Abstract
Perioperative hyperglycemia is a common phenomenon affecting patients both with and without a known prior history of diabetes. Despite an exponential rise in publications and studies of inpatient hyperglycemia over the last decade, many questions still exist as to what defines optimal care of these patients. Initial enthusiasm for tight glycemic control has waned as the unanticipated reality of hypoglycemia and mortality has been realized in some prospective studies. The recent dramatic modification of national practice guidelines to endorse more modest inpatient glycemic targets highlights the dynamic nature of current knowledge as the next decade approaches. This review discusses perioperative hyperglycemia and the categories of patients affected by it. It reviews current recommendations for ambulatory diabetes screening and its importance in preoperative patient care. Finally, it concludes with a review of current practice guidelines, as well as a discussion of future direction and goals for inpatient perioperative glycemic control.
Keywords: diabetes screening, glycemic control, preoperative evaluation, public health
Introduction
It is no longer a stretch to predict that the majority of surgical patients could have an abnormality in glucose control during their hospitalization. National Health and Nutrition Examination Survey (NHANES) statistics have identified 12.9% of the United States ambulatory population aged ≥20 years as having diabetes, 40% of which are undiagnosed.1 An additional 29.5% have one of the following prediabetic conditions: impaired fasting glucose (IFG) or impaired glucose tolerance (IGT).1 Together, fully 42.4% of ambulatory patients have a dysglycemic condition at baseline, with experts estimating this number to be even higher in the inpatient setting.2 With the additional fraction of surgical patients that will experience stress-induced hyperglycemia, a reasonable approach may be to look at the surgical patient as dysglycemic until proven otherwise.
While practitioners and hospitals often have protocols and practice standards in place to manage those known to have diabetes, these previously identified patients are just a fraction of those at risk for abnormal inpatient glycemic control. These other dysglycemic states, particularly patients with undiagnosed diabetes and those with stress-induced hyperglycemia (SIH), may be unanticipated, unrecognized, hard to differentiate from each other in the inpatient setting, and less well understood, thereby presenting a challenge in perioperative care.2,3 This review discusses (1) the spectrum of inpatient hyperglycemic states and (2) current diabetes screening recommendations and their importance in the comprehensive preoperative evaluation. It concludes with recommendations for perioperative glycemic management and future goals of inpatient care.
Inpatient Hyperglycemia: Definitions
The American Diabetes Association (ADA) identifies three categories of inpatient dysglycemia,2 the first being those with a medical history of diabetes. Diabetes mellitus affected 12.9% of the United States ambulatory diabetes population ≥20 years old in 2005–2006, a staggering statistic that has increased from 9.3% in the short time since the 1999–2002 NHANES survey.4 While approximately 5–10% of these patients have type 1 diabetes, the increase in prevalence is almost entirely attributable to the growing epidemic of type 2 diabetes mellitus due to the increased age of the population, as well as lifestyle issues.5 It is important to note that the prevalence of inpatient diabetes is likely much higher than these figures, perhaps as high as 25%.2 In addition, while not formally included in this ADA-designated definition, the prediabetes states of IFG and IGT, as well as gestational diabetes mellitus (GDM), may best be viewed as existing on a continuum with overt diabetes mellitus. These individuals are at extremely high risk for developing diabetes—with as many as 60–70% of patients progressing from GDM or prediabetes to diabetes.6–8 A known history of either IFG and IGT certainly confers an increased risk of in-hospital hyperglycemia and could be considered a subset of this classification.
The second category, unrecognized diabetes, includes patients with inpatient hyperglycemia that persists after discharge.2 Not just a theoretical problem, NHANES data identified 5.1% of the United States ambulatory population as undiagnosed,1 a figure that is likely higher in the inpatient setting.2 However, because inpatient blood glucose values should not be used routinely to make a diagnosis of diabetes,9 this population is difficult to define accurately in the acute care setting. Evidence suggesting that hemoglobin A1c (HbA1c) is a suitable screening test for diabetes10 may simplify the inpatient diagnosis of diabetes if drawn preoperatively. Undiagnosed diabetes is also a longer term problem in that an initial extended period of uncontrolled hyperglycemia, as might be expected in an unrecognized and untreated patient with diabetes, has lasting effects on cardiovascular health and life expectancy even if blood glucose levels are well controlled later.11,12 These facts argue for more robust diabetes screening practices in the ambulatory setting, such as routine HbA1c for those at risk.
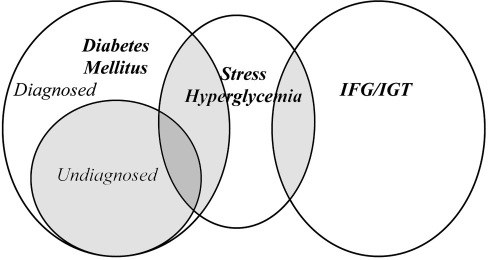
The final category, hospital-related hyperglycemia, or stress-induced hyperglycemia, defines patients with inpatient hyperglycemia that normalizes when the counterregulatory hormone surge and excessive pro-inflammatory state abate.2,13 While hyperglycemia attributed to diabetes and SIH is not mutually exclusive (Figure 1), for purposes of discussion, they are considered as separate entities in this review. First, compared to hyperglycemia due to diabetes, SIH is a particular challenge in inpatient care, as (1) its prevalence and natural history are not well defined and (2) its occurrence is unpredictable and often unanticipated by providers.3 More importantly, there is increasing concern that stress hyperglycemia is different than hyperglycemia secondary to diabetes in that it confers an increased risk of mortality. Rady and colleagues demonstrated higher mortality in patients without diabetes requiring insulin compared to patients with known diabetes (10% vs 6%) in a retrospective single institution intensive care unit (ICU), despite lower average glucose values in the group without diabetes.14 In a similar retrospective study of critical care patients, Egi and colleagues found that higher blood glucose levels were associated with increased mortality in patients without diabetes, but not in patients with known diabetes, suggesting that hyperglycemia in this group may represent a different pathophysiology and natural history than in patients with known diabetes.15 In a general care population. Umpierrez and associates retrospectively found higher mortality in hyperglycemic patients without diabetes compared to those with known diabetes (16% vs 3%), as well as a delay in initiation of insulin.3 An important clinical question remains as to what can be done to ameliorate this increased mortality, and in particular, answer the question of whether all hyperglycemic patients should be treated the same.16,17 This question is complicated because the study group “without diabetes” in each of these three studies is, by definition, a mix of patients with previously undiagnosed diabetes combined with hyperglycemic patients with true SIH. With the recent endorsement of HbA1c ≥6.5% as a diagnostic criterion for diabetes,10 future studies could be able to better define the “nondiabetes” cohort and enable the study of numerous aspects of genuine SIH. Current recommendations for glycemic control do not differentiate patients with diabetes from those with hyperglycemia attributable to stress, but it is not known currently whether glycemic targets should, in fact, be the same.17 Unfortunately, very little is currently known about SIH in general, including its natural history and risk for developing overt diabetes mellitus in the future. Studies of cohorts that survive inpatient SIH are needed to answer this question in order to better characterize this population.
Figure 1.
Relationship of in-hospital hyperglycemia.
Preoperative Screening
Although the majority of elective surgical patients undergo a comprehensive preoperative evaluation prior to surgery, current perioperative recommendations have not advocated testing for diabetes mellitus at this visit.18 However, with the high prevalence of undiagnosed diabetes, case findings should be a public health priority, and every patient visit should be viewed as an opportunity for screening. Adding a simple, inexpensive fasting glucose to other surgery-specific laboratory tests, or HbA1c if fasting values are a challenge, is not only essential to proper perioperative care, but also capitalizes on a screening opportunity for a patient that may not otherwise present for regular routine health care.
The United States Preventive Services Task Force (USPSTF) and the ADA issued the two primary diabetes screening guidelines in the United States.19,20 The USPSTF recommendation (regarded by many as the “gold standard” for evidence-based practice guidelines because of their rigorous standards for guideline development21) advocates diabetes screening for adults with hypertension only, citing insufficient evidence (“I statement”) to recommend screening for other groups.20 The ADA guidelines, based on evidence as well as expert opinion, recommend screening for a much larger population, specifically all adults age 45 or older and in younger overweight patients with at least one additional risk factor (Figure 2).19,22
Figure 2.
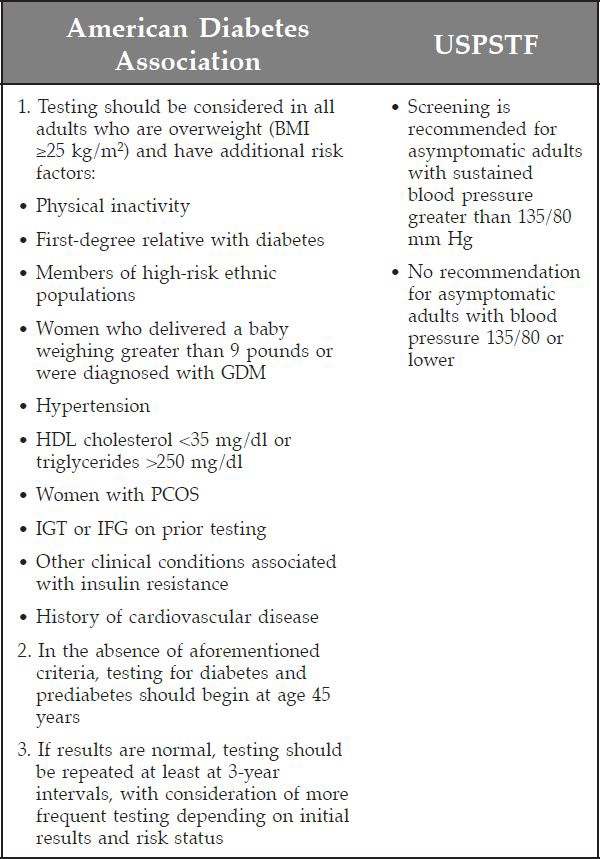
Figure 2. Criteria used to screen for diabetes mellitus: ADA and USPSTF. BMI, body mass index; HDL, high-density lipoprotein; PCOS, polycystic ovarian syndrome. Adapted from Fleisher and colleagues18 and U.S. Preventive Services Task Force,20 with permission from Mayo Clinic Proceedings.
While practice guidelines should clearly be evidence based, they also need to offer providers guidance to make medical decisions when evidence is lacking, or imperfect.23 In the case of diabetes, both the USPSTF and the ADA have acknowledged that the ideal study to support screening, randomizing newly diagnosed diabetes patients to treatment versus observation, is unethical and will not occur. Since this evidence will never be available, it is essential that guidelines reflect available data. Fortunately, the USPSTF issued a statement addressing this issue, as well as provider frustration, with their “I statements” and have provided a new way of recommending screening in cases where evidence is lacking.24 Until a new recommendation is issued by the USPSTF, we advocate adherence to the ADA recommendations for all preoperative patients.
The lack of robust ambulatory diabetes screening practices presents a dilemma in perioperative care. Undiagnosed diabetes is typically not anticipated, which can result in a delay in recognition and treatment, possibly resulting in adverse outcomes.3 Fortunately, if patients have not been screened at their preoperative visit, there are opportunities to risk stratify patients once admitted. First, many patients presenting for elective surgery are fasting, providing an excellent opportunity for preoperative blood glucose testing and risk stratification. In addition, HbA1c, a marker of glycemic control for the prior 3 months, may be an even more valuable inpatient tool to risk stratify hyperglycemic patients without a prior history of diabetes.18 While this test is unreliable in certain populations, such as in patients after blood transfusion or with hemolytic processes or other conditions affecting red blood cell life, it may be useful in many other patient groups. A comprehensive list of factors that may affect HbA1c is available,25 and providers should be aware of these limitations when ordering or interpreting test results. In general, experts have advocated attention to HbA1c values above 6,26 with aHbA1c value of >6.5 endorsed as diagnostic criteria for diabetes.10,16,19 We suggest that any hyperglycemic inpatient without a prior diagnosis of diabetes but with fasting glucose ≥100 mg/dl or random glucose ≥180 mg/dl should have an inpatient HbA1c drawn to determine the presence or absence of diabetes if not done in the preceding 3 months. Given the implication of undiagnosed diabetes on poor wound healing, every effort should be made to establish the diagnosis prior to discharge. Patients with elevated inpatient glucose or HbA1c levels ≥6.0 should be considered high risks for prediabetes10 and should also receive appropriate ambulatory follow-up.
Perioperative Management: Current Recommendations
The Pendulum Swings: Why?
In-hospital hyperglycemia has been the subject of extensive and dynamic publication since the original landmark Leuven “surgical” study was released in 2001.27 In this single-center prospective randomized study of 1548 surgical ICU patients, mortality was reduced in patients given an insulin infusion to control blood glucose to 80–110 mg/dl compared to 180–200 mg/dl. This study, combined with other observational data suggesting adverse outcomes with uncontrolled hyperglycemia,3,28 initiated a cascade of publications, new practice guidelines highlighting the need for intensive insulin therapy, and the belief by many that tight glycemic control (TGC) should be incorporated into quality measurements for hospitals.29,30
However, despite initial enthusiasm for TGC, the resultant large body of literature addressing perioperative glycemic control that has emerged over the latter half of the decade has not been able to confirm these findings and instead has raised concern over a risk of hypoglycemia when this target is attempted.31,32 More recently, the prospective multinational, multidisciplinary Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial in 6104 ICU patients reported increased mortality in patients randomized to achieve a blood glucose level of 81–108 mg/dl versus <180 mg/dl.33 NICE-SUGAR results, combined with mounting evidence produced by other studies since 2001, have caused experts to reconsider their initial enthusiastic endorsement of TGC. Updated, comprehensive recommendations for inpatient glycemic control were issued by the American Association of Clinical Endocrinologists (AACE) and the ADA.16 These guidelines represent a major change from recommendations made just 5 years earlier29 and emphasize the need for “reasonable, achievable, and safe” glycemic goals. These organizations have recommended in-hospital intensive care unit targets to 140–180 mg/dl and have suggested 100–180 mg/dl as a guideline for general care medical and surgical wards.16 These updated guidelines reflect the reality that the implementation of protocols to achieve TGC generally has not achieved the benefit initially hoped for and has also created an unanticipated risk of hypoglycemia that has proved harmful to patients.16
What Factors Have Contributed to Variable Results in the Literature?
While a comprehensive review of existing literature is beyond the scope of this article and has been published elsewhere,34,35 several general principles are worth discussing to illustrate what factors may have contributed to the conflicting results that ultimately resulted in the reversal of expert opinion in 2009.16,36
First, existing studies of perioperative glycemic control have varied by when glycemic control was attempted: preoperatively, intraoperatively, postoperatively, or a combination of time frames.26,37–39 This heterogeneity has resulted in uncertainty in what time frame glycemic control may be most critical and has affected the generalizabilty of results. An additional dilemma is the difference in monitoring practices with regards to both accuracy and variability in point-of-care device used and frequency of measurements obtained.40,41 Variability in insulin delivery to achieve tight glycemic control has also differed by study and has not been standardized across protocols.42 Data also suggest that glucose variability, distinct from actual glucose level, may be deleterious to patient outcomes.43 However, variability could be an unintended consequence of protocols to achieve TGC, as attempts to correct glucose quickly may potentially result in erratic glucose levels and, as a result, poor outcomes.
Definitions of both hypo– and hyperglycemia have also been widely variable across studies. While severe hypo-glycemia has been defined consistently as <40 mg/dl, hypoglycemia has been defined anywhere from 40 to 70 mg/dl in the literature.16,27,31,37,44 Definitions for “control” study arms have also varied and may range anywhere from 150 to 220 mg/dl.45 It follows that a study demonstrating the adverse risk of hyperglycemia at 220 mg/dl may not be comparable to one defining hyperglycemia as greater than 150 mg/dl. This lack of standardization and variability of essentially the definition of the primary end point has affected our ability to derive general conclusions from existing data. In addition, no prospective trials to date have been conducted in non-ICU surgical patients.16,18,19 The safety of TGC in general care patients cannot be inferred from ICU studies due to difference in severity of illness and difference in monitoring capability outside the ICU.
In addition, many existing studies demonstrating adverse outcomes in hyperglycemic perioperative patients have been either retrospective or observational in design. Those studies that have been prospective have often not specifically differentiated patients with diabetes from those with SIH and have drawn conclusions that actually may not be applicable to the entire heterogeneous group studied. More recent awareness of SIH raises the question of whether all hyperglycemia should be treated the same and, more specifically, whether hyperglycemia is causal or instead simply a marker of severity of illness due to an uncontrolled counterregulatory hormone surge.14 If hyperglycemia per se is causing poor outcomes, it would follow that control of hyperglycemia would be beneficial, whereas if it is due to stress and simply a marker of severity of illness, it could be at least in part adaptive and goals for glycemic control might be different. These issues are highlighted by two studies by Gandhi and colleagues in cardiac surgery patients at a single institution. In their retrospective study, hyperglycemia was associated with adverse outcomes,46 yet when they prospectively randomized approximately 400 cardiac surgery patients to achieve intraoperative glucose between 80 and 100 mg/dl or glucose less than 200 mg/dl, there was a significantly increased risk of stroke (p = 0.020) and a trend toward an increased rate of death (p = 0.061) in the tightly controlled group.37 These and other studies3 suggest that caution is warranted before assigning cause and effect and that retrospective data are inherently confounded by this reality. Better identified groups of patients with SIH compared to those with hyperglycemia due to diabetes in prospective randomized trials are needed to help answer these questions.
Finally, many studies have been performed in a specific, well-defined patient population (i.e., cardiac surgery, neurosurgery) with questionable generalizability to other surgical procedures. Other studies, such as the NICE-SUGAR trial, have included a heterogeneous patient population, but may have relatively few patients of a particular type (such as cardiac surgery patients), yet prompt broad, sweeping guidelines for all patient populations. Not just a theoretical problem, it is well known that particular types of surgery pose specific risks, such as risk of stroke and cognitive dysfunction after cardiac surgery.47 In the aforementioned prospective study by Gandhi and colleagues,37 it is not clear that the increased risk of stroke demonstrated in the cardiac surgery patients studied would be apparent in other types of surgeries that place the brain at lesser risk, yet this has not been studied formally in all patient populations.
Clearly, a heterogeneous body of literature exists on perioperative and inpatient glycemic control. More importantly, these many factors illustrate how complicated and variable implementation of protocols to achieve TGC may be in clinical practice and demonstrate why a universal one-size-fits-all plan may have failed to replicate initial promising results.
What Glycemic Targets Are Recommended?
Despite the issues just discussed, it remains clear that both extreme hyperglycemia and hypoglycemia are deleterious to outcomes in ICU patients.48 As indicated previously, the ADA and AACE have revised their recommendations for inpatient glycemic control to reflect new data that have emerged since their previous guidelines, particularly data that have emerged over the latter half of the decade. As mentioned previously, these organizations have recommended ICU targets of 140–180 and 100–180 mg/dl for general care medical and surgical wards.16 It should be noted that while ICU targets reflect evidence from multiple randomized trials, general care guidelines are expert opinion based on observational data only, as there have been no randomized studies in ward patients. Due to this reality, the American College of Cardiology and the American Heart Association have stated that “the usefulness of strict control of blood glucose concentration during the perioperative period is uncertain in patients with diabetes mellitus or acute hyperglycemia who are undergoing noncardiac surgical procedures without planned intensive care admission.”18 In general, we advocate adherence to ADA/AACE recommendations to target reasonable glycemic control with avoidance of extremes in either direction. These guidelines apply to all hyperglycemic patients regardless of etiology, although it is not currently known if SIH and diabetic hyperglycemia should be treated the same.
Should Surgery be Delayed to Optimize Hemoglobin A1c or Acute Hyperglycemia?
Observational data have demonstrated that poor long-term glycemic control (elevated HbA1c) is a marker for adverse outcomes in surgical patients.38,49 However, long-term follow-up of both type 1 and type 2 patients in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study and the United Kingdom Prospective Diabetes Study group, respectively, demonstrated that patients with poorly controlled diabetes mellitus are already known to be at increased risk for morbidity and mortality,11,50 so it is not surprising that poorly controlled patients with diabetes would fare worse in any setting when compared to patients with better glycemic control. As a result, it cannot be concluded from observational data demonstrating adverse surgical outcomes in patients with elevated HbA1c that actually delaying surgery to correct HbA1c will improve surgical outcomes.
Prospective data, however, are limited, and we are aware of only one study in the literature that has addressed this question prospectively. Suto and colleagues studied 87 patients with diabetes undergoing monocular phacoemulsification cataract surgery, 27 of which were willing to undergo rapid correction of HbA1c (10.3 to 7.4%) in the 3 months immediately prior to their surgery.51 Unfortunately, patients in this group demonstrated increased progression of retinopathy compared to both patients with poor control (HbA1c 9.5%) and those with similar, but stable control (HbA1c 7.5%) at time of surgery. As it is believed that rapid glycemic correction can worsen retinopathy, this study cannot be generalized to other surgical populations. However, the randomized prospective Action to Control Cardiovascular Risk in Diabetes Study Group (ACCORD) trial was terminated early due to increased mortality seen in the intensive group that had HbA1c corrected rapidly, by 1.4% over a 4-month period of time.52 While post hoc ACCORD analysis did not demonstrate increased mortality with rapid correction of HbA1c, these studies in general raise questions regarding the safety of intensive glycemic correction for all patients as might be attempted in patients trying to expedite readiness for a surgical procedure, as there are clearly factors still not well understood about targeted, focused glycemic correction. In addition, a delay in surgery is not practical for many patients requiring urgent or emergent procedures. At this point, glycemic correction of elevated HbA1c prior to elective surgery is not recommended.
It is also unclear whether shorter term improvement in glucose control (hours to days) could improve perioperative outcomes. As noted earlier, observational evidence shows that patients with acutely elevated preoperative glucose fare worse than those with normoglycemia,53 although there have been no randomized, prospective trials to date investigating whether acute preoperative glycemic correction carries any benefit. At this time, it seems prudent to control blood glucose to a reasonable level preoperatively, but recommendations for exact targets cannot be made.
What Pharmacologic Agents Are Recommended?
Insulin remains the standard of care for inpatient glycemic control. In the intensive care unit, insulin infusions are the preferred method of administration, as the half-life of intravenous insulin is minutes, allowing for rapid titration in the setting of changing clinical status.2 Insulin infusions are also useful on general care wards when staffing permits, allowing rapid titration at a time when steroids may taper, the counterregulatory hormone surge declines, and diet is advanced.35 However, because insulin infusion is labor-intensive, and is not available on many general care units, subcutaneous insulin, including basal, bolus, and correction, is the recommended alternative to using correction regimens alone. As a general rule, correction or “sliding scale” insulin should not be used as a single modality,54 but may be an appropriate starting point for patients with no previous dysglycemia but at high risk for hyperglycemia, such as in patients starting parenteral nutrition or those placed on steroids.
Oral agents have class-specific limitations for inpatient use and, except for the most stable general care patients approaching discharge, should not be used routinely.16,19 An elevated preoperative HbA1c can provide information that a significant adjustment in preoperative medication may be needed at discharge, including continuing of inpatient insulin for patients that were first placed on it in the hospital. In our experience, restarting oral agents the morning of discharge for patients with good preoperative glucose control seems to work well. This practice ensures both that medications will be tolerated and that the patient will have glycemic coverage postdischarge.
Summary and Future Directions
Inpatient hyperglycemia is a challenging, diverse, yet exceedingly common problem that carries implications for perioperative care. Patients with and without a prior history of diabetes are affected, with unexpected hyperglycemia a difficult issue for inpatient providers to anticipate and treat. Efforts to improve outpatient diabetes screening at the preoperative visit are needed, as well as early identification and risk stratification of perioperative patients in the hospital at high risk for undiagnosed diabetes or stress-induced hyperglycemia. Preoperative fasting blood glucose or HbA1c should be considered for all hospitalized surgical patients who have not recently been screened as an outpatient or have risk factors for diabetes. Any patient found to have an abnormal test in the inpatient setting needs to have an HbA1c drawn in the hospital and should receive follow-up testing in an ambulatory, unstressed state.
Recommended care of the hyperglycemic inpatient has proved to be a swinging pendulum over the past decade. Practice guidelines have been updated to reflect increasing concern over hypoglycemia and increased mortality when protocols to achieve TGC are implemented. Glycemic targets of 140–180 mg/dl for ICU patients and 100–180 mg/dl for general care patients are now recommended. Further studies are needed to determine if poorly controlled patients may benefit if surgery is delayed in order to optimize HbA1c; however, this practice is not currently recommended.
Despite exponentially more publications on inpatient hyperglycemia over the last decade, we have surprisingly been left with far more questions than answers. Future priorities are numerous and include the need to improve ambulatory screening practices so as to have a better defined inpatient population and achieve standardization and optimization of monitoring devices. We also need to investigate the natural history, cause, and treatment of SIH as compared to diabetes patients, better understand the implications of glucose variability, and investigate glycemic targets for general ward patients. As we move into the next decade, it is hoped that we will find answers to these and other questions to optimize outcomes for all perioperative patients.
Abbreviations
- AACE
American Association of Clinical Endocrinologists
- ACCORD
Action to Control Cardiovascular Risk in Diabetes Study Group
- ADA
American Diabetes Association
- GDM
gestational diabetes mellitus
- ICU
intensive care unit
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- HbA1c
hemoglobin A1c
- NHANES
National Health and Nutrition Examination Survey
- NICE-SUGAR
Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation
- SIH
stress-induced hyperglycemia
- TGC
tight glycemic control
- USPSTF
United States Preventive Services Task Force
References
- 1.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–591. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 3.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 4.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National health and Nutrition examination Survey 1999-2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Incidence and prevalence [cited 2009 May 21]. Available from: http://professional.diabetes.org/Disease_Backgrounder.aspx?TYP=6&MID=236.
- 6.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy L, Casas J, Hingavani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 8.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.The International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 12.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meduri GU, Marik PE, Annane D. Prolonged glucocorticoid treatment in acute respiratory distress syndrome: evidence supporting effectiveness and safety. Crit Care Med. 2009;37(5):1800–1803. doi: 10.1097/CCM.0b013e31819d2b43. [DOI] [PubMed] [Google Scholar]
- 14.Rady MY, Johnson DJ, Patel BM, Larson JS, Helmers RA. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80(12):1558–1567. doi: 10.4065/80.12.1558. [DOI] [PubMed] [Google Scholar]
- 15.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;3(8):2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 16.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Practice. 2009;15(4):353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 17.Dungan KM, Braithwaite SS, Preiser J. Stress hyperglycemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Tarkington LG, Yancy CW. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society for Vascular Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):e418–e499. doi: 10.1161/CIRCULATIONAHA.107.185699. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Standards of medical care in diabetes–2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S Preventive Services Task Force. Screening for type2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(11):846–854. doi: 10.7326/0003-4819-148-11-200806030-00007. [DOI] [PubMed] [Google Scholar]
- 21.United States Preventive Services Task Force. About USPSTF [cited 2009 Mar 32]. Available from: http://www.ahrq.gov/clinic/uspstfab.htm.
- 22.Sheehy AM, Coursin DB, Gabbay RA. Back to Wilson and Jungner: 10 good reasons to screen for type 2 diabetes mellitus. Mayo Clin Proc. 2009;84(1):38–42. doi: 10.4065/84.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sniderman AD, Furberg CD. Why guideline-making requires reform. JAMA. 2009;301(4):429–431. doi: 10.1001/jama.2009.15. [DOI] [PubMed] [Google Scholar]
- 24.Petitti DB, Teutsch SM, Barton MB, Sawaya GF, Ockene JK, De Witt T. U.S Preventive Services Task Force. Update on the methods of the U.S. Preventive Services Task Force: insufficient evidence. Ann Intern Med. 2009;150(3):199–205. doi: 10.7326/0003-4819-150-3-200902030-00010. [DOI] [PubMed] [Google Scholar]
- 25.National Glycohemoglobin Standardization Program. Available from: http://www.ngsp.org/prog/index.html. [Google Scholar]
- 26.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93(7):2447–2453. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 27.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 28.Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22(9):1408–1414. doi: 10.2337/diacare.22.9.1408. [DOI] [PubMed] [Google Scholar]
- 29.Garber AJ, Moghissi ES, Bransome ED, Jr., Clark NG, Clement S, Cobin RH, Furnary AP, Hirsch IB, Levy P, Roberts R, Van den Berghe G, Zamudio V American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):77–82. doi: 10.4158/EP.10.1.77. [DOI] [PubMed] [Google Scholar]
- 30.ACE/ADA Task Force on Inpatient Diabetes. American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Endocr Pract. 2006;12(4):458–469. doi: 10.4158/EP.12.4.458. [DOI] [PubMed] [Google Scholar]
- 31.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 32.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 33.NICE-SUGA R Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 34.Lipshutz AK, Gropper MA. Perioperative glycemic control. Anesthesiology. 2009;110(2):408–421. doi: 10.1097/ALN.0b013e3181948a80. [DOI] [PubMed] [Google Scholar]
- 35.Fahy BG, Sheehy AM, Coursin DB. Glucose control in the intensive care unit. Crit Care Med. 2009;37(5):1769–1776. doi: 10.1097/CCM.0b013e3181a19ceb. [DOI] [PubMed] [Google Scholar]
- 36.Bellomo R, Egi M. What is a NICE-SUGAR for patients in the intensive care unit? Mayo Clin Proc. 2009;84(5):400–402. doi: 10.1016/S0025-6196(11)60557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O'Brien PC, Johnson MG, Williams AR, Cutshall SM, Mundy LM, Rizza RA, McMahon MM. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146(4):233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 38.Feringa HHH, Vidakovic R, Karagiannis SE, Dunkelgrun M, Elhendy A, Boersma E, van Sambeek MR, Noordzij PG, Bax JJ, Poldermans D. Impaired glucose regulation, elevated glycated haemoglobin and cardiac ischaemic events in vascular surgery patients. Diabet Med. 2008;25(3):314–319. doi: 10.1111/j.1464-5491.2007.02352.x. [DOI] [PubMed] [Google Scholar]
- 39.Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract. 2006;12(Suppl 3):22–26. doi: 10.4158/EP.12.S3.22. [DOI] [PubMed] [Google Scholar]
- 40.Scott MG, Bruns DE, Boyd JC, Sacks DB. Tight glucose control in the intensive care unit: are glucose meters up to the task? Clin Chem. 2009;55(1):18–20. doi: 10.1373/clinchem.2008.117291. [DOI] [PubMed] [Google Scholar]
- 41.Hoedemaekers CW, KleinGunnewiek JM, Prinsen MA, Willems JL, Van der Hoeven JG. Accuracy of bedside glucose measurement from three glucometers in critically ill patients. Crit Care Med. 2008;36(11):3062–3066. doi: 10.1097/CCM.0b013e318186ffe6. [DOI] [PubMed] [Google Scholar]
- 42.Wilson M, Weinreb J, Hoo GW. Intensive insulin therapy in critical care: a review of 12 protocols. Diabetes Care. 2007;30(4):1005–1011. doi: 10.2337/dc06-1964. [DOI] [PubMed] [Google Scholar]
- 43.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 44.VandenBerghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 45.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 46.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, Schrader LM, Rizza RA, McMahon MM. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80(7):862–866. doi: 10.4065/80.7.862. [DOI] [PubMed] [Google Scholar]
- 47.McKhann GM, Grega MA, Borowicz LM, Jr., Baumgartner WA, Selnes OA. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006;37(2):562–571. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 48.Bagshaw SM, Egi M, George C, Bellomo R. Australia New Zealand Intensive Care Society Database Management Committee; Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med. 2009;37(2):463–470. doi: 10.1097/CCM.0b013e318194b097. [DOI] [PubMed] [Google Scholar]
- 49.Halkos ME, Lattouf OM, Puskas JD, Kilgo P, Cooper WA, Morris CD, Guyton RA, Thourani VH. Elevated preoperative hemoglobin A1C level is associated with reduced long-term survival after coronary artery bypass surgery. Ann Thorac Surg. 2008;86(5):1431–1437. doi: 10.1016/j.athoracsur.2008.06.078. [DOI] [PubMed] [Google Scholar]
- 50.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(250):2643–2653. doi: 10.1056/NEJMoa052187. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suto C, Hori S, Kato S, Muraoka K, Kitano S. Effect of perioperative glycemic control in progression of diabetic retinopathy and maculopathy. Arch Ophthalmol. 2006;124(1):38–45. doi: 10.1001/archopht.124.1.38. [DOI] [PubMed] [Google Scholar]
- 52.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr., Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering therapy in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noordzij PG, Boersma E, Schreiner F, Kertai MD, Feringa HH, Dunkelgrun M, Bax JJ, Klein J, Poldermans D. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156(1):137–142. doi: 10.1530/eje.1.02321. [DOI] [PubMed] [Google Scholar]
- 54.Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT2 trial) Diabetes Care. 2007;30(9):2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]