Abstract
Despite the progressively increasing use of zebrafish (Danio rerio) in research, the most humane method of euthanasia for these fish has not been determined. Contemporary guidance documents state that hypothermia is an unacceptable method of euthanasia. The goal of this study was to compare rapid cooling and tricaine methanesulfonate (MS222) for zebrafish euthanasia. Zebrafish (n = 46) were euthanized by immersion in either an ice-water (4 °C or less) bath or unbuffered MS222 solution (250 mg/L; 25 to 30 °C). Another cohort (n = 10) was exposed to buffered MS222 to determine whether the acidity of the water alone caused distress. The times from exposure until the animals became unable to swim, right themselves, and death were measured, and signs of distress were recorded. Fish then were placed in a ‘recovery tank’ of system water to verify that recovery did not occur. Tissues were examined histologically. The mean time for euthanasia was longer and the number of fish exhibiting signs of distress was greater for fish exposed to MS222 than those exposed to chilled water. In addition, 4 of the 23 fish exposed to MS222 regained consciousness in the recovery tank, whereas none of 23 fish exposed to chilled water recovered. No histopathologic changes or evidence of ice crystal formation were seen in either group. In light of the faster time to death and fewer signs of distress in zebrafish euthanized by rapid cooling, we advocate this method as a humane veterinary practice.
Abbreviation: MS222, tricaine methanesulfonate
In contemporary biomedical research facilities, the use of zebrafish (Danio rerio) has progressively increased in recent years.3 With this increase has come an intensified effort to identify the ideal husbandry, care, and management parameters for this species. One important, yet controversial, issue has involved the selection of humane euthanasia methods for zebrafish. Zebrafish are considered eurythermic animals as they are adaptable to a wide range of temperatures.5 The most commonly used maintenance temperature for zebrafish is 28.5 °C (83 °F), although temperatures between 24 and 30 °C (75 and 86 °F) have been recommended.7,13 Following periods of acclimation, zebrafish can tolerate a much broader temperature range.5 However, acute exposure to temperatures below their thermal neutral zone can cause death in zebrafish due to their inability to quickly acclimate. This natural phenomenon has been used as a method of euthanasia in zebrafish, but the AVMA Guidelines on Euthanasia1 and the report Recognition and Alleviation of Pain and Distress in Laboratory Animals8 from the Institute for Laboratory Animal Research both state that hypothermia (also referred to as rapid cooling) is unacceptable as a method of euthanasia for fish. Although these reports provide no scientific explanation regarding why rapid cooling is considered unacceptable, some speculate that ice crystal formation occurs in tissues during rapid cooling. Currently, the AVMA recommends the use of tricaine methanesulfonate (MS222), decapitation, or injectable methods such as sodium pentobarbital for fish euthanasia.1 These recommendations appear more applicable to larger, nontropical species of fish, because decapitation and sodium pentobarbital injection are impractical for euthanasia of small fish, such as zebrafish. MS222 may be a favored method of euthanasia because, according to 1 set of guidelines, it “causes no signs of stress, such as elevation of blood glucose, cortisol, or catecholamines.”4 However, this statement was not supported by published scientific data.
In mammals, exposure to cold temperatures may result in anesthesia. In humans, exposure to 9 °C (48 °F) is predicted to result in complete nerve conduction blockade. In cats, 20 °C (68 °F) results in the inability of evoked potentials in the central nervous system, and goats at the same temperature do not react to painful peripheral stimuli in the absence of general anesthesia.6 Physiologically, peripheral nerve conduction velocity is highly correlated with temperature, that is, as temperature decreases, nerve conduction velocity also decreases.6 In reptiles and amphibians also, peripheral nerve conduction velocity decreased with decreasing temperature, suggesting that exposure of these species to cold temperatures is anesthetic.6 Other teleost fish actually lack receptors that respond to cold and likely do not experience pain associated with cold.2 Although these studies provide valuable insights into cold exposure in other poikilotherms, one cannot draw direct correlations specific to fish, especially tropical species, from these data.
Rapid cooling affords several advantages as a method of zebrafish euthanasia. These include the ability to euthanize many animals simultaneously, minimization of handling of individual animals (which is necessary when using injectable agents or decapitation), minimal risk of operator error when preparing the euthanasia bath, and reduction in occupational health and safety risk to personnel associated with chemical and physical methods of euthanasia. The goal of our study was to compare the effects of rapid cooling and MS222 (as described by the AVMA Guidelines on Euthanasia) as methods of zebrafish euthanasia.1 We hypothesized that rapid cooling in zebrafish would result in a shorter time between exposure and death than with MS222, would not cause ice crystallization in tissue, and would result in minimal signs of distress. This study is the first to compare these 2 euthanasia methods for zebrafish, and the outcome will be important in assisting institutional animal care and use committees and researchers in the determination of the most appropriate method of euthanasia for zebrafish.
Materials and Methods
Humane care and use of animals.
All zebrafish used in this study were obtained from protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee, and facilities housing these animals were AAALAC-accredited. The fish used were all wildtype strain Tupfel long fin and were approved for use on a mutagenesis protocol but were genetically normal and therefore transferred for use in our study. A specific protocol for comparison of euthanasia methods was approved for these naïve zebrafish.
Housing and husbandry.
Zebrafish were housed in 2-L tanks with approximately 20 fish per tank. The tanks were housed on 8 racks, each holding 64 tanks. The room temperature was maintained at 28 °C (82 °F), and the water temperature was consistently 27 to 28 °C (81 to 82 °F), maintained by temperature controllers and water heaters. All of the tanks on each rack shared a common filtering system and source of reverse-osmosis–purified water (pH = 7.3). Each day, a 20% water change occurred automatically by use of a magnetic float valve and timer. The pH was checked monthly in the water storage tanks and daily in the water system; the pH of the system ranged from 7.2 to 7.8. Bicarbonate was added when the pH dropped lower than 7.2. Bicarbonate, calcium sulfate, and sea salt were added manually when necessary. Ammonia, nitrite, and nitrate levels were measured and found to be undetectable in this housing system. The conductivity of the reverse-osmosis–purified water was about 300 µs, and the system water was maintained between 230 and 350 µs. The recirculating water system (Aqua Schwarz, Göttingen, Germany) consisted of a pump that sent water to a UV filter, followed by a particle filter, to the individual fish tanks, through a biological filter, and back to the pump at a rate of about 3 gallons per minute. Adult fish were fed twice daily with 1 meal of tropical fish flakes (Tetra, Spectrum Brands, Atlanta, GA) and 1 meal of brine shrimp (Artemia salena; Sanders Brine Shrimp, Ogden, UT).
Study design.
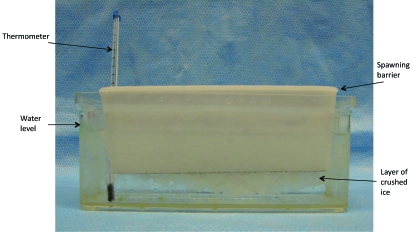
Zebrafish enrolled in the study were older than 6 mo and were euthanized individually by immersion in 1 of 2 water baths. The fish (n = 23 for each solution) were placed in a spawning barrier, which was submerged in a 1-L holding tank (Figure 1) containing approximately equal amounts ice and water at a temperature of 2 to 4 °C (36 to 39 °F; as determined by a bulb thermometer) or in an unbuffered (pH 3.4) solution of MS222 (250 mg/L; Finquel, Argent Laboratories, Redmond, WA) mixed according to the manufacturer's directions with the system water at a temperature of 28 °C (82 °F). The spawning barrier was used to prevent the zebrafish from coming into direct contact with the ice, and was used for the sake of consistency for the zebrafish exposed to MS222. All fish in each group were exposed to the same working euthanasia solution. Once placed in the water bath, fish were visualized under a dissecting microscope. The time from exposure to the chilled water or MS222 until death, which was determined by cessation of opercular movement, was recorded by using a stopwatch. In addition, zebrafish were observed during the exposure period for inability to swim, inability to right themselves, and any notable signs of distress. Signs of distress included rapid opercular movement, piping (the gulping of air at the water surface, usually indicative of hypoxia), twitching, and erratic swimming. Another cohort of fish (n = 10) were exposed to an MS222 solution buffered with sodium bicarbonate (pH = 7.0) to determine whether distress was due to the acidity of the water or to the MS222 solution itself. Two minutes after the last opercular movement, animals were placed in a ‘recovery tank’ of system water to ensure that recovery did not occur and that death of the fish was achieved. All tissues were collected for histologic analysis. Representative tissues from fish euthanized by both rapid cooling and unbuffered MS222 were examined for microscopic evidence of histologic change that may have occurred during the euthanasia process. In addition, 4 zebrafish that had been euthanized by rapid cooling were placed in a –20 °C (–4 °F) freezer for 24 h, fixed, and processed for histologic analysis to determine the appearance after ice crystal formation had occurred. These specimens were compared with tissues from zebrafish euthanized by rapid cooling or unbuffered MS222 to determine whether any changes characteristic of ice crystallization were present.
Figure 1.
Euthanasia equipment. Inside a 1-L holding tank is the spawning barrier (white), which has a metal screen bottom. The tank contains approximately equal amounts of system water mixed with crushed ice (as seen in photo) or a 250 mg/L solution of MS222, also mixed with system water. A thermometer was placed in the tank to monitor the temperature during the experiment.
Statistical analysis.
The Wilcoxon rank test was used to compare the time until loss of righting reflex and time until loss of ability to swim between the 2 groups. An unpaired t test was used to determine whether the time to death after exposure to 1 method was significantly different from that for the other method. Means and standard deviations were calculated for each group. All analysis was conducted by using R 2.7.2 (cran.r-project.org).
Results
Unbuffered MS222 and rapid cooling treatment groups.
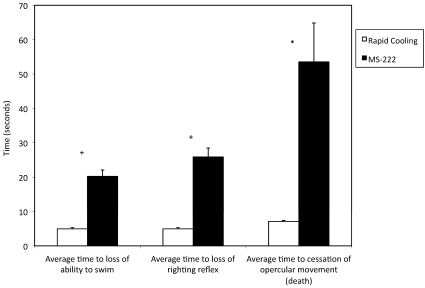
The times from exposure to the water bath until animals lost the ability to swim and lost the righting reflex and opercular movements ceased are shown in Figure 2. For all observations, the differences between unbuffered MS222 and rapid cooling were statistically significant (Figure 2). Signs of distress occurred in 39% (9 of 23) of fish euthanized by rapid cooling, compared with 100% of fish euthanized with unbuffered MS222. All fish exposed to the unbuffered MS222 solution displayed rapid opercular movements; 39% (9 of 23) also exhibited piping behavior (often with multiple consecutive piping incidents), 4% (1 of 23) displayed twitching, and 4% (1 of 23) swam erratically. Of the fish euthanized by rapid cooling, piping was the only behavior observed, at a frequency of 39% (9 of 23), with only 1 piping incident observed per fish. Signs of distress were observed in significantly (P < 0.0001) more fish exposed to unbuffered MS222 than to rapid cooling. Of the zebrafish euthanized with unbuffered MS222, 17.4% (4 of 23) of animals regained consciousness in the recovery tank after exposure to MS222 solution for 2 min past the last observable opercular movement. No animals euthanized by the rapid cooling method recovered.
Figure 2.
Euthanasia by rapid cooling compared with exposure to MS222. The time (s; mean ± SEM) from exposure until the fish lost the ability to swim was 4.90 ± 0.33 s for rapid cooling and 20.20 ±1.86 s for MS222. The time (s; mean ± SEM) from exposure until loss of the righting reflex was 4.90 ± 0.33 s for fish euthanized by rapid cooling and 25.90 ± 2.61 s for those exposed to MS222. The time (s; mean ± SEM) from exposure until death was 7.13 ± 0.27 s for rapid cooling compared with 53.52 ± 11.32 for MS222. For all observations, the difference between rapid cooling and MS222 was statistically significant (+, P < 0.0001; *, P ≤ 0.0005).
Buffered MS222.
All animals exposed to buffered MS222 displayed similar signs of distress as those of the animals exposed to unbuffered MS222. However, piping behavior, twitching, and erratic swimming were displayed by more animals than with the unbuffered solution, and none of the animals exposed to the buffered MS222 displayed rapid opercular movements. Piping and erratic swimming were observed in 80% (8 of 10) and twitching was seen in 90% (9 of 10) of animals exposed to buffered MS222.
Histopathology.
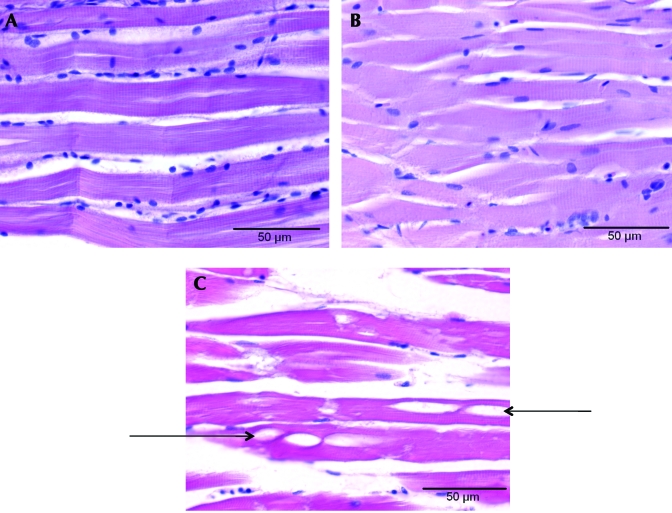
Eighteen animals were examined histologically. Observed changes that were possibly treatment-related included vacuolated neuronal soma, vacuolated epidermal cells, hypercellular epidermal epithelium, swollen alarm cells, vacuolated hepatocytes, fatty infiltration of the pancreas (mostly in the posterior pancreas), increased extramedullary hematopoeisis in the kidneys, and pigmented leptomeninges (primarily occurring dorsally). The incidence and severity of the changes were equivalent in both treatment groups indicating the changes were unrelated to the method of euthanasia. No ice crystallization was observed in the tissue of animals euthanized by rapid cooling. Of the fish that were placed at –20 °C after euthanasia, interfiber and intrafiber vacuoles, representing areas where ice crystallization had occurred (Figure 3), were present in all muscle tissue.
Figure 3.
Photomicrographs of zebrafish musculature. (A) Representative sample of muscle from a zebrafish euthanized with MS222. (B) Representative sample of muscle from a zebrafish euthanized by rapid cooling. (C) Representative sample of muscle from a zebrafish euthanized by rapid cooling and then placed in a –20 °C (–4 °F) freezer. Only (C) had evidence of ice crystal formation (arrows) in the muscle. Hematoxylin and eosin stain; magnification, ×600; bar, 50 µm.
Discussion
The data obtained from this study indicate significant differences between rapid cooling and unbuffered MS222 immersion as methods of euthanasia in zebrafish. As expected compared with MS222 immersion, rapid cooling in zebrafish did not cause ice crystallization in tissue and resulted in a reduced time until confirmed death. In addition, rapid cooling generated fewer indicators of distress. To ensure that the signs of distress actually were due to the MS222 and not to the fact that the MS222 solution produced an acidic environment, an additional group of fish was euthanized with buffered MS222. All animals exposed to buffered MS222 also displayed similar signs of distress as those treated with unbuffered MS222. This finding is consistent with a study performed in channel catfish (Ictalurus punctatus Rafinesque), which found that exposure time and MS222 concentration, but not buffering or pH, had significant effects on the stress responses of the fish (measured by blood glucose and plasma cortisol levels).12 Another study in rainbow trout (Salmo gairdneri) found that exposure to unbuffered MS222 actually decreased the blood glucose level, contrary to what is known to occur during a stressful event.9 Although we interpreted rapid opercular movements and erratic swimming as signs of distress after exposure to MS222, some of this activity may be normal behavioral changes as a fish passes through various anesthetic stages.10 However, neither of these behaviors occurred in animals placed in an ice–water bath. These behaviors could be indicators of distress, but they are observed so frequently with chemical anesthesia in fish that they typically are considered as behavioral responses during some stages of anesthesia.
Histologic examination of tissues from zebrafish euthanized by rapid cooling showed no greater evidence of possible tissue abnormalities than those of fish subjected to MS222 immersion. The guidelines promulgated by the AVMA state that exposure to 4 °C may cause formation of ice crystals on the skin and in tissues, resulting in pain or distress.1 However, considering that the ice–water solution never chills to a temperature of 0 °C (32 °F) due to the solute concentration in the water, the tissues would not cool to a temperature sufficient to cause ice crystallization. In addition, the solutes present in tissue would prevent ice crystal formation, even if the chilled water temperature reached 0 °C (32 °F). In the present study, the skin and deep tissues of fish euthanized by rapid cooling showed no evidence of ice crystal formation and appeared essentially identical to those of fish euthanized with MS222. However, the muscle tissue from the animals deliberately exposed to temperatures below freezing showed marked freeze artifact.
We elected to evaluate MS222 at the concentration of 250 mg/L, which is the minimal concentration suggested by the AVMA for euthanasia of amphibians and fish.1 Given our interest in finding an alternative to the use of MS222 for euthanasia, we did not evaluate higher concentrations that may have had differing outcomes on time until euthanasia in zebrafish. MS222 is known to cause a decrease in pH upon addition to fresh water. We initially chose to use unbuffered MS222 because the AVMA Guidelines on Euthanasia states that only solutions at or exceeding 500 mg/L need to be buffered and because the directions for the commercial MS222 product do not state that the solution needs to be buffered.1 However, in mixing the MS222 for this study, we added buffered system water, which resulted in a pH of 3.4. Even though the system water already contained buffering agents, the MS222 solution remained acidic. Zebrafish commonly are maintained in water with a pH between 7.0 and 8.0, although the optimal pH for zebrafish has yet to be determined.5 Some sources also suggest that fish should remain in MS222 solution for 10 min after the last opercular movement.1,11 Because we wanted to compare the effectiveness of each solution as a method of euthanasia, we removed animals 2 min after the last opercular movement and had a recovery rate of 17.4%. This finding indicates that the time until true death after exposure to MS222 is variable, unlike that after rapid cooling.
Our findings demonstrate that rapid cooling results in more rapid, less distressful, and more effective euthanasia than does MS222, a euthanasia agent advocated by the American Veterinary Medical Association and Institute for Laboratory Animal Research.1,8 Exposure of zebrafish to chilled water caused death more consistently than did exposure to MS222. The results of this study comprise a refinement to zebrafish euthanasia techniques and provide more information on techniques necessary for zebrafish studies for the laboratory animal and biomedical research community.
Acknowledgments
We thank Dr Mary Mullins for providing zebrafish, laboratory space, and materials for this experiment, Dave Cobb for providing invaluable help with performing this experiment, Shannon Chuai for assistance with statistical analysis, and Drs AL Smith and FC Hankenson for helpful review of the manuscript. This study was performed with support from University Laboratory Animal Resources at the University of Pennsylvania.
References
- 1.American Veterinary Medical Association [Internet] 2007. AVMA guidelines on euthanasia, 2007 update. [Cited June 2008]. Available at http://www.avma.org/issues/animal_welfare/euthanasia.pdf
- 2.Ashley PJ, Sneddon LU, McCrohan CR. 2006. Properties of corneal receptors in a teleost fish. Neurosci Lett 410:165–168 [DOI] [PubMed] [Google Scholar]
- 3.Detolla LJ, Srinivas S, Whitaker BR, Andrews C, Hecker B, Kane AS, Reimschuessel R. 1995. Guidelines for the care and use of fish in research. ILAR J 37:159–173 [DOI] [PubMed] [Google Scholar]
- 4.Harrell L. 1992. Handling and euthanasia in production facilities, p 129. In: Schaeffer DO, Kleinow KM, Krulisch L. The care and use of amphibians, reptiles, and fish in research Bethesda (MD): Scientists Center for Animal Welfare [Google Scholar]
- 5.Lawrence C. 2007. The husbandry of zebrafish (Danio rerio): a review. Aquaculture 269:1–20 [Google Scholar]
- 6.Martin BJ. 1995. Evaluation of hypothermia for anesthesia in reptiles and amphibians. ILAR J 37:186–190 [DOI] [PubMed] [Google Scholar]
- 7.Matthews M, Trevarrow B, Matthews J. 2002. A virtual tour of the Guide for zebrafish users. Lab Anim (NY) 31:34–40 [DOI] [PubMed] [Google Scholar]
- 8.National Research Council Committee on Pain and Distress in Laboratory Animals 1992. Recognition and alleviation of pain and distress in laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 9.Soivio A, Nyholm K, Huhti M. 1977. Effects of anaesthesia with MS222, neutralized MS222, and benzocaine on the blood constituents of rainbow trout, Salmo gairdneri. J Fish Biol 10:91–101 [Google Scholar]
- 10.Stoskopf M. 1993. Anesthesia and restraint, p 80–84. In: Fish medicine Philadelphia (PA): WB Saunders [Google Scholar]
- 11.Stoskopf M, Posner LP. 2008. Anesthesia and restraint of laboratory fish, p 519–534. In: Fish R, Danneman PJ, Brown M, Karas A. Anesthesia and analgesia in laboratory animals 2nd ed.San Diego (CA): Academic Press [Google Scholar]
- 12.Welker TL, Lim C, Yildirim-Aksoy M, Klesius PH. 2007. Effect of buffered and unbuffered tricaine methanesulfonate (MS222) at different concentrations on the stress responses of channel catfish, Ictalurus punctatus Rafinesque. J Appl Aquacult 19:1–18 [Google Scholar]
- 13.Westerfield M. 1995. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio), 3rd ed.Eugene (OR): University of Oregon Press [Google Scholar]