Abstract
A growing body of evidence from clinical trials and epidemiological studies has identified elevated resting heart rate as a predictor of clinical events. Proof of direct cause and effect is limited, because current drugs that lower heart rate (eg, beta-blockers) have multiple mechanisms of action. A new class of drug, selective If inhibitors, is under investigation as a ‘pure’ heart rate-reducing medication and will help confirm if there is a causal link between elevated heart rate and cardiovascular outcomes. The present paper reviews the evidence for elevated heart rate as a cardiovascular risk factor and some of the current clinical trials testing this hypothesis.
Keywords: Cardiovascular disease, Clinical outcomes, Heart failure, Heart rate, Myocardial ischemia, Prognosis
Abstract
De plus en plus de données probantes tirées d’essais cliniques et d’études épidémiologiques indiquent qu’une fréquence cardiaque élevée au repos est un prédicteur d’événements cliniques. La preuve de sa cause et de son effet direct est limitée, car les médicaments courants qui réduisent la fréquence cardiaque (p. ex., les bêta-bloquants) ont de multiples mécanismes d’action. Une nouvelle catégorie de médicament, les inhibiteurs If sélectifs, est en cours d’exploration à titre de « pur » médicament réduisant la fréquence cardiaque, ce qui aidera à confirmer s’il existe un lien fortuit entre la fréquence cardiaque élevée et les issues cardiovasculaires. Le présent article analyse les données probantes selon lesquelles une fréquence cardiaque élevée est un facteur de risque cardiovasculaire ainsi que certains essais cliniques courants qui évaluent cette hypothèse.
IS HEART RATE A TRUE CARDIOVASCULAR RISK FACTOR?
A considerable number of epidemiological studies have reported a strong association between elevated heart rate and cardiovascular risk, and this association appears to be independent of other major risk factors for atherosclerosis (1). This association has been consistent and was observed in healthy populations among men and women (2–4), (although in some studies the association is less robust in women [5]), various races (2,4), hypertensive subjects (6,7), patients with coronary artery disease (8,9) and in those with heart failure (10). This increasing evidence suggests that heart rate does not merely predict outcome but that elevated heart rate may be a true cardiovascular risk factor; that is, it may be a causal determinant of cardiovascular disease. Indeed, elevated heart rate meets many of the criteria proposed by Sir Bradford Hill in 1965 (11) for defining a true risk factor. These criteria that define a causal link between an exposure and an outcome of interest are strength of the association, consistency of the association, specificity of the association, temporal relationship, biological gradient, biological plausibility, coherence, evidence from experimentation and analogy.
The notion that elevated heart rate could be causally linked to atherosclerosis also appears biologically plausible, as evidenced by the pathophysiological links described below. Moreover, some studies show a biological gradient – a continuous and graded association between heart rate and cardiovascular risk (6,12). To definitively establish heart rate as a cardiovascular risk factor, evidence that lowering heart rate reduces cardiovascular risk is required. To date, such evidence is available retrospectively from studies of beta-blocker use after myocardial infarction (13,14) and heart failure (15–18).
In addition to lowering heart rate and blood pressure, beta-blockers have other actions that may also account for their benefits. A new class of agents is now available that acts selectively on the sinoatrial node and lowers heart rate without affecting other pathways. Ongoing trials with these agents will more directly test the role of heart rate as a true cardiovascular risk factor.
The present paper will review, in brief, the evidence suggesting that resting heart rate is a cardiovascular risk factor, and that lowering heart rate may have an important role in the prevention and management of cardiovascular diseases.
PATHOPHYSIOLOGICAL MECHANISMS LINKING HEART RATE AND CARDIOVASCULAR DISEASE
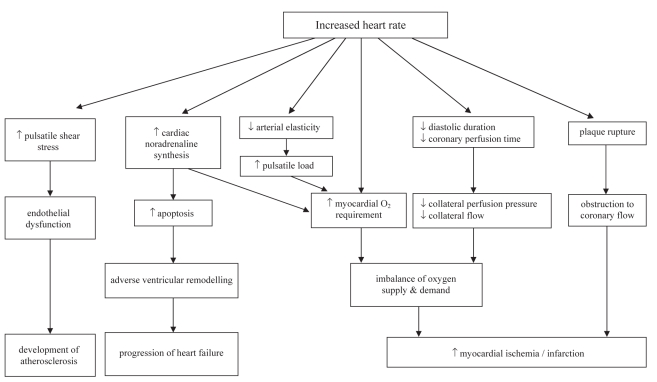
Resting heart rate both contributes to and reflects cardiac pathology. Increased heart rate, due to imbalances of the autonomic nervous system with increased sympathetic activity or reduced vagal tone, has an impact on perfusion-contraction matching, which is the dynamic that regulates myocardial blood supply and function. In the healthy heart, increased metabolism as a result of increased contractile function results in increased myocardial blood flow and, to a lesser degree, increased oxygen extraction. In the presence of coronary artery disease, perfusion-contraction mismatching is localized to areas of inadequate supply. When coronary artery inflow is inadequate to meet demands, contractile and diastolic functions in the affected area are correspondingly reduced (19). An increase in heart rate results not only in an increase in myocardial oxygen demands, but also a potential impairment of supply resulting from a reduction of collateral perfusion pressure and collateral flow (19). This imbalance may promote ischemia, arrhythmias and ventricular dysfunction, as well as acute coronary syndromes, heart failure or sudden death (Figure 1).
Figure 1).
Pathophysiological mechanisms promoted by increased heart rate
With increased heart rate, diastolic perfusion time lessens while myocardial oxygen demand increases. Peak coronary flow increases markedly during diastole, subjecting the coronary arteries to enhanced endothelial shear stress and pulsatile wall stress. The stressed endothelium releases growth hormones (eg, transforming growth factor-beta and insulin-like growth factor-1) and vasoconstrictor peptides (eg, endothelin), and is associated with increased platelet aggregation and a relative deficiency of nitric oxide synthesis. Rapid pulsatile changes appear to increase mechanical damage on the already stressed endothelium. All of these factors encourage the development of atherosclerotic lesions, especially at arterial branches (15).
Prolonged elevated heart rates cause cardiac noradrenaline synthesis to increase (as measured by spillover techniques) and circulating plasma noradrenaline levels to rise. This increase in sympathetic activity and myocardial oxygen requirements may have a direct cytotoxic effect on myocytes, increasing apoptosis with deleterious effects on ventricular remodelling. Elasticity of the larger arteries is reduced by elevated heart rates, resulting in a greater pulsatile arterial load on the heart, consequently increasing the myocardial energy requirements (15). In addition, prolonged elevated heart rates may also cause or exacerbate heart failure (20).
Bradycardia also has adverse effects: it can reduce coronary perfusion pressure, especially in elderly patients with noncompliant arteries and wide pulse pressure, and it can result in nocturnal angina in patients with severe aortic valve regurgitation yet normal coronary arteries (21).
EPIDEMIOLOGICAL ASSOCIATION BETWEEN HEART RATE AND MORBIDITY/MORTALITY
Over the past 30 years, at least 38 studies have looked at the connection between heart rate and cardiovascular or all-cause mortality (5). These studies have covered a wide variety of populations: men and women, black and white, healthy and diseased, and younger and older. After adjusting for risk factors and lifestyle, at least 32 studies show that elevated heart rate is an independent risk factor for mortality and morbidity in healthy people with and without hypertension; it is also an independent risk factor in patients with coronary artery disease, myocardial infarction and heart failure (5,12). Table 1 shows characteristics and representative findings from some of these studies.
TABLE 1.
Selected studies of the relationship between resting heart rate and cardiovascular or all-cause mortality in different patient populations
| Study (reference) | Type | Baseline characteristics | Sample | Follow-up | Selected findings/conclusions |
|---|---|---|---|---|---|
| Chicago (2)* | PC | ||||
| Gas Company | No CHD | 1233 white men (age 40–59 y) | 15 y | Heart rate is significantly related to mortality from cardiovascular and noncardiovascular causes | |
| Western Electric | No CHD | 1899 white men (age 40–55 y) | 17 y | ||
| Heart Assoc | No CHD | 5784 men (age 45–64 y) | 5 y (average) | ||
| Paris Prospective Study 1 (22) | PC | No known or suspected CVD; rSBP ≤180 mmHg | 5713 men (age 45–50 y) | 23 y (mean) | Risk of sudden death from MI was increased in subjects with:
|
| NHEFS (4) | PC | Two races (black, white) | 5136 white men and women; 859 black men and women | 6–13 y white: average 9.9 y black: average 10.3 y |
Race not a major factor, although some variations:
|
| Framingham (6) | PC | Hypertension | 2037 men; 2493 women | 36 y: men: 10,433 person-exams women: 13,001 person-exams |
Independent effect of heart rate on cardiovascular death in persons with hypertension; effect stronger for fatal than nonfatal disease; no confounding by physical activity or by a proxy for pre-existing disease |
| Syst-Eur (7) | PC | Isolated SBP | 4682 men and women; male:female ratio ∼1:2 age ≥63 y | 1–97 m (median 24 m) | Clinic heart rate >79 beats/min significantly predicted all-cause, cardiovascular, noncardiovascular mortality; ambulatory heart rate did not offer additional prognostic information |
| British Regional Heart Study (8) | PC | Ischemic heart disease | 7735 men (age 40–59 y) | 8 y | Heart rate ≥90 beats/min:
|
| CASS (9) | PC | Stable CAD; multiple clinical variables (eg, smoking, hypertension, diabetes, dyslipidemia, inactivity) | 24,913 men and women; male:female ratio ∼ 3:1 age 42–63 y | Median 14.7 y | Women tended to have higher resting heart rate; resting heart rate ≥83 beats/min at baseline conferred significantly higher risk for total mortality and cardiovascular mortality after adjusting for multiple clinical variables; high resting heart rate is a predictor for total and cardiovascular mortality independent of other risk factors in patients with CAD |
| COMET (10) | RT | Heart failure; beta-blocker therapy | 2599 men and women; male:female ratio ∼4:1 age 50–73 y | 4 m after beginning beta-blocker therapy (ie, most subjects were taking maintenance doses) | Survival not related to heart rate before initiation of beta-blocker therapy; heart rate achieved on maintenance beta-blocker therapy has prognostic value |
Includes the Chicago Peoples Gas Company study (Gas Company), the Chicago Heart Association Detection Project in Industry study (Heart Assoc) and the Chicago Western Electric Company study (Western Electric). CAD Coronary artery disease; CASS Coronary Artery Surgery Study; CHD Coronary heart disease; COMET Carvedilol or Metoprolol European Trial; CVD Cardiovascular disease; Framingham The Framingham Study; m months; MI Myocardial infarction; NHEFS NHANES I Epidemiologic Follow-up Study; PC Prospective cohort; rSBP Resting systolic blood pressure; RT Randomized trial; Syst-Eur Systolic Hypertension in Europe Trial; y years
Three studies of normal populations with and without hypertension – the Paris Prospective Study I (22), Hypertension Ambulatory Recording VEnetia STudy (HARVEST) (23) and a review by Aboyens and Criqui (24) – had a combined sample of nearly 180,000 people. They all showed that cardiovascular mortality significantly increases as resting heart rate increases (12). The Framingham study (3) (5070 healthy subjects) measured its subjects’ heart rates every two years. Investigators reported that, in both sexes and at all ages, all-cause, cardiovascular and coronary mortality rates increased progressively in relation to antecedent heart rates; this was, however, more marked in men than in women. The National Health and Nutrition Examination Survey (NHANES) I Epidemiologic Follow-up Study (4) (5995 healthy subjects) concluded that elevated resting heart rate was an independent risk factor for coronary artery disease incidence or death among white and black men and women.
The British Regional Heart Study (8) looked at a mixed (healthy and unhealthy) population of 7735 men aged 40 to 59 years over a period of eight years. In men with no evidence of ischemic heart disease, there was a strong positive association between resting heart rate and age-adjusted rates of all major ischemic heart disease events (fatal and nonfatal), ischemic heart disease-related deaths and sudden cardiac death. After adjustment for age, systolic blood pressure, blood cholesterol, smoking, social class, heavy drinking and physical activity, this association was still significant. In men with pre-existing ischemic heart disease, the association also held but its effect was less pronounced. Investigators noted that elevated heart rate (90 beats/min or greater) is a risk factor, particularly for sudden cardiac death; it is independent of other established coronary risk factors and is most clearly seen in men with no pre-existing ischemic heart disease at the time of the initial examination.
The Coronary Artery Surgery Study (CASS) (9) looked at 24,913 men and women with stable coronary artery disease and one or several clinical variables such as hypertension, diabetes, dyslipidemia and high body mass index. Among the subjects, 4.3% took lipid-lowering drugs, 33.2% smoked while enrolled and 34.8% had sedentary lifestyles. They ranged in age from 50 to 73 years. After 14.7 years (median) of follow-up, the investigators determined that, even after adjusting for those many clinical variables, a resting heart rate of 83 beats/min or greater at baseline conferred significantly higher risk for all-cause and cardiovascular mortality.
The male and female subjects (n=2599, age 50 to 73 years) in the Carvedilol or Metoprolol European Trial (COMET) (10) were patients with heart failure, and they were prescribed beta-blockers with the dose titrated up over several weeks. Most subjects were on a steady maintenance dose at evaluation (four months after enrollment). Heart rate was related to mortality, both at rest and after therapy, although, after multivariate regression, only post-treatment heart rate (at four-month follow-up on maintenance therapy) had prognostic value for all-cause mortality.
These studies support a conclusion that heart rate is a reliable prognostic indicator in both the healthy population and in those with cardiovascular disease.
APPLYING CURRENT KNOWLEDGE INTO PRACTICE
Based on our current knowledge of the connection between elevated heart rate and mortality (both cardiovascular and all-cause), who is likely to benefit from intervention and what form should that intervention take in these target populations?
Angina and acute myocardial infarction
Beta-blockers are well known for their anti-ischemic effects; they slow the heart rate and reduce myocardial contractility with a corresponding reduction of myocardial oxygen demand (25). Mechanistic studies have shown that administering beta-blockers to patients experiencing angina or acute myocardial infarction is associated with significantly improved myocardial oxygen efficiency, which may be responsible for the observed reverse remodelling and infarct-size limitation. Two decades ago, Kjekshus (13) analyzed trials from the 1970s and early 1980s in which beta-blockers were given within 6 h of symptom onset during acute myocardial infarction; results from six acute-intervention studies (total n=1427) showed that a reduction of at least 14 beats/min during infarct evolution was associated with infarct size reduction between 25% and 30% (measured by serum creatine kinase). In the same paper, his analysis of 11 long-term follow-up trials revealed that there is also a relationship between reduction in resting heart rate and nonfatal reinfarctions. The Norwegian Timolol Multicenter Study (14) (n=1884) showed that timolol treatment reduced all-cause mortality by 41.6% and reduced nonfatal reinfarctions by 23.2%; the authors concluded that treatment was a significant predictor of all events, that the mortality rate after acute myocardial infarction is strongly related to the resting heart rate early after infarction in both placebo- and timolol-treated patients, and that the major effect of timolol treatment on mortality may be attributed to the reduction in heart rate. Mauss et al (26) reported that, at hospital discharge after acute myocardial infarction, a patient’s age, left ventricular (LV) ejection fraction and heart rate are univariate risk predictors of event-free survival. Mehta et al (27) recently showed that patients presenting with acute myocardial infarction and shock, and with heart rate greater than 100 beats/min and systolic blood pressure 80 mmHg or less, had 30-day death rates greater than 90%; the authors suggested that this information might be used when determining treatment strategy and counselling patients about their risks.
Heart failure
A 1999 review (15) of several large trials of long-term (six months or greater) pharmacological treatment for heart failure following acute myocardial infarction concluded that beta-blocker treatment had potential to reduce heart rate, increase diastolic time, block sympathetic nervous system activity and thereby reduce mortality. The Cardiac Insufficiency BIsoprolol Study (CIBIS) (16) showed that heart rate change over time had the highest predictive value for survival in patients with LV systolic dysfunction, and that bisoprolol promotes preservation of LV function. The CIBIS II trial (17) examined the relationships between baseline heart rate (BHR), heart rate changes at two months (HRC) and outcomes (mortality and hospitalization for heart failure) in a large (n=2539) sample of patients with mild to moderate symptomatic heart failure. BHR and HRC were significantly related to prognosis, the lowest BHR and the greatest HRC being associated with best survival and reduction of hospital admissions. Beta blockade (with bisoprolol in this study) further improved survival at any level of BHR and HRC and to a similar extent (17). A different beta-blocker (nebivolol) was studied in the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS) with a mean duration of follow-up of 21 months (18). SENIORS involved the most elderly cohort in a randomized beta-blocker study to date, with one-half of the 2128 subjects 75 years of age or older. As expected, a significant reduction in resting heart rate occurred, followed by clinical benefits that became apparent after six months of treatment. It was notable that event curves continued to diverge as the study progressed. The study showed that nebivolol treatment for elderly patients with heart failure (either systolic or diastolic dysfunction) reduced the composite risk of all-cause mortality or hospital admission due to cardiovascular illness. Retrospective analysis of data from the Acute Infarction Ramipril Efficacy (AIRE) study (28) demonstrated that beta-blocker therapy was a significant independent predictor of reduction in the risk of all-cause mortality and severe heart failure.
Taken together, these studies suggest that agents that reduce heart rate – in this case, beta-blockers – are clinically useful for heart failure irrespective of patient age or LV ejection fraction, and they are recommended in current national heart failure guidelines (29,30).
Arrhythmias
A small (n=49) study (31) of right ventricular pacemaker-dependent patients with heart failure and LV systolic dysfunction showed that the benefits of beta blockade can be attenuated or even reversed when pacemakers are set to 80 pulses/min as opposed to 60 pulses/min. The CIBIS II study with bisoprolol did not, however, reduce mortality in patients with atrial fibrillation compared with those in normal sinus rhythm (17).
Cardiovascular prevention
As previously noted in the CASS (9), Framingham (6) and Systolic Hypertension in Europe (Syst-Eur) (7) studies, heart rate is related to prognosis in patients with diabetes, hypertension or dyslipidemia, although it must be pointed out that heart rate modification was not a primary goal, nor was it controlled for on an a priori basis. While it is tempting to think that reduction of heart rate in these populations may improve outcomes, this conclusion is, as yet, unproven.
Target heart rate
It has been shown, for heart failure patients, that greater heart rate reduction is associated with better outcomes; in addition, patients with persistently elevated heart rates derive additional benefit from higher beta-blocker doses (32). The Metoprolol Controlled Release/Extended Release Randomized Intervention Trial in Chronic Heart Failure (MERIT-HF) investigators (33) have recommended aiming for the target beta-blocker dose rather than a target heart rate. Current practice when prescribing heart failure medications is thus to titrate doses up to the maximum recommended unless symptoms impose limits. Recent guidelines (34) recommend caution in introducing a beta-blocker if the heart rate is approximately 50 beats/min to 55 beats/min or less, because lower heart rates tend to be associated with increasing symptoms such as fatigue.
A new class of drugs to reduce heart rate
A new class of agents – selective If inhibitors – is now under investigation (35). The first in its class to be studied is ivabradine, which acts specifically on the sinoatrial node. Administered to rats with heart failure, ivabradine promoted long-term heart rate reduction while improving LV function, increasing stroke volume, and preserving cardiac output despite the heart rate reduction (36). Part of this improvement may be attributable to ivabradine’s modifications in the extracellular matrix and/or function of myocytes as a consequence of long-term heart rate reduction.
The INternatIonal TrIAl on the Treatment of angina with IVabradinE vs atenolol (INITIATIVE) study (37) in humans (n=939) has shown that ivabradine is as effective as atenolol in patients with stable angina. The morBidity-mortality EvAlUaTion of the If inhibitor ivabradine in patients with coronary disease and left ventricULar dys-function (BEAUTIfUL) study, evaluating ivabradine in patients with coronary disease and LV dysfunction, began in January 2005; 10,947 subjects have completed the study, and results are expected in late 2008. On Top Of BB is a four-month randomized, double-blind, parallel-group multicentre study (n=750) that is evaluating the antianginal efficacy and safety of oral ivabradine on top of therapy with atenolol in patients with stable angina pectoris that started in 2005 and has just been completed; results are expected to be published in 2008.
The Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIfT) began in 2006. Each arm of the trial (ivabradine 7.5 mg twice daily versus placebo) will include 2750 patients with a heart rate 70 beats/min or greater and a LV ejection fraction less than 35%. Results should be published in 2010. The eValuation of the IntraVenous If inhibitor ivabradine after ST segment elevation mYocardial infarction (VIVIfY) study will be a phase II pilot trial evaluating the effects of intravenous ivabradine compared with placebo on heart rate and LV dimensions and function in the setting of primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. The study will enroll 75 patients, aged 40 to 80 years, with ST-elevation acute myocardial infarction and treated less than 6 h after symptom onset.
CASE STUDY IN HEART FAILURE
A 49-year-old labourer had been diagnosed with diabetes eight years earlier and was prescribed oral hypoglycemic agents. Three years ago, he developed mild shortness of breath during exertion, which his family physician attributed to obesity, hypertension (150/90 mmHg) and deconditioning. The patient was prescribed a thiazide and had occasionally taken acetaminophen or nonsteroidal anti-inflammatory drugs; the patient said that he never had chest pain. A review of his past office visits showed that his resting heart rate was generally 80 beats/min to 90 beats/min.
He now presented with coughing, fatigue and reduced exercise tolerance, and was admitted to hospital. A physical examination revealed the following: resting heart rate of 126 beats/min, blood pressure of 92/66 mmHg and body mass of 112 kg with abdominal girth of 114 cm. The patient had low pulse volume and displaced LV apex with soft S1 and S2, and with S3 gallop. His jugular venous pressure was 15 cm above the sternal angle (although difficult to see due to his large neck). His chest sounded clear and he had a firm liver edge.
His electrocardiogram showed sinus tachycardia with poor anterior R waves and nonspecific repolarization abnormalities. A chest x-ray showed cardiomegaly with vascular redistribution. Results of blood tests were normal with no evidence of acute myocardial infarction.
The patient was given a diagnosis of decompensated heart failure. He underwent echocardiography, which showed LV ejection fraction of 26% with global hypokinesis, elevated estimated right ventricular systolic pressure (60 mmHg), and moderate tricuspid and mitral regurgitation. On the third day, cardiac catheterization showed 50% stenosis of the mid-left anterior descending artery, 70% stenosis of the proximal left circumflex artery, and 100% occlusion of the right coronary artery with collateralization from the left coronary artery. The patient’s pulmonary artery occlusive pressure was 30 mmHg and his heart rate was 105 beats/min.
The patient was treated with parenteral diuretics and vasodilators. Following stabilization, he was prescribed an angiotensin-converting enzyme inhibitor, a beta-blocker, digoxin, acetylsalicylic acid, a statin and oral diuretics. He slowly recovered. At hospital discharge, his blood pressure was 108/70 mmHg and his heart rate was 94 beats/min. During his exercise test he was able to do four metabolic equivalents without chest pain. Due to this patient’s increased heart rate at hospital discharge, plans were made for possible up-titration of his beta-blocker during follow-up.
This case illustrates the importance of recognizing a patient at high risk before, during and after development of heart failure. In all three stages, this man’s elevated heart rate was an additional indicator of increased risk.
OPPORTUNITIES AND CHALLENGES FOR THE FUTURE
Across a wide range of mammals, slower heart rates are associated with greater longevity (38). Heart rate varies with time of day, emotion, stress and exercise, and is a simple part of every cardiovascular clinical examination. The accumulated weight of evidence linking elevated heart rate to cardiovascular and all-cause mortality, even in apparently healthy individuals, makes a strong case for it to be considered in the assessment of cardiovascular risk.
Defining precisely when a heart rate should be considered ‘elevated’ is not so straightforward. Heart rate assessed from a conventional electrocardiogram carries similar predictive power compared with that determined from the 24 h mean heart rate obtained from Holter recordings (26). An analysis (24) of 18 epidemiological studies showed a mortality excess of 30% to 50% for every 20 beats/min increase at rest. While tachycardia is usually defined as heart rate greater than 100 beats/min, lower rates could also be considered elevated under quiet resting conditions. Spodick et al (39) have suggested that a normal resting heart rate be defined as 50 beats/min to 90 beats/min.
Some current instruments incorporate heart rate as a predictor and/or factor when assessing risk – for example, the Global Registry of Acute Coronary Events (GRACE) risk score (40). The challenge remains in applying epidemiological data to individual persons or patients. Current clinical trials of selective If inhibitors such as ivabradine may help to define further the independent role of heart rate lowering and to quantify its benefit. This new class of drug may complement our clinical armamentarium of other drugs such as beta-blockers and nondihydropyridine calcium channel blockers.
Acknowledgments
The authors acknowledge the assistance of Phillipa Rispin in writing and editing this manuscript.
Footnotes
DISCLOSURE: All authors have received speaker and advisory board honoraria from Servier Canada Inc; JG Howlett and J-C Tardif have received research grants from Servier Canada Inc.
REFERENCES
- 1.Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens. 2004;26:637–44. doi: 10.1081/ceh-200031959. [DOI] [PubMed] [Google Scholar]
- 2.Dyer AR, Persky V, Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: Findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112:736–49. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: The Framingham Study. Am Heart J. 1987;113:1489–94. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 4.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: The NHANES I Epidemiologic Follow-up Study. Am Heart J. 1991;121:172–7. doi: 10.1016/0002-8703(91)90970-s. [DOI] [PubMed] [Google Scholar]
- 5.Palatini P, Benetos A, Julius S. Impact of increased heart rate on clinical outcomes in hypertension: Implications for antihypertensive drug therapy. Drugs. 2006;66:133–44. doi: 10.2165/00003495-200666020-00001. [DOI] [PubMed] [Google Scholar]
- 6.Gillman MW, Kannel WB, Belanger A, D’Agostino RB. Influence of heart rate on mortality among persons with hypertension: The Framingham Study. Am Heart J. 1993;125:1148–54. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 7.Palatini P, Thijs L, Staessen JA, et al. Predictive value of clinic and ambulatory heart rate for mortality in elderly subjects with systolic hypertension. Arch Intern Med. 2002;162:2313–21. doi: 10.1001/archinte.162.20.2313. [DOI] [PubMed] [Google Scholar]
- 8.Shaper AG, Wannamethee G, Macfarlane PW, Walker M. Heart rate, ischaemic heart disease, and sudden cardiac death in middle-aged British men. Br Heart J. 1993;70:49–55. doi: 10.1136/hrt.70.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–74. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 10.Metra M, Torp-Pedersen C, Swedberg K, et al. Influence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: Results from the COMET trial. Eur Heart J. 2005;26:2259–68. doi: 10.1093/eurheartj/ehi386. [DOI] [PubMed] [Google Scholar]
- 11.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjalmarson Å. Heart rate: An independent risk factor in cardiovascular disease. Eur Heart J Suppl. 2007;9(Suppl F):F3–7. [Google Scholar]
- 13.Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol. 1986;57:43F–9F. doi: 10.1016/0002-9149(86)90888-x. [DOI] [PubMed] [Google Scholar]
- 14.Gundersen T, Grøttum P, Pedersen T, Kjekshus JK. Effect of timolol on mortality and reinfarction after acute myocardial infarction: Prognostic importance of heart rate at rest. Am J Cardiol. 1986;58:20–4. doi: 10.1016/0002-9149(86)90234-1. [DOI] [PubMed] [Google Scholar]
- 15.Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J Suppl. 1999;1(Suppl H):H64–9. [Google Scholar]
- 16.Lechat P, Escolano S, Golmard JL, et al. Prognostic value of bisoprolol-induced hemodynamic effects in heart failure during the Cardiac Insufficiency BIsoprolol Study (CIBIS) Circulation. 1997;96:2197–205. doi: 10.1161/01.cir.96.7.2197. [DOI] [PubMed] [Google Scholar]
- 17.Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103:1428–33. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- 18.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 19.Heusch G, Schulz R. The role of heart rate and the benefits of heart rate reduction in acute myocardial ischaemia. Eur Heart J Suppl. 2007;9(Suppl F):F8–F14. [Google Scholar]
- 20.Khasnis A, Jongnarangsin K, Abela G, Veerareddy S, Reddy V, Thakur R. Tachycardia-induced cardiomyopathy: a review of literature. Pacing Clin Electrophysiol. 2005;28:710–21. doi: 10.1111/j.1540-8159.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 21.Basta LL, Raines D, Najjar S, Kioschos JM. Clinical, haemodynamic, and coronary angiographic correlates of angina pectoris in patients with severe aortic valve disease. Br Heart J. 1975;37:150–7. doi: 10.1136/hrt.37.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–8. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 23.Palatini P, Dorigatti F, Zaetta V, et al. Heart rate as a predictor of development of sustained hypertension in subjects screened for stage 1 hypertension: The HARVEST Study. J Hypertens. 2006;24:1873–80. doi: 10.1097/01.hjh.0000242413.96277.5b. [DOI] [PubMed] [Google Scholar]
- 24.Aboyans V, Criqui M. Can we improve cardiovascular risk prediction beyond risk equations in the physician’s office? J Clin Epidemiol. 2006;59:547–58. doi: 10.1016/j.jclinepi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Task Force of the European Society of Cardiology Management of stable angina pectoris. Recommendations of the Task Force of the European Society of Cardiology. Eur Heart J. 1997;18:394–413. doi: 10.1093/oxfordjournals.eurheartj.a015259. [DOI] [PubMed] [Google Scholar]
- 26.Mauss O, Klingenheben T, Ptaszynski P, Hohnloser SH. Bedside risk stratification after acute myocardial infarction: Prospective evaluation of the use of heart rate and left ventricular function. J Electrocardiol. 2005;38:106–12. doi: 10.1016/j.jelectrocard.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Mehta RH, Califf RM, Yang Q, et al. Impact of initial heart rate and systolic blood pressure on relation of age and mortality among fibrinolytic-treated patients with acute ST-elevation myocardial infarction presenting with cardiogenic shock. Am J Cardiol. 2007;99:793–6. doi: 10.1016/j.amjcard.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 28.Spargias CS, Hall AS, Ball SG.Beta-blocker therapy in patients with clinical evidence of heart failure after acute myocardial infarction J Am Coll Cardiol 199831Suppl 132A(Abst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: Diagnosis and management Can J Cardiol 20062223–45.(Erratum in 2006;22:271). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold JM, Howlett JG, Dorian P, et al. Canadian Cardiovascular Society Consensus Conference recommendations on heart failure update 2007: Prevention, management during intercurrent illness or acute decompensation, and use of biomarkers. Can J Cardiol. 2007;23:21–45. doi: 10.1016/s0828-282x(07)70211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thackray SD, Ghosh JM, Wright GA, et al. The effect of altering heart rate on ventricular function in patients with heart failure treated with beta-blockers. Am Heart J. 2006;152:713.e9–13. doi: 10.1016/j.ahj.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Huang RL, Listerman J, Goring J, Giesberg C, Nading MA, Butler J. Beta-blocker therapy for heart failure: Should the therapeutic target be dose or heart rate reduction? Congest Heart Fail. 2006;12:206–10. doi: 10.1111/j.1527-5299.2006.05477.x. [DOI] [PubMed] [Google Scholar]
- 33.Gullestad L, Wikstrand J, Deedwania P, et al. What resting heart rate should one aim for when treating patients with heart failure with a beta-blocker? Experiences from the Metoprolol Controlled Release/Extended Release Randomized Intervention Trial in Chronic Heart Failure (MERIT-HF) J Am Coll Cardiol. 2005;45:252–9. doi: 10.1016/j.jacc.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Heart Failure Society Of America Heart failure in patients with left ventricular systolic dysfunction. J Card Fail. 2006;12:e38–e57. doi: 10.1016/j.cardfail.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 35.DiFrancesco D, Camm JA. Heart rate lowering by specific and selective If current inhibition with ivabradine: A new therapeutic perspective in cardiovascular disease. Drugs. 2004;64:1757–65. doi: 10.2165/00003495-200464160-00003. [DOI] [PubMed] [Google Scholar]
- 36.Mulder P, Barbier S, Chagraoui A, et al. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–9. doi: 10.1161/01.CIR.0000118464.48959.1C. [DOI] [PubMed] [Google Scholar]
- 37.Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K, INITIATIVE Investigators Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–36. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- 38.Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol. 1997;30:1104–6. doi: 10.1016/s0735-1097(97)00246-5. [DOI] [PubMed] [Google Scholar]
- 39.Spodick DH, Raju P, Bishop RL, Rifkin RD. Operational definition of normal sinus heart rate. Am J Cardiol. 1992;69:1245–6. doi: 10.1016/0002-9149(92)90947-w. [DOI] [PubMed] [Google Scholar]
- 40.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]