Abstract
The purpose of the present study was to describe the technique for and findings of ultrasonographic examination of the rat uterus for diagnosis of early and midterm pregnancy. The uterus of anesthetized Wistar rats was examined between days 9 and 16 post coitum by transabdominal real-time ultrasonography by using a 12-MHz linear transducer. Pulsed-waved color Doppler sonography was used to measure the embryonic heart rate. The embryonic vesicles were detected with 25% false-negative diagnosis on day 9, 8% on day 10, and 0% thereafter. By day 12, the embryos were detected with measurable crown-rump length and heart rate. Ultrasonographic evidence of pregnancy in the rat was present by day 9 post coitum. Diagnosis of pregnancy was confirmed by detection of the embryo heart beat by day 12. Embryo characteristics were ultrasonographically measurable between days 9 and 16.
Abbreviation: bpm, beats per minute; CRL, crown–rump length; pc, post coitum
The rat is used in a wide range of fertility and reproductive toxicology studies that optimally would require early diagnosis of pregnancy as well as detailed description of fetal characteristics. Diagnostic approaches currently used for the detection of pregnancy do not fulfill these requirements, especially with regard to the early and midterm gestational period. Transdermal palpation of the uterus is used after the 10th to 12th day post coitum (pc), with variable success.3 In practice, pregnancy in rats is indicated by abdominal enlargement (day 13), mammary development (day 14), nipple enlargement (day 14),3,4 and increased body weight and food consumption and is confirmed by birth.
Ultrasonographic examination of the uterus is a reliable noninvasive method for pregnancy diagnosis in domestic animals. In the absence of a thermal effect, ultrasonography represents no measurable risk for the fetus when used at recommended intensity levels.9 The method has not been well described or applied in small laboratory animal species, such as rats, especially during early stages of pregnancy, due to the small size of the embryonic structures and the high cost of high-frequency (20 to 55 MHz) transducers that may be required to obtain high-definition images. A 1985 study using a 5-MHz linear-array transducer detected the gravid rat uterus from day 9 with 83% accuracy, the fetus from day 14 and the embryo beating heart from day 19 without, though, providing detailed description of their findings or any embryometric measurements.8 In recent studies, rat models of pregnancy pathology, such as preeclampsia or fetal hydrocephalus, involved ultrasonography-based embryometry performed mainly at advanced stages of pregnancy2,10,15.
The aim of the present project was to describe the technique and findings of transabdominal ultrasonography of the uterus for the detection of pregnancy in rats during early and midterm gestation. For this reason, we used a commercially available 12-MHz linear transducer, which is commonly used in human medicine.
Materials and Methods
Animals.
Forty multiparous female Wistar rats (Rattus norvegicus; age, 6 to 8 mo; weight, 300 to 350 g) from our laboratory's inhouse breeding colony were used. They were the 10th inbred generation of Wistar rats obtained from the conventional breeding facility of the Democritos National Center of Physics Research (Athens, Greece). Colony health status was monitored semiannually, and rats were found to be free of Mycoplasma spp., adventitious viruses, respiratory and enteric bacteria, and ecto- and endoparasites. The rats were housed in polycarbonate cages, 3 rats per cage, at 20 to 22 °C, on a 14:10-h light:dark cycle, and were given commercial pelleted diet (4RF25, Mucedola, Milan, Italy) and tap water ad libitum. The facilities were in accordance with Directive 86/609/EEC, which refers to “the protection of animals used for experimental and other scientific purposes.”5
Experimental design.
Female rats were mated with male rats in a 1:1 mating scheme. Female rats underwent general anesthesia and transabdominal ultrasonographic examination of their uterus before mating and on days 8 through 16 pc (the day of copulation, as confirmed by detection of sperm in vaginal smears, was defined as day 0). Half of the female rats was examined on days 8, 10, 12, 14, and 16, whereas the rest were evaluated on days 9, 11, 13, and 15, to reduce potential adverse effects conferred by repeated anesthesia and prolonged exposure to ultrasound. Evidence of pregnancy was described on the basis of the dimensions, shape, and echogenicity of the embryonic vesicles; presence and rate of the embryonic heart beat; and the embryonic crown–rump length (CRL). The longitudinal and transverse diameters of the embryonic vesicles were measured when the largest surface area appeared on the screen. Pulsed-waved color Doppler sonography was used to detect the embryonic heart beat. The embryonic heart rate was calculated in Doppler waveforms and saved for later offline analysis (CorelDraw 14.0, Corel, Ottawa, Canada). The number of cardiac cycles per second was multiplied by 60 to obtain the heart rate expressed as beats per minute (bpm). The number of cardiac cycles per second was calculated by dividing the length corresponding to a 1-s period along the baseline of the blood velocity waveform by the length of the distance between 2 consecutive A waves on the inflow velocity waveform. The CRL was determined as the maximal distance between the crown and tail base with the embryo at sagittal plane. Because it was in compliance with Directive 86/609/EEC, the experimental protocol was approved by the Animal Care and Use Committee of the local veterinary service.5
Mating.
Each female rat was caged with a single male rat of verified fertility (history of fertile mating) until successful copulation. Detection of sperm in vaginal smears collected between 0900 and 1100 the following morning was considered evidence of mating. Smears were examined at a magnification of ×100 (KF2, Carl Zeiss, Hamburg, Germany).
Ultrasonographic examination.
Examination of the uterus was performed transabdominally by using a real-time ultrasonographic machine (HDI 1500, Philips, Best, The Netherlands) equipped with a 5- to 12-MHz linear transducer. The pulse–echo frequency used was 12 MHz at a spatial peak–temporal peak intensity level of 230 W/cm2 for a maximal period of 5 min per examination between days 9 and 16 pc. B-mode axial resolution was 0.3 mm and lateral 1.0 mm. The temporal frame rate was set at 32 Hz. In Doppler mode, the high-pass filter was set at 20 Hz, and the pulsed repetition frequency was set between 4 and 48 kHz in order to detect low to high blood-flow velocities. The spatial peak temporal average was 24 mW/cm2 for a maximal period of 2 min for each litter between days 12 and 16 pc. The sampling gate used was 1.0 mm, and the maximal sweep speed was 150 mm/s.
The rats were placed in dorsal recumbency under general anesthesia (2% sevoflurane in oxygen). The abdominal area was clipped, and ultrasound scanning gel was applied to the tip of the probe. During examination, rectal temperature and heart rate were monitored (Vet Ox 4700 Plus, SDI, Kansas City, MO). Body temperature remained between 36 and 37 °C and the heart rate between 310 and 340 bpm. Scanning progressed from right to left, with the probe oriented perpendicular to the abdominal wall in a sagittal plane, starting at the right inguinal region and rotated, if needed, to the axial plane at the level of the caudal abdomen. Upon detection of evidence of pregnancy, the examination proceeded cranially along the course of the uterine horn.
Sensitivity of the method.
The sensitivity of the method was assessed for each day of examination on the basis of the percentage of animals with false-negative diagnosis of pregnancy. Diagnosis was confirmed by ultrasonographic detection of the embryonic heart beat on successive examinations, as defined earlier, and confirmed by reviewing parturition records.
Results
All pregnant rats gave birth between days 21 and 22 pc. Litter size ranged from 6 to 9 pups.
Sensitivity of the method.
A false-negative diagnosis was made in 25% of animals on day 9 (5 of 20 animals), 8% on day 10 (2 of 25 animals), and 0% thereafter.
Ultrasonographic findings.
The normal uterus was not ultrasonographically detectable in nonpregnant rats (data not shown).
There was no ultrasonographic evidence of pregnancy on day 8 pc.
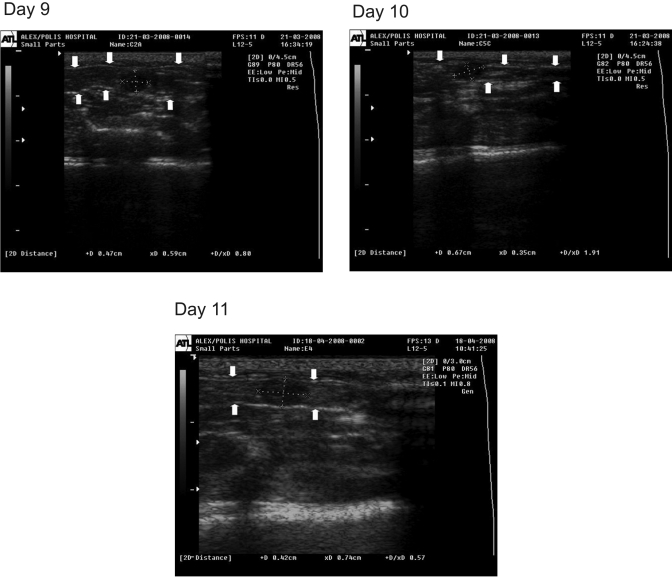
On days 9 through 11, conceptuses were detected as fluid-filled embryonic vesicles, recognizable as round or oval structures of intermediate echogenicity in the lumen of the uterine horn. The ventral and dorsal borders of the uterine wall were depicted at longitudinal section as 2 parallel thin hyperechoic lines adjoining the embryonic vesicles (Figure 1). The dimensions of the embryonic vesicles (longitudinal, transverse, and mean diameters) of the embryonic vesicles from day 9 to day 11 are presented in Table 1.
Figure 1.
Ultrasonographic image of rat uterine horn at longitudinal section on days 9, 10, and 11 of pregnancy. The conceptus is depicted as a hypoechoic round or oval structure (dotted lines indicate the longitudinal and transverse diameters). The ventral and dorsal borders of the uterine wall are seen as hyperechoic lines (arrows).
Table 1.
Dimensions of embryonic vesicles, crown–rump length, and heart rate (mean ± 1 SD) of conceptuses assessed ultrasonographically in pregnant rats
| Day of pregnancy | Embryonic vesicle diameter (cm) |
Crown–rump length (cm) | Heart rate (beats per minute) | No. of rats evaluated | |||
| Longitudinal | Transverse | Average | No. of embryos | ||||
| 9 | 0.37 ± 0.07 | 0.52 ± 0.08 | 0.45 ± 0.05 | not done | not done | 20 | 45 |
| 10 | 0.44 ± 0.07 | 0.56 ± 0.09 | 0.51 ± 0.06 | not done | not done | 25 | 50 |
| 11 | 0.51 ± 0.10 | 0.71 ± 0.06 | 0.61 ± 0.06 | not done | not done | 20 | 45 |
| 12 | 0.55 ± 0.05 | 0.79 ± 0.04 | 0.67 ± 0.02 | 0.43 ± 0.08 | 170.0 ± 9.7 | 20 | 45 |
| 13 | 0.58 ± 0.08 | 0.83 ± 0.12 | 0.71 ± 0.06 | 0.45 ± 0.09 | 169.8 ± 15.8 | 20 | 45 |
| 14 | 0.69 ± 0.08 | 0.95 ± 0.08 | 0.82 ± 0.06 | 0.50 ± 0.09 | 193.6 ± 12.5 | 20 | 45 |
| 15 | 0.78 ± 0.14 | 1.24 ± 0.12 | 0.98 ± 0.14 | 0.82 ± 0.13 | 214.7 ± 20.3 | 20 | 45 |
| 16 | 0.87 ± 0.06 | 1.55 ± 0.15 | 1.21 ± 0.08 | 1.11 ± 0.11 | 223.6 ± 27.6 | 20 | 45 |
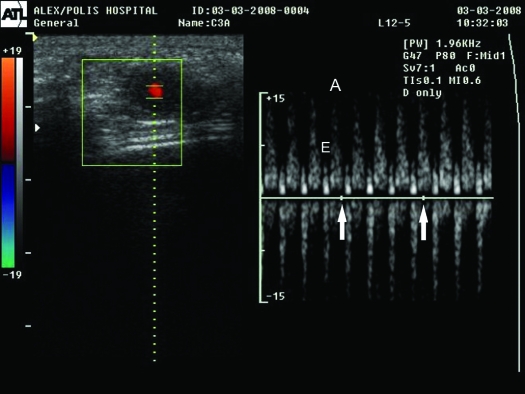
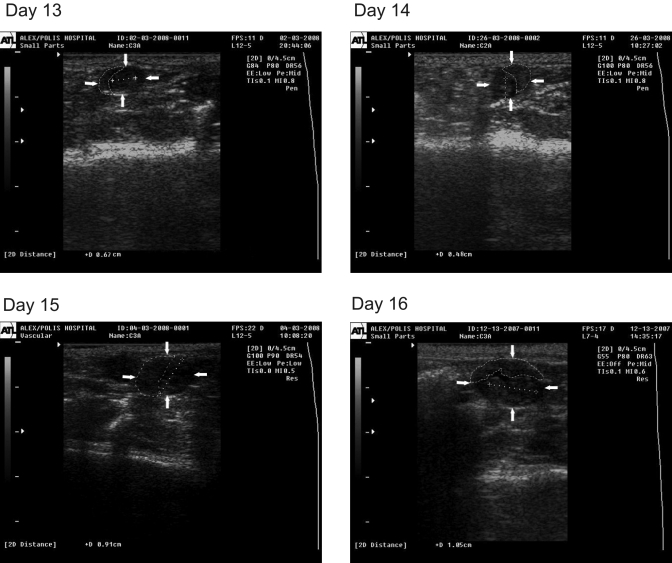
By day 12, embryos were detectable within oval-shaped embryonic vesicles, with measurable heart rate (Table 1, Figure 2) and CRL (Table 1, Figure 3). Both parameters were increased with gestational age. The placenta was identified by day 13 as a hyperechoic structure of ellipsoidal shape (Figure 3).
Figure 2.
Ultrasonographic image of rat pregnancy on day 12. The heart rate is measured by using pulsed-waved color Doppler sonography. The heart is depicted in red. The inflow pattern is seen as the deflection above the baseline with the smaller E-wave (E) and the larger A-wave (A) components. The outflow pattern is seen as the deflection below the baseline. The number of A waves on the inflow pattern per 1-s period (distance between 2 arrows on the baseline) is multiplied by 60 to obtain the heart rate.
Figure 3.
Ultrasonographic image of rat pregnancy on days 13, 14, 15, and 16. The embryo is identified as an hyperechoic structure within the embryonic vesicle (arrows). The dotted line indicates CRL; the dashed line indicates the placenta.
Differential diagnosis.
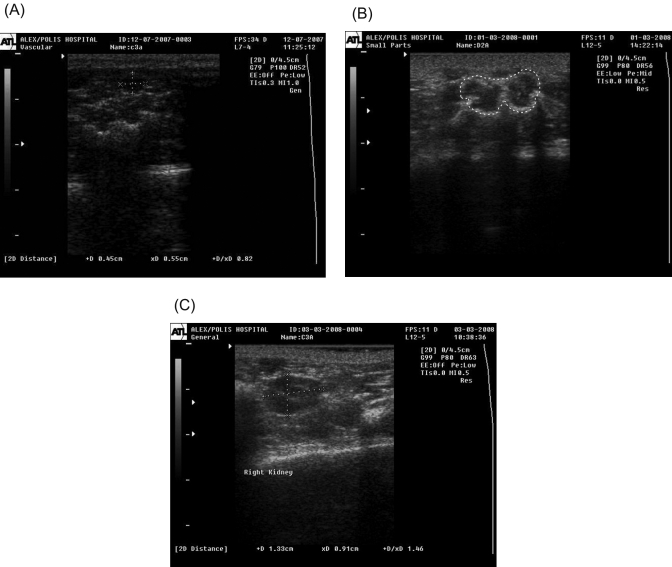
Embryonic vesicles were differentiated from other round or oval structures (fecal stools, psoas major muscles, kidneys) of similar echogenicity that were adjacent to the uterus.
Early (day 9 or 10) embryonic vesicles were differentiated from fecal stools detected at the lateral abdominal area. Fecal stools were identified as round or oval structures whose shape did not change on pressure of the scanning probe on the abdominal wall. In contrast, pressure from the probe altered the shape of the embryonic vesicles. The echogenicity of fecal stools ranged from intermediate to high, in contrast to the lower echogenicity of the fluid-filled embryonic vesicles (Figure 4 A).
Figure 4.
Differential diagnosis of ultrasonographically detected rat pregnancy. (A) Ultrasonographic image of rat fecal stool. Dotted lines demarcate the longitudinal and transverse diameters of the fecal stools. (B) Ultrasonographic image of psoas major muscles at transverse section (demarcated by dotted line) in the rat. (C) Ultrasonographic image of rat kidney. Dotted lines demarcate its longitudinal and transverse diameters.
Two adjacent embryonic vesicles with dimensions corresponding to a gestational age of 10 to 14 d could be differentiated from the ultrasonographic image of the psoas major muscles in transverse section. These muscles lay along the midline of the caudal abdomen and appeared as structures with an irregularly oval (butterfly-like) shape in transverse section and intermediate echogenicity with a hyperechoic circumference (Figure 4 B). From days 12 to 14, an exclusion criterion for pregnancy was the absence of embryonic structures.
By approximately day 15, embryonic vesicles could be differentiated from kidneys, which were located in the lateral midabdomen as oval structures at longitudinal section with a clearly distinguishable outer hypoechoic cortical region, hyperechoic medullary region, and central hypoechoic collecting system (Figure 4 C). The pulse of the renal artery was measurable, and its rate was equal to the mother's heart rate (310 to 340 bpm).
Because of the proximity of the embryonic vesicles to one another, their large number, and the curvature of the uterine horns as pregnancy developed, individual embryos might not have been counted or counted twice. Therefore, an accurate estimation of the number of fetuses, as confirmed by litter size at birth, was not possible.
Discussion
This study describes the technique for and findings of transabdominal ultrasonographic examination of the uterus in anesthetized pregnant rats between days 9 and 16 of gestation. Ultrasonographic evidence of pregnancy was present by day 9, and confirmation of the diagnosis was possible on day 12 by detection of the embryo heart beat.
Our methodologic approach included determination of the day of copulation (defined as day 0). During the 4- to 5-d estrous cycle of rats under a 14:10-h light:dark photoperiod with lights-off at 1900, rats ovulate at 2400 to 0200 during the night of estrus and copulate several times from the proestrus evening to the estrus morning.11 It is advantageous to determine the day of copulation because the examiner will seek evidence of pregnancy in a short time window. Conversely, in the absence of detailed mating records, the age of pregnancy may be assessed by an experienced examiner, based on ultrasonographic image dimensions.
Apart from induction of a light plane of anesthesia and clipping of the abdominal area, no specific preparation of the animals was required for ultrasonographic examination. Keeping the urinary bladder full, which facilitates ultrasonographic examination of the reproductive tract in other species, is not possible in the rat since handling of a conscious rat usually induces urination.17
A common, commercially available linear transducer of 12 MHz frequency was used for the ultrasound examination. The resolving power of the present transducer allowed reliable identification of embryonic vesicles and embryonic structures of not less than 4 mm in length. According to data from embryologic studies,7 rat blastocysts descend to the uterine lumen on day 4 pc. The blastocysts are then distributed to their sites of implantation which takes place by the end of day 5. During day 6, the blastocysts increase in size and elongate. According to our findings, amniotic fluid-filled embryonic vesicles were not ultrasonographically detectable until day 9, when the amniotic cavity starts to form.7
On day 11, the embryo CRL is approximately 2 mm. On day 12, the body of the embryo is extremely curved, and the CRL is approximately 4.5 mm.7 By that time, the embryo was ultrasonographically detectable with a similar CRL. On day 13, the embryo is still of spiral shape, while it begins to straighten by day 14. The CRLs on days 14, 15, and 16 are 8.5, 11.0 and 13.5 mm, respectively.7 However, CRLs obtained by our ultrasonographic measurements were lower than the reported embryologic data. This difference might be attributable to curvature of the embryo body during these days. Another possible explanation is variation of embryo size depending on the number of fetuses gestated.
The placenta was ultrasonographically identifiable by day 13. The rat placenta attains full size on day 16.7
Pregnancy was confirmed by detection of the embryonic heart beat by day 12. The use of Doppler sonography allowed the accurate measurement of heart rate, which gradually increased from day 12 to day 16. According to embryologic data, the first contractions of the heart can be observed at about the middle of day 9, whereas circulation starts on day 10.7 Conventional ultrasound systems at frequencies between 7.5 and 15 MHz have been used to measure the embryonic heart rate in mice as well,6,14,18 in which the heart beat was detected as early as day 10 of gestation.6 However, modern obstetric ultrasound devices operating at high frequencies (20 to 55 MHz), known as ultrasound biomicroscopes, can be used to obtain high-resolution fetal images practically from implantation to term.19
Despite the evolution in ultrasound technology, which has substantially upgraded the quality of fetal images and allowed considerably earlier diagnosis of physiologic and pathologic phenomena during pregnancy, there has been an extensive debate regarding the potential side effects on the forming embryo. Exposure of the pregnant uterus to ultrasound may lead to temperature elevation, depending on factors such as the intensity of the scan, ultrasound frequency, dwell time along the beam axis, width of the beam, tissue properties, length of the scan, and stage of pregnancy.13 Hyperthermia has been shown to be teratogenic in all animal species tested, including the rat, and is a suspected teratogen for humans.16 According to the Bioeffects Committee of the American Institute of Ultrasound Medicine (AIUM), fetal exposure from an in-situ temperature rise to greater than or equal to 41 °C is considered hazardous.1 The committee recommended that diagnostic exposures should not result in a temperature rise of greater than 1 °C above normal physiologic levels. According to the vast majority of animal studies, ultrasound exposure at clinically relevant diagnostic levels does not result in adverse maternal pregnancy outcome, significant morphologic alterations in term fetuses, or perinatal or postnatal adult neurophysiologic abnormalities. Therefore, provided that the causative factors related to hyperthermia are controlled, the procedure is safe.9 In the present study, to minimize the time of exposure of fetuses to ultrasound, pregnant rats were examined on alternating days, and only 2 to 3 embryos per litter were scanned per examination. In addition, body temperature was monitored, and no elevation beyond 1 °C above baseline temperature was recorded.
Recommendations and safety limits regarding the use of ultrasound during pregnancy have been based on studies using conventional devices that produce low acoustic outputs, whereas modern diagnostic ultrasound devices make use of substantially increased acoustic outputs. Therefore, concern is rising regarding the safety of these high-frequency ultrasound devices, and further evaluation of the potential risks to the fetus is required.12
According to the findings of the present study, real-time ultrasonography assisted by pulsed-waved color Doppler sonography provided a reliable noninvasive method for the detection of early and midterm pregnancy in the rat, as well as for the measurement of embryo characteristics, including embryonic vesicle dimensions, heart rate, and CRL. Ultrasonograph-based diagnosis of pregnancy may improve the management of reproduction in rat colonies by minimizing the period between successive matings when infertile copulations have taken place, thus providing labor-, time-, space-, and cost-saving benefits. This advantage is particularly important for ‘valuable’ rat colonies, such as those of transgenic animals. Furthermore, the method may be useful in reproductive toxicology and embryo development studies that require the earliest possible diagnosis of pregnancy or monitoring of embryo development in utero.
The cost of the ultrasonographic device is a limitation of the method presented. However, this device can be portable and shared among collaborating laboratories. Ultrasonographic images of higher definition that would increase the sensitivity and accuracy of the method might require using a probe of higher frequency. Finally, rats were kept under light plane of general anesthesia to provide restraint for examination. This practice constituted an additional potentially stressful manipulation for the pregnant animal and a potential hazard for the embryos. The design of a special restraint device that would permit free access to the abdominal area in conscious rats might overcome this limitation.
In conclusion, ultrasonographic examination of the uterus provided a reliable noninvasive method for pregnancy diagnosis in the rat. Evidence of pregnancy was present by day 9 pc, and confirmation of diagnosis was possible by detection of the embryonic heart beat by day 12. Ultrasonographically based embryometry was performed from day 9 to 16.
References
- 1.American Institute of Ultrasound in Medicine 1987. Safety considerations for diagnostic ultrasound. Newsletter of the American Institute of Ultrasound in Medicine Bioeffects Committee Laurel (MD): American Institute of Ultrasound in Medicine [Google Scholar]
- 2.Babapour B, Oi S, Boozari B, Tatagiba M, Bleck J, Hussein S, Samii M. 2005. Fetal hydrocephalus, intrauterine diagnosis, and therapy considerations: an experimental rat model. Childs Nerv Syst 21:365–371 [DOI] [PubMed] [Google Scholar]
- 3.Bennet JP, Vickery BH. 1970. Rats and mice. : Hafez ESE. Reproduction and breeding techniques for laboratory animals, p299–315 Philadelphia (PA): Lea and Febiger [Google Scholar]
- 4.Bivin WS. The rat. 1986. : Morrow DA. Current therapy in theriogenology 2. Diagnosis, treatment, and prevention of reproductive diseases in small and large animals, p1015–1021 London (UK): WB Saunders [Google Scholar]
- 5.European Union 1986. Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Official Journal L 358:1–28 [Google Scholar]
- 6.Gui YH, Linask KK, Khowsathit P, Huhta JC. 1996. Doppler echocardiography of normal and abnormal embryonic mouse heart. Pediatr Res 40:633–642 [DOI] [PubMed] [Google Scholar]
- 7.Hebel R, Stromberg MW. 1986. Anatomy and embryology of the laboratory rat. Worthsee (Germany): BioMed Verlag [Google Scholar]
- 8.Inaba T, Inoue A. 1985. Use of echography in rats for pregnancy diagnosis. Nippon Juigaku Zasshi 47:523–525 [DOI] [PubMed] [Google Scholar]
- 9.Jensh RP, Brent RL. 1999. Intrauterine effects of ultrasound: animal studies. Teratology 59:240–251 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Lemke RP, Greer JJ. 2001. Ultrasound measurements of fetal breathing movements in the rat. J Appl Physiol 91:316–320 [DOI] [PubMed] [Google Scholar]
- 11.Maeda K, Ohkura S, Tsukamura H. 2000. Physiology and reproduction, p 145–176. In: Krinke GJ. The laboratory rat London (UK): Academic Press [Google Scholar]
- 12.Miller DL. 2008. Safety assurance in obstetrical ultrasound. Semin Ultrasound CT MR 29:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MW, Nyborg WL, Dewey WC, Edwards MJ, Abramowicz JS, Brayman AA. 2002. Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: implications of new standards on tissue heating. Int J Hyperthermia 18:361–384 [DOI] [PubMed] [Google Scholar]
- 14.Spurney CF, Leatherbury L, Lo CW. 2004. High-frequency ultrasound database profiling growth, development, and cardiovascular function in C57BL/6J mouse fetuses. J Am Soc Echocardiogr 17:893–900 [DOI] [PubMed] [Google Scholar]
- 15.Verlohren S, Niehoff M, Hering L, Geusens N, Herse F, Tintu AN, Plagemann A, LeNoble F, Pijnenborg R, Muller DN, Luft FC, Dudenhausen JW, Gollasch M, Dechend R. 2008. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension 51:547–553 [DOI] [PubMed] [Google Scholar]
- 16.Warkany J. 1986. Teratogen update: hyperthermia. Teratology 33:365–371 [DOI] [PubMed] [Google Scholar]
- 17.Weiss J, Taylor GR, Zimmermann F, Nebendahl K. 2000. Collection of body fluids, p 485–510. In: Krinke GJ. The laboratory rat London (UK): Academic Press [Google Scholar]
- 18.Yu Q, Leatherbury L, Tian X, Lo CW. 2008. Cardiovascular assessment of fetal mice by in utero echocardiography. Ultrasound Med Biol 34:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou YQ, Foster FS, Qu DW, Zhang M, Harasiewicz KA, Adamson SL. 2002. Applications for multifrequency ultrasound biomicroscopy in mice from implantation to adulthood. Physiol Genomics 10:113–126 [DOI] [PubMed] [Google Scholar]