Abstract
The D1-D2 heterodimer in the reaction center core of phototrophs binds the redox plastoquinone cofactors, QA and QB, the terminal acceptors of the photosynthetic electron transfer chain in the photosystem II (PSII). This complex is the target of the herbicide atrazine, an environmental pollutant competitive inhibitor of QB binding, and consequently it represents an excellent biomediator to develop biosensors for pollutant monitoring in ecosystems. In this context, we have undertaken a study of the Chlamydomonas reinhardtii D1-D2 proteins aimed at designing site directed mutants with increased affinity for atrazine. The three-dimensional structure of the D1 and D2 proteins from C. reinhardtii has been homology modeled using the crystal structure of the highly homologous Thermosynechococcus elongatus proteins as templates. Mutants of D1 and D2 were then generated in silico and the atrazine binding affinity of the mutant proteins has been calculated to predict mutations able to increase PSII affinity for atrazine. The computational approach has been validated through comparison with available experimental data and production and characterization of one of the predicted mutants. The latter analyses indicated an increase of one order of magnitude of the mutant sensitivity and affinity for atrazine as compared to the control strain. Finally, D1-D2 heterodimer mutants were designed and selected which, according to our model, increase atrazine binding affinity by up to 20 kcal/mol, representing useful starting points for the development of high affinity biosensors for atrazine.
Keywords: reaction center, photosystem II, Chlamydomonas reinhardtii, herbicides, site-directed mutagenesis, binding energy calculations
Introduction
The widespread use of herbicides in agriculture, industry, and urban areas poses a threat to the entire ecosystem. Therefore, a huge interest has currently arisen for the development of biosensors that can provide reliable in situ rapid measurement of herbicides concentration in the environment. Because of its effectiveness and low cost, atrazine (6-chloro-N2-ethyl-N4-isopropyl-1,3,5-triazine-2,4-diamine) is one of the most widely used herbicides throughout the world, but also a persistent contaminant in aquatic ecosystems.1 Adverse effects of atrazine also affect human health.2 In fact, atrazine is an endocrine disruptor and co-relates with breast and reproductive disorders in mammalians.3 Atrazine at present is widely used in USA and, although it has been withdrawn from the market in several European countries, analogous molecules (as terbuthylazine differing only by side chain methyl groups) are currently used.4 Furthermore, although banned in Europe in 1980, atrazine is still present as a contaminant in several surface and ground water sites at concentrations higher than imposed by EU directives.
Atrazine inhibits photosynthesis by interrupting the photosynthetic electron transport chain at the photosystem II (PSII) site.5 PSII is a multi-subunit pigment-protein complex embedded in the thylakoid membrane of oxygen-evolving phototrophs that supports light-driven oxidation of water to molecular oxygen and plastoquinone reduction. The scaffold of the PSII reaction center (RC) is formed by two protein subunits, D1 and D2, each composed of five transmembrane α-helices (named from A to E) with their N- and C-termini exposed to the stromal and luminal sides, respectively.6,7 All the photosynthetic redox active components are located within the D1-D2 heterodimer, including the plastoquinones QA and QB6,7 (PQs).
Photonic energy is captured by the antenna systems and sequentially transferred via a pheophytin molecule, from a specific chlorophyll (Chl) a molecule in the RC to the primary acceptor QA, a single-electron carrier tightly bound to the D2 protein. Thereafter, electron transfer occurs from QA− to the secondary plastoquinone QB, bound to the D1 subunit, via a non-haem iron.8 In contrast to QA, QB is a two-electron carrier and while the partially reduced QB− form is tightly bound to the D1 protein in a loop connecting the D and E helices (the QB pocket), the fully reduced and protonated QBH2 form has a relatively low affinity for the hydrophobic pocket within D1. Consequently, another molecule of the plastoquinone pool displaces QBH2 occupying the D1 binding pocket.6,7
X-ray diffraction studies of the PSII from Thermosinechoccus elongatus showed that QB is hydrogen-bonded with one keto oxygen to the Nδ atom of His215, and with the other keto oxygen to the hydroxyl group of Ser264 and the backbone NH group of Phe265. The binding is also stabilized by ring stacking of the quinone head group with the phenyl side chain of Phe255 and by hydrophobic interactions with Phe211, Met214, Phe265, Leu271, and Phe274. In addition, there are a number of D2 protein residues, which have been identified as part of the QB binding cavity, though they do not appear to directly contact QB.6,7 Notably, the quinone binding cavities of QA and QB binding sites are in close proximity and mutations of one of the two binding sites are likely to cause conformational changes in the other, thereby changing their binding affinity. Within the D2 protein, the quinone QA is sandwiched between Trp253 and Leu267, and the keto oxygens are hydrogen-bonded to the Nδ atom of His214 and the backbone NH group of Phe261 (both from D2 protein). Several other hydrophobic side chains define the QA binding cavity. Among these, Leu209, Phe257, Phe270, and Val274 are in close contact with QA.6,7 Atrazine inhibits the electron flow from QA to QB by binding to the D1 protein.8 In particular, atrazine competes with the free QB pool for displacing QBH2 and binding to the D1 protein.9,10 The displacement of the electron mediator QB from the D1 protein prevents the oxidation of reduced QA, leading to the interruption of the electron flow and, consequently, resulting in plant's death. The D2 protein contains an extended loop between helices D and de at the reducing side of PSII. Characterization of D2 mutants of the cyanobacterium Synechocystis sp. PCC 6803 has indicated that the length and amino acid composition of the D-de loop are not critical for basic PSII functions, although most of the residues in that region are phylogenetically conserved. It has been shown using herbicide binding and electron flow inhibition measurements that modifications in the D-de loop of the D2 protein modify the interaction of some PSII-directed herbicides with their binding niche.9,10 Several results in various organisms suggest a close functional association between the D-de loop of the D2 protein and the QB/herbicide-binding environment, which is viewed as being coordinated mostly by residues of the D1 protein.11
Recently, isolated complexes of D1-D2 proteins have received great attention for application as biomediators in biosensor technology for environmental herbicide monitoring.12 The application of QB binding pocket as a biosensing element requires high specificity and binding affinity.13–15 In this context, we have undertaken an in silico study to predict mutations within the D1-D2 heterodimer which improve its specificity, sensitivity, and binding affinity for atrazine. For this purpose, we have chosen the D1 and D2 proteins from C. reinhardtii, as improvements of its chloroplast engineering make this organism ideal for the generation of a number of D1 and D2 site-directed mutants.16 In detail, taking advantage of the high sequence homology observed between T. elongatus D1 and D2 proteins and the corresponding proteins from C. reinhardtii (87% and 89% amino acid sequence identity, respectively), the three-dimensional structure of the latter proteins has been homology modeled. On the basis of this model, a series of D1 and D2 mutants has been generated in silico and the atrazine affinity of wild type and mutant proteins has been predicted by binding energy calculations to identify mutations able to increase PSII affinity for atrazine. The computational approach has been successfully validated through comparison with the available experimental data and through generation and functional characterization of the D1 mutant S268C. The results obtained allowed to pinpoint D1 and D2 residues which make a strong contribution to atrazine binding and to design mutants for the development of high affinity biosensors for atrazine.
Results and Discussion
Homology modeling of D1 and D2 proteins of C. reinhardtii
Because of the importance of the QB pocket in photosynthesis and in agrofood applications, models have been previously built using methods of molecular mechanics and notions of contact-surface between atoms which account for the experimentally functional state of D1 protein mutants in the region of QB.17–19 Taking advantage of the recently determined crystal structures of T. elongatus PSII,6,7 we have undertaken a new study to predict mutations within both the D1 and D2 proteins, which improve specificity, sensitivity, and binding affinity for atrazine of the D1-D2 heterodimer for biosensing applications.
A BLAST20 search of the PDB database using the amino acid sequences of the D1 and D2 proteins from C. reinhardtii retrieved the amino acid sequences of the corresponding proteins from T. elongatus. For both proteins, a very high degree of sequence identity is observed (87% and 89% for the D1 and D2 proteins, respectively) with 94% sequence similarity; no insertions or deletions were found (Fig. 1). Thus, the three-dimensional structure of D1 and D2 proteins from T. elongatus (PDB code: 2AXT)7 were used as templates to model the three-dimensional structure of the corresponding proteins from C. reinhardtii. The resulting models after energy minimization are shown in Figure 2.
Figure 1.

Amino acid sequence alignment between the D1 (A) and D2 (B) proteins from C. reinhardtii and T. elongatus. Conserved residues are shaded.
Figure 2.
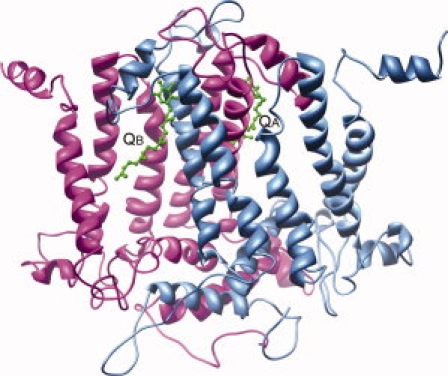
Schematic representation of the modeled three-dimensional structures of C. reinhardtii D1 and D2 proteins. QA and QB molecules, marked by labels, are shown in ball-and-stick representation and colored in green.
Docking studies of atrazine on D1-D2 experimentally characterized mutants
To validate the computational approach undertaken in the present study, docking simulations were performed on mutants of the D1-D2 proteins for which experimental data concerning resistance to the herbicides atrazine and metribuzin, i.e., A251I, F255Y, and L275F are available.21,22
Docking studies of atrazine and plastoquinone were performed on 189 different rotamer structures of the C. reinhardtii D1 A251I mutant. Results obtained with the five best rotamer structures (Table I) indicate that binding energy for atrazine increases several kcal/mol in the mutant protein (indicating lower affinity), in agreement with the experimental evidence that mutation A251I in the D1 protein confers herbicide resistance and photoautotrophic growth.21
Table I.
Comparison of the Binding Energy Values for Atrazine Between Mutant A251I Structures and Wild Type
| Eapo | EQA | EATZ | Ecomplex | Ebind | |
|---|---|---|---|---|---|
| 11,426.4 | 40.2 | 3.6 | 11,421.0 | −49.2 | Wild type |
| 11,424.9 | 40.2 | 3.6 | 11,439.9 | −28.8 | A251I _1 |
| 11,424.9 | 40.2 | 3.6 | 11,440.6 | −28.1 | A251I _2 |
| 11,424.2 | 40.2 | 3.6 | 11,429.6 | −38.4 | A251I _3 |
| 11,426.2 | 40.2 | 3.6 | 11,432.4 | −37.6 | A251I _4 |
| 11,424.8 | 40.2 | 3.6 | 11,438.5 | −30.1 | A251I _5 |
All energy values are in kcal/mol. Numbers following A251I indicate the rotamer number.
Eapo, energy of the apo protein; EQA, energy of plastoquinone A; EATZ, energy of atrazine; Ecomplex, energy of the complex;
Ebind, binding energy.
Results obtained by docking studies of atrazine on F255Y and L275F mutant structures (Table II) indicated that the first mutation leads to a strong increase in the binding energy value for atrazine (lower affinity), while the latter leads to a decreased binding energy value (higher affinity). This is in line with the experimental evidence that mutation F255Y confers resistance to atrazine, but not to metribuzin, while mutation L275F confers resistance to metribuzin, but not to atrazine.22
Table II.
Comparison of the Binding Energy Values for Atrazine Between Mutant F255Y and L275F Structures and Wild Type
| Eapo | EQA | EATZ | Ecomplex | Ebind | |
|---|---|---|---|---|---|
| 11,426.4 | 40.2 | 3.6 | 11,421.0 | −49.2 | Wild type |
| 11,427.8 | 40.2 | 3.6 | 11,432.0 | −39.6 | F255Y |
| 11,445.7 | 40.2 | 3.6 | 11,436.2 | −53.3 | L275F |
All energy values are in kcal/mol.
Eapo, energy of the apo protein; EQA, energy of plastoquinone A; EATZ, energy of atrazine; Ecomplex, energy of the complex;
Ebind, binding energy.
Figure 3 shows a view from the bulk solvent of the molecular details of the atrazine binding site in the wild type and in the A251I, F255Y, and L275F mutants docked complexes. It is interesting to note how the atrazine molecule binds in a more external position in all the three mutants with respect to the wild type. However, introduction of the bulky hydrophobic Phe sidechain in the L275F mutant seems to be able to compensate for this effect, most likely through an increase of the contact surface with atrazine thus increasing van der Waals and hydrophobic interactions.
Figure 3.
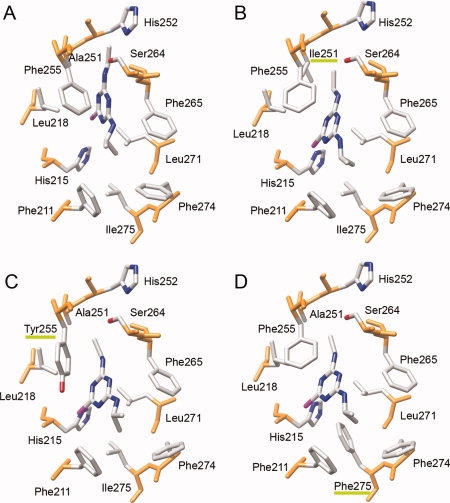
View along the membrane plane of the molecular details of the atrazine binding site in wild type D1 (A), A251I (B), F255Y (C), and L275F (D) D1 mutants docked complexes. Green bars underline mutated residues labels.
Design of mutants with increased atrazine binding affinity
To select mutations that potentially increase the affinity of C. reinhardtii D1-D2 protein for atrazine, residues within 4.5 Å distance from QA and QB binding sites were selected and subjected to mutation into the other 19 possible amino acids. This procedure selected 18 active site residues in D1 (F211, M214, L218, V219, Y246, I248, H252, F255, I259, F260, A263, F265, N266, S268, L271, H272, F274, and L275) and 12 active site residues in D2 (I213, T217, V218, Y244, M246, A249, N250, W253, A260, F261, S262, and L267). Rotamer generation procedure yielded 3213 rotamer structures for the QB binding site and 2268 rotamer structures for the QA binding site. QA and QB were initially docked in both D1 and D2 separately and binding energies were calculated. Subsequently, atrazine was docked in D1 with QA bound in D2 of wild type protein and binding energies were calculated.
The binding energy calculated for the natural substrates QA in D2 and QB in D1 for wild type was −53.1 kcal/mol (Table III), whereas for the natural substrate QA in D2 and atrazine in D1 for wild type was −49.2 kcal/mol (Table I). These binding energy values were taken as a reference for comparison with binding energies calculated for D1 and D2 mutants and selection of the best mutant rotamers.
Table III.
Binding Energy Values for the Natural Substrates QA and QB in D2 and D1 Wild Type Proteins
| Eapo | EQA | EQB | Ecomplex | Ebind |
|---|---|---|---|---|
| 11,426.4 | 40.2 | 40.6 | 11,454.1 | −53.1 |
All energy values are in kcal/mol.
Eapo, energy of the apo protein; EQA, energy of plastoquinone A; EATZ, energy of atrazine; Ecomplex, energy of the complex;
Ebind, binding energy.
In detail, the natural PQs were docked in all the rotamer mutant structures generated (see Methods section) and the corresponding binding energies were calculated. From comparison with the wild type binding energies, 375 best rotamers were selected for the QB binding site and 281 best rotamers for the QA binding site (data not shown). Atrazine was then docked in all above generated mutants of D1, with QA docked in D2, selecting mutant rotamer structures which displayed better binding energy than the wild type. Conversely, QA was docked in D2 mutant rotamer structures with atrazine docked in wild type structure of D1. Results obtained for the best mutant rotamer structures of D1 and D2 are shown in Tables IV and V.
Table IV.
Binding Energies of QA in Wild Type and Atrazine in D1 Mutants
| Eapo | EQA | EATZ | Ecomplex | Ebind | Mutant |
|---|---|---|---|---|---|
| 11,414.2 | 40.2 | 3.6 | 11,400.3 | −57.7 | F274D |
| 11,416.9 | 40.2 | 3.6 | 11,403.2 | −57.5 | F274T |
| 11,432.8 | 40.2 | 3.6 | 11,421.9 | −54.7 | I248W |
| 11,413.3 | 40.2 | 3.6 | 11,403.5 | −53.6 | F260L |
| 11,445.7 | 40.2 | 3.6 | 11,436.2 | −53.3 | L275F |
| 11,426.6 | 40.2 | 3.6 | 11,417.5 | −52.9 | L218Q |
| 11,445.3 | 40.2 | 3.6 | 11,437.2 | −51.9 | L275P |
| 11,425.4 | 40.2 | 3.6 | 11,417.4 | −51.8 | S268C |
| 11,446.9 | 40.2 | 3.6 | 11,439.0 | −51.7 | A263F |
| 11,426.6 | 40.2 | 3.6 | 11,418.8 | −51.6 | I259M |
| 11,425.5 | 40.2 | 3.6 | 11,417.9 | −51.4 | M214H |
| 11,425.1 | 40.2 | 3.6 | 11,417.6 | −51.3 | V219A |
| 11,438.1 | 40.2 | 3.6 | 11,431.5 | −50.4 | L271W |
| 11,413.3 | 40.2 | 3.6 | 11,407.0 | −50.1 | F274H |
| 11,414.9 | 40.2 | 3.6 | 11,408.8 | −49.9 | Y246K |
| 11,431.0 | 40.2 | 3.6 | 11,425.3 | −49.5 | L218T |
| 11,412.9 | 40.2 | 3.6 | 11,407.3 | −49.4 | F274N |
| 11,415.4 | 40.2 | 3.6 | 11,409.9 | −49.3 | F255C |
All energy values are in kcal/mol.
Eapo, energy of apo protein; EQA, energy of plastoquinone A; EATZ, energy of atrazine; Ecomplex, energy of complex; Ebind, binding energy.
Table V.
Binding Energies of QA in D2 Mutants and Atrazine in Wild Type D1
| Eapo | EQA | EATZ | Ecomplex | Ebind | Mutant |
|---|---|---|---|---|---|
| 11,430.4 | 40.2 | 3.6 | 11,386.9 | −87.3 | L267V |
| 11,422.4 | 40.2 | 3.6 | 11,411.6 | −54.6 | M246K |
| 11,430.1 | 40.2 | 3.6 | 11,419.4 | −54.5 | N250E |
| 11,436.5 | 40.2 | 3.6 | 11,426.0 | −54.3 | H214I |
Eapo, energy of apo protein; EQA, energy of plastoquinone A; EATZ, energy of atrazine; Ecomplex, energy of complex; Ebind, binding energy.
The mutations identified in D1 include: A263F, I248W, I259M, L218Q, L218T, L271W, L275F, L275P, M214H, F255C, F260L, S268C, F274D, F274H, F274N, F274T, F274T, Y246K, and V219A, while those in D2 include: N250E, L267V, and M246K. Analysis of the three-dimensional structure of wild type and mutant docking complexes indicates that the orientation of atrazine in D1 binding site plays crucial role in increased binding affinity. Atrazine establishes several van der Waals, hydrophobic, Pi-stacking and hydrogen bonding interactions with the active site residues. As an example, the interactions observed in wild type and in the D1 F274D mutant docking complexes of atrazine are detailed in Tables VI and VII and in Figure 4. Analysis of the interactions of atrazine with wild type as well as mutant proteins allows to identify residues such as F211, H215, L218, A251, H252, F255, G256 S264, F265, S268, L271, F274, and L275 which play an important role in herbicide binding. Hydrogen bonding also plays vital role in structural stability and photoinhibition. For the wild type, important interactions are those taking place between the ethylamino chain of atrazine and the hydroxymethyl group of S264 (2.26 Å distance) and between the aromatic ring nitrogen of atrazine and the backbone amide nitrogen of F265 (1.76 Å distance).
Table VI.
Interactions Between Atrazine and Wild Type D1 Residues
| Type of interaction | Contributing residues |
|---|---|
| vdW interactions | F211, H215, L218, A51, H252, F255, S264, F265, L271, F274, and L275 |
| Hydrophobic interactions | F211, H252, F255, G256, S264A, F265, L271, and L275 |
| Pi-stacking interaction | F255 |
| Hydrogen bonding | S264 [2.29 Å], F265 [1.74 Å] |
Table VII.
Interactions Between Atrazine and F274D Mutant D1 Residues
| Type of interaction | Contributing residues |
|---|---|
| vdW interactions | F211, H215, L218, A251, H252, F255, G256A, S264, F265, L271, D274, and L275 |
| Hydrophobic interactions | F211, H252, F255, G256, S264, F265, L271, and L275 |
| Pi-Stacking interaction | F255 |
| Hydrogen bonding | S264 [2.27 Å], F265 [1.76 Å] |
Figure 4.
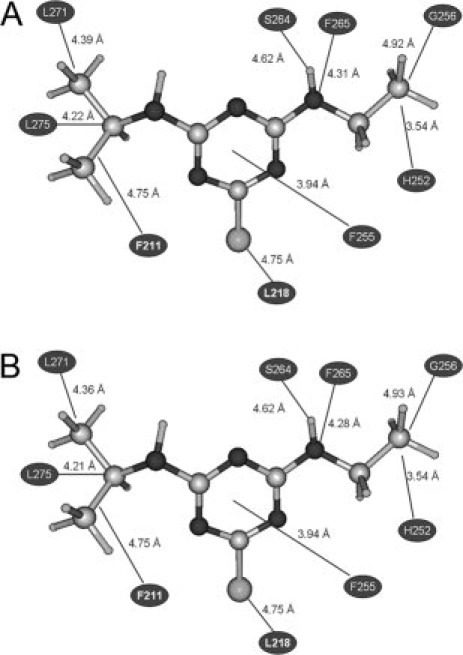
Graphical representation of the interactions observed between atrazine and wild type (A) and F274D (B) D1 residues.
Design of combined D1-D2 mutants with increased atrazine binding affinity
The mutants selected on the basis of the computational procedure described earlier were then combined to select the best mutant combinations for increased atrazine binding affinity. The results of this procedure, shown in Table VIII for the best combined mutants, indicate that in our model carefully selected point mutations of D1 and D2 proteins can lead to an increase in atrazine binding affinity of up to 20 kcal/mol. A complete list of all the combined mutants and their relative atrazine binding affinity is given in Supporting Information. It is evident from the analysis of data reported in Table VIII that increasing the polar nature of the molecular environment of atrazine leads to a substantial increase in binding affinity. In fact eight out of the 10 best mutants reported involve mutation of a hydrophobic D1 residue into a polar or charged one (Leu218 to Gln or Thr; Phe274 to Asn, Asp, or His; Phe255 to Cys). This is not surprising, given the higher polar nature of atrazine as compared to the natural D1 ligand QB. However, the approach here described allows an almost exhaustive search of the best molecular environment for specific and tight binding of atrazine.
Table VIII.
Binding Energies of QA and Atrazine in Combined Mutants of D1 and D2
| Eapo | EQA | EATZ | Ecomplex | Ebind | Combined mutants (D1 and D2) |
|---|---|---|---|---|---|
| 11,426.4 | 40.2 | 3.6 | 11,421.0 | −49.2 | Wild type |
| 11,438.7 | 40.2 | 3.6 | 11,414.4 | −68.1 | L218Q and N250E |
| 11,442.3 | 40.2 | 3.6 | 11,418.9 | −67.2 | L218T and N250E |
| 11,424.4 | 40.2 | 3.6 | 11,401.5 | −66.7 | F274N and N250E |
| 11,457.3 | 40.2 | 3.6 | 11,435.3 | −65.8 | L275F and N250E |
| 11,435.4 | 40.2 | 3.6 | 11,414.3 | −64.9 | F255C and N250E |
| 11,425.7 | 40.2 | 3.6 | 11,405.2 | −64.3 | F274H and N250E |
| 11,421.9 | 40.2 | 3.6 | 11,402.4 | −63.3 | F274D and M246K |
| 11,442.5 | 40.2 | 3.6 | 11,423.3 | −63.0 | I248W and M246K |
All energy values are in kcal/mol.
Eapo, energy of apo protein; EQA, energy of plastoquinone A; EATZ, energy of atrazine; Ecomplex, energy of complex; Ebind, binding energy.
These results lead to the identification of a limited set of mutations that can significantly increase atrazine binding affinity and thus represent an important step towards the exploitation of D1-D2 proteins for the development of highly specific herbicide biosensors.12
Experimental validation of the computational procedure—D1 S268C site-directed mutant production
To further validate the computational approach that led to the identification of candidate mutations potentially able to increase atrazine affinity of the D1 protein, one of the candidate mutants was selected for experimental characterization. Among these residues, S268 is particularly interesting in that it forms part of the QB binding pocket and at the same time is located in the close vicinity of the non-haem iron site where electron transfer occurs from QA− to QB. In particular, the Cys residue in D1 S268C mutant lies at the D1-D2 interface and is predicted to strongly interact with D1 His272, a non-haem iron ligand important for electron transfer. The experimental characterization of the S268C mutant would have the double advantage of validating the computational approach described in the present article while at the same time probing the robustness of the electron transfer path from QA− to QB. In addition, the efficiency of electron transfer from QA− to QB and the stability of the D1-D2 heterodimer are also essential for the exploitation of PSII for biosensor applications. Thus, the mutation S268C was chosen for the experimental characterization.
The S268C mutation was generated in the pSH5 plasmid containing the complete intronless psbA gene encoding the D1 protein, developed to facilitate site-directed mutagenesis as previously reported.23 After confirmation of the produced lesion by sequencing of the entire region subjected to PCR amplification, the mutagenized plasmid was shot into the Del1 mutant of the green alga C. reinhardtii carrying a defined deletion in the chloroplast encoded psbA gene.24 The transformation restored the full psbA gene by homologous recombination between the PCR fragments and the deleted psbA gene, generating a photosynthetically active mutant.
Functional characterization of the D1 S268C mutant compared to control IL strain
Figure 5(A) shows the time course of the photoheterotrophic growth of S268C mutant and control reference IL strains in TAP medium under 50 μmol photons/m2/s of continuous white light. The relative growth of the mutant and control strains, as measured by OD750 after 5 days of growth, is shown in Table IX. In the early logarithmic phase (OD750 < 0.2), the S268C mutant and the control reference strain IL have doubling time of 8.0 and 8.7 h, respectively; subsequently, in the later growth phases, differences were slightly visible. The cell culture of the control strain IL reached the same optical density of the S268C mutant, corresponding to OD750 value of 2.5, after 5 days. In Figure 5(B), the time courses of total chlorophyll accumulation in the control reference IL and S268C mutant strains are also reported. Chlorophyll concentration values after 5 days of growth are slightly different, being 49.4 μg/mL in IL and 41.5 μg/mL in S268C mutant (Table IX).
Figure 5.

Time course of growth and chlorophyll accumulation of C. reinhardtii strains. Cells were growth in TAP medium at 25°C, with 150 RPM and illuminated continuously with 50 μmol photons/m2/s white light. (A) Growth curves determined by measuring the optical density (OD) of the cell suspension at 750 nm. (B) Total content of chlorophylls determined by measuring the optical density (OD) of cell suspension in 80% acetone at 652 nm. Control, IL (closed rhombuses); S268C mutant (open squares). Average values, n = 3, ± SE. The error bars for S268C are smaller than the symbols. P-values < 0.05 were calculated as reported in Methods.
Table IX.
Growth Comparison, Total Content of Chlorophylls (μg/mL), and Steady-State Oxygen Evolution Activity for Wild Type and S268C Mutant Strains
| Photoheterotrophic growth |
O2 Evolution activity |
|||||
|---|---|---|---|---|---|---|
| Strain | OD750 (After 5 days) | Wild type (%) | Growth rate (Doubling time−1) wild type (%) | μg/mL of Chlorophylls (After 5 days) | μM O2/mg Chl/h | Wild type (%) |
| IL | 2.49 ± 0.10 | 100 | 100 | 49.4 ± 1.4 | 94.8 ± 1.9 | 100 |
| S268C | 2.50 ± 0.03 | 92 | 110–115 | 41.5 ± 0.1 | 96.8 ± 2.3 | 102 |
Oxygen evolution activity was measured in tap medium in the presence of NaHCO3 10 mM at 24°C with 350 μmol photons/m2/s of red led light. Average values ± SE are reported.
P-values < 0.05 were calculated as reported in methods.
Table IX also reports the measured steady state oxygen evolution activity, determined on S268C mutant and control IL cultures during the exponential growth phase (16–20 h) with OD750 ≈ 0.4–0.5. The rates of oxygen evolution measured in IL and S268C strains were 94.8 and 96.8 μM O2/mg Chl/h, respectively. These values are very similar indicating that the two C. reinhardtii strains have a similar PSII activity in the saturating light intensity conditions tested (350 μmol photons/m2/s of red led light).
Determination of herbicide effect on PSII photochemical quantum yield
Since our purpose was to produce Chlamydomonas mutants as probes for the development of optical biosensors,25 we studied in detail the response of the S268C mutant by fluorescence analyses. Chlorophyll fluorescence is a physiological parameter routinely used to measure the photochemical efficiency of PSII. Measurement of chlorophyll fluorescence is a reliable and non-invasive method to determine a set of parameters linked to various stress factors, including the activity of the herbicides.26,27 On the basis of the typical shape of the fluorescence rise, Strasser and Strasser28 developed a test providing adequate information about the structure and function of photosynthetic apparatus. Among the measurable parameters, the relative variable fluorescence VJ depends on the redox state of QA, in particular, the value 1−VJ refers to the rate of reoxidation of QA− with respect to its reduction. If the reoxidation of QA− is inhibited in presence of the herbicide, the value 1−VJ changes in a way that is correlated to the herbicide concentration. Therefore, the [1−VJ] parameter was selected to characterize the photosynthetic performance of algal strains in the presence of atrazine at concentrations ranging from 10−10 to 10−5M or in its absence. IL and S268C Chlamydomonas cultures strains were analyzed by the fluorescence induction technique performed by measuring emission in the range of 0.01–1000 ms. As an example, Figure 6 shows the fluorescence induction pattern of IL and S268C strains in the presence or absence of atrazine at 10−7M. The results indicated that both strains were affected by the herbicide and in particular, the S268C strain accumulates higher levels of QA− compared to IL.
Figure 6.
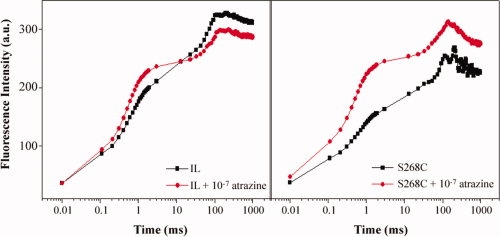
Fluorescence induction curves of IL and S268C strains in the presence or absence of atrazine. Measurements were carried out in Chlamydomonas cultures containing equal amounts of chlorophyll (20 μg) after 10 min incubation in the light with an atrazine concentration of 10−7M. Fluorescence induction measurements were carried out after 10 min of dark incubation. FI a.u, fluorescence induction arbitrary units. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fluorescence measurements allowed the determination of the dose-response curves of C. reinhardtii IL and S268C strains to atrazine and the calculation, on the basis of the 1−VJ parameter (see Methods), of the biomediator affinity and sensitivity, which are fundamental parameters for the development of biosensors. These data are reported in Table X. The affinity of the S268C mutant has been estimated by the I50 value as the concentration of the target material that determines the 50% inhibition of the reaction. The obtained results indicated that the S268C mutant displays an affinity value for atrazine more than one order of magnitude higher as compared to the IL control strain (Table X). Likewise, we expressed the sensitivity of the biomediator with the LoD parameter which is the least amount of the target material that produces a detectable signal. The calculation was done following the definition reported in the legend to Table X and reference therein. Again, the results clearly indicate an increase of one order of magnitude in the mutant as compared to the control strain (Table X).
Table X.
Values of Atrazine I50 for IL and S268C PSII
| I50 (Affinity) (M) | LoDa (Sensitivity) (M) | SE (M) | |
|---|---|---|---|
| IL | 4.02 × 10−7 | 1.00 × 10−8 | 2.44 × 10−8 |
| S268C | 3.76 × 10−8 | 1.00 × 10−9 | 1.77 × 10−9 |
The LOD was determined on the basis of 99% confidence intervals, which, assuming a normal distribution, corresponds to 2.6 × standard error of the measurement (σ). Using then the modified relationship for the Langmuir absorption isotherm, LOD can be calculated as LOD = 2.6 × σ × I50/(100 − 2.6 × σ) as reported by Koblizek et al.29
Atrazine incubation determines maximum inhibition of the PSII activity in the S268C D1 mutant at 5 × 10−8M herbicide concentration, while the control IL PSII activity is only slightly inhibited (data not shown), providing an experimental confirmation that indeed S268C mutation is able to increase D1 affinity for atrazine without affecting the efficiency of electron transfer from QA− to QB. The observed increase in atrazine affinity is in fair agreement with the predicted binding energy value. In fact, atrazine I50 value for S268C mutant is more than one order of magnitude lower than that for the IL control, a value which compares fairly well with the 2.6 kcal/mol difference in the atrazine binding energy value calculated between control IL and S268C mutant (refer Tables II and IV).
The usefulness of the approach described in this study has been further investigated through development of a whole-cell based biosensor, capable of real time response on critical changes caused by herbicides, which exploits the increased sensitivity for atrazine of the D1 S268C mutant. A net increase of the detection sensitivity of about one order of magnitude is observed for a range of herbicides including triazines and urea derivatives, confirming a major importance of this amino acid site for the binding of photosynthetic herbicides. Biosensor development details and analysis of its performance are described in a separate publication.25
Methods
Homology modeling of D1 and D2 proteins of C. reinhardtii
The three-dimensional structure of C. reinhardtii D1 and D2 proteins was homology modeled using as templates the crystal structures of the corresponding proteins of T. elongatus (PDB code: 2AXT)7. T. elongatus D1 and D2 proteins display 87% and 89% amino acid sequence identity with C. reinhardtii D1 and D2 proteins, respectively (see Fig. 1, Results section). The template structures were cleaned with respect to missing residues, improper bond lengths and overall geometry and optimized to 0.1 kcal/(mol × Å) using MMFF94 force field.30 Homology modeling was performed using VLifeMDS 3.0.31 This is a standard molecular modeling program which simply uses the sidechain replacement method where no insertions and deletions are observed between the target and template sequences. Subsequently, gaps in the target sequence are excised and loops are inserted. For loop building, starting and ending anchoring residues are selected to search the PDB database for loops satisfying the distance criteria within the adjoining secondary structures. However, in this case the T. elongatus template D1 and D2 proteins display ∼95% sequence similarity with C. reinhardtii D1 and D2 proteins and no insertions and deletions are observed in the sequence alignment. Thus, no loop search procedure was required and the final models were obtained optimizing the energy of the initial C. reinhardtii D1 and D2 protein models up to 0.1 kcal/(mol × Å) using MMFF94 force field to remove bad contacts. A distance dependent dielectric constant was used for electrostatic terms during MMFF94 energy calculations. The minimization procedure implemented in VLifeMDS software applies an optimizer that uses Powell method till the energy gradient is >10 kcal/(mol × Å), thereafter it shifts to conjugate gradient method till it reaches 1 kcal/(mol × Å) and subsequent minimization below this gradient is done using Broyden-Fletcher-Goldfarb-Shanno (BFGS) method to achieve the desired convergence.
Docking studies
For docking studies, residues surrounding the QA and QB binding sites of D1 and D2 proteins, respectively, were substituted into all the other 19 possible amino acids modeled in different conformations through a rotamer generation procedure. Residues within 4.5 Å distance from the natural plastoquinone were selected for mutation and rotamer generation. This procedure selected 19 active site residues in D1 protein: F211, M214, H215, L218, V219, Y246, I248, H252, F255, I259, F260, A263, F265, N266, S268, L271, H272, F274, and L275. Similarly, 13 active site residues were selected in the D2 molecule: I213, H214, T217, V218, Y244, M246, A249, N250, W253, A260, F261, S262, and L267. D1 H215 and D2 H214 were excluded from the mutant generation procedure as these residues are essential for binding of the non-haem iron which mediates electron transfer from QA to QB. Each residue mutation generates ∼189 rotamers of the remaining 19 residues. Thus, a total of 17 single site mutants in the QB binding site produced 3213 rotamer structures, whereas 12 single site mutants in the QA binding site produced 2268 rotamer structures. The rotamers were generated with dihedral angles of 60°, 180°, and −60°, for sp3-sp2 hybridized bonds, +90° and −90° for aromatics and 0° and 180° for carboxylates and amides. However, for torsions around Cα–Cβ bonds, six rotamers with torsion angles incremented with a step of 60° were generated. For each possible mutation, the best rotamer of both QB and QA binding site residues was selected on the basis of binding energy calculations results. In the docking procedure used in this study, all the rotamers of a given mutated residue were generated and resulting protein structures were energy minimized till the energy gradient was lower than 0.1 kcal/(mol × Å). The resulting most stable conformer was used for docking of atrazine in the active site.
Docking of ligands and energy-minimized natural PQs into both the QA and QB binding pockets of the D1 and D2 mutants molecules were performed by energy minimization of the ligand-free (apo) protein, of the ligand and of the protein-ligand complex to a gradient RMS of 0.1 kcal/(mol × Å), using MMFF94 force-field as implemented in VLifeMDS 3.0.31 The binding energy was then calculated as: Ebinding = Ecomplex − (Eapo + Eligand). It must be noted that MMFF94 force field has been demonstrated to allow fairly accurate prediction of binding affinities with an error in the range of ±10% (see Ref.30 and references therein).
During the docking study, various low energy conformers of atrazine were docked into the active site. However, it is known from experimental evidence that the ethylamino side chain of atrazine orients towards S264 and can form a hydrogen bond with the hydroxymethyl group of serine.32 Further, the aromatic ring nitrogen is hydrogen-bonded to the backbone amide nitrogen of F265.32 S264 and F265 not only participate in atrazine binding but also in the binding of the native plastoquinone at the QB-site.33–35 In the present study, the docking of atrazine in the active site was performed and poses were chosen in which atrazine was within interacting distance with S264 and F265. The resulting docking poses were energy minimized to a gradient of 0.1 kcal/(mol × Å) and binding energies were calculated to select the most stable poses for each mutation.
QA and QB were initially docked in both D1 and D2 separately and binding energies were calculated. Subsequently, atrazine was docked in D1 with QA bound in D2 of wild type protein and binding energies were calculated. Further, the natural PQs were docked in the 3213 rotamer structures generated in the QB binding site and in the 2268 rotamer structures generated in QA binding site and the corresponding binding energies were calculated. From comparison with wild type binding energies, 375 best rotamers were selected for the QB binding site and 281 best rotamers for the QA binding site. Atrazine was docked in all above generated mutants of D1 and in those mutant rotamer structures which displayed higher binding energy than the wild type. To further validate if a mutation in D1 actually improved atrazine binding, QA was docked in wild type D2 and the calculated binding energy was compared with the corresponding wild type system. The same protocol was followed for the D2 binding site, in which atrazine was docked in wild type rotamer structures of D1 and QA was docked in the mutants of D2. The energies were compared to the corresponding wild type system. In a further study, 629 mutant structures were generated in all possible combinations of favorable mutants of D1 as well as of D2. These combined mutants were explored for favorable binding of atrazine in D1 active site and QA in D2 active site.
Strains and culture conditions
C. reinhardtii strains were grown photoheterotrophically in Tris-Acetate-Phosphate (TAP) or photoautotrophically with aeration by sterile air containing 3% (v/v) CO2 in high-salt (HS) media,36 at 50 μmol/m2/s fluorescent white light intensity, 25°C temperature, and 150 rpm agitation. Cells used for measuring photosynthetic growth rates, oxygen evolution, and fluorescence kinetics were harvested at mid-exponential growth phase (up to 2 × 107 cells/mL). Growth curves were determined by optical density (OD) measurements at 750 nm, at the indicated times, using a Perkin Elmer Lambda Bio 40 spectrophotometer (Perkin Elmer, Norwakl, CT).
The C. reinhardtii IL strain containing the intronless psbA gene represented the control reference strain. Its derivative Del1 mutant carrying a defined deletion in the psbA gene, was used as a recipient strain to obtain D1 site-directed mutants by particle gun transformation.24
Site-directed mutagenesis and chloroplast transformation procedures
Site-directed mutagenesis of the psbA gene encoding the D1 protein was performed as previously described37,38 by PCR using the mutagenic primers:
-
5′-GCTTCTTTCAACAACTGTCGTTC −3′
and
5′-GAACGACAGTTGTTGAAAGAAGC-3′ to substitute serine 268 with cysteine (S268C mutant). Base substitutions are underlined.
PCR fragments were amplified from the plasmid pSH5 containing the complete intronless psbA gene and the 3′-flanking regions. The introduction of the mutation was confirmed by sequence analysis. As reported by Dauville et al.,23 the specific PCR-generated psbA fragments were directly precipitated onto tungsten particles and delivered into the host by particle gun. Following transformation, photosynthetically active colonies grown in liquid HS medium were selected and analyzed.
Determination of chlorophyll content
The chlorophyll content was calculated by the Lichtenthaler method,39 after extraction of whole cells with 80% acetone.
Oxygen evolution measurements
Oxygen evolution activity was measured at 25°C by exposing cells to saturating irradiance (350 μmol/m2/s) in a Clark-type oxygen electrode connected to a YSI Biological Oxygen Monitor (model 5300, Yellow Springs, OH). To ensure that oxygen evolution was not limited by the carbon source available to the cells, 100 mL of 0.5 M NaHCO3, pH 7.4 was added to a 2.5 mL aliquot of the culture prior to oxygen evolution measurements.40 Measurements were performed with the O2 electrode, beginning with the registration of dark respiration in the cell suspension, followed by measurement of the light-saturated rate of O2 evolution. The rate of each process was recorded for 5 min. To compare the relative photon yield of photosynthesis between the different samples, the same Chl concentration (15 mg/L) was loaded in the oxygen electrode chamber. Each strain was assayed in three separate cultures, with an average of three measurements for each culture.
Fluorescence analyses
Measurements of chlorophyll fluorescence is a reliable and non-invasive method to determine a set of parameters linked to various stress factors, including the activity of specific herbicides.41,42 From the shape and the form of the fluorescence transition curve (or Kautsky curve), in fact, it is possible to derive various fluorescence parameters that provide information on the photochemical efficiency of PSII. The fluorescence parameter 1−VJ was used to determine the herbicide effect, and it was calculated by the following equation28:
where F0, Fmax, and F2ms are the initial fluorescence, the maximum fluorescence, and the fluorescence after 2 ms, respectively.
Measurements were carried out by the Plant Efficiency Analyser, PEA (Hansatech, King's Lynn, Norfolk, UK). The excitation was carried out at 650 nm wavelength and the fluorescence emission recorded at 720 nm wavelength. The instrument automatically calculated the fluorescence parameters F0, Fmax, Fv, Fv/Fmax, Tm, and Area over fluorescence curve between F0 and Fmax.
Measurements were performed on C. reinhardtii strains IL and S268C in the absence (control) and in the presence of increasing amounts of atrazine, after 10 min of incubation at 50 μmol/m2/s light intensity, 25°C temperature, and 150 rpm agitation. Before each measurement, samples were kept in the dark for 10 min to reach the steady dark adapted state of PSII. Values reported are the average of at least five experiments.
Statistics
All statistical tests were performed using analysis of variance (ANOVA). The statistical significance of differences was evaluated by P-level. P Values have been calculated comparing fluorescence and oxygen evolution values measured in mutant and control strains.
Conclusions
Results presented in this article indicate that the computational approach used, though based on a simplified representation of the PSII, is able to capture the essential thermodynamics of herbicides binding to PSII. Through this approach, D1-D2 combined mutations potentially able to increase the protein affinity for atrazine by several orders of magnitude have been designed. This has been verified experimentally by the production and functional characterization of the S268C D1 mutant which is able to increase the affinity for atrazine by over an order of magnitude while leaving unchanged physiological parameters as well as the efficiency of electron transfer from QA− to QB via the non-hem iron and oxygen evolution capability. The usefulness of the designed mutants for biosensor applications has also been demonstrated using the S268C D1 mutant to develop a whole-cell optical based biosensor, which is characterized by a net increase of the detection sensitivity for a range of herbicides with respect to wild type PSII.25 Studies are ongoing for the further exploitation of the designed mutants in biosensor technology.
References
- 1.Trajkovska V, Petrovska-Jovanovic S, Cvetkovski M. Development and optimization of a method for the determination of simazine, atrazine and propazine using solid-phase extraction and HPLC/GC. J Serb Chem Soc. 2001;66:199. [Google Scholar]
- 2.Rowe AM, Brundage KM, Schafer R, Barnett JB. Immunomodulatory effects of maternal atrazine exposure on male Balb/c mice. Toxicol Appl Pharmacol. 2006;214:69–77. doi: 10.1016/j.taap.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan WQ, Yanase T, Morinaga H, Gondo S, Okabe T, Nomura M, Hayes TB, Takayanagi R, Nawata H. Herbicide atrazine activates SF-1 by direct affinity and concomitant co-activators recruitments to induce aromatase expression via promoter II. Biochem Biophys Res Commun. 2007;355:1012–1018. doi: 10.1016/j.bbrc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 4.Giardi MT, Pace E. Photosynthetic proteins for technological applications. Trends Biotechnol. 2005;25:253–267. doi: 10.1016/j.tibtech.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Moreland DE. Mechanisms of action of herbicides. Annu Rev Plant Physiol. 1980;31:597–638. [Google Scholar]
- 6.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 7.Loll B, Kern J, Zouni A, Saenger W, Biesiadka J, Irrgang KD. The antenna system of photosystem II from Thermosynechococcus elongatus at 3.2 Å resolution. Photosynth Res. 2005;86:175–184. doi: 10.1007/s11120-005-4117-0. [DOI] [PubMed] [Google Scholar]
- 8.Diner BA, Petrouleas V. Q400, the non-heme iron of the photosystem II iron-quinone complex. A spectroscopic probe of quinone and inhibitor binding to the reaction center. Biochim Biophys Acta. 1987;895:107–125. [Google Scholar]
- 9.Kless H, Oren-Shamir M, Ohad I, Edelman M, Vermaas W. Protein modifications in the D2 protein of photosystem II affect properties of the QB/herbicide-binding environment. Z Naturforsch [C] 1993;48:185–190. doi: 10.1515/znc-1993-3-413. [DOI] [PubMed] [Google Scholar]
- 10.Kless H, Oren-Shamir M, Malkin S, McIntosh L, Edelman M. The D-E region of the D1 protein is involved in multiple quinone and herbicide interactions in photosystem II. Biochemistry. 1994;33:10501–10507. doi: 10.1021/bi00200a035. [DOI] [PubMed] [Google Scholar]
- 11.Wilski S, Johanningmeier U, Hertel S, Oettmeier W. Herbicide binding in various mutants of the photosystem II D1 protein of Chlamydomonas reinhardtii. Pest Biochem Physiol. 2006;84:157–164. [Google Scholar]
- 12.Giardi MT, Piletska EV. Biotechnological applications of photosynthetic proteins: biochips, biosensors and biodevices, landes bioscience. Georgetown: Springer Publishers; 2006. ISBN 1-58706-249–6. [Google Scholar]
- 13.Giardi MT, Guzzella L, Euzet P, Rouillon R, Esposito D. Detection of herbicide subclasses by an optical multibiosensor based on an array of photosystem II mutants. Environ Sci Technol. 2005;39:5378–5384. doi: 10.1021/es040511b. [DOI] [PubMed] [Google Scholar]
- 14.Giardi MT, Esposito D, Leonardi C, Mattoo AL, Margonelli A, Angeli G. Biosensor and method for the monitoring of pollutants. 2006. EU patent 01830148:1–2204.
- 15.Breton F, Euzet P, Piletsky SA, Giardi MT, Rouillon R. Integration of photosynthetic biosensor with molecularly imprinted polymer-based solid phase extraction cartridge. Anal Chim Acta. 2006;569:50–57. [Google Scholar]
- 16.Xiong L, Sayre RT. Engineering the chloroplast encoded proteins of chlamydomonas. Photosynth Res. 2004;80:411–419. doi: 10.1023/B:PRES.0000030458.98624.37. [DOI] [PubMed] [Google Scholar]
- 17.Xiong J, Subramaniam S, Govindjee Modeling of the D1/D2 proteins and cofactors of the photosystem II reaction center. Implications for herbicide and bicarbonate binding. Prot Sci. 1996;5:2054–2073. doi: 10.1002/pro.5560051012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svensson B, Vass I, Cedergren E, Styring S. Structure of donor side components in photosystem II predicted by computer modeling. EMBO J. 1990;9:2051–2059. doi: 10.1002/j.1460-2075.1990.tb07372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobolev W, Edelman M. Modeling the quinone-B binding site of the photosystem-II reaction center using notions of complementarity and contact-surface between atoms. Proteins. 1995;21:214–225. doi: 10.1002/prot.340210304. [DOI] [PubMed] [Google Scholar]
- 20.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 21.Sinning I, Michel H, Mathis P, Rutherford AW. Characterization of 4 herbicide-resistant mutants of Rhodopseudomonas viridis by genetic analysis, electron paramagnetic resonance and optical spectroscopy. Biochemistry. 1989;28:5544–5553. doi: 10.1021/bi00439a031. [DOI] [PubMed] [Google Scholar]
- 22.Trebst A. The three dimensional structure of the herbicide binding niche on the reaction center polypeptides of photosystem II. Z Naturforsch [C] 1987;42:742–750. [Google Scholar]
- 23.Dauvillee D, Hilbig L, Preiss S, Johanningmeier U. Minimal extent of sequence homology required for homologous recombination at the psbA locus in Chlamydomonas reinhardtii chloroplasts using PCR-generated DNA fragments. Photosynth Res. 2004;79:219–224. doi: 10.1023/B:PRES.0000015384.24958.a9. [DOI] [PubMed] [Google Scholar]
- 24.Johanningmeier U. Construction of a Chlamydomonas reinhardtii mutant with an intronless psbA gene. Plant Mol Biol. 1993;22:91–99. doi: 10.1007/BF00038998. [DOI] [PubMed] [Google Scholar]
- 25.Scognamiglio V, Raffi D, Lambreva M, Rea G, Tibuzzi A, Pezzotti G, Johanningmeier U, Giardi MT. Chlamydomonas reinhardtii genetic variants as probes for fluorescence sensing system in detection of pollutants. Anal Bioanal Chem PMID. 2009:19238365. doi: 10.1007/s00216-009-2668-1. [DOI] [PubMed] [Google Scholar]
- 26.Campbell D, Hurry V, Clarke AK, Gustafsson P, Öquist G. Chlorophyll fluorescence analysis of cyanobacterial Photosynthesis and acclimation. Microbiol Mol Biol Rev. 1998;62:667–683. doi: 10.1128/mmbr.62.3.667-683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorigo U, Leboulanger C. A pulse-amplitude modulated fluorescence-based method for assessing the effects of photosystem II herbicides on freshwater periphyton. J Appl Phycol. 2001;13:509–515. [Google Scholar]
- 28.Strasser BJ, Strasser RJ. Photosynthesis: from light to biosphere. In: Mathis P, editor. Vol. 5. The Netherlands: Kluwer Academic; 1995. pp. 977–980. [Google Scholar]
- 29.Koblizek M, Maly J, Masojidek J, Komenda J, Kucera T, Giardi MT, Mattoo AK, Pilloton R. A biosensor for the detection of triazine and phenylurea herbicides designed using Photosystem II coupled to a screen printed electrode. Biotechnol Bioeng. 2002;78:110–116. doi: 10.1002/bit.10190. [DOI] [PubMed] [Google Scholar]
- 30.Halgren TA. MMFF VII. Characterization of MMFF94, MMFF94s, and other widely available force fields for conformational energies and for intermolecular-interaction energies and geometries. J Comput Chem. 1999;20:730–748. doi: 10.1002/(SICI)1096-987X(199905)20:7<730::AID-JCC8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 31.Barve V, Ahmed F, Adsule S, Banerjee S, Kulkarni S, Katiyar P, Anson CE, Powell AK, Padhye S, Sarkar FH. Synthesis, molecular characterization, and biological activity of novel synthetic derivatives of chromen-4-one in human cancer cells. J Med Chem. 2006;49:3800–3808. doi: 10.1021/jm051068y. [DOI] [PubMed] [Google Scholar]
- 32.Oettmeier W. Herbicide resistance and supersensitivity in photosystem II. Cell Mol Life Sci. 1999;55:1255–1277. doi: 10.1007/s000180050370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wildner GF, Heisterkamp U, Trebst A. Herbicide cross-resistance and mutations of the psbA gene in chlamydomonas reinhardtii. Z Naturforsch. 1990;45:1142–1150. [Google Scholar]
- 34.Oettmeier W, Hilp U, Draber W, Fedtke C, Schmidt RR. Structure-activity relationships of triazinone herbicides on resistant weeds and resistant Chlamydomonas reinhardtii. Pest Sci. 1991;33:399–409. [Google Scholar]
- 35.Tietjen KG, Kluth JF, Andree R, Haug M, Linding M, Muller KH. The herbicide binding niche of photosystem II—a model. Pest Sci. 1990;31:65–72. [Google Scholar]
- 36.Harris EH. The chlamydomonas sourcebook: a comprehensive guide to biology and laboratory use. In: Harris E, editor. Academic Press; 1989. pp. 25–66. San Antonio. [DOI] [PubMed] [Google Scholar]
- 37.Johanningmeier U, Sopp G, Brauner M, Altenfeld U, Orawski G, Oettmeier W. Herbicide resistance and supersensitivity in Ala250 or Ala251 mutants of the D1 protein in Chlamydomonas reinhardtii. Pestic Biochem Physiol. 2000;66:9–19. [Google Scholar]
- 38.Preiss S, Schrader S, Johanningmeier U. Rapid, ATP-dependent degradation of a truncated D1 protein in the chloroplast. Eur J Biochem. 2001;268:4562–4569. doi: 10.1046/j.1432-1327.2001.02383.x. [DOI] [PubMed] [Google Scholar]
- 39.Lichtenthaler H. Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–381. [Google Scholar]
- 40.Melis A, Neidhardt J, Benemann JR. Dunaliella salina (chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J Appl Phycol. 1999;10:515–525. [Google Scholar]
- 41.Misra AN, Srivastava A, Strasser RJ. Utilization of fast chlorophyll a fluorescence technique in assessing the salt/ion sensitivity of mung bean and Brassica seedlings. J Plant Physiol. 2001;158:1173–1181. [Google Scholar]
- 42.Christensen MG, Teicher HB, Streibig JC. Linking fluorescence induction curve and biomass in herbicide screening. Pest Manag Sci. 2003;59:1303–1310. doi: 10.1002/ps.763. [DOI] [PubMed] [Google Scholar]


