Abstract
The role of new and emerging biomarkers in risk prediction has become a topic of significant interest and controversy in recent times. Currently, available models for risk prediction are reasonably good yet still misclassify a not insignificant portion of the population. The sheer number of new potential risk markers is daunting, and it is difficult to assess the importance of each one over and above the traditional risk factors. Endothelial function is one potential biomarker of risk that has been extensively studied. However, while it has demonstrated some utility in risk prediction, its use in daily clinical practice is yet to be clearly defined. The present review assesses the prognostic significance of measures of endothelial function.
Keywords: Endothelial function, Risk prediction
Abstract
Le rôle des biomarqueurs nouveaux et émergents sur la prédiction du risque est récemment devenu un sujet de grand intérêt et de controverse. Les modèles actuels de prédiction du risque sont raisonnablement bons, mais ils proposent la classification erronée d’une forte proportion de la population. Le nombre même de nouveaux marqueurs de risque potentiels est décourageant, et il est difficile d’évaluer l’importance de chacun d’eux en plus des facteurs de risque classiques. La fonction endothéliale est un biomarqueur potentiel de risque qui a fait l’objet de nombreuses études. Cependant, même s’il a démontré une certaine utilité de prédiction du risque, son utilisation dans la pratique clinique quotidienne n’est pas encore clairement définie. La présente analyse permet d’évaluer la signification pronostique de la fonction endothéliale.
The wealth of available epidemiological data has led to the development of many risk stratification models in cardiovascular disease, perhaps the most prominent of which is the Framingham model (1,2). However, there is no risk model that perfectly predicts cardiovascular risk at an individual level, and thus, there have been many attempts to refine existing risk models through the addition of further factors. Traditional risk scoring systems evaluating risk factors such as smoking, hypertension and diabetes are reasonably effective; however, nearly one-quarter of cardiovascular events occur in people with minimal identifiable risk (3). Furthermore, the prevalence of all of these risk factors is nearly the same in those with and without disease (4).
The potential risk modifier that has received the most attention of late is serum high-sensitivity C-reactive protein, which has demonstrated some additive value in refining risk in the Framingham and Physicians Health Study risk models (5,6). However, there are many other potential biomarkers available and under investigation for their utility in risk prediction, including coagulation markers, genetics, various lipids subfractions and, more recently, imaging modalities. Of particular interest to vascular biologists is the use of measures of endothelial function as a biomarker of risk.
Endothelial function is a major contributor to overall vascular health and plays an important role in clinical expression of significant vascular disease (7). Endothelial dysfunction has been found to be in association with many disease states, including all major Framingham risk factors, and dysfunction occurs before the development of overt cardiovascular disease (8–15). Furthermore, vascular dysfunction has been demonstrated to be predictive of adverse outcomes following vascular surgical or percutaneous coronary intervention (16,17). However, its role in risk assessment for an individual patient is yet to be defined. Before it can even be considered to be of value as a bio-marker, several questions must be answered:
Is endothelium function reasonably associated with the pathophysiology of heart disease?
Can it be reproducibly measured?
Can an abnormality be detected in healthy asymptomatic subjects?
Are there data to suggest that measuring endothelial function adds to the ability to detect risks independent of established risk factors? (18).
These issues are addressed in more detail in the present review.
ENDOTHELIAL FUNCTION
Is endothelial function reasonably associated with the pathophysiology of heart disease?
Healthy endothelium is vital as a regulator of vascular homeostasis. It sits as a monolayer of endothelial cells lining the lumen of the vasculature, extending from large conduit vessels down to the intravasculature microcirculation (19). As such, it is exposed to any and all systemic stressors, undergoing constant injury and subsequent repair (19,20), and has the ability to exert vascular control. The endothelium has myriad roles that demonstrate its centrality in the development of atherosclerosis (7). This understanding has evolved since the initial description of endothelium-derived relaxing factor (21).
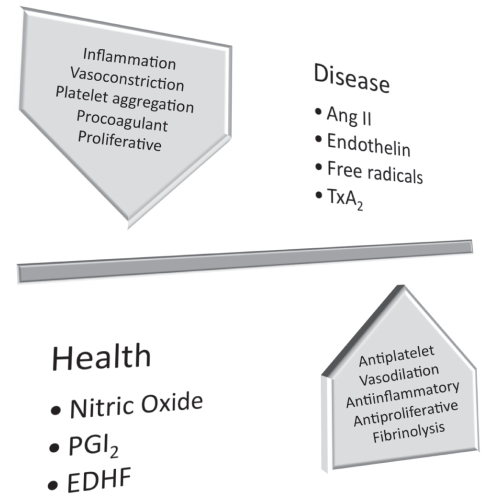
The endothelium has many functions beyond simply serving as an inert, selectively permeable barrier between the circulation and the vessel wall (Figure 1). It plays a role in vasomotion (and thus responses to ischemia), platelet function (adhesion and aggregation), inflammation, coagulation and fibrinolysis, smooth muscle cell proliferation and mediating endothelial progenitor cell activity. Healthy endothelial function relies on the production of nitric oxide (NO), prostaglandin I2 and endothelium-derived hyperpolarizing factor; all of these vasoactive substances support endothelial function through their effect on vasodilation, but also by being anti-inflammatory, anti-proliferative and anticoagulatory (via heparin and protein C and S), and by promoting fibrinolysis (via tissue plasminogen activator) (22) and diminishing platelet aggregation (via NO and prostacyclin) (23). In disease, the role of endothelins, angiotensin II and free radicals become dominant, promoting vasoconstriction (via diminished NO), smooth muscle cell proliferation (via mitogenic substances such as endothelin-1) (24) and inflammation, while also resulting in platelet aggregation and coagulation. In moving from health to disease, the endothelium loses its antiatherogenic characteristics, and abnormal responses to injury develop. Perturbations in endothelial function are a very early sign of atherosclerotic disease (25,26).
Figure 1).
The role of the endothelium in health and disease. ANG II Angiotensin II; EDHF Endothelium-derived hyperpolarizing factor; PGI2 Prostaglandin I2; TxA2 Thromboxane A2
The relationship between cardiovascular risk factors and endothelial dysfunction is thought to be mediated via the common pathway of increased oxidative stress, although the exact mechanisms are different for various pathologies. Diabetes, hypertension, hyperlipidemia and smoking are all known to cause increased oxidative stress, and all have been demonstrated to lead to endothelial dysfunction.
While numerous molecules play a role in endothelial function, the most prominent and central one is NO. NO bioavailability is reduced in the presence of reactive oxygen species that are generated at times of oxidative stress (27). NO bioavailability is further reduced at times of stress owing to oxidation and subsequent depletion of tetrahydrobiopterin (28). This results in the uncoupling of NO synthase, and therefore a disruption in the healthy balance of the nitroso-redox system, resulting in impaired endothelial function (28). Tetrahydrobiopterin supplementation has been demonstrated to improve endothelial function in patients with high levels of oxidative stress, such as patients with diabetes (29) or hypercholesterolemia (30–32).
There are currently no treatments directed toward primarily improving endothelial function, because its utility as a clinical end point has not been established and it is not measured outside of the research setting. However, the roles of several cardiovascular medications in improving endothelial dysfunction have been well documented. Of particular importance are 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) and angiotensin-converting enzyme inhibitors. It bas been demonstrated that low-density lipoprotein reduction with statins improves endothelial function in both the coronary and peripheral vascular beds (33–35); furthermore, this improvement has been associated with favourable cardiovascular outcomes. Angiotensin-converting enzyme inhibition has been demonstrated to have similar beneficial effects (36,37).
These medications are obviously well established to reduce cardiovascular events, which perhaps highlights the importance of endothelial function in such events. Still, there is no evidence to suggest that treatment with a goal of improving endothelial function results in an improvement in cardiovascular health and a reduction in CV events.
How do we measure endothelial function? can it be reproducibly measured?
There are many published mechanisms by which to assess endothelial function, varying from very invasive, such as intracoronary assessment, to noninvasive, such as brachial artery flow-mediated dilation (FMD) (Table 1).
TABLE 1.
Summary of modalities for assessing endothelial function
| Microvasculature |
|---|
| Pulse arterial tonometry |
| Coronary blood flow – Doppler |
| Coronary blood flow – positron emission tomography |
| Forearm impedance plethysmography |
| Pulse wave analysis (applanation tonometry) |
| Cardiac magnetic resonance |
| Laser Doppler flowmetry of the skin |
| Hyperemic velocity post occlusion |
|
Conduit vessel |
| Flow-mediated dilation |
| Quantitative coronary angiography |
| Flow-mediated constriction |
The first assessments of endothelial function were made in the coronary circulation (38,39). Using coronary angiography and intra-vascular ultrasound, the responses of the coronary circulation to endothelium-dependent stimuli (such as acetylcholine) in terms of coronary diameter and blood flow can be directly measured. This is still considered to be the gold standard in terms of endothelial function assessment. However, this method is obviously invasive, time intensive and expensive. Outside of use in patients already undergoing angiography, it is limited. It is especially impractical for any long-term study requiring serial measurement.
Atherosclerosis is not a disease limited to the coronary arteries. It is a systemic and diffuse disease, and we can therefore assess endothelial function in any vascular bed and extrapolate our findings systemically (19). Thus, methods have been established to assess endothelial function in the peripheral circulation. The relationship between coronary and peripheral measures of endothelial function is modest to good (40).
The brachial artery is one that holds particular appeal for peripheral endothelial assessment. It is easily and consistently accessible across patient populations. Initial assessments of endothelial function using the forearm were done using similar techniques as the intracoronary assessments: it involved direct cannulation of the brachial artery and infusion of vasoactive substance. The change in volume of the forearm was then assessed as a measure of forearm microvascular function. This technique of impedance plethysmography was demonstrated to be reproducible and easily taught, and was used extensively in small studies of endothelial function (41). Impairment in endothelial function demonstrated by this technique has been shown to be predictive of cardiovascular events in a small cohort of patients (14,42).
However, less invasive methods of assessing endothelial function in the forearm have been developed. Specifically, newer methods no longer require intervention at all: with the use of ultrasound imaging, dilation of the brachial artery in response to ischemic stress (FMD) can be assessed. FMD is a well-accepted and validated modality for assessing endothelial function (43,44). This technique has been used with very good reproducibility and low interobserver variability (45). In addition, recently, there has been interest in the hyperemic stimulus itself as a marker of endothelial health (46). A recent study by Huang et al (16) demonstrated that both FMD and hyperemic velocity were predictive of cardiovascular events in a cohort of patients with peripheral arterial disease referred for vascular surgery. However, this technique requires vasoactive medications to be held, as well as time and significant skill to perform.
Another noninvasive way to measure endothelial function is by using peripheral arterial tonometry (PAT) devices (47). This newly developed device resembles a pulse oximeter and is placed on the index finger tip in a similar manner. Measurements of pulse volume amplitude are thereby taken from both fingers. Similar to FMD, a blood pressure cuff is placed on the right arm, and PAT is measured before cuff inflation and subsequently following deflation. A measure of pulse amplitude is thereby obtained in a hyperemic state, which is reported as a ratio compared to the pulse volume amplitude in the nonstressed arm (PAT ratio). Impairment in hyperemic pulse response (lower PAT ratio) has been associated with impaired endothelial function. In a relatively large sample (1957 patients) of Framingham Third Generation Cohort participants, PAT ratio was shown to be affected by numerous traditional cardiovascular risk factors, including diabetes, smoking and lipid measures (48). This lends some credence to its proposed validity. An advantage of this technique includes its relative ease of use with shallow learning curve, but it is still limited by the length of time studies take as well as the need to avoid vasoactive medications and to fast prior to undergoing study.
Although the use of PAT has recently been validated in reasonably larger populations, its role in vasomotor assessments of endothelial function is not yet secure. The pathophysiology underlying impaired digital hyperemic responses to stress is not clearly defined. Furthermore, while PAT has been demonstrated to be associated with other peripheral measures of endothelial function, such as FMD, the association is modest at best (47). Further study and time are required to truly assess its utility; to that end, there are a number of very large studies underway studying patients from the Framingham study, among others, with PAT to answer some of these lingering questions.
Beyond assessing coronary vasoreactivity, FMD and PAT, there are multitudinous other methods by which to measure endothelial function. These include other methods of assessing vasomotion, imaging modalities (cardiac magnetic resonance, positron emission tomography and nuclear techniques), measures of arterial compliance (applanation tonometry), and measurement of markers of oxidative stress and inflammation (endothelin-1, NO). While each of these techniques holds particular appeal, each also has technique-specific pitfalls. At present, vasomotor assessments of endothelial function with the described techniques are the most broadly described and applied. The relative importance and utility of each of these measures is yet to be determined.
Does endothelial function predict cardiovascular risk?
There have been a number of studies that have assessed the ability of endothelial function to predict cardiovascular risk (Table 2). While earlier studies attempting to demonstrate a relationship between endothelial dysfunction were limited to high-risk populations and involved small numbers of patients, there are newer studies looking at larger, younger populations demonstrating a similar result.
TABLE 2.
Studies demonstrating a relationship between endothelial function and prognosis
| Author | Patient population | Conclusions |
|---|---|---|
|
Coronary vasomotion | ||
| Al Suwaidi et al (38) | Mild coronary atherosclerosis (n=157) | Predictive of increased rates of myocardial events |
| Schachinger et al (49) | Chest pain (n=147) | Independently predictive of increased rates of myocardial events |
| Hollenberg et al (59) | Postcardiac transplant (n=73) | Predictive of CV events |
| Halcox et al (60) | Patients with and without CAD (n=308) | Independent predictor of CV events |
| Targonski et al (61) | Patients with mild CAD (n=503) | Independent predictor of cerebrovascular events |
|
Impedance plethysmography | ||
| Perticone et al (14) | Untreated hypertensives (n=225) | Predictive of increased rates of myocardial events in step-wise modelling |
| Heitzer et al (42) | Patients with CAD (n=281) | Independent predictor of CV events |
| Fichtlscherer et al (62) | Patients with ACS (n=198) | Response to acetylcholine predictive of events |
|
Flow-mediated dilation | ||
| Modena et al (10) | Post-menopausal female hypertensives (n=400) | Lack of improvement in endothelial dysfunction with antihypertensives associated with CV events |
| Rossi et al (15) | Postmenopausal women (n=2264) | FMD predictive of CV events beyond traditional risk factors |
| Yeboah et al (51) | Elderly cohort (n=2792) | FMD predictive of CV events beyond traditional risk factors |
| Gokce et al (63) | Elective vascular surgery patients (n=187) | FMD independently predictive of CV events |
| Brevetti et al (64) | Patients with peripheral vascular disease (n=131) | ABI predictive of CV events |
| Chan et al (65) | Patients in cardiac rehabilitation (n=152) | FMD associated with CV events |
| Karatzis et al (66) | Patients with NSTEMI (n=98) | FMD independently predictive of CV events |
| Patti et al (17) | Patients postcoronary stent (n=136) | FMD predictive of restenosis |
| Shimbo et al (52) | Multiethnic population with varied levels of risk (n=842) | FMD predictive of outcomes, but not in multivariate analysis |
|
Reactive hyperemia | ||
| Huang et al (16) | Vascular surgery patients (n=267) | RH and FMD independently predictive of CV events beyond traditional risk factors |
Table adapted from Mancini (58). ABI Ankle-brachial index; ACS Acute coronary syndrome; CAD Coronary artery disease; CV Cardiovascular; FMD Flow-mediated dilation; NSTEMI Non-ST elevation myocardial infarction; RH Reactive hyperemia
Endothelial function in those with established disease
Endothelial function has been demonstrated to be predictive of poor outcomes in those with established disease. In the first study of its kind, Schachinger et al (49) demonstrated a higher incidence of cardiovascular events in patients with abnormal coronary vasomotion and mild coronary disease. This provided the first evidence of the prognostic significance of coronary vascular dysfunction. This result was confirmed by others (38).
A similar study was conducted as part of the Women’s Ischemia Syndrome Evaluation trial (50); in that study of 163 women referred for clinically indicated coronary angiogram, coronary dilation in response to acetylcholine was independently predictive of cardiovascular events during a median follow-up period of 48 months. In contrast, a Dutch study of 277 patients referred for first coronary angiogram for suspected coronary artery disease showed that there was no difference in rates of events by response to intracoronary acetylcholine infusion (39).
In a study of 136 patients with single-vessel coronary disease undergoing percutaneous coronary intervention, depressed FMD was found to be a strong predictor of in-stent restenosis (17). This effect was significant, even after adjustment for other cardiovascular risk factors, including diabetes and hypercholesterolemia. In fact, preserved endothelial function was found to have a negative predictive value of 96% for excluding restenosis.
Endothelial function in high-risk patients
Endothelial function has been established to predict risk in those with established cardiovascular disease – but beyond that, it has proven utility in predicting risk in those with risk factors alone. Furthermore, plasticity in endothelial function in terms of response to cardiovascular risk factor treatment has been demonstrated to be predictive of risk.
Beyond looking at FMD as a measure of endothelial function, Huang et al (16) demonstrated that lower reactive hyperemia is associated with increased cardiovascular risk in a high-risk group of 267 patients with peripheral vascular disease referred for surgery. The predictive value of reactive hyperemia was incremental beyond that of FMD alone in this population.
Postmenopausal hypertensive women have been demonstrated to have abnormal endothelial function (10,15). Based on this observation, Modena et al (10) set out to determine whether standard hypertension treatments are of any benefit at the level of the endothelium. A total of 400 hypertensive postmenopausal women were enrolled, and all were documented to have impaired FMD at baseline. FMD was reassessed after six months of antihypertensive therapy, and the majority of the women had significant improvement – with the remaining demonstrating no change. Those who had improvement in their endothelial function also had a significant reduction in clinical cardiovascular events, suggesting that improvements in endothelial function portends improved prognosis.
Can an abnormality be detected in healthy, asymptomatic subjects?
The real role for endothelial function in risk assessment may be in reclassifying patients who are low or medium risk and do not yet have any established cardiovascular disease. These are patients who provide a diagnostic and treatment dilemma. There are now several large studies evaluating the additive value of assessment of endothelial function over and above traditional risk factors in predicting risk. A recently published study by Yeboah et al (51) examined the relationship between FMD and subsequent cardiovascular events. A total of 2792 adults with a mean age of 78.6 years from the Cardiovascular Health Study had FMD assessed at baseline and were followed for five years. Patients with FMD greater than the sex-specific median had significantly better cardiovascular event-free survival than those with FMD at or below the median. FMD remained predictive of events, above and beyond the traditional risk factors. However, it added only approximately 1% to the prognostic accuracy of the model. Thus, while FMD is a statistically significant predictor of cardiovascular risk, it is still not apparently a clinically important predictor.
A similar study of a more diverse, younger population was conducted recently by Shimbo et al (52). For this study, 842 patients free of stroke or myocardial infarction were selected from a multiethnic prospective cohort study, the Northern Manhattan Study. While FMD was found to be predictive of cardiovascular events, this relationship was not statistically significant once multivariate analysis including all traditional risk factors was conducted.
Whether a biomarker can detect early stages of disease before any clinically overt manifestations is a question of utmost importance when assessing risk ‘prediction’. If testing can only detect an abnormality once a disease state has actually been established, it is obviously of doubtful clinical or practical importance in risk stratification. Larger studies are ongoing to determine whether these measures are practical and helpful in larger cohorts, especially those including younger patients.
One ongoing study from our institution, the Firefighters And Their Endothelium (FATE) study, is a prospective study of a relatively young population of 1585 male firefighters from Canada (53). At enrollment, none of the subjects had overt cardiovascular disease. Several measures of vascular health were taken at baseline, including serum biomarkers (CRP, homocysteine, adhesion molecules), carotid intimal medial thickness and FMD measurements. Results from this study should be available shortly; the questions we may be able to answer include the applicability of FMD in predicting outcomes in a young, healthy population, and the relative importance of different measures of vascular health in predicting outcomes. In addition, there are further ongoing epidemiological studies looking at the utility of endothelial function measurement in predicting risk in young healthy and diverse populations, including the Framingham Study (46), the National Institutes of Health-sponsored Multi-Ethnic Study of Atherosclerosis (MESA) study, the Young Finnish Study and the Uppsala Seniors study (54–56).
Are there any data to suggest that measuring endothelial function adds to the ability to detect risks independent of established risk factors?
At present, there is insufficient evidence to suggest that endothelial function should be added to the daily clinical armamentarium for risk prediction at an individual patient level. It is clearly associated with cardiovascular disease and has been demonstrated to be dysfunctional long before the development of overt cardiovascular disease. It is also demonstrated to be modifiable through the use of medications whose place in the treatment of atherosclerotic disease has been well established. However, given the high predictive value of current models, it would be difficult to add any further predictors that would be of global significance or of much additive value. As noted, the best published study in this area demonstrated that FMD only improved risk prediction by approximately 1% (51). Ongoing studies should shortly address the utility of these measures in younger subjects at intermediate risk of atherosclerosis complications, the population in which biomarkers may play an important role.
IMPLICATIONS
The role of endothelial function as a biomarker may be twofold: persistence in its importance in clinical trials and research, and perhaps in helping to reclassify young patients at low or moderate risk. It has a key role as a research tool in vascular biology and has helped to further our understanding of atherosclerotic disease. It has and will be useful in the evaluation of new treatments for cardiovascular disease. Before it becomes practical for use on a more regular basis, methods for assessment need to be simplified and more widely available. Further work needs to be done to determine the utility of a measure of endothelial function in identifying patients who are in need of risk modification when traditional markers are unable to do so, analogous to how measurement of CRP has identified a new cohort of patients who may benefit from statin therapy (46). At present, there is work yet to be done to determine the clinical importance and utility of endothelial function for both patients and clinicians.
Acknowledgments
BMJ is a Clinical Research Fellow of the Alberta Heritage Foundation for Medical Research, and a Tomorrow’s Research Cardiovascular Health Professionals (TORCH) Trainee. TJA is a Senior Scholar of the Alberta Heritage Foundation for Medical Research.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: The Framingham study. Am J Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 3.Khot UN, Khot MB, Bajzer C, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:891–7. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, Knoll M, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891–7. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PW, Pencina MJ, Jacques P, Selhub J, D’Agostino R, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circulation: Cardiovasc Qual Outcomes. 2008;1:92–7. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction. The Reynolds Risk Score for Men. Circulation. 2008;118:2243–51. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mensah GA. Healthy endothelium: The scientific basis for cardiovascular health promotion and chronic disease prevention. Vascul Pharmacol. 2007;46:310–4. doi: 10.1016/j.vph.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am. 2008;37:685–711. doi: 10.1016/j.ecl.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perticone F, Maio R, Tripepi G, Zoccali C. Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation. 2004;110:821–5. doi: 10.1161/01.CIR.0000138745.21879.27. [DOI] [PubMed] [Google Scholar]
- 10.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–10. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 11.Linder L, Kiowski W, Buhler FR, Lüscer TF. Indirect evidence for the release of endothelium-derived relaxing factor in the human forearm circulation in vivo: Blunted response in essential hypertension. Circulation. 1990;81:1762–7. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- 12.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–34. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Bee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–16. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 14.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–6. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 15.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Huang AL, Silver AE, Shvenke E, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–9. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patti G, Pasceri V, Melfi R, et al. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–5. doi: 10.1161/01.CIR.0000151308.06673.D2. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 19.Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation. 2005;111:363–8. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 20.Lerman A. Restenosis: Another “dysfunction” of the endothelium. Circulation. 2005;111:8–10. doi: 10.1161/01.CIR.0000152694.27996.3E. [DOI] [PubMed] [Google Scholar]
- 21.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 22.Oliver JJ, Webb DJ, Newby DE. Stimulated tissue plasminogen activator-release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol. 2005;25:2470–9. doi: 10.1161/01.ATV.0000189309.05924.88. [DOI] [PubMed] [Google Scholar]
- 23.Karsan A, Harlan JM. The blood vessel wall In: Hoffman R, ed Hematology: Basic Principles and Practice. 4th edn. Philadelphia: Elsevier; 2005. pp. 1921–5. [Google Scholar]
- 24.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–9. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. Atherosclerosis is an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 26.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–9. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 27.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–58. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–44. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 29.Pieper GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Stroes E, Kastelein JJ, Cosentino F, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–6. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyss CA, Koepfli P, Namdar M, et al. Tetrahydrobiopterin restores impaired coronary microvascular dysfunction in hypercholesterolemia. Eur J Nucl Med Mol Imaging. 2005;32:84–91. doi: 10.1007/s00259-004-1621-y. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda Y, Teragawa H, Matsuda K, Yamagata T, Matsuura H, Chayama K. Tetrahydrobiopterin restores endothelial function of coronary arteries in patients with hypercholesterolaemia. Heart. 2002;87:264–9. doi: 10.1136/heart.87.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The Effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–93. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 34.Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–7. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 35.Dupuis J, Tardif J-C, Cernacek P, Theroux P. Cholesterol reduction Rapidly Improves Endothelial Function After Acute Coronary syndromes: The RECIFE (Reduction of Cholesterol in Ischemia and Function of the Endothelium) trial. Circulation. 1999;99:3227–33. doi: 10.1161/01.cir.99.25.3227. [DOI] [PubMed] [Google Scholar]
- 36.Anderson TJ, Elstein E, Haber H, Charbonneau F. Comparative study of ACE-inhibtion, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) J Am Coll Cardiol. 2000;35:60–6. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- 37.Mancini GBJ, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease: The TREND (Trial on Reversing ENdothelial Dysfunction) study. Circulation. 1996;94:258–65. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 38.Al Suwaidi J, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 39.Asselbergs FW, Monnink SH, Jessurun GA, et al. Assessing the prognostic value of coronary endothelial function in patients referred for a first coronary angiogram. Am J Cardiol. 2004;94:1063–7. doi: 10.1016/j.amjcard.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 40.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 41.Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–53. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 43.Anderson TJ. Prognostic significance of brachial flow-mediated vasodilation. Circulation. 2007;115:2373–5. doi: 10.1161/CIRCULATIONAHA.107.697045. [DOI] [PubMed] [Google Scholar]
- 44.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 45.Donald AE, Charakida M, Cole TJ, et al. Non-invasive assessment of endothelial function – which technique? J Am Coll Cardiol. 2006;48:1846–50. doi: 10.1016/j.jacc.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 46.Vita JA, Keaney JF, Jr, Larson MG, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004;110:3604–9. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- 47.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 48.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–74. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasomotor dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 50.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: Results From the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–5. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 51.Yeboah J, Crouse JR, Hsu F-C, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation. 2007;115:2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 52.Shimbo D, Grahame-Clarke C, Miyake Y, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis. 2007;192:197–203. doi: 10.1016/j.atherosclerosis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Anderson TJ, Roberts AC, Hildebrand K, et al. The fate of endothelial function testing: Rationale and design of the Firefighters And Their Endothelium (FATE) study. Can J Cardiol. 2003;19:61–6. [PubMed] [Google Scholar]
- 54.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 55.Juonala M, Viikari JS, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: The cardiovascular risk in young Finns study. Circulation. 2004;110:2918–23. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 56.Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005;25:2368–75. doi: 10.1161/01.ATV.0000184769.22061.da. [DOI] [PubMed] [Google Scholar]
- 57.Ridker PM, Danielson E, Fonseca F, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 58.Mancini GB. Vascular structure versus function: Is endothelial dysfunction of independent prognostic importance or not? J Am Coll Cardiol. 2004;43:624–8. doi: 10.1016/j.jacc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 59.Hollenberg SM, Klein LW, Parrillo JE, et al. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–6. doi: 10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- 60.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 61.Targonski P, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–9. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 62.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary systems: Further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926–32. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- 63.Gokce N, Keaney JF, II, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 64.Brevetti G, Silvestro A, Schiano V, Chiarello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease. Circulation. 2003;108:2093–8. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 65.Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol. 2003;42:1037–43. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 66.Karatzis EN, Ikonomodis I, Vamvakou GD, et al. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol. 2006;98:1424–8. doi: 10.1016/j.amjcard.2006.06.043. [DOI] [PubMed] [Google Scholar]