Abstract
Female Sprague–Dawley rats (n = 100; age, 3 wk) were fed diets that included a vitamin premix and either albumin or milk powder. Rats fed the albumin diet gained weight more slowly than did the other group. Between 19 and 28 wk of being fed the albumin diet, 12 rats died of bacterial cystitis and pyelonephritis. In addition, 2 more rats from the same dietary group developed peritonitis after ovariohysterectomy. Examination of the 44 rats fed the albumin diet that completed the 34-wk experiment revealed pyelonephritis in 68%, cystitis in 66%, urolithiasis in 27%, and nephrolithiasis in 5%. Squamous metaplasia of the transitional epithelium was present in all 44 rats, although other epithelia were histologically normal. Vitamin A deficiency was diagnosed after analyses of blood and liver samples. Analysis of the vitamin premix revealed approximately 25% of the expected amount of vitamin A. Because the milk powder contained sufficient vitamin A, deficiency did not occur in rats fed the milk powder diet. The major consequences of vitamin A deficiency in the rats were squamous metaplasia, bacterial infection, and calculus formation within the urinary tract. This report illustrates the importance of careful formulation and storage of vitamin premixes used in experimental diets. Vitamin A deficiency should be considered in rats with decreased weight gain and urinary tract disease even if ocular lesions are not present.
Vitamin A is necessary for normal epithelial maturation, vision, and immune function.21 Deliberately feeding rats vitamin-A–free diets typically results in weight loss and high mortality.2,10 Other reported signs of deficiency include corneal keratinization and ulceration, respiratory and skin infections, salivary gland enlargement, and urinary tract disease.2,4,19
Accidental feeding of vitamin-deficient diets can occur by using defective vitamin sources, adding insufficient vitamin to the diet, or by allowing vitamin degradation by incorrect storage prior to feeding. Although vitamin A is well recognized to degrade if stored inappropriately,22 there are no recent reports of rats being accidentally fed diets deficient in vitamin A. In this case report, rats fed diets containing low levels of vitamin A showed a different clinical presentation than that previously reported to occur in rats fed vitamin A-free diets.2,19 The present report demonstrates the importance of strict quality control to ensure that the expected vitamin concentration is present within an experimental diet.
Case Report
As part of an investigation into diet and bone mineralization, female Sprague–Dawley rats (n = 100; age, 3 wk; Massey University Small Animal Production Unit, Palmerston North, New Zealand) were fed 1 of 2 diets. Forty rats received diet containing 63% whole-milk powder and 29.5% starch (milk group), whereas the remaining 60 rats received a diet that contained a balanced protein content with 24.7% albumin, 10.9% soya oil, and 51% starch (albumin group). The milk-powder diet contained 18.3 kJ/g, and the albumin diet contained 18.8 kJ/g. Both diets were based on the AIN93G diet, and both contained 1% of a vitamin premix based on AIN93VX.18 This premix was purchased from an outside source and was stated to contain 250,000 IU/kg vitamin A to provide 2500 IU/kg within the formulated diet. Both diets also contained choline chloride. Animals were housed in accordance with the Guide for the Care and Use of Laboratory Animals,16 and all procedures were approved by the Massey University Animal Ethics Committee. Rats were housed individually in a room that was maintained at 22 °C on a 12:12-h light:dark cycle. Food was changed daily and provided ad libitum until week 20, when food was limited to 20 g per rat daily. Water was provided in water bottles that were changed weekly. The rats were from a colony that was free of internal and external parasites as well as rat coronavirus, rat parvovirus, Theiler murine encephalomyelitis virus, pneumonia virus of mice, and Mycoplasma pulmonis as determined by serologic testing.
Animals were weighed weekly throughout the experiment, and differences between the groups were investigated using ANOVA models (SPSS version 16.0 for Windows, SPSS, Chicago IL). Initially the mean weight of rats in the albumin group (55.5 g) was not significantly different from that of rats in the milk group (55.4 g). However, after 5 wk of receiving the experimental diets, rats in the albumin group (171.5 g) were significantly (P = 0.008) lighter than rats in the milk group (179.6 g). Rats in the milk group remained significantly heavier throughout the 34-wk study.
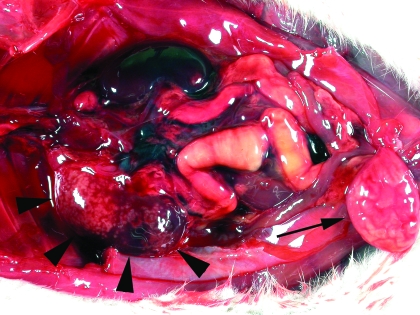
After 19 wk of the experiment, a rat in the albumin group was observed to be depressed and anorexic; the rat was found dead 1 d later. Necropsy examination revealed that the rat was in moderate body condition with some adipose reserves remaining. The most significant findings were present in the urinary system. Here, 1 kidney was markedly enlarged, with a renal pelvis that was dilated by viscous white fluid that extended as radiating streaks into the renal cortex. Both ureters were distended by white floccular fluid, which also was present in the distended bladder (Figure 1). Histologic examination of both kidneys revealed renal pelvises expanded by neutrophilic inflammation that included cellular debris, aggregates of keratinized squamous cells, and bacteria (Figure 2). The inflammation and necrosis extended into the surrounding medulla and cortex, and cavitation was prominent in 1 kidney. Neutrophilic inflammation containing aggregates of keratin debris also was present in both ureters and the bladder. The transitional epithelium throughout the urinary tract showed moderate squamous metaplasia. Pure growth of Escherichia coli was cultured from a sample of kidney. A diagnosis of ascending cystitis and pyelonephritis was made. At this time, the squamous metaplasia was considered to have developed in response to the bacterial infection.
Figure 1.
Urinary tract from a rat that died after a brief clinical illness. Note the multiple pale foci within the renal cortex (arrowheads). These pale areas were confirmed to represent pyelonephritis histologically. Marked dilation of both ureters and the bladder (arrow) is visible.
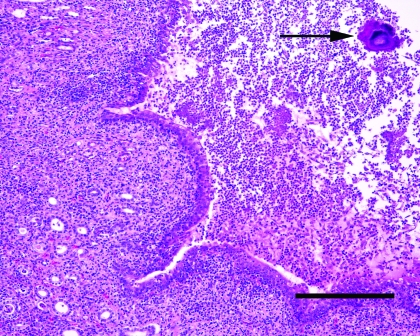
Figure 2.
Kidney from the rat pictured in Figure 1. Note expansion of the renal pelvis by large numbers of degenerate neutrophils and fibrin. Large colonies of bacteria are visible within the exudate (arrow). The transitional epithelium lining the pelvis shows mild squamous metaplasia. Inflammatory cells are also visible within surrounding tubules confirming the presence of a pyelonephritis. Hematoxylin and eosin stain; bar, 200 μm.
All rats were ovariohysterectomized after 20 wk of feeding. Two days after surgery, 2 rats from the albumin group were found dead. A malfunction of the heating system had resulted in a brief drop in temperature and, when necropsy examination did not reveal a cause of death, hypothermia was suspected. Two additional rats from the albumin group died 5 and 7 d after ovariohysterectomy. Necropsy examination of both rats revealed fibrin, neutrophils, and bacteria within the abdominal cavity. Peritonitis due to bacterial contamination during surgery was suspected. Because 3 rats had died of bacterial infections, all rats were treated with enrofloxacin (5 mg/kg SC every 12 h; Baytril, Bayer AG, Leverkusen, Germany) for 6 d as a precautionary measure.
Two days later, 21 wk after the experiment started, 2 more rats from the albumin group were found dead. A further 2 rats from the albumin group died 3 wk later. Similar to that in the earliest rat, necropsy of all rats revealed bilateral pyelonephritis and cystitis with prominent dilation of the renal pelvises, ureters, and bladder. Squamous metaplasia of the transitional epithelium was prominent within the renal pelvis, ureter, and bladder, with desquamated epithelial cells and keratin debris visible within the inflammatory exudate.
The development of urinary tract infection in 5 rats from the same dietary group suggested a predisposing cause. Vitamin A deficiency has previously been reported to cause squamous metaplasia of the urinary tract and bacterial infections of the bladder and kidney.4 To investigate possible deficiency of vitamin A, the vitamin premix, albumin diet, a postmortem liver sample, and 2 plasma samples from rats within the albumin group were analyzed by high-performance liquid chromatography by using standards with known vitamin A content.16 Vitamin A could not be detected in either the vitamin premix or the diet. Analysis of the liver sample revealed 0.3 µg/g vitamin A compared with reported normal hepatic vitamin A concentrations of 100 to 160 µg/g.9,24 Plasma vitamin A concentrations of 0.07 and 0.12 µmol/L (normal, 1.9 to 2.3 µmol/L)1,9 were obtained. These results confirmed that the rats were vitamin-A–deficient due to inadequate vitamin A in the experimental diet. The vitamin premix had been formulated 1 mo prior to the start of the study and stored in a ‘walk-in’ refrigerator at 4 °C. The premix was contained in a clear plastic bag that allowed exposure to light when the refrigerator door was open and when the refrigerator light was turned on. The premix was added to new batches of diet that were made at approximately 1-mo intervals and then stored in sealed black bags at 4 °C prior to usage. Because vitamin A degrades when exposed to light,22 the failure to detect vitamin A in the premix or diet was considered due to poor storage of the premix throughout the first 25 wk of the experiment. Because the premix was purchased from an external source, little consideration was given to the premix not containing the expected quantity of vitamin A.
To correct the deficiency, a new vitamin premix was purchased from the same source, and new diets were made. However, 3 d after the introduction of the new diet, 2 more rats from the albumin group died of bacterial pyelonephritis and cystitis. Again, squamous metaplasia of the transitional epithelium was a prominent histologic feature. Three weeks after the new diet was introduced, 2 more rats died with similar gross and histologic lesions, and an additional rat died 2 wk later; in addition to pyelonephritis, this rat also had multiple uroliths. The study was concluded 6 wk later after a total of 34 wk. During the 34-wk experiment, 16 of the 60 rats fed the albumin diet died, compared with no deaths among the 40 rats in the milk group.
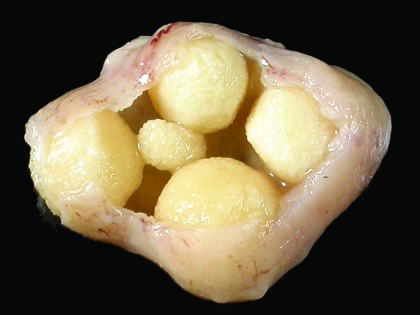
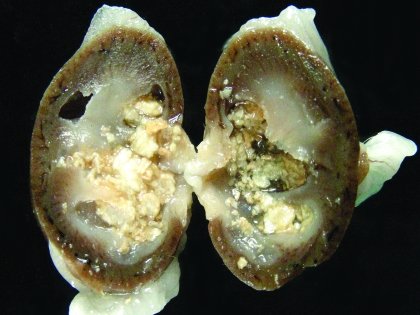
At the conclusion of the experiment, complete necropsies were performed on the remaining 44 rats in the albumin group as well as 6 rats from the milk group. Gross examination of rats in the albumin group revealed 12 (27%) had visible uroliths (Figure 3) and 2 (5%) had nephroliths (Figure 4). Histologic examination revealed bilateral pyelonephritis in 19 (43%) rats and unilateral pyelonephritis in 11 (25%) rats. The pyelonephritis was characterized by large numbers of neutrophils, degenerate cells, and keratin debris present within the renal pelvis. Large colonies of bacteria were visible admixed with inflammatory exudate in the kidneys from 7 (16%) rats. All rats with urinary calculi also had pyelonephritis. Microscopic foci of tubular mineralization were visible within the renal medullae of 10 (23%) rats, including the 4 rats with visible urinary calculi. The ureters from 20 (45%) rats contained squamous metaplasia, neutrophils, lymphocytes, and plasma cells (Figure 5). Cystitis was detected histologically in 29 (66%) rats. Mild to marked squamous metaplasia of the transitional epithelium was visible within all rats from the albumin group. As has previously been reported to occur in mice,15 variation in the severity of squamous metaplasia throughout the transitional epithelium in individual rats. Epithelial changes were not visible within sections of cornea, lacrimal gland, salivary gland, stomach, lingual papillae, or vagina from any of the rats examined. Necropsy examination of 6 rats from the milk group did not reveal significant lesions.
Figure 3.
Urinary bladder from a vitamin-A–deficient rat that did not show clinical evidence of disease. Note the presence of multiple large calculi within the bladder.
Figure 4.
Kidney from a vitamin-A–deficient rat that did not show clinical evidence of disease. Note the dilation of the renal pelvis and cavitation visible within the renal cortex. The pelvis contains multiple nephroliths, and foci of mineralization are scattered throughout the renal parenchyma.
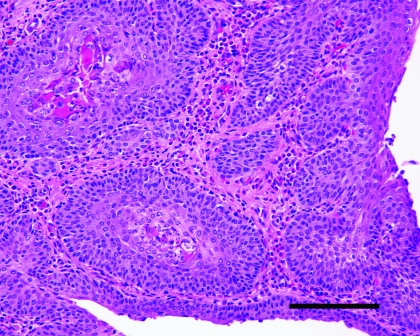
Figure 5.
Ureter from a vitamin-A–deficient rat that did not show clinical evidence of disease. Note the marked squamous metaplasia of the transitional epithelium. Keratin is visible within the epithelium, and moderate numbers of inflammatory cells are present. Hematoxylin and eosin stain; bar, 50 μm.
Because changing to diets made with new vitamin premix had not prevented further rat deaths or resolved the squamous metaplasia of the transitional epithelium, the replacement vitamin premix, which had been frozen and stored in the dark, was analyzed as before. It contained 70,000 IU/g vitamin A rather than the expected 250,000 IU/g. As the premix constituted 1% of the experimental diets, this would have resulted in a final vitamin A concentration of 700 IU/kg—well below the 1100 to 2500 IU/kg required to prevent deficiency.19 The low concentration of vitamin A within both premixes suggests either an error in the formulation of the premixes or that the vitamin A in the premix had degraded prior to delivery.
Discussion
Vitamin A regulates epithelial cell growth and differentiation, enables production of visual pigment, is necessary for normal function of the immune system, and influences skeletal development.21,24 When rats are fed diets that contain no vitamin A the most commonly reported clinical signs of deficiency are weight loss, corneal keratinization and ulceration, respiratory or skin disease, and salivary gland enlargement.2,10,19 An 80% mortality rate due to inanition or bacterial infection of the skin or respiratory tract is expected after 15 wk.2,19 In the present case report, the first observed sign of vitamin A deficiency was reduced growth rate. Although failure to gain weight is a consistent sign of vitamin A deficiency in rats, the mechanism of this effect is uncertain.7,19
Two vitamin premixes were used during this experiment. No vitamin A was detected in the original premix, but it had been stored at 4 °C and regularly exposed to light rather than being frozen and kept in the dark as recommended. Therefore, any vitamin A initially present within the original premix might have degraded prior to analysis. The replacement vitamin premix contained 70,000 IU/kg vitamin A rather than the stated 250,000 IU/kg. Because the same company formulated both the original and replacement premixes, both likely initially contained a similar concentration of vitamin A. This scenario suggests that rats in the albumin group received small amounts of vitamin A throughout most of the experiment. This hypothesis is supported by the detection of vitamin A, albeit at low concentrations, within samples of plasma and liver taken after 25 wk of feeding the deficient diet. In addition, unlike rats fed diets that contain no vitamin A,2 rats in the present case report continued to gain weight slowly throughout the experiment, and the majority survived 34 wk of deficiency.
In contrast to studies of rats fed vitamin A-free diets, the predominant cause of mortality in the present case report was urinary tract infection. Urinary tract disease as the predominant sign of vitamin A deficiency appears to be rare in rats, but it has been reported previously.4 Rats in the previous study were repeatedly supplemented with low doses of vitamin A to prevent death due to inanition.4 After 22 wk of deficiency, 25% of the rats had died of urinary tract infection accompanied by marked squamous metaplasia of the urinary tract.4 In the present case report, all vitamin A-deficient rats had squamous metaplasia that was confined to the transitional epithelium. The restriction of the squamous metaplasia to the transitional epithelium suggests that this epithelial type is the most susceptible to vitamin A deficiency in rats. Squamous metaplasia can predispose animals to bacterial infection.13 The development of squamous metaplasia only in the transitional epithelium is the likely reason that bacterial infections were only observed within the urinary tract in the presently described rats. Evidence from the previous study4 and this case report suggests that less severe vitamin A deficiency in rats results in a predominance of disease within the urinary tract.
Although both diets contained the defective vitamin premix, deficiency only occurred in rats within the albumin group. This result occurred because the milk powder contained in the milk diet contained 15,500 IU/kg vitamin A. Therefore, this diet contained 9729 IU/kg vitamin A, well above the 1100 to 2500 IU/kg required to prevent deficiency.19 In contrast, because none of the ingredients within the albumin diet contained vitamin A, deficiency developed within this dietary group.
After 34 wk of vitamin A deficiency, 32% of the rats had developed urinary calculi. Urinary calculus development occurred in rats fed a vitamin A-deficient diet for 18 wk.7 In addition, feeding a diet that was both calculogenic and deficient in vitamin A for 22 wk resulted in urinary calculi in 86% of the rats.12 The mechanisms by which vitamin A deficiency causes calculus formation in rats are unclear. Vitamin A deficiency alters the composition of urine.8,25 In addition, squamous metaplasia of the urinary tract can result in keratin debris which promotes calculus formation.13 In the present case report, calculi were not apparent in rats that died before 28 wk of deficiency. After 34 wk of deficiency, all evaluated rats had squamous metaplasia of the urinary tract. Therefore it is difficult to determine whether calculi formed due to squamous metaplasia, the altered composition of urine, or a combination of both.
The original AIN76 diet was modified in 1993 in part to reduce the development of urinary calculi.18 Therefore, calculi are expected to be rare when using the AIN93 diet.18 However, replacement of levorotary (l) choline bitartrate with the synthetic racemic (dl) choline bitartrate has been shown to induce urinary calculi in rats fed the AIN-93 diet.3,14,17 This difference is probably due to the comparative insolubility of dl-choline bitartrate, which results in tubular precipitation and subsequent crystalluria.6 In addition, inadequate mixing of AIN93 diets may predispose animals to calculus formation due to isolated areas containing high concentrations of selenium.17 Incorrect formulation resulting in diets deficient in vitamin B6 also can predispose rats to urinary calculus formation.5
Two vitamin-A–deficient rats developed peritonitis shortly after ovariohysterectomy. Vitamin A deficiency can impair many components of the immune system.20,23,24 Therefore, although surgical contamination was the likely source of the bacteria, perhaps an otherwise insignificant bacterial contamination resulted in fatal peritonitis due to an impaired immune system. However, only 2 rats developed peritonitis, and it could be a coincidence that both were vitamin A-deficient. Likewise, the extent to which immune function impairment predisposed the deficient rats to urinary tract infections is unknown.
Although the clinical signs and histologic lesions in the rats in this case report were consistent with vitamin A deficiency, definitive diagnosis required analysis of samples from the affected rats. Analysis of the diet and vitamin premixes allowed the cause of the deficiency to be determined. However, although vitamin A deficiency can be confirmed, the possibility that the rats had multiple additional vitamin or mineral deficiencies cannot be definitively excluded.
This case report illustrates the need for stringent quality controls on the formulation and storage of vitamin premixes. A faulty vitamin premix can, as in this case, result in a diet that fails to meet the requirements of the animal, causing a deficiency syndrome. However, faulty vitamin premixes can also compromise experimental results. In the present report, the incorrect vitamin A content of the premix was detected because there was no additional source of vitamin A within the albumin-based diet. If both diets had contained an additional source of vitamin A, deficiency would not have developed, but the low vitamin A within the premix could have influenced the experimental results.
In conclusion, the vitamin A deficiency in the described rats presented clinically as failure to gain weight followed by increased urinary tract infections. Histologic examination revealed squamous metaplasia that was confined to the urinary tract. In these rats, the amount of vitamin A in the diet appeared sufficient to prevent more well-recognized symptoms of deficiency such as inanition, ocular lesions, and respiratory tract infection.19 Therefore, vitamin A deficiency should be considered even in the absence of these changes. Analysis of diet and tissue samples is required for definitive diagnosis of vitamin A deficiency. This case highlights the importance of rigorous standards in the production and storage of vitamin premixes and formulated diets.
References
- 1.Audouin-Chevallier I, Higueret P, Pallet V, Higueret D, Garcin H. 1993. Dietary vitamin A modulates the properties of retinoic acid and glucocorticoid receptors in rat liver. J Nutr 123:1195–1202 [DOI] [PubMed] [Google Scholar]
- 2.Beaver DL. 1961. Vitamin A deficiency in the germ-free rat. Am J Pathol 38:335–357 [PMC free article] [PubMed] [Google Scholar]
- 3.Bollen AM, Baskin CR, Treuting PM. 2006. Urolithiasis in rats consuming a dl bitartrate form of choline in a purified diet. Comp Med 56:245.[author reply 245–246] [PubMed] [Google Scholar]
- 4.Cohen SM, Wittenberg JF, Bryan GT. 1976. Effect of avitaminosis A and hypervitaminosis A on urinary bladder carcinogenicity of N-(4-(5-nitro-2-furyl)-2-thiazolyl)formamide. Cancer Res 36:2334–2339 [PubMed] [Google Scholar]
- 5.Di Tommaso L, Tolomelli B, Mezzini R, Marchetti M, Cenacchi G, Foschini MP, Mancini AM. 2002. Renal calcium phosphate and oxalate deposition in prolonged vitamin B6 deficiency: studies on a rat model of urolithiasis. BJU Int 89:571–575 [DOI] [PubMed] [Google Scholar]
- 6.Down WH, Sacharin RM, Chasseaud LF, Kirkpatrick D, Franklin ER. 1977. Renal and bone uptake of tartaric acid in rats: comparison of L(+) and DL forms. Toxicology 8:333–346 [DOI] [PubMed] [Google Scholar]
- 7.Gershoff SN, McGandy RB. 1981. The effects of vitamin A-deficient diets containing lactose in producing bladder calculi and tumors in rats. Am J Clin Nutr 34:483–489 [DOI] [PubMed] [Google Scholar]
- 8.Grases F, Garcia-Gonzalez R, Genestar C, Torres JJ, March JG. 1998. Vitamin A and urolithiasis. Clin Chim Acta 269:147–157 [DOI] [PubMed] [Google Scholar]
- 9.Green MH, Green JB. 1994. Vitamin A intake and status influence retinol balance, utilization and dynamics in rats. J Nutr 124:2477–2485 [DOI] [PubMed] [Google Scholar]
- 10.Heaton FW, Lowe JS, Morton RA. 1957. Aspects of vitamin A deficiency in the rat. Biochem J 67:208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kancha RK, Anasuya A. 1990. Effect of vitamin A deficiency on urinary calculus formation in rats. J Clin Biochem Nutr 8:51–60 [Google Scholar]
- 12.Kane A, Kumar V. 2005. Environmental and nutritional pathology, p 415–468. In: Kumar V, Abul K, Fausto N. Robins and Cotran pathologic basis of disease Philadelphia (PA): Elsevier Saunders [Google Scholar]
- 13.Kankesan J, Vanama R, Renlund R, Thiessen JJ, Ling V, Rao PM, Rajalakshmi S, Sarma DS. 2003. Source of a micronutrient in a semi-synthetic basal diet as a causative factor in inducing urinary calculi in rats and its inhibition by PSC 833, a potent inhibitor of P-glycoprotein. Comp Med 53:444–447 [PubMed] [Google Scholar]
- 14.Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng FM, Wu XR, Shapiro E, Sun TT. 2005. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol 171:835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMurray CH, Blanchflower WJ. 1979. Application of a high-performance liquid chromatographic fluorescence method for the rapid determination of α-tocopherol in the plasma of cattle and pigs and its comparison with direct fluorescence and high-performance liquid chromatography–ultraviolet detection methods. J Chromatogr 178:525–531 [DOI] [PubMed] [Google Scholar]
- 16.Institute of Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 17.Newland MC, Reile PA, Sartin EA, Hart M, Craig-Schmidt MC, Mandel I, Mandel N. 2005. Urolithiasis in rats consuming a DL bitartrate form of choline in a purified diet. Comp Med 55:354–367 [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC., Jr 1993. AIN93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN76A rodent diet. J Nutr 123:1939–1951 [DOI] [PubMed] [Google Scholar]
- 19.Rogers AE. 1979. Nutrition, p 138–146. In: Baker HJ, Lindsey JR, Weisbroth SH. The laboratory rat San Diego (CA): Academic Press [Google Scholar]
- 20.Smith SM, Levy NS, Hayes CE. 1987. Impaired immunity in vitamin A-deficient mice. J Nutr 117:857–865 [DOI] [PubMed] [Google Scholar]
- 21.Weber F. 1983. Biochemical mechanisms of vitamin A action. Proc Nutr Soc 42:31–41 [DOI] [PubMed] [Google Scholar]
- 22.Whited LJ, Hammond BH, Chapman KW, Boor KJ. 2002. Vitamin A degradation and light-oxidized flavor defects in milk. J Dairy Sci 85:351–354 [DOI] [PubMed] [Google Scholar]
- 23.Wiedermann U, Hanson LA, Holmgren J, Kahu H, Dahlgren UI. 1993. Impaired mucosal antibody response to cholera toxin in vitamin A-deficient rats immunized with oral cholera vaccine. Infect Immun 61:3952–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. 1993. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology 80:581–586 [PMC free article] [PubMed] [Google Scholar]
- 25.Zile M, DeLuca HF, Ahrens H. 1972. Vitamin A deficiency and urinary calcium excretion in rats. J Nutr 102:1255–1258 [DOI] [PubMed] [Google Scholar]