Abstract
Genetic and environmental factors interact to regulate body weight. Overall, the heritability of obesity is estimated at 40% to 70%. More than 244 genes have been found to strongly affect adiposity when overexpressed or deleted in mice. These genes can be considered in four broad categories: regulation of food intake by molecular signalling in the hypothalamus and hindbrain by signals originating in adipose tissue, gut and other organs; regulation of adipocyte differentiation and fat storage; regulation of spontaneous exercise activity; and effect on basal and postprandial thermogenesis. Rare variants in the coding sequences of major candidate genes account for an obese phenotype in 5% to 10% of individuals.
Keywords: Candidate gene, Genetics, Obesity
Abstract
Les facteurs génétiques et environnementaux interagissent pour réguler le poids corporel. Dans l’ensemble, on estime l’héritabilité de l’obésité à entre 40 % et 70 %. On a découvert que plus de 244 gènes ont un effet important sur l’adiposité lorsqu’ils sont surexprimés et ou supprimés chez les souris. Ces gènes peuvent être divisés entre quatre grandes catégories : la régulation de l’apport alimentaire par la signalisation moléculaire dans l’hypothalamus et le rhombencéphale au moyen de signaux en provenance des tissus adipeux, de l’estomac et d’autres organes, la régulation de la différenciation adipeuse et du stockage de gras, la régulation de l’activité physique spontanée et l’effet de la thermogenèse basale et postprandiale. De rares variantes des séquences de codage des principaux gènes candidats représentent un phénotype d’obésité chez 5 % à 10 % des individus.
Obesity results from a combination of environmental and genetic factors (Figure 1). The most convincing evidence for a genetic component to obesity comes from twin and adoption studies (1–4). In studies (1) in which body fat content was measured (either as body mass index [BMI] or skinfold thickness), the comparison of obesity in monozygotic twins with obesity in dizygotic twins indicated heritability quotients ranging from 0.4 to 0.98 (where 0 = no inheritance and 1.0 = complete inheritance of the trait). Although the environment shared by monozygotic twins is more similar than the environment shared by dizygotic twins, the heritability of BMI is not different in identical twins reared together or apart. A genetic component to obesity has also been confirmed in adoption studies (3). These comparisons indicate that the genetic transmission of obesity is at least as large as the nongenetic transmission. Finally, genetic segregation analyses (5) in extended families suggest that approximately 30% to 50% of the obesity phenotype is inherited, and there is evidence for a major recessive gene or genes with an allele frequency of 0.3. A number of candidate genes for obesity have been identified (5,6), and the importance of some of these has been confirmed in genetically engineered mice.
Figure 1).
Early insights into the genetic etiology of obesity. Reproduced from reference 56
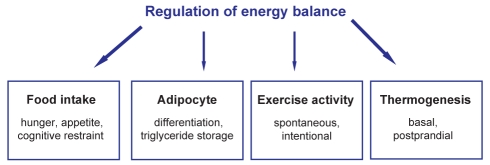
Overall, the genetic origins of obesity can be considered in three broad areas (Figure 2). First, genes coding for proteins that regulate food intake at the level of the hypothalamus (eg, centrally produced molecules, such as proopiomelanocortin [POMC]-derived alpha-melanocyte-stimulating hormone, which signals via the melanocortin-4 receptor [MC4R] [7,8], or peripheral molecules, such as leptin [9], ghrelin and peptide YY [PYY]3–36) appear to have an effect on obesity. Defects at this level are likely to predominate in obesity phenotypes associated with relative hyperphagia. Such patients may lose weight readily in response to energy restriction and may benefit most from pharmacological agents that suppress appetite. Second, at the level of the adipocyte, genetic variation in several genes that regulate preadipocyte differentation (eg, peroxisome proliferatoractivated receptor-gamma [PPAR γ]), triglyceride synthesis (eg, diacylglycerol acyltransferase [DGAT]-1) and lipolytic potential (eg, beta-adrenergic receptors and perilipin) have been associated with a propensity to obesity in animals and humans, and may affect susceptibility to weight gain in the absence of significant hyperphagia. Third, genes that regulate mitochondrial biogenesis and/or adaptive thermogenesis (10) may alter the propensity to gain or lose weight, and may be therapeutic targets in obese subjects resistant to weight loss.
Figure 2).
Regulation of energy balance. Overall, genes that may contribute to obesity susceptibility can be considered in four broad areas. These include genes that regulate food intake, participate in adipogenesis and triglyceride storage, affect spontaneous activity, and influence basal and postprandial energy expenditure via effects on mitochondrial proton leak and adaptive thermogenesis
REGULATION OF FOOD INTAKE
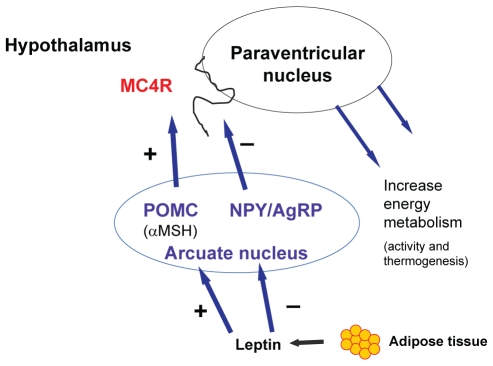
The arcuate nucleus of the hypothalamus comprises two sets of neurons with opposing effects on food intake and energy balance. Activation of the agouti-related peptide (AgRP) and neuropeptide Y neurons increases food intake, whereas activation of the POMC and cocaine- and amphetamine-related transcript neurons results in the release of alpha-melanocyte-stimulating hormone, which binds to the MC4R in the paraventricular nucleus (PVN) to inhibit food intake and increase energy expenditure (11–13) (Figure 3). Mutations in the MC4R gene are a well-described cause of monogenic obesity (14–16). These neurons communicate with neurons in other areas of the brain to transmit signals through the nucleus tractus solitarius. Leptin is a key adipocyte-derived hormone that signals through the POMC/MC4R pathway (6). Leptin is produced in adipose tissue in proportion to the size of fat stores. An increase in body weight increases the production of leptin, which binds to leptin receptors, leading to the inhibition of AgRP/neuropeptide Y neurons and the activation of POMC and cocaine- and amphetamine-related transcript neurons (11). Human subjects who are homozygous for mutations in the leptin gene display severe early-onset obesity, which responds to leptin replacement (6). This pathway, which regulates an important feedback loop to integrate feeding behaviour with body energy stores, is apparently downregulated in chronic obesity. The etiology of leptin resistance is not clear but may be due to alterations in the transport of leptin into the brain, or to an altered number and affinity of leptin receptors (17). Members of the suppressor of cytokine signalling family of proteins are now believed to play a role in the development of leptin resistance because of their ability to inhibit leptin and insulin signalling pathways (18). The targeted disruption of a number of genes in this pathway in mouse models (Lep, Lepr, Pomc, Mc4r and Mc3r) resulted in an alteration in obesity susceptibility (5), and there is evidence for a role of each of these genes in human obesity (19).
Figure 3).
Hypothalamic regulation of food intake. The arcuate nucleus of the hypothalamus comprises two sets of neurons with opposing effects on food intake and energy balance. Activation of the agouti-related peptide (AgRP) and neuropeptide Y (NPY) neurons increases food intake, whereas activation of the proopiomelanocortin (POMC) and cocaine-and amphetamine-related transcript neurons results in the release of alpha-melanocyte-stimulating hormone (αMSH), which binds to the melanocortin-4 receptor (MC4R) in the paraventricular nucleus to both inhibit food intake and increase energy expenditure. Leptin production by fat cells is proportional to fat cell mass. Leptin signals in the arcuate nucleus of the hypothalamus to downregulate (−) the orexigenic NPY and AgRP neurons, and to upregulate (+) the appetite-suppressing POMC neurons
Several additional genes have been associated with monogenic obesity in mice. Single-minded homologue-1 (SIM1) is a transcription factor required for development of the PVN. Sim1 heterozygous mice show a reduction in cellularity of the PVN, hyperphagia and early-onset obesity (20). Unlike in MC4R mutant mice, energy expenditure is not decreased. In humans, profound obesity has been associated with a balanced translocation that disrupts the SIM1 gene (21). Decreased expression of the brain-derived neurotropic factor (BDNF) was reported to affect eating behaviour (22). BDNF and the neurotropic tyrosine kinase receptor B are expressed in the ventromedial hypothalamus and may be downstream effectors of MC4R signalling. Severe obesity was described in a child with a chromosomal inversion in a region encompassing the BDNF gene (23).
A number of gut peptides also signal through hypothalamic pathways to regulate food intake (5,24). Ghrelin is released by the stomach and duodenum, and stimulates AgRP neurons in the arcuate nucleus to increase food intake (25). In contrast, functional studies (26) both in humans and in rodents indicate a potentially important role for PYY in decreasing food intake. Following food intake and in proportion to meal size, PYY is secreted into the bloodstream from L cells in the gastrointestinal tract in two forms, PYY1–36 and PYY3–36, and binds preferentially to neuropeptide Y2 receptors in the arcuate nucleus of the hypothalamus. In both obese and lean human subjects, PYY3–36 infusion markedly decreases food intake (26). The potential genetic contribution of this gene to human body weight through DNA resequencing in extremely lean and obese populations was recently explored, and a novel PYY Q62P variant was identified (27). This gene’s segregation with severe obesity was shown in a small kindred, and its functional significance was demonstrated in feeding studies in mice (27).
ROLE OF THE ADIPOCYTE IN SUSCEPTIBILITY TO OBESITY
The ability to enhance preadipocyte differentiation and increase adipocyte number to cope with fat storage requirements may also be important in the development of obesity. PPAR γ upregulates expression of several important adipocyte genes regulating triglyceride synthesis and storage. In contrast, PPAR γ decreases expression of the leptin gene. Polymorphic variation in PPAR γ has been related to both obesity and insulin sensitivity. The common Pro12Ala polymorphism in PPAR γ has lower transcriptional activity and, hence, reduced capacity to promote adipogenesis. Subjects bearing this polymorphism have a twofold decrease in the risk of obesity (28,29). In contrast, a Pro155Gln mutation in PPAR γ accelerates the differentiation of adipocytes and has been associated with obesity (30). The lipogenic capacity of the adipocyte is under hormonal and substrate control. Tumour necrosis factor-alpha and leptin decrease lipogenesis, whereas insulin promotes triglyceride synthesis. Triglyceride synthesis in mammals is catalyzed by DGAT1 and DGAT2. The DGAT1 knockout mouse is lean and resistant to diet-induced obesity. Energy expenditure and activity are increased. These animals exhibit a specific reduction in white adipose tissue and their adipocytes are smaller. DGAT1-deficient mice exhibit enhanced insulin and leptin sensitivity, likely due to increased thermogenesis (31–34).
The structural integrity of the lipid droplet and susceptibility to lipolysis may also affect susceptibility to obesity or leanness (35). Lipolysis of triglyceride stores is regulated by catecholamines and sympathomimetic stimuli via beta-adrenergic receptors, resulting in activation of the cyclic AMP/protein kinase A (cAMP/PKA) cascade, phosphorylation and translocation of hormone-sensitive lipase (HSL) from the cytoplasm to the lipid droplet. Various polymorphisms in the beta2- and beta3-adrenergic receptors have been reported in relationship with obesity (36–39). The HSL knockout mouse exhibits male sterility and adipocyte hypertrophy but not obesity, as well as normal basal lipolysis but blunted response to catecholamines (40). Polymorphic variation in HSL has not been consistently related to body weight in humans. In contrast with HSL, genetic inactivation of adipose tissue triglyceride lipase increases fat stores in mice (41). Perilipin A is the major lipid droplet protein, and is required for maximal cAMP/PKA-stimulated lipolysis. The perilipin null mouse is obesity resistant (42,43), exhibiting an increased basal lipolysis and increased basal metabolic rate, possibly due to a futile cycle of lipogenesis and lipolysis.
ADAPTIVE THERMOGENESIS
In understanding obesity, it is important to note that most overweight individuals gain an average of 1 kg per year over a lifetime, equivalent to a very small per diem excess energy balance (approximately 20 kcal). On the other hand, individuals who remain lean all their lives apparently avoid this small, gradual accumulation of energy as fat. This suggests that the ability to increase energy output in response to alterations in food intake and exercise (adaptive thermogenesis) and/or the ability to expand adipose tissue stores in response to caloric excess may also be important factors in differentiating obesity-susceptible and obesity-resistant individuals. In accordance with this hypothesis, recent studies (5) in mouse models demonstrate that disruption of genes coding for proteins important in these processes markedly alters the susceptibility to obesity, even in the absence of changes in food intake. Adaptive thermogenesis is the process whereby energy is dissipated in the form of heat in response to cold exposure or excess energy intake, and occurs primarily in brown adipocytes and skeletal muscle (44). A number of processes are involved, including an increase in the number of mitochondria and in the activity of the electron transport system. Members of the PPAR γ family of transcriptional coactivators play an important role in the control of cellular energy pathways and mitochondrial biogenesis (45). Activation of the cAMP-dependent PKA pathway increases energy expenditure via enhancement of adaptive thermogenesis and insulin sensitivity (46). Genetic variation in PKA isoforms has been shown to have a role in regulating energy balance and adiposity (47). The winged helix/forkhead transcription factor forkhead box C2 (FOXC2) has been shown to increase the sensitivity of the cAMP/PKA pathway through increased expression of beta3-adrenergic receptors and lowered activation threshold due to an increased level of the R1alpha subunit of PKA. FOXC2-overexpressing mice are lean and show increased responsiveness to insulin due to sensitization of this pathway. FOXC2 also increases the expression of a number of genes associated with enhanced mitochondrial biogenesis (48).
GENETIC APPROACHES TO OBESITY
Genetic approaches to obesity have included linkage analysis and association studies (19). Genome scan technologies have been valuable in the search for new genetic causes of a particular phenotype because no assumption is made regarding the role of a particular gene. A variable number of markers across the genome can be used to determine segregation with the phenotype of interest, often in large, extended kindreds with affected and unaffected members. However, if linkage to a particular locus is found, these regions may harbour many potentially important genes. Linkage analysis has been most successful for mapping genes responsible for single-gene disorders. Thus far, single mutations in one of 11 genes have been found to account for 2% to 4% of cases of severe early-onset obesity. These include mutations in the leptin (LEP), leptin receptor (LEPR) and MC4R genes (19,49).
Association studies are generally considered a more powerful approach to studying complex disease. The vast majority of obesity cases are polygenic, due to the cumulative effects of common variants in a large number of genes interacting with environmental factors. Candidate genes, or variants in or near candidate genes, are selected for study if they have a known role in metabolism, or if they have been identified based on naturally occurring mutations, linkage analysis, or gene deletion or overexpression studies in animals. The candidate gene approach is dependent on a priori knowledge about a gene or a variant. The numbers can be staggering. There are 244 genes that affect obesity when mutated or overexpressed in mice (5). Two approaches are generally used. The first approach is to determine common single nucleotide polymorphisms (SNPs) (common variants present at approximately every 700 to 1000 base pairs) and haplotypes (group of SNPs) for a particular gene. It is feasible to include promoter SNPs as these are added to the SNP database. Alternatively, if resources permit, the second approach is to resequence entire coding regions of a particular gene. This is useful for finding rare variants with potentially important effects but less useful for determining common variants that affect complex disease. Sequencing is also expensive and is usually limited to exons, such that promoter and intronic variants, which may have a major effect on gene expression, will be missed. To identify potential genetic contributors to this phenotype, the coding exons and splice junctions of 58 genes were recently resequenced in 379 obese (mean BMI 49.0 kg/m2) and 378 lean (mean BMI 19.4 kg/m2) individuals (54). This 96-megabase pair survey included 21 genes associated with monogenic forms of obesity in humans or mice, as well as 37 genes with strong biological plausibility based on the role of their gene products in key metabolic pathways. It was found that the monogenic obesity-associated gene group was enriched for rare, nonsynonymous variants unique to the obese versus lean population. Importantly, computational analysis predicted a greater fraction of deleterious variants within the obese cohort (54). These data support the premise that multiple rare alleles contribute to variation in obesity susceptibility in the general population.
Dense genome-wide SNP scans using 500,000 or 1,000,000 SNP chips will overcome many of the limitations of earlier scan technology and will be useful in the search for obesity-related genes in very large case-control association studies (50–52). These require large sample sizes and iteration of positive findings in multiple data sets because of the high false-discovery rates inherent in multiple comparisons. The contribution of common variants in candidate genes is often modest and contingent on environmental effects and other susceptibility genes. In contrast with studies in genetically defined mouse strains, the genetic and environmental diversity in human populations has made replication of positive SNP-obesity associations difficult (53). Notably, in a recent resequencing study (54), none of the 37 sequenced common variants that were previously reported to associate with BMI, including an SNP near the INSIG2 gene (55), showed a significant frequency difference between the original obese and lean groups.
REFERENCES
- 1.Bodurtha JN, Mosteller M, Hewitt JK, et al. Genetic analysis of anthropometric measures in 11-year-old twins: The Medical College of Virginia Twin Study. Pediatr Res. 1990;28:1–4. doi: 10.1203/00006450-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald A, Stunkard A. Body-mass indexes of British separated twins. N Engl J Med. 1990;322:1530. doi: 10.1056/NEJM199005243222113. [DOI] [PubMed] [Google Scholar]
- 3.Stunkard AJ, Sorensen TI, Hanis C, et al. An adoption study of human obesity. N Engl J Med. 1986;314:193–8. doi: 10.1056/NEJM198601233140401. [DOI] [PubMed] [Google Scholar]
- 4.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322:1483–7. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 5.Rankinen T, Zuberi A, Chagnon YC, et al. The human obesity gene map: The 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 6.Flier JS. Obesity wars: Molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 8.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 9.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 10.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–60. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 11.Broberger C. Brain regulation of food intake and appetite: Molecules and networks. J Intern Med. 2005;258:301–27. doi: 10.1111/j.1365-2796.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- 12.Flier JS. AgRP in energy balance: Will the real AgRP please stand up? Cell Metab. 2006;3:83–5. doi: 10.1016/j.cmet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Shinyama H, Masuzaki H, Fang H, Flier JS. Regulation of melanocortin-4 receptor signaling: Agonist-mediated desensitization and internalization. Endocrinology. 2003;144:1301–14. doi: 10.1210/en.2002-220931. [DOI] [PubMed] [Google Scholar]
- 14.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 15.Farooqi IS, Yeo GS, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106:271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–62. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity (Silver Spring) 2006;14(Suppl 5):254S–8S. doi: 10.1038/oby.2006.319. [DOI] [PubMed] [Google Scholar]
- 18.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17:365–71. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. 2005;6:221–34. doi: 10.1038/nrg1556. [DOI] [PubMed] [Google Scholar]
- 20.Michaud JL, Boucher F, Melnyk A, et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet. 2001;10:1465–73. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- 21.Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–8. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- 22.Xu B, Goulding EH, Zang K, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray J, Yeo GS, Cox JJ, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55:3366–71. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badman MK, Flier JS. The gut and energy balance: Visceral allies in the obesity wars. Science. 2005;307:1909–14. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 25.Flier JS, Maratos-Flier E. The stomach speaks – ghrelin and weight regulation. N Engl J Med. 2002;346:1662–3. doi: 10.1056/NEJM200205233462112. [DOI] [PubMed] [Google Scholar]
- 26.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 27.Ahituv N, Kavaslar N, Schackwitz W, et al. A PYY Q62P variant linked to human obesity. Hum Mol Genet. 2006;15:387–91. doi: 10.1093/hmg/ddi455. [DOI] [PubMed] [Google Scholar]
- 28.Altshuler D, Hirschhorn JN, Klannemark M, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 29.Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–7. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 30.Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–9. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 31.Anand A, Chada K. In vivo modulation of Hmgic reduces obesity. Nat Genet. 2000;24:377–80. doi: 10.1038/74207. [DOI] [PubMed] [Google Scholar]
- 32.Chen HC, Farese RV., Jr DGAT and triglyceride synthesis: A new target for obesity treatment? Trends Cardiovasc Med. 2000;10:188–92. doi: 10.1016/s1050-1738(00)00066-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen HC, Ladha Z, Farese RV., Jr Deficiency of acyl coenzyme a:diacylglycerol acyltransferase 1 increases leptin sensitivity in murine obesity models. Endocrinology. 2002;143:2893–8. doi: 10.1210/endo.143.8.8941. [DOI] [PubMed] [Google Scholar]
- 34.Chen HC, Smith SJ, Ladha Z, et al. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002;109:1049–55. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 36.Meirhaeghe A, Helbecque N, Cottel D, Amouyel P. Beta2-adrenoceptor gene polymorphism, body weight, and physical activity. Lancet. 1999;353:896. doi: 10.1016/S0140-6736(99)00251-2. [DOI] [PubMed] [Google Scholar]
- 37.Meirhaeghe A, Helbecque N, Cottel D, Amouyel P. Impact of polymorphisms of the human beta2-adrenoceptor gene on obesity in a French population. Int J Obes Relat Metab Disord. 2000;24:382–7. doi: 10.1038/sj.ijo.0801168. [DOI] [PubMed] [Google Scholar]
- 38.Oberkofler H, Esterbauer H, Hell E, Krempler F, Patsch W. The Gln27Glu polymorphism in the beta2-adrenergic receptor gene is not associated with morbid obesity in Austrian women. Int J Obes Relat Metab Disord. 2000;24:388–90. doi: 10.1038/sj.ijo.0801180. [DOI] [PubMed] [Google Scholar]
- 39.Ukkola O, Rankinen T, Weisnagel SJ, et al. Interactions among the alpha2-, beta2-, and beta3-adrenergic receptor genes and obesity-related phenotypes in the Quebec Family Study. Metabolism. 2000;49:1063–70. doi: 10.1053/meta.2000.7708. [DOI] [PubMed] [Google Scholar]
- 40.Osuga J, Ishibashi S, Oka T, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA. 2000;97:787–92. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–7. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Botas J, Anderson JB, Tessier D, et al. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;26:474–9. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 43.Tansey JT, Sztalryd C, Gruia-Gray J, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–9. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowell BB. Adaptive thermogenesis: Turning on the heat. Curr Biol. 1998;8:R517–20. doi: 10.1016/s0960-9822(07)00336-3. [DOI] [PubMed] [Google Scholar]
- 45.Finck BN, Kelly DP. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKnight GS, Cummings DE, Amieux PS, et al. Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent Prog Horm Res. 1998;53:139–59. [PubMed] [Google Scholar]
- 47.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Nature. 1996;382:622–6. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 48.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–73. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 49.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–62. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 51.Jorgenson E, Witte JS. A gene-centric approach to genome-wide association studies. Nat Rev Genet. 2006;7:885–91. doi: 10.1038/nrg1962. [DOI] [PubMed] [Google Scholar]
- 52.Wang WY, Barratt BJ, Clayton DG, Todd JA. Genome-wide association studies: Theoretical and practical concerns. Nat Rev Genet. 2005;6:109–18. doi: 10.1038/nrg1522. [DOI] [PubMed] [Google Scholar]
- 53.Swarbrick MM, Waldenmaier B, Pennacchio LA, et al. Lack of support for the association between GAD2 polymorphisms and severe human obesity. PLoS Biol. 2005;3:e315. doi: 10.1371/journal.pbio.0030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahituv N, Kavaslar N, Schackwitz W, et al. Medical sequencing at the extremes of human body mass. Am J Hum Genet. 2007;80:779–91. doi: 10.1086/513471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herbert A, Gerry NP, McQueen MB, et al. A common genetic variant is associated with adult and childhood obesity. Science. 2006;312:279–83. doi: 10.1126/science.1124779. [DOI] [PubMed] [Google Scholar]
- 56.Osler W. Textbook of Medicine. 4th edition. New York: D Appleton and Company; 1901. [Google Scholar]