Liver disease has emerged as one of the major causes of morbidity and mortality among patients infected with the human immunodeficiency virus (HIV), particularly in regions where highly active antiretroviral therapy (ART) is widely available. This dramatic change in disease epidemiology is attributable to a complex interaction between etiologic factors that appear to increase the rate of hepatic fibrosis and accelerate progression to end-stage liver disease. Key factors include ART related hepatoxicity, frequent coinfection with hepatitis B and C, and possibly the direct interaction of HIV virus or soluble protein viral products that interact with hepatocytes and other liver resident cell types. Additionally, there is some evidence that gut permeability is altered during active HIV replication, which affects the complex mix of toxins and growth factors present in the portal circulation. It is critical that hepatologists maintain a strong knowledge base to provide the best possible guidance to HIV-infected patients and their healthcare providers.
To this end, the second international forum on HIV and Liver Disease was convened in Jackson Hole, WY in September 2008. The first forum, held two years earlier was previously summarized in Hepatology, and has been widely cited by experts in the field.1 However, the fast moving nature of this critical health issue led to development of a second meeting, supported by grants from three institutes of the National Institutes of Health (NIAID, NIDDK, NIAAA) and by unrestricted grants provided by the pharmaceutical industry. As before, the meeting sought to bring together basic and clinical researchers representing multiple disciplines including hepatology, infectious diseases, epidemiology, virology, and drug development as well as governmental experts in health policy, research and research funding. This document provides a summary of key presentations and highlights the current state of knowledge and future directions this field will take.
Epidemiology and Natural History (Brooks, Sterling, Rimland, Tuma)
HIV prevalence in the United States is increasing due to the stable incidence of HIV (estimated at 53,600 cases/year in 2006) and the longer life expectancy attributable to widespread use of effective antiretroviral therapies. This pattern permits non-HIV defining processes to predominate as major causes of morbidity and mortality. New infection with HIV is primarily transmitted from persons who do not know that they are HIV infected, and this observation represents a significant change compared to historical data regarding HIV transmission2 . Furthermore, HIV disproportionately affects African-Americans, Hispanics, men who have sex with men (MSM) and those living in the southern U.S. The rate of new infection in MSMs appears to be increasing.3, 4 Recent C.D.C recommendations to broaden screening for HIV may result in an increase in new cases referred to the hepatologist or gastroenterologist.
Shared mechanisms of transmission lead to high coinfection rates with both hepatitis C virus (HCV) and hepatitis B virus (HBV) among those with HIV infection. However, rates of infection are highly variable and depend on the nature of shared risk. Current estimates of HCV disease burden suggest that between 250,000 and 300,000 individuals in the U.S. are coinfected with HCV and HIV.5, 6 Worldwide, rates of coinfection are highly variable. In sub-Saharan Africa rates of HCV/HIV may be as low as 2-3% of the HIV infected population.6 This reflects the predominant mode of HIV transmission, heterosexual exposure, which is relatively inefficient for HCV viral spread. In contrast, reports of acute HCV infection among MSMs appear to be increasing. Small but significant outbreaks of acute HCV have been reported in major cities in Europe and the U.S.7, 8 Epidemiological investigation reveals a high risk association with anal-receptive sexual activity including “fisting”, suggesting that anal mucosal trauma is a key element of HCV transmission. The reason that the increase in acute HCV among gay men is being seen now remains contentious. Some investigators believe that widespread use of ART has created a permissive atmosphere reminiscent of 1980's bathhouse behavior, with multiple sexual exposures in a short window of time. Others do not believe that behaviors have changed, and the perception of increased incidence represents an ascertainment bias. It was suggested that HIV spread in the 1980s was divided into two distinct epidemics, one in men who have sex with men (MSM) and the other in injection drug users (IDU). The MSM spread predominated in the 1980s and these patients did not have high concomitant rates of HCV infection. Over the years, the risk behaviors of high risk sexual activity and injection drug use merged leading to higher prevalence of HCV in the MSM population and contributing to the current acute HCV outbreaks. Additionally, there is evidence that non-injection methamphetamine use may be associated with cases of acute HCV transmission.9 This hypothesis will need to be studied in more detail and represents an important area of epidemiologic research.
The natural history of HCV is altered when HIV is present. We have known for some time that low CD4 counts (<200 cells/mm3) are associated with more rapid progression of hepatic fibrosis, development of cirrhosis and time to appearance of decompensated liver disease.10-13 Recent data suggests that HIV viral load may be an important and independent factor in accelerated disease progression as well. These data are primarily derived from large treatment or observational cohort studies (e.g. SMART,EUROSIDA, GESIDA) which show decreased progression to end-stage liver disease when HIV viral loads are low or undetectable.14, 15 Clearly, it is difficult to separate the effect of CD4 from HIV viral load as these are highly related covariates, but study of large cohorts does permit some insight into the independent effects of these variables. Furthermore, there are biological data regarding HIV's effect on cells residing in the liver that support the epidemiologic associations. These data are described more fully in the section on Pathogenesis below. Unpublished data presented by Dr. Tuma demonstrated that rates of progression to cirrhosis in an ART-treated Spanish cohort did not differ significantly from those with HCV alone. Similarly, Dr. Rimland provided data from a cohort in Atlanta, Georgia (USA) that liver-related deaths among HCV/HIV coinfected patients are decreasing. Taken together, these data suggest a role for effective HIV treatment in HCV/HIV coinfected patients, but additional supporting data is clearly needed. Despite these observations, several meeting participants noted that abnormal liver enzyme levels in HCV/HIV coinfected patients often discourage continuation of potentially hepatotoxic ART agents leading to undertreatment.16 Dr. Sterling presented data regarding the high frequency of serum aminotransferase abnormalities among those with HIV infection absent of known viral coinfections, or use of hepatotoxic drugs.17 It is clear that hepatology input regarding the etiology, significance and severity of the underlying liver disease is a critical element in the comprehensive management of HIV-infected patients.
Pathogenesis of Liver Disease in HIV-Infected Patients (Koziel, Green, Glesby, McClain, Martin, Zamor, Blackard)
The observation of more rapidly progressive liver disease in HCV/HIV coinfection despite the fundamental role of the immune response in chronic viral disease pathogenesis suggests a paradox, since the depletion of CD4 cells might be expected to attenuate such responses in advancing HIV disease. However, available evidence suggests that HIV coinfection is attended by immune dysregulation rather than depletion. For instance, while there appears to be no clear quantitative differences in intrahepatic CD4 or CD8 T cell responses against HCV, there are qualitative differences, including increases in IL-10 secretion compared with HCV monoinfection.18 Another important contributor to accelerated HCV-related liver disease is alteration of the fibrogenic cytokine environment. In this regard, it has been demonstrated that HCV-induced TGF-β secretion by hepatocytes is augmented by addition of recombinant HIV envelope protein gp120 or HIV infection itself, implying that HIV is capable of altering the hepatocyte cytokine environment without necessarily directly infecting hepatocytes.19 There is additional experimental evidence that HIV may also impact hepatic stellate cells, the prime mover of hepatic fibrogenesis.20 HIV may also increase fibrogenesis indirectly through promotion of hepatocyte apoptosis.21 Finally, HIV may alter the hepatocyte environment indirectly through promotion of microbial translocation, immune activation, and alteration of local levels of TNF-α, which promotes hepatocyte injury.22
Alcohol has been demonstrated to accelerate viral liver injury. Ethanol is well known to induce direct hepatic injury through its principal metabolite, acetaldehyde, but also induces steatosis through alterations in the hepatic oxidation-reduction state. Oxidative stress also induces mitochondrial injury, which predisposes to hepatocyte apoptosis. Alcohol may also enhance the injury associated with microbial translocation, promoting production of TNF-α. These findings suggest a direct interaction between HIV infection and alcoholic liver disease pathogenesis.23, 24
Hepatic steatosis can also be observed under nonalcoholic conditions as well, and is particularly frequent among HIV infected persons. Steatosis has been suggested to have an association with accelerated rates of HCV fibrosis progression.25. Persons infected with HIV have multiple predispositions to hepatic steatosis, including use of protease inhibitors and nucleoside analogs, hyperlipidemia, lipodystrophy with increased visceral fat deposition, and insulin resistance, though the prevalence may not be more than in HCV monoinfection.26 HCV infection itself contributes to insulin resistance and indirectly to steatosis. In the case of genotype 3 infection, HCV can directly promote steatosis. Reversal of steatosis has been considered when antiviral therapy for HCV fails or cannot be tolerated. In this regard, the use of insulin sensitizing agents may have a role in not only ameliorating steatosis, but also in improving antiviral response rates, since elevated insulin resistance is associated with diminished interferon response. However, this concept has not been proven in clinical trials. A clinical trial investigating whether pioglitazone pre-treatment of previously treated HCV/HIV non-responder subjects improves retreatment response is actively enrolling in the AIDS Clinical Trials Group (ACTG) at this time.
Antiretroviral therapy can be associated with acceleration of hepatic injury as well, further fomenting hepatic disturbances among HIV-infected persons. Hepatotoxicity can be observed with all classes of ART, and grade 3-4 elevations of ALT can be observed in about 5% of patients in patients receiving nucleoside RTIs, non-nucleoside RTIs, and protease inhibitors. NRTIs, in particular, because they bind mitochondrial DNA polymerase-γ, increase the risk for mitochondrial toxicity, promoting apoptosis and microvesicular steatosis, though not all NRTIs demonstrate similar levels of toxicity. Stavudine and didanosine are particularly troubling in this regard, but their use has been replaced in most practice settings with lower toxicity agents. In addition, immune reconstitution injury can be observed in persons with chronic HBV infection who experience resurgent immune responses. Immune reconstitution may occur with HCV as well; however, this entity has been more difficult to distinguish, since immune responses to HCV are attenuated in general.
The direct effects of HIV on the liver remain unclear, but will constitute an important area of research activity as the field moves forward.27 There are data suggesting that HIV can directly (infection) and indirectly (gp 120 binding) interact with hepatocytes, stellate cells, and Kuppfer cells. Furthermore, it seems likely that active infection of intrahepatic CD4 cells with HIV also occurs. Details regarding HIV tropism and specific adaptations remain to be explored.
Finally, there is recent recognition of “new” forms of liver disease in HIV-infected patients. Several groups around the world have noted cases of nodular regenerative hyperplasia or hepato-portal sclerosis.28, 29 In many cases, these were thought to represent a form of cryptogenic cirrhosis, but appropriate biopsy or evaluation of the liver following liver transplantation provided the correct diagnosis. The etiology remains uncertain. Some investigators suggest a strong association with prior use of didanosine (ddI), but the nearly ubiquitous use of this agent in patients during the late 1990s and mid-2000 raises the possibility that this is a spurious finding. Research is required to determine if persistent mitochondrial injury can lead to this pathologic finding.
Recommendations for HIV treatment (Feinberg, Soriano)
The widespread use of highly active antiretroviral therapy (HAART) has dramatically changed the prognosis of HIV infection since its introduction in the mid 1990s. During the early HAART era, encouraging responses led to the treatment of nearly all infected individuals, following the principle of “hit hard, hit early”.30 However, appreciation of the short and long-term side effects of the oldest antiretrovirals, particularly of “d-drugs” such as didanosine, thymidine analogs (zidovudine and stavudine) and first-generation protease inhibitors (e.g, ritonavir (full dose) and indinavir), prompted reconsideration of when to start treatment, and favoured a delay until CD4 counts reached a critical threshold (200 cells/μl) below which the risk of opportunistic infections was significantly increased. The recent availability of newer antiretroviral agents (see Table 1), many of them with a safer toxicity profile and/or belonging to new drug families (e.g, integrase inhibitors, CCR5 antagonists) along with a better appreciation of the systemic damage caused by uncontrolled HIV replication has favoured a return to an earlier introduction of HAART. Today, HIV treatment intervention is recommended in all HIV patients with CD4 counts below 350 cells/μl as well as in many patients with even higher CD4 counts. (see Table 2).31 A seminal study published in 2009 suggests that early initiation of ART before the CD4 count falls below 500 cell/mm3 is associated with improved survival.32 A growing body of literature suggests that virtually all patients with HBV/HIV coinfection who require HBV treatment should be initiated on full antiretroviral therapy (see HBV below). Some experts also recommend earlier treatment of HIV in the setting of HCV.
Table 1.
Current approved antiretroviral drugs
| NRTI Nucleoside reverse transcriptase inhibitors (block reverse transcriptase) |
NNRTI Non-nucleoside reverse transcriptase inhibitors (block reverse transcriptase) |
PI Protease inhibitors (block protease) |
INI Integrase inhibitors (block integrase) |
Entry Inhibitors (block HIV from entering cells) |
|---|---|---|---|---|
Zidovudine (AZT, ZDV)
Didanosine (ddI)
Stavudine (d4T)
Lamivudine (3TC)
Emtricitabine (FTC)
Tenofovir (TDF)
Abacavir (ABC)
|
Nevirapine (NVP)
Efavirenz (EFV)
Etravirine (ETR)
|
Saquinavir (SQV/r)
Lopinavir (LPV/r)
Fosamprenavir (fAPV/r)
Atazanavir (ATV/r)
Tipranavir (TPV/r)
Darunavir (DRV/r)
|
Raltegravir
|
Enfuvirtide (INN)
Maraviroc
|
Table 2.
Situations in which antiretroviral therapy may be considered in HIV patients with CD4 counts above 350 cells/μl
|
Including Kaposi's sarcoma, non-Hodgkin lymphoma, anal carcinoma, and cervical cancer
Including myocardial infarction, peripheral artery disease, and stroke
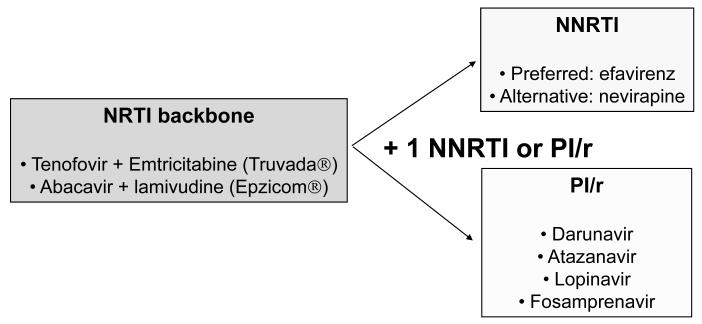
The current preferred initial antiretroviral regimens outlined by the U.S. Department of Health (DHHS: www.aidsinfo.nih.gov) or European AIDS Clinical Society guidelines combine any of two co-formulated nucleoside reverse transcriptase inhibitors (NRTI) with either a non-nucleoside reverse transcriptase inhibitor (NRTI), (e.g. efavirenz) the first choice, or a ritonavir-boosted protease inhibitor (see Figure 1).33 Some experts support initial use of raltegravir following its recent FDA approval for treating naive patients. Using these recommended regimens, around 75% of patients reach undetectable plasma viremia (HIV-RNA <50 copies/ml) at 1 year. However, with time, a steadily growing proportion of patients experience viral rebound mainly as result of poor adherence and selection of drug-resistant viruses. When this occurs, drug resistance testing is recommended and a switch in antiretroviral regimen must be advised in order to regain complete viral suppression.34 Rescue regimens must be built using antiretrovirals with no cross-resistance to prior agents and ideally must include compounds belonging to different drug classes (e.g., raltegravir or maraviroc) and/or with high genetic barrier to resistance (e.g., darunavir/ritonavir).
Figure 1.
Preferred initial antiretroviral regimens
HCV infection in HIV patients (Blackard, Ray, Chung, Fleischer, Butt)
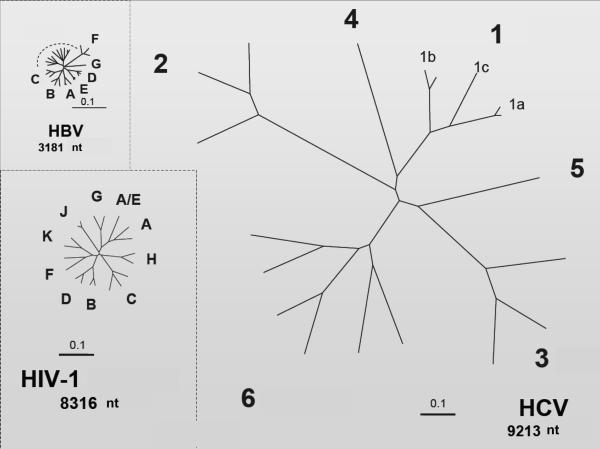
Although both HIV and HCV are RNA viruses and share some similar features in the replication cycle, the HCV genetic material is not integrated into the infected hepatocyte chromosomes, as occurs with proviral HIV DNA in infected lymphocytes. Furthermore, the relative genetic diversity of HCV is much higher than HIV or HBV (see Figure 2) This largely explains why HCV can be eradicated with therapy while HIV infection persists lifelong despite successful suppression of viral replication with antiretroviral therapy. An intriguing observation is that HIV seems to enter and productively infect various liver cell types, while on the other hand extrahepatic replication of HCV, mainly in lymphocytes, has already been reported.35 At this time it is unclear to what extent ectopic replication of viruses in these compartments might modify the course and clinical manifestations in HIV/HCV coinfected individuals.36
Figure 2.
Relative genetic diversity of HIV, HCV, and HBV
(figure 2 used with permission from Stuart C. Ray, M.D.,Associate Professor of Medicine, Division of Infectious Diseases, Johns Hopkins University School of Medicine)
Current treatment paradigms have remained largely intact over the last two years. Most patients are treated with a combination of pegylated interferon alfa and weight-based ribavirin, though weight-based therapy has not been approved by regulatory agencies in the U.S. Preliminary data from ACTG 5178 (SLAM-C) which utilized weight-based ribavirin showed much higher early viral response rates (56% vs 41%) when compared to historical controls who received ribavirin at a dose of 800 mg/day.37 The PRESCO trial also supported use of weight-based ribavirin(1000 mg/day for patients ≤75 kg; 1200 mg/day for those >75 kg).38 Though neither trial was randomized in terms of ribavirin dosing, both studies supported the relative safety of the weight-based regimen. The results of a large multicenter trial of weight-based vs fixed dose ribavirin in HCV/HIV coinfected subjects are pending at this time. Data were presented suggesting that rapid viral response (RVR, HCV viral negative at week 4 of therapy) was a potent predictor of sustained viral response (SVR) in coinfected patients. However, there was little enthusiasm for shortened duration of treatment even in the setting of RVR unless tolerability was an issue. There was discussion of the role of maintenance therapy, and shortly after this meeting the initial results of the SLAM-C protocol were presented at the Conference on Retroviruses and Opportunistic Infections (CROI). . The findings did not support use of pegylated interferon maintenance therapy in HCV/HIV coinfection. The SLAM-C study did identify racial disparities in HCV treatment response, with lower rates of efficacy seen in African-American and Hispanic subjects.37
The advent of new direct antivirals against HCV is eagerly awaited for HIV/HCV coinfected patients, in whom current standard therapy with pegylated interferon plus ribavirin provides clearance in less than one third of HCV genotype 1 carriers, which unfortunately are the most prevalent.39 The new compounds for HCV, however, may face particular challenges in the coinfected population in whom the risk of drug resistance might be increased due to higher viral loads and lower activity of interferon. Furthermore, there is a high potential for interaction and interference with antiretroviral drugs due to shared P450 metabolism profiles for many experimental agents. Despite these concerns, the FDA Antiviral Drug Advisory committee stipulated that studies in HCV/HIV coinfected patients must be initiated prior to approval of an NDA in HCV monoinfected subjects due to the significant disease burden and rapid progression observed in HCV/HIV coinfected patients.40
Data from multiple sources suggest that only a fraction of subjects with HCV/HIV coinfection actually receive treatment for HCV. The low rate of hepatitis C therapy among HIV/HCV coinfected patients in many US cohorts41, especially in those including veterans42, contrasts with treatment rates of 40% in Western Europe.43 Differences in patient population, genotype distribution (higher genotype 2 and 3 in Europe), access to medication, and perhaps variable eligibility criteria appear to account for this observation, but it seems clear that further evaluation of this disparity is warranted.
Chronic hepatitis B in HIV patients (Soriano, McGovern, Martin)
About 10% of HIV patients worldwide show persistent serum HBsAg. The rate is higher in Southeast Asia than in Western countries. Progression to end-stage liver disease occurs faster in HIV/HBV-coinfected patients44, characteristically in the absence of significant elevated liver enzymes, since inflammatory phenomena are ameliorated in the liver of HIV individuals, despite the paradoxically accelerated nature of fibrogenesis.
There are 8 HBV genotypes, each of them including multiple subtypes. Variability in HBV is constrained by the small length of the genome and overlapping of open reading frames. However, recombination and coinfection events may still challenge immune and therapeutic control of HBV.45
In contrast with lamivudine or adefovir, entecavir and tenofovir appear to show a very high genetic barrier to resistance, though entecavir use is problematic in patients with prior lamivudine exposure due to the clinical consequences of hepatitis B viral breakthrough. The benefit of tenofovir in HIV/HBV-coinfected patients has been widely proven, with more than 80% of patients with undetectable HBV viremia at 5 years, accompanied with significant improvements in biochemical liver function tests and amelioration of liver fibrosis assessed using non-invasive markers. While the recognition of a moderate anti-HIV activity for entecavir limits the use of this drug in HIV/HBV coinfected patients with detectable HIV46, the potent dual activity of tenofovir against HIV and HBV makes this drug, especially when co-formulated with emtricitabine (Truvada®) or used with lamivudine, the recommended choices for coinfected patients.45 Another anti-HBV agent, telbivudine, is a nucleoside analogue with excellent HBV activity, but with a resistance profile similar to lamivudine. There are scattered case reports of HIV activity and as with entecavir, use of this agent in HBV/HIV coinfected patients without other concomitant HAART therapy cannot be recommended at this time.
Advanced Liver Disease, Portal Hypertension and Liver Transplantation in HIV (Green, Ragni, Stock, Marks)
In addition to accelerated fibrogenesis, there may be additional clinical features that distinguish HIV-related liver disease. A growing number of reports have identified scattered cases of portal hypertension in the absence of frank cirrhosis, accompanied by nodular regenerative hyperplasia (NRH), in persons with HIV and no other clear liver disease cofactors. Characteristic clinical findings included splenomegaly, ascites and thrombocytopenia, along with development of gastroesophageal varices. Several of these patients underwent liver transplantation.29 The most common predisposition was the receipt of didanosine, which raises the specter of a vascular reaction induced by the drug or its metabolites that provokes the hyperplastic response characteristic of NRH.47 Further study is required to tease out the true frequency and causal factors underlying this disorder.
For those persons with fully decompensated, end stage liver disease (ESLD), an important question is when to refer for liver transplantation. Anecdotal reports suggest that infectious disease providers do not routinely include measures like INR in their routine laboratory evaluation of HIV-infected patients. Few studies have addressed prognostic variables and their utility in predicting outcomes among ESLD patients with HIV. The multicenter NIH-supported HIV-Transplant Network has recently demonstrated that while detectable HIV viral load and CD4 count are associated with increased transplant wait list mortality, MELD score was the only independent predictor of outcome. Moreover, preliminary analysis suggests that wait list mortality did not appear to be higher among HIV+ persons than in HIV negative listees matched for indication and MELD score. Thus, it would appear that MELD is an equally accurate tool in HIV+ transplant listees as it is in the HIV(−) population and should be used to monitor HIV-infected patients with cirrhosis. Why then the concern about the slippery slope of liver disease deterioration in HIV+ ESLD? One possibility is that the decline may be more evident from the compensated to the decompensated state; however, once MELD scores become more advanced, the pace of deterioration may become more congruent.48 Further prospective study is needed to delineate the clinical factors that accompany the steep decline.
Data are now accumulating in the U.S. and Europe regarding outcomes of liver transplantation in HIV-infected patients. Of the transplants that have been performed, roughly half have been performed for HCV-associated ESLD, the other half for HBV-related ESLD. Uniformly, HIV-specific outcomes have been excellent, with virtually complete control of HIV viremia with HAART, and no progression to AIDS observed among persons who have been able to continue ART postoperatively. With the use of immunoprophylaxis with hepatitis B immune globulin (HBIG) and antiviral prophylaxis with lamivudine or adefovir, the success rates of HBV-related ESLD have been excellent and on par with HIV(−) outcomes. In contrast, the 3 year survival rates for those undergoing orthotopic liver transplantation for HCV ESLD have been lower in both the U.S. and Europe, and compare somewhat unfavorably with HIV(−) outcomes. The major reason for the decrement in graft and patient survival has been aggressive recurrent HCV. Curiously, in a small but striking number of patients, spontaneous clearance of HCV has been observed. The factors that underlie this spontaneous conversion have not been elucidated. Clearly, much work is required to understand the host, graft and viral factors that underlie the widely divergent outcomes of HIV+ persons undergoing liver transplant for HCV ESLD.
Biopsychosocial Issues (Samet, Angelino, McClain, Bhatti )
The management of liver disease in the setting of HIV must take into account social, psychiatric and cultural issues that impact disease transmission, prevention, diagnosis and treatment. While most people are risk averse, a high proportion of patients with HIV infection are “risk takers”, and their behavior is manifest by actions that provide immediate gratification with little concern for consequences. These behaviors lead to increased risk of HIV, HCV and HBV as well as comorbid drug and alcohol toxicities. Animal studies have clearly demonstrated the powerful addictive potential of agents such as cocaine, and this group of patients is highly susceptible to drug experimentation and subsequent addiction. Behavioral modification techniques seem to be critical in the management of such patients, both in terms of addiction management and in the ability to successfully maintain and/or complete antiretroviral or time-limited HCV therapy. The most effective therapeutic manipulations involve development of a reward/punishment system that utilizes the existing behavioral patterns to achieve a desirable outcome.
Research Issues: Opportunities and Barriers (Brobst, Sherman)
There is significant ongoing interest in the generation of new research activity in the area of HIV and liver disease. This interest crosses several institutes of the NIH and has led development of multiple RFAs and PA that are accessible on the NIH websites for each institute. In addition, several institutes support career development awards to provide early to mid-career funding to investigators with an interest in this field. There is considerable interest in the development of studies that make use of samples and data derived from existing cohorts including the AIDS Clinical Trials Group (ACTG), the Multicenter AIDS Cohort Study (MACS), the Women's Interagency HIV Study (WIHS) and others. Barriers to research include the relatively low rate of grant funding, though this may be somewhat mitigated by increased funding due to the appropriation of economic stimulus dollars. Furthermore, an increasingly strict regulatory environment has increased regulatory paperwork related to patient-oriented research.
Many key questions remain unanswered with regard to development and management of liver disease in the setting of HIV infection. The development of new agents for hepatitis C represents both an opportunity and an increased level of complexity as issues of HCV viral mutation and selection, drug toxicity and drug interactions emerge. Furthermore, it has become clear that few HCV/HIV coinfected patients are actually deemed treatment candidates, and the formula for successful multidisciplinary interventions that include all infected patients remains elusive. The direct and indirect role of HIV and its soluble proteins on hepatic cells and function remains largely unknown, and issues of gut translocation of endotoxin and the effect on the liver remain speculative musings at this time. Provocative epidemiologic data suggest that HIV control may be the cornerstone of liver disease prevention, but this hypothesis must be supported by elucidation of pathophysiologic mechanisms that underlie these observations. Hepatitis B therapy seems more manageable, but issues of long-term selection and resistance remain concerning and mandate serious consideration of new strategies for management.
Acknowledgements
The project described was supported by Award Number R13 AI 071925 from the National Institute of Allergy and Infectious Diseases (NIAID), and co-funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID, NIDDK, NIAAA or the National Institutes of Health.
Unrestricted educational grants from pharmaceutical sponsors to support this meeting were provided by Roche, Gilead Sciences, Vertex Pharmaceuticals, Bristol Meyers Squibb, Merck, Tibotec, and Valeant. CME credit and content oversight were provided by the University of Wisconsin School of Medicine and Public Health.
References
- 1.Sherman KE, Peters M, Koziel MJ. HIV and liver disease forum: conference proceedings. Hepatology. 2007;45:1566–77. doi: 10.1002/hep.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks G, Millett GA, Bingham T, Bond L, Lauby J, Liau A, Murrill CS, Stueve A. Understanding Differences in HIV Sexual Transmission among Latino and Black Men who have Sex with Men: The Brothers y Hermanos Study. AIDS Behav. 2008 doi: 10.1007/s10461-008-9380-6. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan PS, Hamouda O, Delpech V, Geduld JE, Prejean J, Semaille C, Kaldor J, Folch C, Op de Coul E, Marcus U, Hughes G, Archibald CP, Cazein F, McDonald A, Casabona J, van Sighem A, Fenton KA. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996-2005. Ann Epidemiol. 2009;19:423–31. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Hall HI, Geduld J, Boulos D, Rhodes P, An Q, Mastro TD, Janssen RS, Archibald CP. Epidemiology of HIV in the United States and Canada: current status and ongoing challenges. J Acquir Immune Defic Syndr. 2009;51(Suppl 1):S13–20. doi: 10.1097/QAI.0b013e3181a2639e. [DOI] [PubMed] [Google Scholar]
- 5.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US Adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 6.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, Vogel M, Baumgarten A, Chaix ML, Fisher M, Gotz H, Matthews GV, Neifer S, White P, Rawlinson W, Pol S, Rockstroh J, Coutinho R, Dore GJ, Dusheiko GM, Danta M. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danta M, Dusheiko GM. Acute HCV in HIV-positive individuals - a review. Curr Pharm Des. 2008;14:1690–7. doi: 10.2174/138161208784746761. [DOI] [PubMed] [Google Scholar]
- 9.Fierer DS, Uriel AJ, Carriero DC, Klepper A, Dieterich DT, Mullen MP, Thung SN, Fiel MI, Branch AD. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–6. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, Poynard T. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 11.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman D. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clinical Infectious Diseases. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 12.Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, Moore RD, Afdhal NH, Thomas DL. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. Aids. 2007;21:2209–16. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 13.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, Calvo G, Monforte A, Rickenbach M, Ledergerber B, Phillips AN, Lundgren JD. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 14.Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, Rodriguez-Torres M. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Montes ML, P J, Lopez-Dieguez M, Tural C, Quereda C, Ortega E, Arranz A, von Wichmann M, Barquilla E, Arribas J, GESIDA . CROI. Montreal, CANADA: 2009. Survival of HIV/HIC co-infectged patients with compensated liver cirrhosis: Effects of HCV therapy; p. 105. [Google Scholar]
- 16.Mocroft A, Phillips AN, Soriano V, Rockstroh J, Blaxhult A, Katlama C, Boron-Kaczmarska A, Viksna L, Kirk O, Lundgren JD. Reasons for stopping antiretrovirals used in an initial highly active antiretroviral regimen: increased incidence of stopping due to toxicity or patient/physician choice in patients with hepatitis C coinfection. AIDS Res Hum Retroviruses. 2005;21:743–52. doi: 10.1089/aid.2005.21.743. [DOI] [PubMed] [Google Scholar]
- 17.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci. 2008;53:1375–82. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham CS, Curry M, He Q, Afdhal N, Nunes D, Fleming C, Horsburgh R, Craven D, Sherman KE, Koziel MJ. Comparison of HCV-specific intrahepatic CD4+ T cells in HIV/HCV versus HCV. Hepatology. 2004;40:125–32. doi: 10.1002/hep.20258. [DOI] [PubMed] [Google Scholar]
- 19.Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, Kim SS, Borges CB, Shao RX, Chung RT. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803–11. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Tuyama A, Hong F, Schecter A, Mosoian A, Chen B, Chen P, Klotman M, Bansal M. HIV entry and replication in stellate cells promotes cellular activation and fibrogenesis: implications for hepatic fibrosis in HIV/HCV co-infection; The 58th Annnual Meeting of the American Association for the Study of Liver Diseases; Boston, MA. November 2-6, 2007; 2007. [Google Scholar]
- 21.Balasubramanian A, Koziel M, Groopman JE, Ganju RK. Molecular mechanism of hepatic injury in coinfection with hepatitis C virus and HIV. Clin Infect Dis. 2005;41(Suppl 1):S32–7. doi: 10.1086/429493. [DOI] [PubMed] [Google Scholar]
- 22.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 23.Barve SS, Kelkar SV, Gobejishvilli L, Joshi-Barve S, McClain CJ. Mechanisms of alcohol-mediated CD4+ T lymphocyte death: relevance to HIV and HCV pathogenesis. Front Biosci. 2002;7:d1689–96. doi: 10.2741/A872. [DOI] [PubMed] [Google Scholar]
- 24.Dong Q, Kelkar S, Xiao Y, Joshi-Barve S, McClain CJ, Barve SS. Ethanol enhances TNF-alpha-inducible NFkappaB activation and HIV-1-LTR transcription in CD4+ Jurkat T lymphocytes. J Lab Clin Med. 2000;136:333–43. doi: 10.1067/mlc.2000.110104. [DOI] [PubMed] [Google Scholar]
- 25.Marks KM, Petrovic LM, Talal AH, Murray MP, Gulick RM, Glesby MJ. Histological findings and clinical characteristics associated with hepatic steatosis in patients coinfected with HIV and hepatitis C virus. J Infect Dis. 2005;192:1943–9. doi: 10.1086/497608. [DOI] [PubMed] [Google Scholar]
- 26.Halfon P, Penaranda G, Carrat F, Bedossa P, Bourliere M, Ouzan D, Renou C, Tran A, Rosenthal E, Wartelle C, Delasalle P, Cacoub P. Influence of insulin resistance on hepatic fibrosis and steatosis in hepatitis C virus (HCV) mono-infected compared with HIV-HCV co-infected patients. Aliment Pharmacol Ther. 2009;30:61–70. doi: 10.1111/j.1365-2036.2009.03995.x. [DOI] [PubMed] [Google Scholar]
- 27.Blackard JT, Sherman KE. HCV/ HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat. 2008;15:323–30. doi: 10.1111/j.1365-2893.2008.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiano TD, Kotler DP, Ferran E, Fiel MI. Hepatoportal sclerosis as a cause of noncirrhotic portal hypertension in patients with HIV. Am J Gastroenterol. 2007;102:2536–40. doi: 10.1111/j.1572-0241.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 29.Dinh MH, Stosor V, Rao SM, Miller FH, Green RM. Cryptogenic liver disease in HIV-seropositive men. HIV Med. 2009 doi: 10.1111/j.1468-1293.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 30.Ho DD. Time to hit HIV, early and hard. N Engl J Med. 1995;333:450–1. doi: 10.1056/NEJM199508173330710. [DOI] [PubMed] [Google Scholar]
- 31.Hammer SM, Eron JJ, Jr., Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 32.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, Hogg RS, Deeks SG, Eron JJ, Brooks JT, Rourke SB, Gill MJ, Bosch RJ, Martin JN, Klein MB, Jacobson LP, Rodriguez B, Sterling TR, Kirk GD, Napravnik S, Rachlis AR, Calzavara LM, Horberg MA, Silverberg MJ, Gebo KA, Goedert JJ, Benson CA, Collier AC, Van Rompaey SE, Crane HM, McKaig RG, Lau B, Freeman AM, Moore RD. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clumeck N, Pozniak A, Raffi F. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of HIV-infected adults. HIV Med. 2008;9:65–71. doi: 10.1111/j.1468-1293.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch MS, Gunthard HF, Schapiro JM, Brun-Vezinet F, Clotet B, Hammer SM, Johnson VA, Kuritzkes DR, Mellors JW, Pillay D, Yeni PG, Jacobsen DM, Richman DD. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47:266–85. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 35.Blackard J, Hiasa Y, Smeaton L, Jamieson D, Rodriguez I, Mayer K, Chung R. Compartmentalization of hepatitis C virus (HCV) during HCV/HIV coinfection. Journal of Infectious Diseases. 2007;195:1765–1773. doi: 10.1086/518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44:15–22. doi: 10.1002/hep.21283. [DOI] [PubMed] [Google Scholar]
- 37.Sherman KE, A J, Butt A, Goodman Z, umbleja T, Alston B, Koziel M, Peters M, Sulkowski M, Chung R, ACTG A5178 Study Team . CROI. Boston, MA: 2008. Sustained long-term antiviral maintenance with pegylated interferon in HCV/HIV coinfected patients: Early viral response and effect on fibrosis in treated and control subjects; p. 92. [Google Scholar]
- 38.Nunez M, Miralles C, Berdun MA, Losada E, Aguirrebengoa K, Ocampo A, Arazo P, Cervantes M, de Los Santos I, San Joaquin I, Echeverria S, Galindo MJ, Asensi V, Barreiro P, Sola J, Hernandez-Burruezo JJ, Guardiola JM, Romero M, Garcia-Samaniego J, Soriano V. Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res Hum Retroviruses. 2007;23:972–82. doi: 10.1089/aid.2007.0011. [DOI] [PubMed] [Google Scholar]
- 39.Shire NJ, Welge JA, Sherman KE. Response rates to pegylated interferon and ribavirin in HCV/HIV coinfection: a research synthesis. J Viral Hepat. 2007;14:239–48. doi: 10.1111/j.1365-2893.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- 40.Sherman KE, Fleischer R, Laessig K, Murray J, Tauber W, Birnkrant D. Development of novel agents for the treatment of chronic hepatitis C infection: Summary of the FDA Antiviral Products Advisory Committee recommendations. Hepatology. 2007;46:2014–20. doi: 10.1002/hep.21985. [DOI] [PubMed] [Google Scholar]
- 41.Butt AA, Khan UA, Shaikh OS, McMahon D, Dorey-Stein Z, Tsevat J, Lo Re V., 3rd Rates of HCV treatment eligibility among HCV-monoinfected and HCV/HIV-coinfected patients in tertiary care referral centers. HIV Clin Trials. 2009;10:25–32. doi: 10.1310/hct1001-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut. 2007;56:385–9. doi: 10.1136/gut.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maida I, Soriano V, Ramos B, Rios P, Gonzalez-Lahoz J, Nunez M. Characteristics and prospects for hepatitis C therapy of an HIV-HCV coinfected population followed at a reference HIV center. HIV Clin Trials. 2005;6:329–36. doi: 10.1310/25KL-0VTL-JWXP-FE6Y. [DOI] [PubMed] [Google Scholar]
- 44.Thio CL, Seaberg EC, Skolasky R, Jr., Phair J, Visscher B, Munoz A, Thomas DL. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 45.Soriano V, Vispo E, Bottecchia M, Sheldon J, Tuma P, Garcia-Samaniego J, Barreiro P. Management of hepatitis B virus co-infection on and off antiretroviral therapy. Curr HIV/AIDS Rep. 2008;5:86–93. doi: 10.1007/s11904-008-0014-4. [DOI] [PubMed] [Google Scholar]
- 46.McMahon MA, Jilek BL, Brennan TP, Shen L, Zhou Y, Wind-Rotolo M, Xing S, Bhat S, Hale B, Hegarty R, Chong CR, Liu JO, Siliciano RF, Thio CL. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614–21. doi: 10.1056/NEJMoa067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maida I, Garcia-Gasco P, Sotgiu G, Rios MJ, Vispo ME, Martin-Carbonero L, Barreiro P, Mura MS, Babudieri S, Albertos S, Garcia-Samaniego J, Soriano V. Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome. Antivir Ther. 2008;13:103–7. [PubMed] [Google Scholar]
- 48.Stock PG. Rapid deterioration of HIV co-infected patients waiting for liver transplantation is not predicted by MELD. Liver Transpl. 2005;11:1315–7. doi: 10.1002/lt.20539. [DOI] [PubMed] [Google Scholar]