Abstract
The frequency at which mouse cages are changed has important implications for the animals, animal care personnel, and facility managers. The objective of this study was to determine how bedding volume and the interval between changes affect microenvironmental conditions, health, and behavior of mice housed in individually ventilated cages (IVC). A total of 15 cages (n = 5 cages per bedding volume) housing ICR female mice (n = 5 animals per cage) were monitored for 17 d. Parameters monitored included clinical evaluation of each animal, appearance of the cage, fecal corticosterone levels, bedding weight, and mouse mass. Atmospheric analysis was performed daily to determine intracage ammonia cage humidity and temperature on a daily basis. Mice were videotaped for 10 min on days 1, 8, and 15, and videos were analyzed for abnormal behaviors. On day 17, 1 mouse from each cage was euthanized, and bronchoalveolar lavage was performed. Statistical differences in parameters were most often noted between low- and high-volume bedding groups. Correlation between visual appearance and actual intracage environmental conditions and mouse health and behavior at specific time points indicated cages that appear dirty to most observers did not have measurably adverse effects on the animals for any of the many parameters evaluated in this study. This study demonstrated that a 2-wk interval between cage changes for ICR female mice housed in IVC caging (with approximately 90 air changes per hour) and aspen chip bedding did not significantly affect measures of animal well-being in this study. This lack of effect occurred despite the appearance of excessive soiling by the 2-wk time point.
Abbreviation: ACPH, air changes per hour; BAL, bronchoalveolar lavage; HVBG, high-volume bedding group; IVC, individually ventilated cage; LVBG, low-volume bedding group; MVBG, medium-volume bedding group; PID, photoionization detector
Cage changes, although an essential element for appropriate laboratory mouse husbandry, can be disruptive to mice. This required maintenance activity disrupts their scent cues and arrangements of nesting materials while placing them into a ‘new’ environment at an interval typically defined at the discretion of the facility management to meet general guidelines. The frequency of cage changes varies among facilities, and this variation may adversely affect scientific studies. Multiple studies have been initiated to investigate parameters that inform a scientifically based approach to rodent cage change interval,4,7,8,10,11, 12,27,35,42,46 leading to reports documenting that the type of caging, type and volume of bedding, air changes, strain, and sex all can influence microenvironmental parameters. In addition, cage changes may affect the behavior and health of mice, indices that have not typically been measured in association with the microenvironment.
The laboratory mouse is the most genetically characterized and most common research animal, with an estimated population of 30 million.13,25 In recent years, animal facilities have been under growing public and governmental regulatory pressure to increase provisions for animal welfare. Many of the standards in mouse housing that are currently in place were based solely on common industry practices at the time of inception.
Rodents are exposed to many physiologic stressors during the time of cage changing in laboratory facilities.3 The intent of changing cages is to keep mice and humans healthy, provide a suitable microenvironment, and ideally reduce stress (which is often done by providing what humans perceive as a clean environment).35 However, because of the scent-driven nature of mice and the fact their pheromone laden-cages are disrupted at the time of bedding replacement, cage changing could be considered to be stressful for the cage occupants.3,11,47 Animal users and caretakers therefore must weigh the stress induced by frequent bedding changes for ‘dirty cages’ against the realization that the perceptions of humans and mice may differ regarding what constitutes a dirty cage.5,25
The vivarium management that oversees husbandry practices and cost containment of laboratory mice also are affected by the cage-changing practices.7,46 Exposure of laboratory animal personnel to laboratory animal allergens, infectious agents, and rodent pathogens increases during the cage-changing procedure. Therefore, in many instances, cage changes should be minimized to an interval that has no scientifically proven negative implications for the welfare of mice in order to help human occupational health.
The Guide for the Care and Use of Laboratory Animals states, “The frequency is a matter of professional judgment of animal care personnel based on consultation with the investigator.”21 The factors to consider for cage-changing intervals should be number and size of animals, size of the primary enclosure, urinary and fecal output, wetness of bedding, and experimental conditions.21 Many facilities have implemented cage-change intervals of 3 to 7 d with the aim of keeping these values within the recommended limits. However, frequent cage changing in rodents has been reported to cause negative behavior (decreased resting), including increases in aggression and decreases in ease of handling (consisting of vocalizing, biting, and struggling) while also having a negative effect on body mass in mice.4,7,8 The Guide has not been updated since 1996, thereby making timely and relevant input from science all the more necessary.
Recently, AALAS released a performance-based criterion document 1 stating, “Proper husbandry practices and housing should be the safeguard for the wellbeing of the mice but also a prerequisite for sound scientific methodology. Inadequacies can skew scientific findings and render particular research useless, while good husbandry minimizes variations that can modify an animal's response to experimentation.”
The husbandry and housing of mice should be controlled to remove variables in scientific studies. In an age of mouse genetic standardization and specificity in other husbandry practices (including light cycles, feed, and water quality), cage changing potentially can affect many aspects of a study. Consistent husbandry may contribute to animal welfare goals by potentially reducing the number of animals.22 Housing systems are an important element in the wellbeing of laboratory animals, and, consequently, influence the outcomes of animal experiments.
Although aspects of animal microenvironmental parameters have been evaluated during variable cage-changing frequencies (Figure 1), systematic simultaneous examination of microenvironment, health, and welfare relative to bedding volume and cage change frequencies has not been defined by past work. The studies described here were designed to address this void and provide a scientific basis for development of bedding volume and cage change guidelines for mice housed in individually ventilated caging (IVC).
Figure 1.
Summary of literature regarding cage-change frequency for rodents.
Environmental parameters analyzed in this study consisted of daily intracage ammonia levels, temperature, and humidity. Indices that were assessed in mice at specific time points throughout the study included fecal corticosterone, body mass, cage biomass, behavioral analysis, and bronchoalveolar lavage (BAL). Cages were photographed at specific time points to correlate visual appearance with other measured indices, because we have noted that appearance of bedding is often the primary human motivator for cage changing frequency.
Materials and Methods
Animals.
Female ICR mice (n = 80; age, 9 wk) were obtained from a commercial vendor (Harlan, Madison, WI) and housed in a semibarrier animal room. According to results of health surveillance programs performed by the vendor and research institution, the mice were free from infections with mouse hepatitis virus, Mycoplasma pulmonis, cilia-associated respiratory bacillus, parvovirus, minute virus of mice, pneumonia virus of mice, epizootic diarrhea of infant mice, adenovirus, ectromelia virus, rotavirus, lymphocytic choriomeningitis virus, cytomegalovirus, polyoma virus, Sendai virus, and Helicobacter spp. The cages contained autoclaved aspen bedding chips (Harlan Teklad) at the 3 different volumes described later, pelletted food (Harlan Teklad 8640), and ad libitum access to water through water bottles.
All research adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals.21 The protocol was approved by the Colorado State University Institutional Animal Care and Use Committee and was performed in an AAALAC-accredited facility.
Experimental design.
Each cage contained 5 mice, cages were grouped according to bedding volume, and all the cages were located within the same rack (Table 1). A total of 17 cages were used for the study: 2 controls and 5 cages at each of the 3 bedding volumes: low-bedding volume group (LVBG), 250 mL of bedding; medium-volume bedding group (MVBG), 400 mL bedding; high-volume bedding group (HVBG), 550 mL bedding (Table 1). The bedding was changed, and environmental, health, and behavioral parameters were measured for 17 d. Internal control cages (n = 2) each contained a medium volume of bedding. One cage had 5 mice and was maintained on the 7-d cage-change cycle during the experiment, whereas the other was on the experimental 17-d cage-changing cycle but housed no mice. Cage location on the rack was chosen at random, and 17 of the 56 cage locations on 1 side of the rack were occupied.
Table 1.
Categories, volumes of bedding, mice, and cages per group used during the study. * One MVBG control cage housed no mice.
| Bedding category |
|||
| Low (LVBG) | Medium (MVBG) | High (HVBG) | |
| Bedding mass | 40 ± 0.10 g | 70 ± 0.10 g | 100 ± 0.10 g |
| Bedding volume | 250 mL | 400 mL | 550 mL |
| No. of cages | 5 | 7 | 5 |
| No. of ICR mice | 25 | 30 | 25 |
| No. of mice per cage | 5 | 5* | 5 |
Throughout the study, mice were housed in commercially available caging [19.56 cm × 30.92 cm × 14.93 cm (7.70 in. × 12.17 in. × 5.88 in.); #9 cages, Thoren Caging, Hazelton, PA]. In addition, the caging manufacturer (Thoren Caging) provided a #9 cage airflow measurement setup for our trial. The measuring system consisted of a #1 mouse cage with a precision hot-wire anemometer (model 4045, TSI, Shoreview MN) that was calibrated before shipment. The flowmeter was connected such that it measured the airflow across the partition built into the testing cage. The blowers on the rack were unplugged initially, and the magnehelic gauges were zeroed prior to calibration. The rack then was turned on, the pressure of the air supply set to 0.30 in. water, and the exhaust air pressure set to 0.25 in. water. The testing cage was placed randomly in 40 of the 112 positions in the rack. The reading obtained from the testing cage was reported in L/min and converted to air changes per hour (ACPH) by using the following equation, which was provided by the manufacturer and accounts for the volume of the #9 cage used in our study (6.4 L).
To obtain cage microenvironmental samples, 17 cages were modified so that the air inside cages could be accessed while they were housed in the rack, to minimize disturbance to the mice. This modification consisted of drilling a hole 1.9 cm in diameter 4 cm from the floor in the front middle portion of each cage. This location was chosen to allow sampling cage air at the level of the mouse's nose and therefore represented a sample that would be respired during normal mouse activity. A male adapter (Swagelok, Solon, OH) and a connector, which had a screw cap to prevent cage air from escaping, were installed in the hole to create a sampling port. The cage rack was housed under positive pressure ventilation.
Ammonia.
Ammonia concentration was measured during the study by using a photoionization detector (PID; MiniRae2000 Portable VOC Monitor, RAE Systems, San Jose, CA) that was calibrated with 100 ppm isobutylene (Calibration Gas Mixture, NorLAB, Boise, ID) before use. Measurement accuracy of this device can range from ± 2 ppm of reading. The PID measured the concentration of various gases in air every 3 to 5 s and reports the maximal concentration (in ppm). The macroenvironment was always sampled first by placing the PID in the center of the room, and then the 17 individual cages were sampled.
The same sampling methodology was used for each cage during the study. The material exclusion cap was removed from the sampling port, and then the PID probe was inserted approximately 15 cm into the cage. The PID probe used for ammonia sampling was smaller in diameter than the sampling port and therefore could be inserted through the port and into each cage. The probe was removed, purged, and cleaned after each sample to remove any internal or external debris such as bedding or feces. Microenvironmental air from each cage was tested for 5 min daily.
In addition to using the PID, the ammonia concentrations in the room and all cages were measured on day 17 by using an alternate method (Kwik-Draw Pump and Ammonia Detector Tubes, Mine Safety Appliances Company, Pittsburgh, PA). The tubes were discarded after single use. The reported uncertainty of these measurements is 15% to 25%, depending on ammonia levels. In this passive sampling device, reactive glass-fiber filters impregnated with citric acid trap and indicate ammonia concentrations. The tube was placed through the sampling port, and the pump action withdrew samples of microenvironmental air from each cage. This alternative methodology was used to verify results obtained from the PID on the final day of the study.
Temperature and humidity.
Temperature and humidity were measured by using a hygrometer (model 11-661-18, Traceable Calibration Control Company, Fisher Scientific, Friendswood, TX), which was calibrated (Instruments Traceable to National Institute of Standards and Technology certificate number 4185-1440578) prior to shipping. Measurements were reported by the manufacturer to be accurate within 2% RH and 1°. The readings were cross-checked with the stationary wall hygrometer (Traceable Humidity–Temperature with Dual Min–Max Memories, VWR International, West Chester, PA), which was used only for the in-room measurements. The macroenvironment always was measured first, followed by measuring each of the 17 individual cages. The device sampled air for a minimum of 3 min before recording the relative humidity in percent and temperature in degrees Fahrenheit.
The device was placed within a hose connected externally by means of an airtight seal to the sampling port to measure temperature and humidity of the microenvironment; 1 sampling hose was used throughout the study. Because the cages were housed under positive-pressure ventilation, cage air flowed out through the sampling port and into the tube when the exclusion cap was removed. The tube was purged between cage samples, and any debris was removed. The room's daily minimal and maximal temperatures and percentages of humidity were recorded by using another stationary wall hygrometer, according to the standard operating procedure for this institution.
Fecal corticosterone.
To assess the response of the hypothalamic–pituitary–adrenal axis to altered husbandry practices, the immunoreactive corticosterone metabolites in feces of the ICR female mice used in this study were quantified. Fecal pellets were collected from the 5 mice in each cage, analyzed by cage, and then were pooled by bedding-volume group for analysis.
All 5 animals from each cage were placed in an empty cage for 20 min at 1000 for fecal collection on days 0, 7, 10, 14, and 17. Fecal pellets were collected and frozen at 0 °C until later processing. Fecal samples were extracted according to the protocol provided by the manufacturer of the enzyme immunoassay kit (Correlate-EIA kit, Assay Designs, Ann Arbor, MI) used. On the basis of previous assays with this kit,9 samples were diluted 50:1 before analysis. This kit contains internal controls, and a standard curve was calculated to determine sample values.
Cage mass.
Before being placed in the 17 cages, bedding (Sani-Chips 7090, Harlan, Madison, WI) was autoclaved and then weighed on a scale (model FP 6200, A and D Weighing, San Jose, CA) accurate to 0.01 g. The scale was tared daily and recalibrated annually. Table 1 shows the initial bedding masses for the cages. After bedding was added, each cage (without the wire top, mice, cage card, and lid) was weighed on days 0, 7, 10, 14, and 17. By subtracting the initial (day 0) mass of the cage from those on subsequent days, the accumulation of biomass over the cage-changing testing period was calculated. Cages were grouped according to the initial amount of bedding—that is, low-, medium-, and high-volume bedding groups (LVBG, MVBG, HVBG, respectively; Table 1).
Mouse body mass.
The same electronic scale that was used to weigh the bedding was used to weigh the 80 mice over the course of the study. Masses of individual mice on days 0, 7, 14, and 17 were recorded. Each mouse was placed in a weigh boat that had been tared previously, body mass was recorded, and the mouse was returned to the home cage. During the weighing process, mice were assessed for gross abnormalities in appearance, such as hair loss and fight wounds. They also were assigned a numeric body condition score, as described elsewhere.45
Photographs of bedding.
Photographs (model D70s, Nikon, Tokyo, Japan) were taken of the 17 mouse cages on days 0, 7, 10, 14, and 17 while mice were in the fecal collection containers. The photographs were used to document ‘cage dirtiness.’ The wire top was removed during photographs so that the bedding could be seen. The cage number and date of photograph were noted within each photo for later reference but are masked in the publication photographs.
Behavioral assessment.
Mice were videotaped within the cage approximately 2 h after the start of the room dark cycle. This recommended time for behavioral assessment represents an active period for mice, because they are known crepuscular animals.13,38 Each of the cages that housed mice were videotaped (model CCD-RRV67, Steady Shot Handy Cam, Sony, Park Ridge, NJ) for approximately 10 min between 2000 and 2100 on days 0, 8, and 15 by using a night-vision setting. The camera was placed so that the lateral side of the cage could be seen in its entirety. The videos then were scored by manually entering data into a custom database program (Access, Microsoft, Redmond, WA) with the ability to calculate time spent in stereotypic and aggressive behaviors while counting dominance displacement instances displayed in the video.
BAL.
Microenvironmental alterations after prolonged periods without bedding changes can manifest as disease of the respiratory tract.41 Although prolonged exposure to high ammonia concentrations can cause histologic changes to the respiratory tract,35 early detection of pathologic changes associated with disease or inflammation can be aided by BAL sampling.26 One randomly chosen mouse from each cage was euthanized in accordance with the 2007 AVMA Guidelines on Euthanasia and underwent BAL followed by cytologic evaluation. The samples were evaluated in 3 groups according to bedding volume, with 5 BAL samples from each group for a total of 15 samples.
After euthanasia, the trachea was exposed and intubated with a 20-gauge cannula at the level of the tracheal bifurcation. A 3.0-mL syringe was connected to the tracheal cannula, and the lungs were washed with 2.0 mL Ca2+- and Mg2–-free PBS at 4 °C. A minimum of 1.5 mL BAL fluid was recovered from each mouse. The cell counts in the recovered fluid were determined as the average of two counts obtained by using a hemocytometer. In addition, direct smears were made and stained with Diff-Quick (Andwin Scientific, Addison, IL) to confirm the hemocytometer counts. Cytospin slides were prepared to assess cellular morphology by centrifuging 10 drops of BAL fluid at 200 rpm (6.5 × g) for 5 min. A reviewer blinded to experimental group calculated cellular counts and evaluated morphology.
Statistical analysis.
All statistical analysis was performed using SAS version 9.1 for Windows (SAS Institute, Cary, NC). Individual measurements were combined into groups by bedding level for all statistical comparisons. Ammonia data were analyzed by using a mixed-linear model for repeated measures (ANOVA), with day as a within-cage factor and volume as a between-cages factor. Temperature and humidity were included as within-cage covariates. After goodness-of-fit indices for several covariance structures were compared, a first-order autoregressive structure was chosen to model the covariance over time within cage.
Temperature data were analyzed by using a mixed-linear model for repeated measures (ANOVA), with day as a within-cage factor and volume as a between-cages factor. After goodness-of-fit indices for several covariance structures were compared, a compound symmetric structure was used to model the covariance over time within cage.
Humidity data were analyzed by using a mixed-linear model for repeated measures (ANOVA), with day as a within-cage factor and volume as a between-cages factor. After goodness-of-fit indices for several covariance structures were compared, a first-order autoregressive structure was chosen to model the covariance over time within cage.
Cage mass, mouse body mass, and BAL samples were all analyzed according to bedding volume group by using ANOVA with a significance level of 0.05. Similarly, fecal corticosterone samples were analyzed according to bedding volume group by using ANOVA with a significance level of 0.05, but they were also modeled by using linear regression. Behavioral assessment statistical analysis used calculated mean values for relevant behaviors, which then were scored and evaluated for trends according to bedding volume group. A complete list of variables measure, frequency of sampling, and methodology used to obtain data is given in Table 2.
Table 2.
Measured variables, frequencies of sampling, and sampling methodologies used during the study
| Measured variable | Frequency | Methodology |
| Ammonia | Daily; day 17 | photo-ionization detector; pump and NH3 detection tubes |
| Temperature and humidity | Daily | Hygrometer |
| Body mass | Days 0, 7, 14, 17 | 0.1-g scale |
| Dermatitis, wounds, alopecia | Daily | Observational scoring |
| Bedding mass | Days 0, 7, 10, 14, 17 | 0.1-g scale |
| Fecal corticosterone | Days 0, 7, 10, 14, 17 | ELISA |
| Behavioral assessment | Days 0, 8, 15 | 10-min recording |
| Photograph of bedding | Days 0, 7, 10, 14, 17 | Still photograph |
| Pulmonary cytology | Day 17 | Terminal bronchoalveolar lavage |
Results
ACPH.
The cage rack was tested for calculation of average ACPH prior to housing any mice in the study. The measurements ranged from 78.3 to 98.1 ACPH (mean ± SEM, 90.5 ± 4.1 ACPH) for the 40 randomly sampled positions of the 112 total possible positions on both sides of the rack.
Ammonia.
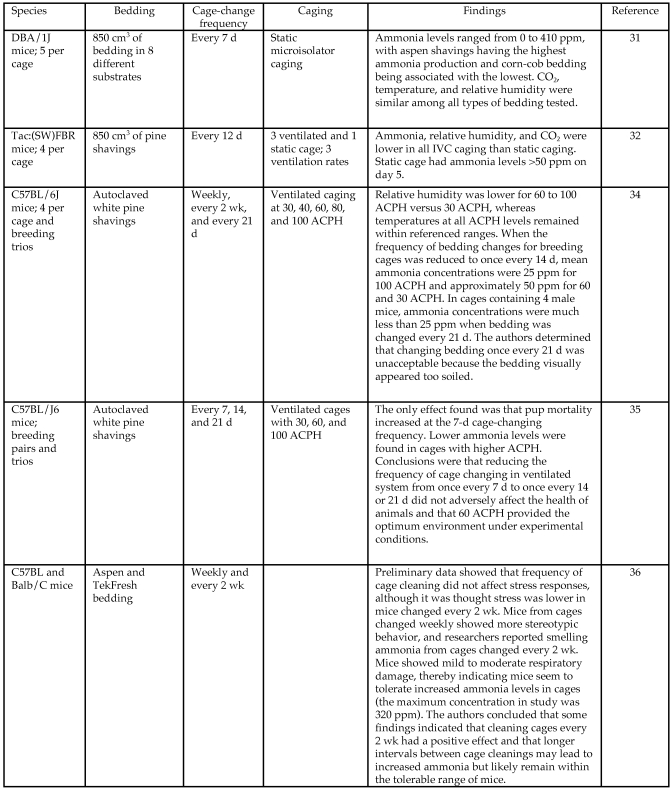
All 17 cages and the room had ammonia values of 0 ppm for the first 11 d. The mouse room and 7 of the 15 cages did not have measurable quantities of ammonia throughout the course of the study. Figure 2 suggests a relationship between bedding volume and ammonia changes over time (bedding volume × ammonia interaction). Adjusting for bedding volume explained most of the variability in ammonia levels of cages for days 11 to 17, but significant (P < 0.001) cage-to-cage variability existed. The highest intracage ammonia level was in the LVBG (4.8 ppm) and was recorded on days 16 and 17.
Figure 2.
Ammonia concentrations (ppm, parts per million) over time. Average NH3 levels in each cage–bedding-volume group and room daily during the study. Each bedding-volume group had 5 cages. The numeric values reflect averages of the 5 cages in each bedding group. Note that no ammonia was detected until day 12.
Temperature and humidity were not significant predictors of ammonia concentration. Days after last change, bedding volume, and the day × bedding volume interaction were all highly significant (P < 0.001; Table 3). Post hoc tests indicated that bedding volume was significantly (P < 0.05) associated with ammonia level on days 13 to 17 but not on days 11 or 12. The association between day and ammonia level was significant only among cages within LVBG.
Table 3.
Results of statistical analysis
| Effect | P | F | |
| Ammonia | Day | 0.0009 | 4.71 |
| Volume | 0.0005 | 11.25 | |
| Day × Volume | <0.0001 | 3.98 | |
| Humidity | 0.0750 | 3.27 | |
| Temperature | 0.6003 | 0.28 | |
| Temperature | Day | <0.0001 | 4.29 |
| Volume | <0.0001 | 21.47 | |
| Day × Volume | 0.0008 | 1.89 | |
| Humidity | Day | 0.9473 | 0.51 |
| Volume | 0.0405 | 3.61 | |
| Day × Volume | 0.7655 | 0.84 |
The data were obtained by using an F test by means of type 3 tests of fixed effects.
Temperature.
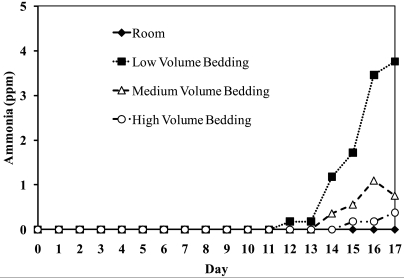
Cage temperature was significantly associated with day, bedding volume, and the day × bedding volume interaction (P < 0.001 for all comparisons). Across all days, the only significant difference by volume was between room and LVBG; there was no difference among the 3 bedding-volume groups (Table 3). Average temperature on days 0, 1, 2, 4, and 15 differed significantly (P < 0.05) by bedding volume.
Cage temperature did not differ statistically as a function of bedding volume, and with the exception of the first 2 d, significant day-to-day differences in temperature were not detected for the bedding-volume groups or the room (Figure 3). The daily room temperature was lower than the average cage temperature for all 3 groups on each of the 17 d (P < 0.05).
Figure 3.
Temperature (°F) of cage groups over time. Average temperatures (°F) in each cage–bedding-volume group and room daily during the study. Each line represents the average value of the 5 cages in each bedding group.
Humidity.
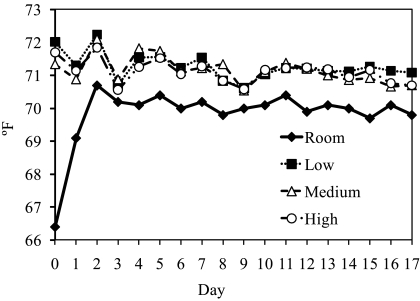
Humidity did not vary significantly by day (P = 0.9473). Although bedding volume was a significant (P = 0.0405) predictor of humidity, the association between volume and humidity did not vary significantly from day to day (P = 0.7655; Table 3). When average humidity was compared across volume levels by using the Tukey post hoc test, the only significant (P < 0.05) difference was between the room and LBVG (Figure 4).
Figure 4.
Relative humidity (% RH) from each cage–bedding-volume group and room daily during the study. Each line represents the average value of the 5 cages in the bedding group.
Fecal corticosterone.
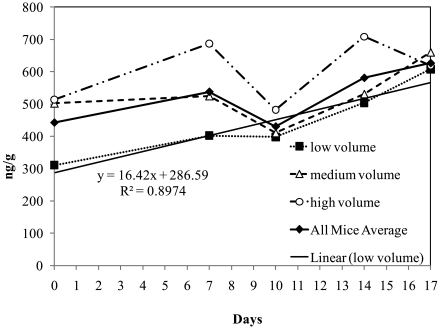
Fecal corticosterone values were determined and converted to nanograms per gram feces by using a standard curve. Significant statistical differences at a 95% confidence interval were not detected at any time point. The 5 cages within each bedding-volume group had relatively large standard deviations of fecal corticosterone level after calculation of the mean, with a covariance of 39.9%. Average day 0 fecal corticosterone for all groups (442 ng/g) was statistically equivalent to the levels detected on day 10 (430 ng/g). No statistically significant trends were detected in fecal corticosterone concentration over time.
Graphically, fecal corticosterone levels for LVBG appeared to increase over time (y = 16.4 x + 286.6), with R2 = 0.89 (Figure 5). However, statistical tests (ANOVA) did not support statistically significant changes in fecal corticosterone over time in LVBG, MVBG, or HVBG. The overall mean for all cages in the study, regardless of bedding volume, is indicated as ‘all mice average’ in Figure 5.
Figure 5.
Average fecal corticosterone levels of the groups housed in each bedding-volume group and for all animals. Samples were taken at 5 time points during the study: days 0, 7, 10, 14, and 17.
Cage mass.
The mass of the cage, bedding, and waste from the mice was calculated on days 0, 7, 10, 14, and 17. Cages were grouped by the bedding volume, and the average cage mass was calculated and reported as the difference between days 17 and 0. Gains in mass were 150 ±13 g, 127 ±7 g, and 149 ±12 g for LVBG, MVBG, and HVBG, respectively. These gains were not statistically different at the 95% confidence level.
Mouse body mass.
The difference in mouse body mass between days 17 and day 0 by bedding group was: LVBG 2.08 ±1.17 g, MVBG 1.79 ±1.00 g, and HVBG 1.67 ± 0.88 g. Only 1 mouse lost weight (0.26 g; LVBG) from day 0 to 17. Mouse weight gains across groups did not differ at the 95% confidence interval. Mice exhibited no evidence of fight wounds or changes in body condition score, nor were barbering, alopecia, or fight wounds observed during the 17-d period.
Photographs of bedding.
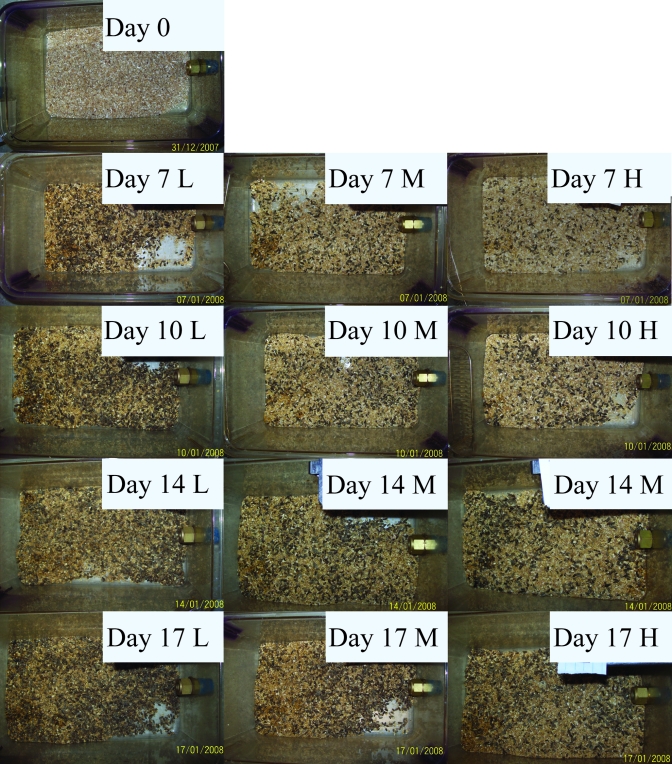
Photographs of representative cages from each group on days 0, 7, 10, 14, and 17 are presented in Figure 6. The cages reached what most personnel likely would consider ‘too dirty’ by appearance on day 14. Cages in LVBG seemed to progress to the unacceptably ‘dirty’ state faster than those in the other 2 bedding groups, most likely because of the smaller starting volume of clean bedding.
Figure 6.
Photographs of cages from each bedding volume at days 0, 7, 10, 14, and 17 without mice or cage top. Cage cards are masked. L, low-volume bedding group; M, medium-volume bedding group; H, high-volume bedding group.
Behavioral assessment.
Cages were grouped into LVBG, MVBG, and HVBG for data analyses. Behaviors identified included circling repetitively, tail biting, rolling on back for submission, and actively chasing cagemates. The behaviors of individual mice markedly affected the average number of behavioral abnormalities due to the small total number of behaviors observed. In addition, the behaviors that changed substantially were only noted in a few individual mice, and significant overall differences among bedding groups were not detected.
Aggressive behavior and instances of dominance displacement were recorded. None of the behaviors noted differed significantly among bedding-volume groups. In general, detrimental behaviors did not clearly increase over time for any of the bedding groups, although further studies are needed to evaluate cage effect versus cage change interval.
BAL.
Samples of BAL fluid from each mouse were evaluated with a hemocytometer to obtain the quantitative cell population in the lungs. Respiratory epithelial cells were excluded from the total nucleated cell counts. The total nucleated cellular counts from each mouse were grouped according to bedding volume for analysis. Cell counts did not differ significantly among groups (LVBG, 19.2 ± 13.3 cells/µL; MVBG, 23.8 ± 15.8 cells/µL; HVBG, 26.6 ± 22.2 cells/µL); a trend of increasing white blood cell count with increasing bedding volume was not statistically significant. To assess the morphology of cells present in the total nucleated cell counts, cytospin preparations were used. Bedding volume again was used in grouping samples and the results are seen on the Table 4. There was no statistical difference in cell types among the samples of BAL fluid from the 3 bedding-volume groups in this study. In addition, none of the BAL samples showed any evidence of acute inflammation or pathology.
Table 4.
Cell type distributions and count from concentrated bronchoalveolar lavage samples (cytospin preparations)
| % of total nucleated cell count |
||||
| Bedding group | Total no. nucleated cells (per μL) | Neutrophils | Lymphocytes | Monocytes and macrophages |
| Low-volume | 19.2 ± 13.3 | 2.3 ± 0.5 | 5.3 ± 0.5 | 92.5 ± 0.6 |
| Medium-volume | 23.8 ± 15.8 | 3.8 ± 2.9 | 2.0 ± 2.5 | 94.2 ± 5.0 |
| High-volume | 26.6 ± 22.2 | 5.0 ± 2.8 | 1.2 ± 1.8 | 93.8 ± 3.8 |
Data are given as mean ± SEM for each bedding group.
Discussion
This study provides data relevant to laboratory mouse husbandry practices in a setting that is similar to that of many institutions. Over the past 30 y, mouse housing has changed dramatically in design, engineering, regulatory oversight, and animal model sophistication. Conversely, it is possible that many institutions have not updated their care and husbandry practices in several decades and may be awaiting revised regulatory guidelines. In the current study, aspects of welfare and health in mice were assessed during a 17-d cage-changing cycle. This study was designed to generate information that may provide a basis for performance-based standards and associated regulations. Variables related to mouse health were assessed to determine whether the prolonged cage-change intervals would compromise the general wellbeing of mice.
ICR female mice were chosen because of their large size and were housed at maximal cage density in hope of creating the ‘dirtiest’ cage microenvironment possible during an extended cage-change frequency. The 10-wk average weight for an outbred, ICR female mouse is approximately 30 g; that for age-matched inbred strains of mice (C57/BL6, Balb/c, and FVB) is approximately 20 g.17 These data suggest that the waste generation during the current experiment would meet or exceed housing conditions for most laboratory mouse stocks or strains. A common sequela of a prolonged cage-changing interval is an increase in intracage ammonia. It is the author's experience that human detection of the odor can often serve as the primary motivator for initiating cage cleaning. The ammonia odor threshold for human detection is at 0.04 ppm, a value substantially lower than the American Conference of Industrial Hygienists recommended exposure limits.6 Past studies have shown that ammonia production rate is influenced by cage-cleaning interval, temperature, humidity, bedding type, and mouse density.14,39,40
Ammonia is a severe irritant to the respiratory tract, skin, and mucous membranes of the eyes.31 At room temperature, ammonia is a colorless gas with a distinctive pungent odor easily detected by humans.30 The primary source of ammonia in rodent housing is conversion of urea to ammonia by urease. Ammonia gas is released from urine by urease, which can be endogenous in bedding or produced by fecal bacteria.27The effects of ammonia on laboratory-housed rodents are not elucidated clearly in the literature. Exposure to ammonia has been implicated in many pathologic processes in rodents, including thickened tracheal mucosa and corneal opacities, and is thought to interact synergistically with pathogens such as Mycoplasma and Bordatella.39,48
Currently, upper-level ammonia exposure guidelines are not available for rodents; for humans, the 8-h time-weighted average exposure limit is 25 ppm, with a maximal exposure of 50 ppm,2 and it is generally accepted these human limits should not be exceeded for laboratory-housed rodents. Although exposure to ammonia may influence rodent health, precise exposure and tolerable ranges are unknown.42 Because feral Mus are adapted to life in tunnels below ground with little ventilation, equating their exposure limits to those of humans may be inappropriate.24,43 Studies have documented that rodents have a stronger preference for location than ammonia concentrations when given the option to inhabit various cage locations with varying concentrations of ammonia, suggesting a relatively high tolerance for environmental ammonia exposure.16
Cage and room ammonia levels were monitored daily over the course of this study by using a photoionization detector. Ammonia was not detected until day 12, and no level was greater than 4.8 ppm at day 17 . The validity of the data from the photoionization detector was tested by using an alternate method on the same day (day 17). The average difference between the 2 methodologies was 0.42 ppm. We hypothesize that LVBG cages had the highest levels of ammonia due to the rapid saturation of bedding by animal waste. This situation aids bacterial proliferation, resulting in greater ammonia production.
Despite our use of large female mice to maximize urine and fecal output and potential ammonia production, ammonia levels reported in the current study were lower than those in other studies using ventilated caging.32,34-36 This result may reflect variations in data collection methods or the high rate of airflow (90.5 ± 4.1 ACPH) in the cages used in this study. Our results are consistent with several other studies demonstrating that ammonia concentrations do not exceed levels that result in adverse effects on mice,16,34,35 even if the cage change interval is extended to 17 d, as in the current study.
Temperatures measured in this study remained within the boundaries suggested in the Guide for laboratory-housed mice.21 No temperature differences were found between bedding-volume groups, and no temporal relationship between temperature and day after changing was noted. The volume of bedding did not alter temperature within the cage, suggesting that ambient temperatures have a larger effect on cage temperature than does the amount of bedding present. Other factors that may alter temperature are the type of bedding and size of the animals, which were not measured in this study. In the wild, the stability of soil temperature allows rodents to avoid temperature fluctuations.43
Current guidelines state that the relative humidity of the microenvironment for rodents should remain within 30% to 70%.21 Relative humidity at levels significantly exceeding 35% have been shown to dramatically promote ammonia generation rates in static mouse caging.27 Other authors have shown lower relative humidity in IVC caging when compared with static caging.32 In the present study, relative humidity levels ranging between 36% and 55% were associated with negligible ammonia. The humidity levels reported here are consistent with those of facilities using conditioned air to maintain ambient room humidity within levels recommended in the Guide and those that house mice in IVC—all husbandry practices in the present study were consistent with those of ‘modern’ laboratory animal holding rooms. Throughout this study, humidity in the room and the cages stayed within recommended ranges. Cages of LVBG consistently had a statistically significant higher relative humidity (P < 0.05) than did other bedding-volume groups and the room. Although the MVBG had the lowest numerical values for humidity, it was not statistically different from any of the other groups. This finding might have resulted from complete saturation of the bedding when smaller bedding volumes were used, resulting in constant moisture. Perhaps MVBG had lower humidity levels than HVBG because the larger volume of bedding can pile up, form mounds, and heap in corners, preventing air flow over the deeper bedding and resulting in decreased desiccation of bedding substrates.
Corticosterone levels have been widely used as a physiologic parameter reflecting the health and welfare of animals and are used to measure reactions to standard husbandry routines.15,29 Significant changes in physiologic parameters considered to reflect stress were associated with handling, including the lifting of an animal and moving it to another cage.3 Because glucocorticoids exhibit regular and episodic changes over time in blood (circadian variations and pulsatile secretion patterns), hormone levels assessed by plasma analysis represent a very narrow time frame and therefore are not representative of basal levels.44 In contrast, circulating hormones are excreted in feces over time and thus fecal hormone levels represent an average over an extended timespan.44 Further, the fecal steroid assay is not invasive.18 Measuring fecal cortisol metabolites as an indicator of adrenocortical activity in animals offers the advantage of a simple sampling technique that does not interfere with the study results. The use of fecal corticosterone analysis enables long-term, longitudinal studies,29 and commercially available assay kits can be used to assay this parameter in mice.49
Fecal corticosterone levels reported for rodents range from 4000 to 5000 ng/g (mice) and 100 to 900 ng/g (rats, voles).9,18,20 This broad range of described levels can be attributed to strain and species differences, dissimilar methodology in extraction protocols or processing, and the situation the animal experienced due to procedures performed during the measurement period. Because no standard fecal corticosterone levels exist for mice, we examined baseline levels and their trends over the 17 d of this study. Levels obtained in this study (212 to 1190 ng/g) were within the ranges cited earlier.
Other authors, although not demonstrating a statistically significant trend, have postulated that plasma corticosterone levels tended to be lower when cage changing intervals were longer.35 In the current study, mice housed in cages with medium or high volumes of bedding had final corticosterone levels approximately equal to those found at baseline. Corticosterone values were not statistically different among bedding-volume groups at day 17. Further studies may prove or disprove the existence of any trends with time. No significant differences in fecal corticosterone were detected between days 7 and 14 in any of the bedding-volume groups. Beneficial future studies include measuring fecal corticosterone levels in other strains of mice housed under conditions identical to those in this study, establishing basal levels of fecal corticosterone for different strains and circumstances, and measuring the response to a known stressor for positive-control comparisons.
Reducing the number of cage changes results in energy savings and decreased total waste generated, an important consideration in implementing ‘Green Initiatives.’ Waste volume generation was examined to determine the effect of bedding volume by measuring biomass over the 17-d change period.
Body weight is a useful nonspecific indicator of mouse health because it is noninvasive, and negative changes likely would suggest a detrimental housing environment. All cage averages and bedding-volume group averages for body weight increased over the 17-d course of the study, and only 1 mouse among the 80 lost weight. Significant differences in body weight were not detected across bedding-volume groups, and evidence of poor health was not associated with any of the bedding volumes during the 17-d cage-changing cycle.
The incidence of aggressive behavior and development of stereotypies in rodents after cage changing has been documented;47 therefore, prolonging the cage-change interval may be beneficial in terms of decreasing atypical or deleterious behaviors. Statistically significant differences in mouse behaviors were not observed over the 17 d after cage change. No increases in negative behaviors were measured during 15-min observation periods during the active cycle. The relatively short observation periods likely would not have revealed subtle differences, and the behavior of specific mice markedly influenced the overall groups analyses in light of the small number of mice that contributed the majority of the total behaviors observed. However, other parameters evaluated during this study (weight, fecal corticosterone, health) support the conclusion that the prolonged change interval did not negatively alter mouse health.
A previous study did not detect pulmonary lesions associated with alterations in cage ventilation rates, cage changing frequencies, or ammonia levels approaching 400 ppm.35 Cytology of BAL fluid can be used to detect early changes within the lung that either precede or confirm morphologic changes in respiratory disease. Quantitative BAL measurements can aid identification of potential beneficial or adverse effects of novel drugs designed for the treatment of respiratory disease.19,23,28,33 Cell counts are one of the most informative BAL measurements. The number of lymphocytes in BAL fluids is generally very low in rats and mice. Typical total leukocyte counts in BAL fluid are usually less than 2 × 109/L. Neutrophils are rare in normal BAL fluids; BAL neutrophilia is a sensitive marker of an inflammatory response, whereas eosinophilia can be indicative of allergic reactions.26
Neither BAL quantitative cell counts nor cell phenotype differed among bedding-volume groups. Markers of acute pulmonary inflammation were not detected in any of the animals. These results suggest that any effect of a prolonged change-cage interval on pulmonary immunocyte subsets is not detectable after a single 17-d cycle; however, more animals should be evaluated over longer time periods to definitively establish that pulmonary cytology is unaffected by change interval.
After prolonged time between cage changes, researchers and staff note that cages are ‘dirty.’ Although the interpretation of photographs is subjective, the accumulation of fecal material is the primary means by which ‘dirtiness’ is assessed visually by humans. Cages with different bedding volumes showed little discernable difference on day 7 of the study. However, between days 7 and 17, LVBG cages appeared to have excess feces and little nonsoiled bedding remaining in the cage compared with other groups. Conversely, the HVBG still contained a nesting area and visibly clean bedding on day 17, although all cages were soiled to the extent that most persons likely would consider the caging excessively ‘dirty’ by day 14. This finding is important in that, despite the fact that no other detrimental effect of extended cage changing was detected during this study by using objective data assessments, this subjective parameter still forms staff and investigator opinion about the welfare of the animals in caging that has not been changed weekly (or more frequently).
Bedding volume proved to be a significant (P < 0.001) predictor of ammonia within the microenvironment (Table 3). In regard to intracage temperature, bedding volume played no role between groups. Bedding volume also was a significant (P = 0.041) predictor of intracage humidity (Table 3). Bedding-volume groups did not differ significantly in terms of fecal corticosterone, cage mass, mouse body mass, behavior, or BAL analysis. Visual perception of dirtiness was more pronounced in LVBG cages compared with those of other bedding volumes.
In summary, none of the parameters evaluated in this study indicated a detrimental effect related to prolonged cage-change interval in terms of health status, welfare, and microenvironmental conditions. Data reported here suggest that changing bedding every 14 d in IVC housing (with 5 female ICR mice per cage and appropriate ACPH) while using a medium volume of aspen chip bedding (400 mL) may present a balance between maintaining microenvironmental hygiene, minimizing disturbances to mice, and minimizing biomass accumulation. Of note, the ‘dirtiness’ index remains a dominant indicator motivating cage changing. Because humans perceive that mice housed in a cage with a high density of fecal pellets are living in an undesirable environment, this factor may require consideration when establishing cage-change guidelines.
Acknowledgments
The views, opinions, and findings expressed in the manuscript are those of the authors, not Colorado State University or East Carolina University. Funding for this project was provided by the Vice President for Research at Colorado State University. Special thanks to Cara Olsen (USHUS) for statistical analysis and Aimee Oke (CSU) for manuscript preparation.
References
- 1.AALAS 2008. [Internet] Performance- based criteria as the basis for determining laboratory animal housing standards. [Cited 10 Nov 2008] Available at http://www.aalas.org/association/position_statements.aspx#housing
- 2.American Conference of Governmental Industrial Hygienists 2007. Threshold limit values (TLVs) and biological exposure indices (BEI). Cincinnati (OH): ACGIH [Google Scholar]
- 3.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51 [PubMed] [Google Scholar]
- 4.Beynen AC, van Tintelen G. 1990. Daily change of cage depresses mass gain in mice. Z Versuchstierkd 33:106–107 [PubMed] [Google Scholar]
- 5.Bohannan J. 2002. Can a mouse be standardized? Science 298:2320–2321 [DOI] [PubMed] [Google Scholar]
- 6.Brown JA. c1993. [Internet] Haz-Map: Information on hazardous chemicals and occupational diseases. Bethesda (MD): NIH; [Cited 10 Nov 2008]. Available at http://hazmap.nlm.nih.gov/index.html [DOI] [PubMed] [Google Scholar]
- 7.Burn C, Peters A, Mason G. 2006. Acute effects of cage cleaning at different frequencies on laboratory rat behaviour and welfare. Anim Welf 15:161–171 [Google Scholar]
- 8.Burn CC, Peters A, Day MJ, Mason GJ. 2006. Long-term effects of cage-cleaning frequency and bedding type on laboratory rat health, welfare, and handleability: a cross-laboratory study. Lab Anim 40:353–370 [DOI] [PubMed] [Google Scholar]
- 9.Cavigelli SA, Guhad FA, Ceballos RM, Whetzel CA, Nevalainen T, Lang CM, Klein LC. 2006. Fecal corticoid metabolites in aged male and female rats after husbandry-related disturbances in the colony room. J Am Assoc Lab Anim Sci 45:17–21 [PubMed] [Google Scholar]
- 10.Cisar CF, Jayson G. 1967. Effects of frequency of cage cleaning on rat litters prior to weaning. Lab Anim Care 17:215–217 [PubMed] [Google Scholar]
- 11.Duke JL, Zammit TG, Lawson DM. 2001. The effects of routine cage-changing on cardiovascular and behavioral parameters in male Sprague–Dawley rats. Contemp Top Lab Anim Sci 40:17–20 [PubMed] [Google Scholar]
- 12.Flynn RJ, Poole CM. 1969. Frequency of cage cleaning and the survival of mice. ANL7635. ANL Rep Dec:64. [PubMed] [Google Scholar]
- 13.Fox JG, Anderson LC, Lowe FM, Quimby FW. 2002. Laboratory animal medicine, 2nd ed New York (NY): Academic Press [Google Scholar]
- 14.Gamble MR, Clough G. 1976. Ammonia build-up in animal boxes and its effect on rat tracheal epithelium. Lab Anim 10:93–104 [DOI] [PubMed] [Google Scholar]
- 15.Godfrey D, Silverman J. 2009. Effects of a fire alarm strobe light on fecal corticosterone metabolite concentrations in mice. Lab Anim (NY) 38:61–68 [DOI] [PubMed] [Google Scholar]
- 16.Green AR, Wathes CM, Demmers TG, Clark JM, Xin H. 2008. Development and application of a novel environmental preference chamber for assessing responses of laboratory mice to atmospheric ammonia. J Am Assoc Lab Anim Sci 47:49–56 [PMC free article] [PubMed] [Google Scholar]
- 17.Harlan Laboratories [Internet] Research models: US growth curves. [Cited 29 May 2009]. Available at http://www.harlan.com/research_models_and_services/research_models_by_product_type/outbred_mice/icr.hl
- 18.Harper JM, Austad SN. 2000. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool 73:12–22 [DOI] [PubMed] [Google Scholar]
- 19.Henderson RF. 2005. Use of bronchoalveolar lavage to detect respiratory tract toxicity of inhaled material. Exp Toxicol Pathol 57Suppl 1:155–159 [DOI] [PubMed] [Google Scholar]
- 20.Hunt C, Hambly C. 2006. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group-housed males. Physiol Behav 87:519–526 [DOI] [PubMed] [Google Scholar]
- 21.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 22.Ishii T, Yoshida K, Hasegawa M, Mizuno S, Okamoto M, Tajima M, Kurosawa T. 1998. Invention of a forced-air-ventilated microisolation cage and rack system—environment within cages: temperature and ammonia concentrations. Appl Anim Behav Sci 59:115–123 [Google Scholar]
- 23.Kilinc G, Kolsuk EA. 2005. The role of bronchoalveolar lavage in diffuse parenchymal lung diseases. Curr Opin Pulm Med 11:417–421 [DOI] [PubMed] [Google Scholar]
- 24.Krohn TC, Hansen AK. 2002. Carbon dioxide concentrations in unventilated IVC cages. Lab Anim 36:209–212 [DOI] [PubMed] [Google Scholar]
- 25.Latham N, Mason G. 2004. From house mouse to mouse house: the behavioural biology of free-living Mus musculus and its implications in the laboratory. Appl Anim Behav Sci 86:261–289 [Google Scholar]
- 26.Mathers R, Evans G, Bleby J, Tornow T. 2007. Total and differential leucocyte counts in rat and mouse bronhcoalveolar lavage fluids using the Sysmex XT2000iV. Comp Clin Pathol 16:29–39 [Google Scholar]
- 27.Memarzadeh F, Harrison PC, Riskowski GL, Henze T. 2004. Comparison of environment and mice in static and mechanically ventilated isolator cages with different air velocities and ventilation designs. Contemp Top Lab Anim Sci 43:14–20 [PubMed] [Google Scholar]
- 28.Meyer KC. 2004. The role of bronchoalveolar lavage in interstitial lung disease. Clin Chest Med 25:637–649 [DOI] [PubMed] [Google Scholar]
- 29.Mostl E, Palme R. 2002. Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74 [DOI] [PubMed] [Google Scholar]
- 30.National Institute for Occupational Safety and Health 1974. Criteria for a recommended standard: occupational exposure to ammonia. US DHHS Publ No. 74-136 Cincinnati (OH): National Institute for Occupational Safety and Health [Google Scholar]
- 31.Perkins SE, Lipman NS. 1995. Characterization and quantification of microenvironmental contaminants in isolator cages with a variety of contact beddings. Contemp Top Lab Anim Sci 34:93–98 [PubMed] [Google Scholar]
- 32.Perkins SE, Lipman NS. 1996. Evaluation of microenvironmental conditions and noise generation in 3 individually ventilated rodent caging systems and static isolator cages. Contemp Top Lab Anim Sci 35:61–65 [PubMed] [Google Scholar]
- 33.Rajamaki MM, Jarvinen AK, Saari SA, Maisi PS. 2001. Effect of repetitive bronchoalveolar lavage on cytologic findings in healthy dogs. Am J Vet Res 62:13–16 [DOI] [PubMed] [Google Scholar]
- 34.Reeb C, Jones R, Bearg D, Bedigan H, Myers D, Paigen B. 1998. Microenvironment in ventilated animal cages with differing ventilation rates, mice populations, and frequency of bedding changes. Contemp Top Lab Anim Sci 37:43–49 [PubMed] [Google Scholar]
- 35.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73 [DOI] [PubMed] [Google Scholar]
- 36.Reed B, Hawkins P, Latham N, Westwood K, van Driel K, Battram C, Golledge H, Farmer AM, Osborne N, Jennings M, Hubrecht R. 2008. Report of the 2006 RSPCA/UFAW Rodent Welfare Group meeting. Lab Anim (NY) 37:216–222 [DOI] [PubMed] [Google Scholar]
- 37.Riskowski GL, Harrison PC, Memarzadeh F. 2006. Mass generation rates of ammonia, moisture, and heat production in mouse cages with 2 bedding types, 2 mouse strains, and 2 room relative humidities. ASHRAE Trans 112:134–144 [Google Scholar]
- 38.Roedel A, Storch C, Holsboer F, Ohl F. 2006. Effects of light- or dark-phase testing on behavioural and cognitive performance in DBA mice. Lab Anim 40:371–381 [DOI] [PubMed] [Google Scholar]
- 39.Schaerdel AD, White WJ, Lang CM, Dvorchik BH, Bohner K. 1983. Localized and systemic effects of environmental ammonia in rats. Lab Anim Sci 33:40–45 [PubMed] [Google Scholar]
- 40.Serrano LJ. 1971. Carbon dioxide and ammonia in mouse cages: effect of cage covers, population, and activity. Lab Anim Sci 21:75–85 [PubMed] [Google Scholar]
- 41.Silver S, McGrath F. 1948. A comparison of acute toxicities of ethylene imine and ammonia to mice. J Ind Hyg Toxicol 30:7–9 [PubMed] [Google Scholar]
- 42.Silverman J, Bays DW, Cooper SF, Baker SP. 2008. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci 47:57–62 [PMC free article] [PubMed] [Google Scholar]
- 43.Studier EH, Baca TP. 1968. Atmospheric conditions in artificial rodent burrows. Southwest Nat 13:401–410 [Google Scholar]
- 44.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 45.Ullman-Cullere MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323 [PubMed] [Google Scholar]
- 46.Van Loo PL, Van der Meer E, Kruitwagen CL, Koolhaas JM, Van Zutphen LF, Baumans V. 2004. Long-term effects of husbandry procedures on stress-related parameters in male mice of 2 strains. Lab Anim 38:169–177 [DOI] [PubMed] [Google Scholar]
- 47.Van Loo PL, Van Zutphen LF, Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab Anim 37:300–313 [DOI] [PubMed] [Google Scholar]
- 48.Van Winkle TJ, Balk MW. 1986. Spontaneous corneal opacities in laboratory mice. Lab Anim Sci 36:248–255 [PubMed] [Google Scholar]
- 49.Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. 2000. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275 [DOI] [PubMed] [Google Scholar]