigpen JE, Setchell KRD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB. 2007. Variations in the phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD1 mice and F344 rats but not in CD Sprague–Dawley rats. Environ Health Perspect 115:1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thigpen JE, Setchell KDR, Saunders HE, Haseman JK, Grant MG, Forsythe DB. 2004. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR J 45:401–416 [DOI] [PubMed] [Google Scholar]
- 34.Tobin G, Stevens KA, Russell RJ. 2007. Nutrition, p 321–383. Fox JG, Newcomer CE, Smith AL, Barthold SW, Quimby FW, Davisson MT. The mouse in biomedical research, vol 3. San Diego (CA): Elsevier [Google Scholar]
- 35.Waldemarson AH, Hedenqvist P, Salomonsson AC, Haggblom P. 2005. Mycotoxins in laboratory rodent feed. Lab Anim 39:230–235 [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. 2005. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA 102:9960–9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss B, Stern S, Cernichiari E, Gelein R. 2005. Methylmercury contamination of laboratory animal diets. Environ Health Perspect 113:1120–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]