Abstract
Large granular lymphocyte (LGL) leukemia is a clonal proliferative disease of T and natural killer (NK) cells. Interleukin (IL)-15 is important for the development and progression of LGL leukemia and is a survival factor for normal NK and T memory cells. IL-15 alters expression of Bcl-2 family members, Bcl-2, Bcl-XL, Bim, Noxa, and Mcl-1; however, effects on Bid have not been shown. Using an adoptive transfer model, we show that NK cells from Bid-deficient mice survive longer than cells from wild-type control mice when transferred into IL-15-null mice. In normal human NK cells, IL-15 significantly reduces Bid accumulation. Decreases in Bid are not due to alterations in RNA accumulation but result from increased proteasomal degradation. IL-15 up-regulates the E3 ligase HDM2 and we find that HDM2 directly interacts with Bid. HDM2 suppression by short hairpin RNA increases Bid accumulation lending further support for HDM2 involvement in Bid degradation. In primary leukemic LGLs, Bid levels are low but are reversed with bortezomib treatment with subsequent increases in LGL apoptosis. Overall, these data provide a novel molecular mechanism for IL-15 control of Bid that potentially links this cytokine to leukemogenesis through targeted proteasome degradation of Bid and offers the possibility that proteasome inhibitors may aid in the treatment of LGL leukemia.
Introduction
Large granular lymphocyte (LGL) leukemias are rare lympho-proliferative diseases defined by clonal amplification of CD3+ T cell or CD3− natural killer (NK) cell lineages. Clinical manifestations include splenomegaly, fatigue, recurrent bacterial infections, and neutropenia, which is prevalent in a majority of T-LGL leukemia patients (1).
Cytokines in LGL leukemias have been studied (2–4), and interleukin (IL)-15, a member of the IL-2 family, has been implicated in LGL leukemia through transgenic mouse studies (5). IL-15 stimulates T-LGL leukemia cell proliferation and cytotoxicity (4); however, it functions in other pathogenic states such as central nervous system leukemia relapse in acute lymphoblastic leukemia and in autoimmune diseases (6–9).
IL-15 was initially identified as a T-cell growth factor (10) but is critical for NK cell survival, proliferation, and cytotoxicity (11–13). IL-15 interacts with the IL-15 receptor, which is composed of a high-affinity IL-15–specific chain, IL-15 receptor α, the IL-2/IL-15Rβ chain, which is shared by IL-2, and the γccommon chain used by all IL-2 family cytokines for signal transduction. Mice deficient in IL-15, IL-2/IL-15Rβ, or IL-15Rα- have severely decreased numbers of NK cells, γ/δ T cells, CD8+ T cells, and CD8+ memory cells (13–15), supporting a role for IL-15 in both naive and memory CD8+ T cell homeostasis as well as NK cell homeostasis.
Carson and colleagues initially implicated IL-15 in cell survival by showing that IL-15 could sustain NK cells in serum-free medium (12). All other family members, except IL-2, cannot promote NK cell survival, although they share the common γc signaling chain. The molecular mechanism for this difference is at least partially due to IL-15 maintenance of Bcl-2 in NK cells (12). Mouse knockout/transgenic studies show that adoptively transferred wild-type NK cells are depleted in IL-15−/− mice and this effect is reversed by the transfer of Bcl-2 transgenic NK cells (16, 17).
IL-15 is a central factor in autoimmunity and cancer. Thus, we focused our attention on NK cells, which affect autoimmune function, and LGL leukemia cells. In NK cells, we report that IL-15 specifically reduces Bid, a critical apoptotic factor and BH3-only subgroup member of the Bcl-2 family, by a proteasome-dependent mechanism. We show that T-LGL and NK-LGL leukemias have significantly reduced Bid accumulation that can be reversed by inhibition of either IL-15 or the proteasome pathway. The reversal in Bid accumulation preludes increased leukemic cell death, suggesting a potential link between IL-15 and BH3-only proteins in clonal leukemic cell survival and the pathogenesis of some human hematologic malignancies.
Materials and Methods
Mice
Bid−/− mice on a C57Bl6 background have been described previously (18, 19). IL-15−/− mice were obtained from Drs. Yutaka Tagaya and T.A. Waldmann. Experiments were done under protocols approved by Animal Use and Care Committees at NIH in accordance with NIH guidelines as outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH Publication 86-23, 1985).
Human cell isolation, culture, and treatments
Highly purified NK and T cells were obtained from peripheral blood mononuclear cells (PBMC) as described previously (20). Cells were cultured in RPMI 1640 (BioWhittaker) supplemented with 10% FCS or human AB serum, 2 mmol/L l-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin and grown in 5% CO2 at 37°C. 293T cells were maintained in DMEM with 10% fetal bovine serum, penicillin, and streptomycin. Treatments: IL-15, IL-21 (Peprotech), IL-2 (Hoffmann-La Roche), and IL-18 (Research Diagnostics) were added at 10 to 20 ng/mL, 100 ng/mL, 100 IU/mL, and 100 ng/mL, respectively. Lactacystin (Calbiochem/EMD) and bortezomib/Velcade/PS-341 (Millennium Pharmaceuticals) were used at final concentrations of 5 μmol/L and 2.5 to 5 mmol/L, respectively. PYR-41 was provided by Dr. Allan Weissman (National Cancer Institute-Frederick) and used at 50 μmol/L concentration (21). Cycloheximide (Sigma-Aldrich) was used at concentrations of 0.1 to 10 μg/mL. Cell signaling pathways were evaluated using the following inhibitors: PD98059 and SB203580 (Calbiochem/EMD) and LY294002 (Biosource/Invitrogen).
Adoptive cell transfer
Freshly isolated splenic NK cells from wild-type and Bid−/− mice were labeled with 5 μmol/L CFSE (room temperature, 8 min). Cells were washed once with RPMI 1640 containing 10% FCS and then two washes with HBSS. Cells were resuspended in HBSS and equal numbers (2.5 × 106-5 × 106 per mouse) were injected into tail veins of IL-15+/+ and IL-15−/− mice. Mice were sacrificed 24 h later, and splenic and hepatic lymphocytes were isolated and stained with PE anti-NKp46 (R&D Systems) and PerCP-Cy5.5 anti-mouse CD5 (eBioscience). Flow cytometry analysis determined the number of CFSE-positive transferred NK cells in each organ.
TA-Bid-GFP expression construct design and transduction
cDNA from reverse-transcribed human NK cell RNA was cloned into pcDNA3.1/CT-GFP-TOPO (Invitrogen) to create a TA-Bid-GFP expression vector. Construction PCR primers used: forward CCACCATGGACTGTGAGGTCAACAACGG and reverse GGTCCATCCCATTTCTGGCTAAG.
DNA clones were isolated and sequenced before experimental use. LGL leukemia cells were transduced by Amaxa nucleofector system for primary T cells with TA-Bid-GFP, pd4EGFP-Bid, and control pd4EGFP (BD Clontech) vectors.
Protein isolation and Western analysis
Total cellular protein isolation, Western analysis, and protein detection were done as described previously (20). Blots were probed with rabbit anti-Bid (BD Pharmingen) and goat anti-actin (Santa Cruz Biotechnology) antibodies. Other Bcl-2 family members, p38, Akt, and p42/44, were detected with antibodies from Cell Signaling Technology. HDM-2 detection used antibodies from BD Pharmingen and Zymed/Invitrogen.
Lentivirus production and transduction
Self-inactivating pLKO.1 short hairpin RNA (shRNA) constructs for HDM2 (Open Biosystems) were used to generate vesicular stomatitis virus-pseudotyped lentiviral vectors as described previously (22). Briefly, HDM2-shRNA constructs and non-silencing shRNA pLKO.1 vectors were transfected into 293FT cells using FuGENE 6 (Roche) according to the manufacturer's instruction. 293A cells were transduced by lentivirus in the presence of 8 μg/mL polybrene (Sigma-Aldrich) for 12 h. Seventy-two hours post-transduction, cells were placed on 5 μg/mL puromycin (Sigma-Aldrich) for 4 days and then harvested and analyzed for HDM2 knockdown efficiency by Western analysis.
Leukemia samples, patients, and preparation of PBMC
Leukemia samples obtained from 10 T-LGL and 5 NK-LGL donors met the clinical criteria of LGL leukemia with increased numbers of CD3+, CD8+, or CD57+ lymphocytes for T-LGL leukemia increased CD3−/CD16+ and CD3−/CD56+ lymphocytes in NK-LGL leukemia (23). All patients had chronic disease and were clinically stable and untreated at the time of sample acquisition. Informed consents were signed by patients before the use of their cells for the experiments according to the protocols approved by the Institutional Review Board of the Penn State Cancer Institute. Peripheral blood specimens from LGL patients were collected, and buffy coat samples from age- and gender-matched normal donors were obtained from the blood bank of Milton S. Hershey Medicine Center at College of Medicine, Penn State University. Buffy coat PBMCs were isolated by Ficoll-Hypaque gradient separation as described previously (24). Cell viability as determined by trypan blue was >95%.
Apoptosis assay
LGL and normal PBMCs were treated with bortezomib at 2.5 and 5 nmol/L, respectively. After 24 and 48 h, two-color flow cytometry was done following 7-amino-actinomycin D (7AAD) and Annexin V-FITC (EMD Biosciences) staining. For GFP-Bid-transfected cells, apoptosis was measured by Annexin V-PE-7AAD (EMD Biosciences) cell staining 24 h post-transfection. Percent-specific apoptosis was calculated as follows: Apoptosis (%) = (% Annexin V positive in the assay well - % Annexin V positive in the control well) × 100 / (100 - % Annexin V positive in the control well). Technical triplicates were used for each treatment per experiment.
Results
IL-2 family of cytokines regulates Bid expression
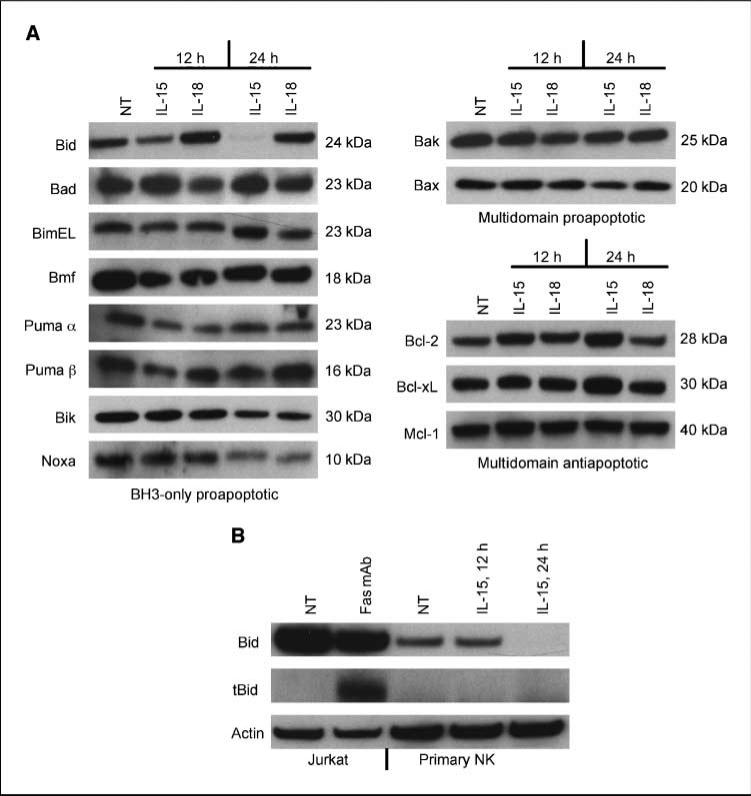
The γc signaling chain cytokines contribute to murine T and NK cell survival through control of the proapoptotic and antiapoptotic proteins Bcl-2, Bax, Bim, Noxa, and Mcl-1 (12, 25, 26). We extended this knowledge to human cells by stimulating NK cells with IL-15 and examining proapoptotic and antiapoptotic Bcl-2 family expression. IL-15 slightly up-regulated the multidomain antiapoptotic factor Bcl-2 but had little to no effect on Bcl-xL or Mcl-1, nor did it affect the proapoptotic proteins Bax and Bak (Fig. 1A). With the exception of Bid, BH3-only family members were not dramatically altered. IL-15 slightly down-regulated Noxa as well as the α and β forms of Puma, but this effect was not specific, as changes in these proteins were observed in cells treated with IL-18, a non-γc signaling cytokine. In contrast, IL-15 dramatically affected Bid and this effect was specific as evidenced by the inability of IL-18 to alter Bid (Fig. 1A). By 12 h IL-15 treatment, Bid was reduced by ~50% and by 24 h was virtually absent (Fig. 1A). However, we did observe slower decay rates in some donors (compare 12 h treatment points in Fig. 1A and B). Nevertheless, in all donors, Bid was reduced to >90% by 24 h.
Figure 1.
Expression of Bcl-2 family members in primary human NK cells. A, primary human NK cells were stimulated with 20 ng/mL IL-15 or 100 ng/mL IL-18 for 12 and 24 h. Protein extracts were separated by SDS-PAGE and Western analysis was done using anti-Bcl-2 family antibodies. B, extracts from anti-Fas monoclonal antibody (Millipore). Jurkat cells were compared with extracts from IL-15–treated primary human NK by Western analysis using an anti-Bid polyclonal antibody. Blots were reprobed with anti-actin as a control. Representative of four individual donors.
To determine if other γc signaling cytokines had similar effects on Bid, we treated primary human NK cells with IL-2 and IL-21 and compared their actions to IL-15. All cytokines down-regulated Bid, but the effect was not immediate, as IL-15 alone reduced Bid by 12 h, whereas all three cytokines effectively diminished Bid by 24 h (Supplementary Fig. S1A).
In vivo, IL-15 is complexed at the cell membrane with IL-15Rα and is presented in trans to cells expressing the IL-2/IL-15Rβ and γc chains (27). Our results indicated that noncomplexed IL-15 down-regulated Bid; however, we asked if a more physiologic form of IL-15 could alter Bid expression. For this, an IL-15/IL-15Rα complex was added to primary human NK cells and we found that concentrations of ≥500 pg reduced Bid levels (Supplementary Fig. S1B). These results show that both free and complexed forms of IL-15 are effective at reducing Bid.
Reductions in full-length Bid are concomitant with increases in truncated Bid (tBid; ref. 28). To ascertain if tBid is required for NK-associated decreases in Bid, we treated NK cells with IL-15 for 12 and 24 h and compared tBid accumulation in these cells to Jurkat cells treated with an anti-Fas monoclonal antibody for 12 h. As expected, large increases in tBid were observed in monoclonal antibody-treated Jurkat cells; however, IL-15 had no effect on tBid in NK cells even after 24 h when full-length Bid was absent (Fig. 1B). This suggests that IL-15 elimination of Bid in NK cells does not require Bid cleavage.
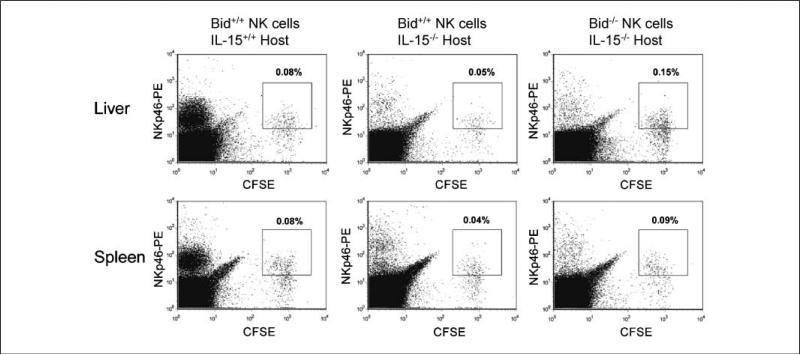
IL-15 is critical for NK cell survival as shown by loss of adoptively transferred wild-type NK cells in IL-15−/− mice (16). To determine if Bid plays a role in NK cell survival, CFSE-labeled primary NK cells from Bid−/− mice were adoptively transferred into IL-15−/− mice. First, we confirmed the requirement of IL-15 by NK cells for survival by adoptively transferring CSFE labeled Bid+/+ NK cells into IL-15−/− mice and found 40% to 50% reduction in Bid+/+ NK cells in the livers and spleens of IL-15−/− mice compared with IL-15+/+ mice (Fig. 2). In contrast, adoptively transferred NK cells from Bid−/− mice restored the NK cell population in IL-15−/− mice to levels equal to or greater than that observed in the IL-15+/+ mice. These results suggest that both IL-15 and Bid play active roles in NK cell survival.
Figure 2.
Detection of CFSE-labeled NK cells in IL-15+/+ and IL-15−/− mice. CFSE-labeled Bid+/+ NK cells were transferred into IL-15+/+ and IL-15−/− mice. Additionally, CFSE-labeled Bid−/− NK cells were transferred into IL-15−/− mice. The amount of NKp46+CFSE+ double-positive NK cells in liver and spleen was evaluated 24 h later by fluorescence-activated cell sorting analysis. The percentage of CFSE+NKp46+ double-positive cells is represented by boxes within each dot blot. Representative of two independent experiments each with six individual recipient mice.
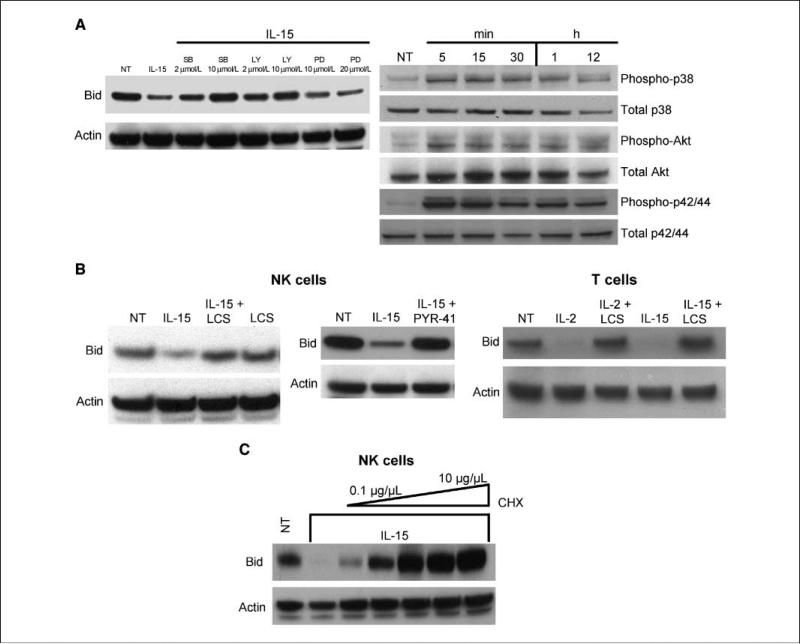
To determine the pathway(s) involved in Bid regulation, we treated primary human NK cells with IL-15 in the presence of the phosphatidylinositol 3-kinase inhibitor LY294002 or a mitogen-activated protein kinase inhibitor, PD98059. As a control, IL-15 was added to SB203580-pretreated cells because p38 is not thought to be a major downstream γc signaling kinase (29). We found that LY294002 effectively reversed the decrease in Bid, but PD98059 had no effect, showing that the phosphatidylinositol 3-kinase/Akt, but not the mitogen-activated protein kinase, pathway plays an active role in Bid regulation (Fig. 3A). Surprisingly, the p38 inhibitor completely reversed the Bid diminution, suggesting that, in human NK cells, IL-15 uses this pathway to alter Bid expression.
Figure 3.
Signaling and cellular pathways involved in IL-15 control of Bid. A, primary human NK cells were pretreated with LY294002 (LY), PD98059 (PD), or SB203580 (SB) for 1 h before IL-15 addition. Cells were cotreated for 12 h, lysed, and analyzed by Western blot using a polyclonal anti-Bid antibody. Kinetics of kinase phosphorylation in primary human NK cells treated with IL-15 for the indicated times was examined by Western blot using phosphospecific anti-p38, anti-Akt, and anti-p42/p44 antibodies. Blots were quenched and reprobed with anti-p38, Akt, and p42/p44 polyclonal antibodies. Representative of one of three experiments using cells from individual donors. B, primary human NK and PHA-activated T cells were cotreated with IL-2, IL-15, and lactacystin or the ubiquitin-conjugating enzyme inhibitor PYR-41 for 12 h. Protein lysates were analyzed by Western blot using a polyclonal anti-Bid antibody. Blots were reprobed with a goat polyclonal anti-actin antibody. C, NK cells were treated with increasing concentrations of cycloheximide (0.1-10 μg/mL) for 12 h and cell lysates were analyzed by Western blot using an anti-Bid antibody. All the experiments were done on at least three individual donors. LCS, lactacystin; CHX, cycloheximide.
We then examined p38, Akt, and extracellular signal-regulated kinase p42/p44 phosphorylation kinetics and found that all pathways were activated within 5 min IL-15 addition to the NK cells (Fig. 3A). Interestingly, the phosphorylated target proteins were evident for up to 12 h, showing that IL-15 can sustain long-term activation of signaling cascades in human NK cells.
We next asked if transcriptional changes accounted for the decline in Bid and found no change in Bid mRNA accumulation following IL-15 treatment (Supplementary Fig. S2). We then focused on post-translational control mechanisms and asked whether IL-15 altered Bid through proteasomal degradation. Primary human NK cells were treated with IL-15 in the presence and absence of lactacystin, an irreversible 20S proteasome inhibitor. As observed previously, IL-15 reduced Bid and lactacystin reversed this effect but did nothing when added alone (Fig. 3B). Further support for ubiquitin-proteasome pathway involvement came from our finding that PYR-41 inhibition of ubiquitin-activating enzyme completely reversed Bid degradation (Fig. 3B). Proteasome-associated depletion of Bid was not specific to NK cells, as purified healthy donor T cells also exhibited IL-2- and IL-15–mediated Bid degradation (Fig. 3B).
Bid decay is not rapid (Fig. 1); thus, IL-15 may not act directly on Bid but instead mediate its effect through synthesis of an essential degradation factor. To test this hypothesis, we treated primary human NK cells with IL-15 in the presence of increasing concentrations of the translational inhibitor cycloheximide. Western analysis showed that Bid reduction is blocked by cycloheximide in a concentration-dependent fashion with effective dose ranges from 0.1 to 10 μg/mL (Fig. 3C). Collectively, these data suggest that Bid is regulated by a proteasomal decay mechanism that requires new protein synthesis.
Bid interacts with the E3 ligase HDM2
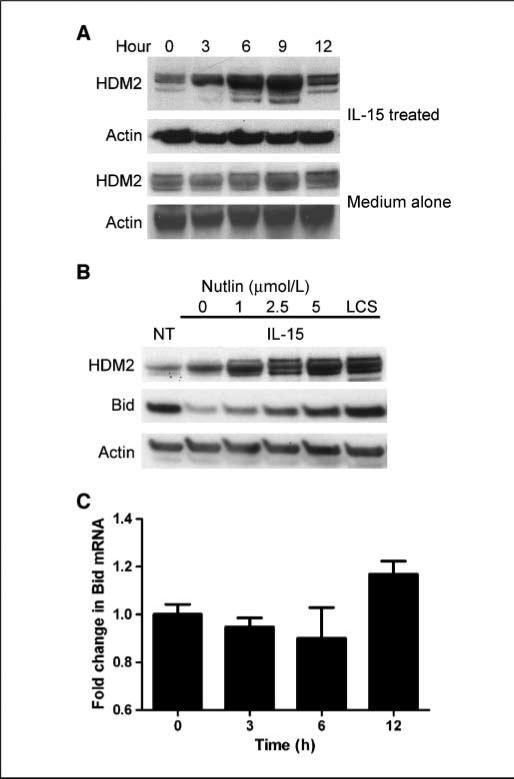
Bid is involved in multiple cellular processes and may have functions similar to p53 (30, 31). In humans, p53 is controlled by the E3 ubiquitin ligase mouse double-minute 2 protein (HDM2), so we sought to determine whether Bid and p53 require/use the same ubiquitin-proteasome-associated factors. Gene array and quantitative PCR analysis showed that IL-15 up-regulated HDM2 mRNA (data not shown). We next asked if IL-15 elevated HDM2 protein levels in NK cells. Increases in HDM2 were observed by 3 h IL-15 treatment and continued to increase for up to 9 h (Fig. 4A). Interestingly, HDM2 levels diminished by 12 h, a time coordinate with Bid disappearance in NK cells. In addition, we cultured NK cells in medium alone and found that HDM2 remains constant, further supporting a role for IL-15 regulation of HDM2.
Figure 4.
IL-15 alters HDM2 expression. A, primary NK cells were treated with IL-15 or medium alone for the indicated times. Cell lysates were analyzed by Western blot using a mouse anti-HDM2 antibody. Blots were reprobed with goat anti-actin. B, primary human NK cells were treated with IL-15 and increasing concentrations of nutlin-3 or with lactacystin for 12 h. Cell lysates were analyzed by Western blot using mouse anti-HDM2 and polyclonal anti-Bid antibodies. Blots were reprobed with a goat anti-actin antibody. C, nutlin-3 was added to NK cells at a final concentration of 5 μmol/L and quantitative PCR for Bid expression was done on reverse-transcribed DNA at the indicated times. Fold change in Bid mRNA levels relative to 0 h nutlin-3 addition. Values ± SE were calculated using Relative Quantification Software (Roche Applied Science). Representative of three individual donors.
We next asked if nutlin-3, a specific HDM2 inhibitor, blocks Bid degradation in the presence of IL-15 and found that nutlin-3 reversed Bid decay in a concentration-dependent fashion (Fig. 4B). Additionally, as reported previously, nutlin-3 enhanced HDM2 levels (32). HDM2 is partially regulated by autoubiquitination and proteasome-dependent degradation. To ascertain if proteasome inhibition coordinately increases HDM2 and Bid, we treated cells with lactacystin and found that both proteins were elevated (Fig. 4B). This shows that proteasome activity influences the level of both factors in NK cells. One byproduct of nutlin-3 treatment is p53 activation (32), which in turn alters Bid gene transcription (33). To determine if nutlin-3 has effects on Bid gene expression, quantitative PCR was done on RNA isolated from NK cells. At all times examined, Bid mRNA accumulation was relatively unchanged with only a modest 1.2-fold increase observed after 12 h (Fig. 4C).
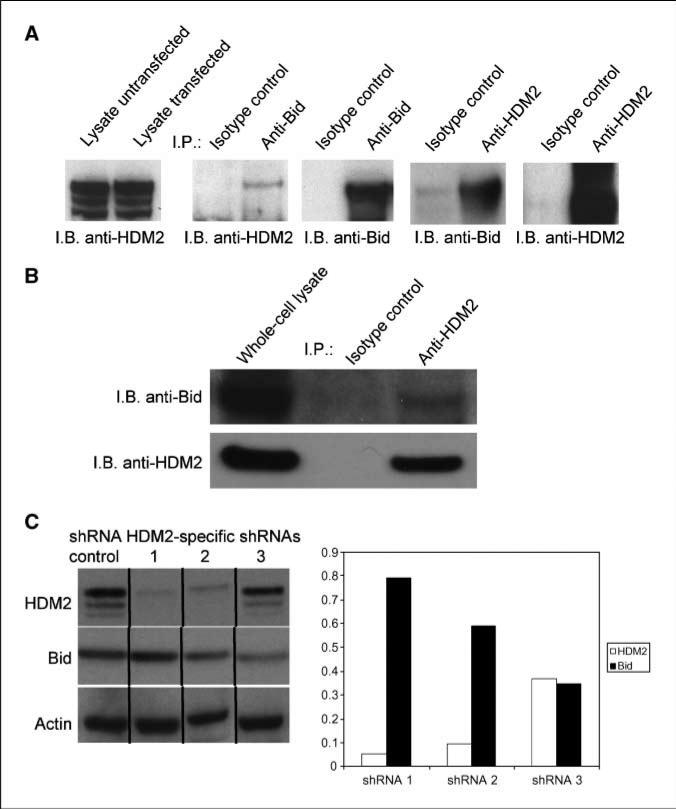
HDM2 is a well-known p53-E3 ligase. Increases in HDM2 preceded reductions in Bid, which led us to askif HDM2 could function as a Bid-E3 ligase. To ascertain if Bid and HDM2 associate, 293T cells were transfected with a human TA-Bid-GFP expression construct. Western analysis showed equivalent amounts of HDM2 expression in both untransfected and transfected 293T cells (Fig. 5A, lanes 1 and 2). Cell lysates were immunoprecipitated with an anti-human Bid antibody and immunoblotted with anti-HDM2. HDM2 was easily identifiable in Bid immunoprecipitates, whereas no HDM2 was pulled down with an isotype control antibody (Fig. 5A, lanes 3 and 4). Next, a HDM2 reverse immunoprecipitation was done and HDM2 again coprecipitated with Bid (Fig. 5A, lane 8). To determine if endogenous Bid and HDM2 interact, lysates from primary human NK cells were immunoprecipitated with anti-HDM2 and immunoblotted with anti-Bid. As seen with the Bid-GFP overexpression system, HDM2 coprecipitated with Bid (Fig. 5B).
Figure 5.
HDM2 interacts with Bid. A, 293T cells were transfected with a TA-Bid-GFP construct with an efficiency estimate of 70% to 80%. Cells were treated with lactacystin for 12 h and lysates were prepared and immunoprecipitated using rabbit polyclonal anti-Bid and control antibodies. Immunoprecipitations were washed and bound proteins were resolved by SDS-PAGE and immunoblotted using mouse anti-HDM2 (lanes 3 and 4). A reverse immunoprecipitation was done using mouse anti-HDM2 antibody for protein pull-down and rabbit anti-Bid antibody for Western analysis (lanes 7 and 8). Blots were reprobed with rabbit polyclonal Bid and HDM2 antibodies to show efficient immunoprecipitation and immunoblotting (lanes 6 and 10). Aliquots of transfected and untransfected cell lysates were analyzed to ascertain levels of endogenous HDM2 (lanes 1 and 2). Representative of four individual transfections. B, primary human NK cells were lysed and immunoprecipitated with anti-HDM2 followed by Western analysis of Bid as described in A. C, 293A cells infected with lentiviral expressing scrambled control or HDM2-specific shRNAs were placed on puromycin selection for 4 d. Cells were lysed and protein extracts were analyzed by Western blot using mouse anti-HDM2 and rabbit anti-Bid antibodies. Results are arranged to show decreasing inhibition of HDM2 by different shRNAs. Densitometric analysis of the bands was done using Total Lab Analysis Software version 2.01 (Nonlinear Dynamics). All experiments were done at least two times.
We next asked if alterations in HDM2 levels affected Bid accumulation by infecting 293A cells with HDM2-specific shRNA lentiviruses. Western blot results are arranged to show decreasing inhibition of HDM2 by different shRNAs (Fig. 5C). The data reveal an inverse relationship between HDM2 and Bid with greater decreases in Bid occurring as levels of HDM2 increase. Overall, the data presented in Figs. 4 and 5 show both a physical interaction between Bid and HDM2 and a functional relationship between these proteins that is controlled by the amount of HDM2 present in the cell.
Bid in NK and T-LGL leukemias
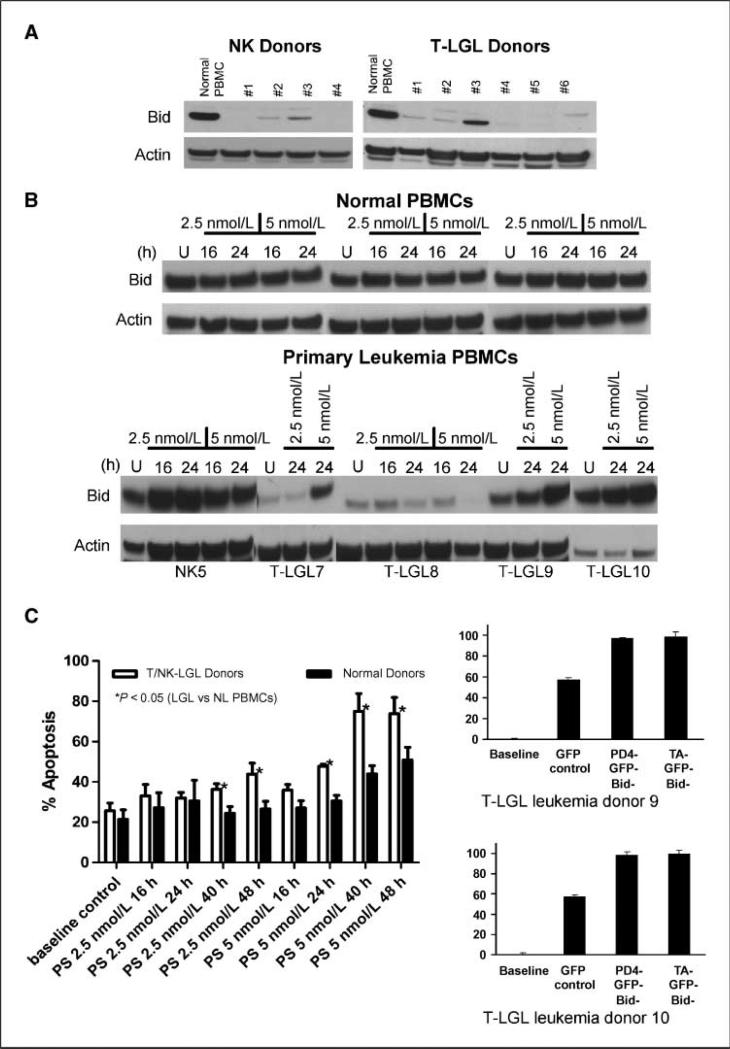
NK leukemia cell lines are useful models for understanding NK cell biology; however, the NK cell lines NK92, NKL, and NK3.3 have reduced Bid levels even in the absence of cytokine (data not shown). This led us to ask whether primary NK or T cell leukemias have diminished levels of Bid. Extracts from NK-LGL cells, T-LGL cells, and PBMCs were compared by Western analysis and the results revealed that normal PBMCs expressed high levels of Bid. In contrast, cells from 10 leukemia donors had significantly reduced or an absence of Bid (Fig. 6A). IL-15 alteration of Bid in leukemia cells was examined by treating low-level Bid-expressing cells with IL-15Rα and IL-15 neutralizing antibodies. IL-15 alone significantly reduced Bid in these cells and this result was reversed by blocking both IL-15 and IL-15Rα (Supplementary Fig. S3). To determine if proteasome inhibition augmented Bid accumulation, PBMCs from normal and leukemia donors were treated with the proteasome inhibitor bortezomib for 16 and 24 h. Results show that Bid is present and remains unchanged in bortezomib-treated normal donors (Fig. 6B). In contrast, bortezomib increases Bid in cells isolated from four of five LGL donors (Fig. 6B; compare NK5, LGL7, LGL9, and LGL10 with LGL8), although the concentration of bortezomib required for the increase varied, indicating sensitivity differences between donor cells.
Figure 6.
Human NK-LGL and T-LGL leukemias with reduced Bid expression are susceptible to bortezomib-induced cell death. A, PBMCs from one healthy, four NK-LGL, and six T-LGL leukemia donors were lysed in Triton X-100. SDS-PAGE fractionated protein extracts were analyzed by Western blot using a rabbit polyclonal anti-Bid antibody. Blots were reprobed with goat anti-actin. B, PBMCs from three healthy, one NK-LGL, and four T-LGL leukemia donors were isolated and treated with the indicated bortezomib concentrations for 16 or 24 h. Protein extracts were analyzed by Western blot as described above. C, PBMCs from healthy and LGL leukemia donors were treated with bortezomib at the indicated concentrations and times. Apoptosis was evaluated by Annexin V-FITC and 7AAD staining (left). Samples assays were in triplicate. P < 0.05, statistical significance determined using the Student's t test. Leukemia cells from T-LGL donors 9 and 10 were transduced with Bid expression constructs, pD4-GFP-Bid, TA-GFP-Bid, and a GFP-only construct, pd4EGFP (right). Apoptosis was analyzed 24 h later by Annexin V-PE and 7AAD staining. All samples were assayed in triplicate.
We next asked if bortezomib induced LGL leukemia cell apoptosis. PBMCs from normal and LGL leukemia donors were treated with bortezomib for the times indicated and apoptosis was assayed by Annexin V and 7AAD staining (Fig. 6C, 1eft). Normal donor cells were highly resistant to bortezomib, and only at the higher dose (5 nmol/L) and longer incubation periods (40 and 48 h) was apoptosis apparent. In contrast, LGL leukemia cells were more sensitive and had significant cell death at bortezomib concentrations of 2.5 and 5 nmol/L. Additionally, percent apoptosis was significantly different between normal and LGL leukemia cells. For example, after 40 h of 5 nmol/L bortezomib treatment, normal and leukemia donor cell apoptotic measurements were 45% and 80%, respectively.
We next asked if increased Bid expression was coordinate with increased LGL leukemia cell death. T-LGL leukemia cells from two donors were transduced with a commercially available Bid-GFP expression construct, pD4-GFP-Bid, as well as a Bid-GFP (TA-GFP-Bid) construct that we created. Annexin V and 7AAD staining indicated that the GFP control transduced cells had a somewhat elevated level of apoptosis, which was due to the transduction method used. Nevertheless, increases in Bid resulted in elevated cell death that was ~2-fold greater than that observed in the GFP control cells (Fig. 6C, right). These results suggest that there is a strong correlation between Bid levels and susceptibility of LGL cells to apoptosis.
Discussion
BH3-only family members including Bid are essential to apoptosis. Our data show that the IL-2 cytokine family strongly reduces Bid levels in primary human NK cells by protein degradation. Ubiquitin-mediated decay of Bid has been established, as previous studies showed proteasomal degradation of the NH2- and COOH-terminal portions of cleaved Bid (28, 34). Our study does not provide evidence of tBid degradation; thus, we believe that IL-15 promotes elimination of the full-length protein in NK cells.
NK cells are highly dependent on IL-15 for survival, which Huntington and colleagues showed involves down-regulation of Bim gene transcription and up-regulation of Mcl-1 in mice (35). In contrast, we see only IL-15–specific effects on Bid. As our studies were conducted primarily on human cells, it is possible that species differences account for these differing outcomes. We show that Bid is the only Bcl-2 family member affected by IL-15, and this effect is long-term, as Bid is virtually absent after 24 h IL-15 treatment. Decreases in Bid were found to involve both p38 and phosphatidylinositol 3-kinase pathways, and this was surprising, as IL-15 is not regarded as a primary p38 activator. We also found that the Bid decline was protracted with changes not observed until 12 h after IL-15 addition. HDM2, an E3 ligase important for p53 degradation, was a likely candidate for mediating this effect, as IL-15 induced a transient increase in HDM2 accumulation that peaked between 6 and 9 h but fell concurrently with the elimination of Bid. Western blot analysis and coimmunoprecipitation showed that endogenous HDM2 could specifically bind Bid in primary human NK cells. This is significant because HDM2 is best known as a p53-associated protein. However, evidence has revealed functional similarities between p53 and Bid with regard to apoptotic initiation at the mitochondria (36, 37); thus, it is not unreasonable to assume a role for HDM2 in Bid regulation.
We find that IL-15 enhances both HDM2 gene and HDM2 protein expression. How IL-15 performs this function is not entirely known, but it may act through Akt to activate transcriptional factors that contribute to HDM2 gene activation. Post-transcriptionally, Akt can activate HDM2 through phosphorylation of Ser166 and Ser186 (38). Our data show that IL-15 activates Akt for up to 12 h in primary NK cells. Thus, Akt may dually contribute to the synthesis and activity of HDM2, leading to increased Bid degradation.
Our initial work examined Bid expression in primary human NK cells. We extended our study to human NK cell lines to find that these cells had little to no Bid expression. As these cell lines were derived from various leukemia donors, we wondered whether Bid played a role in leukemia pathogenesis, especially because Bid−/− mice develop a disorder resembling chronic myelomonocytic leukemia (39). This phenomenon is purported to be coordinate with DNA damage and unabated cell cycle progression (31); however, this finding is now in question (40).
In NK-LGL and T-LGL leukemia cells, bortezomib induction of Bid precedes apoptosis. This is curious because apoptosis requires proteasomal degradation of the NH2-terminal portion of cleaved Bid in advance of COOH-terminal tBid fragment activation (34). Several factors may explain this conundrum. First, the effective bortezomib dose sufficient for Bid increases ranged from 2.5 to 5 nmol/L. Second, Bid was increased by 24 h treatment, but apoptosis was significant by 40 h. Recent data show that, in hematologic malignant cells, bortezomib IC50 doses range from 1 to >1,000 nmol/L (41). Interestingly, our effective dose was in the lower range of this estimate. Moreover, the half-life of bortezomib is estimated to be 9 to 15 h. This suggests that decreases in bortezomib may yield the drug ineffective by 40 h, allowing for NH2-terminal portion of cleaved Bid degradation and COOH-terminal tBid fragment activity to proceed. Bortezomib-induced increases in Bid coincided with increased Fas and TRAIL-independent apoptosis (data not shown). In fact, bortezomib increased cell surface expression of DR4, a TRAIL death receptor decoy (data not shown). The inability of death receptors to account for the apoptosis may be explained by the putative role of Bid in DNA damage and repair. This combined with hematopoietic cell intolerance to attenuated cell cycling results in rapid apoptosis (42). Thus, it is possible that elevated Bid expression in LGL leukemia cells promotes S-phase cell cycle block and death. Alternatively, bortezomib blocks Bax degradation (43) that can lead to increased tBid cleavage and activity through the action of effector caspases.
Bortezomib is an effective antitumor agent that acts through the BH3-only proteins Bim and/or Bik, in collaboration with TRAIL (44), and can also work through Noxa in a p53-independent manner (45). Our findings extend this proteasome target group to include the BH3-only protein Bid. The correlation between Bid and apoptosis in LGL leukemia is striking; however, the exact mechanism by which Bid functions in these cells is still unknown. It is apparent that the family of BH3-only proteins plays a critical role in the development of a variety of malignancies (46, 47). Knowledge of the interplay between BH3-only proteins and chemotherapeutic agents such as bortezomib will aid in the development of strategies to overcome apoptotic resistance in neoplastic cells.
Supplementary Material
Acknowledgments
Grant support: Intramural Research Program of the National Cancer Institute/NIH, Center for Cancer Research and National Cancer Institute/NIH contract N01-CO-12400.
We thank Dr. Gillian Whittaker, Michael Sanford, and Thomas Wolfe for technical assistance and advice, Drs. Allan Weissman, Giorgio Trinchieri, and Dan McVicar for scientific discussion and article review, and Drs. Tom A. Waldmann and Yutaka Tagaya for providing the IL-15−/− mice.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Liu JH, Wei S, Lamy T, et al. Chronic neutropenia mediated by Fas ligand. Blood. 2000;95:3219–22. [PubMed] [Google Scholar]
- 2.Gentile TC, Loughran TP., Jr. Interleukin-12 is a costimulatory cytokine for leukemic CD3+ large granular lymphocytes. Cell Immunol. 1995;166:158–61. doi: 10.1006/cimm.1995.0018. [DOI] [PubMed] [Google Scholar]
- 3.Zambello R, Trentin L, Cassatella MA, et al. IL-12 is involved in the activation of CD3+ granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Br J Haematol. 1996;92:308–14. doi: 10.1046/j.1365-2141.1996.d01-1495.x. [DOI] [PubMed] [Google Scholar]
- 4.Zambello R, Facco M, Trentin L, et al. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood. 1997;89:201–11. [PubMed] [Google Scholar]
- 5.Fehniger TA, Suzuki K, Ponnappan A, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–31. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cario G, Izraeli S, Teichert A, et al. High interleukin-15 expression characterizes childhood acute lymphoblastic leukemia with involvement of the CNS. J Clin Oncol. 2007;25:4813–20. doi: 10.1200/JCO.2007.11.8166. [DOI] [PubMed] [Google Scholar]
- 7.Losy J, Niezgoda A, Zaremba J. IL-15 is elevated in sera of patients with relapsing-remitting multiple sclerosis. Folia Neuropathol. 2002;40:151–3. [PubMed] [Google Scholar]
- 8.McInnes IB, Leung BP, Sturrock RD, et al. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat Med. 1997;3:189–95. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 9.Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–66. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 11.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carson WE, Fehniger TA, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–43. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki H, Duncan GS, Takimoto H, et al. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor β chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MA, Bush JE, Fehniger TA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–8. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 17.Ranson T, Vosshenrich CA, Corcuff E, et al. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–93. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 18.Ni HM, Chen X, Chen L, et al. The impact of genetic background and Bid on the phenotype of Bcl-2-deficiency in mice. Apoptosis. 2008;13:53–62. doi: 10.1007/s10495-007-0147-8. [DOI] [PubMed] [Google Scholar]
- 19.Yin XM, Wang K, Gross A, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–91. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 20.Hodge DL, Subleski JJ, Reynolds DA, et al. The proinflammatory cytokine interleukin-18 alters multiple signaling pathways to inhibit natural killer cell death. J Interferon Cytokine Res. 2006;26:706–18. doi: 10.1089/jir.2006.26.706. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Kitagaki J, Dai RM, et al. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007;67:9472–81. doi: 10.1158/0008-5472.CAN-07-0568. [DOI] [PubMed] [Google Scholar]
- 22.Moffat J, Grueneberg DA, Yang X, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Semenzato G, Zambello R, Starkebaum G, et al. The lymphoproliferative disease of granular lymphocytes: updated criteria for diagnosis. Blood. 1997;89:256–60. [PubMed] [Google Scholar]
- 24.Epling-Burnette PK, Zhong B, Bai F, et al. Cooperative regulation of Mcl-1 by Janus kinase/stat and phosphatidylinositol 3-kinase contribute to granulocyte-macrophage colony-stimulating factor-delayed apoptosis in human neutrophils. J Immunol. 2001;166:7486–95. doi: 10.4049/jimmunol.166.12.7486. [DOI] [PubMed] [Google Scholar]
- 25.Deng G, Podack ER. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci U S A. 1993;90:2189–93. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaled AR, Li WQ, Huang J, et al. Bax deficiency partially corrects interleukin-7 receptor α deficiency. Immunity. 2002;17:561–73. doi: 10.1016/s1074-7613(02)00450-8. [DOI] [PubMed] [Google Scholar]
- 27.Dubois S, Mariner J, Waldmann TA, et al. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 28.Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J Biol Chem. 2000;275:21648–52. doi: 10.1074/jbc.M001083200. [DOI] [PubMed] [Google Scholar]
- 29.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol Invest. 2004;33:109–42. doi: 10.1081/imm-120030732. [DOI] [PubMed] [Google Scholar]
- 30.Kamer I, Sarig R, Zaltsman Y, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Zinkel SS, Hurov KE, Ong C, et al. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–91. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 33.Sax JK, Fei P, Murphy ME, et al. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4:842–9. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 34.Tait SW, de Vries E, Maas C, Keller AM, D'Santos CS, Borst J. Apoptosis induction by Bid requires unconventional ubiquitination and degradation of its N-terminal fragment. J Cell Biol. 2007;179:1453–66. doi: 10.1083/jcb.200707063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huntington ND, Puthalakath H, Gunn P, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–63. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leu JI, Dumont P, Hafey M, et al. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–50. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Zhu H, Xu CJ, et al. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhou BP, Liao Y, Xia W, et al. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 39.Zinkel SS, Ong CC, Ferguson DO, et al. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17:229–39. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufmann T, Tai L, Ekert PG, et al. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell. 2007;129:423–33. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Wiberg K, Carlson K, Aleskog A, et al. In vitro activity of bortezomib in cultures of patient tumour cells-potential utility in haematological malignancies. Med Oncol. 2008 Nov 18; doi: 10.1007/s12032-008-9107-6. Epub. [DOI] [PubMed] [Google Scholar]
- 42.Liebermann DA, Hoffman B, Steinman RA. Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene. 1995;11:199–210. [PubMed] [Google Scholar]
- 43.Liu FT, Agrawal SG, Gribben JG, et al. Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood. 2008;111:2797–805. doi: 10.1182/blood-2007-08-110445. [DOI] [PubMed] [Google Scholar]
- 44.Nikrad M, Johnson T, Puthalalath H, et al. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bikand Bim. Mol Cancer Ther. 2005;4:443–9. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez Y, Verhaegen M, Miller TP, et al. Differential regulation of Noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- 46.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei MC. Bcl-2-related genes in lymphoid neoplasia. Int J Hematol. 2004;80:205–9. doi: 10.1532/ijh97.04110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.