Abstract
Accurate monitoring of glucose in the perioperative environment has become increasingly important over the last few years. Because of increased cost, turnaround time, and sample volume, the use of central laboratory devices for glucose measurement has been somewhat supplanted by point-of-care (POC) glucose devices. The trade-off in moving to these POC systems has been a reduction in accuracy, especially in the hypoglycemic range. Furthermore, many of these POC devices were originally developed, marketed, and received Food and Drug Administration regulatory clearance as home use devices for patients with diabetes. Without further review, many of these POC glucose measurement devices have found their way into the hospital environment and are used frequently for measurement during intense insulin therapy, where accurate measurements are critical. This review covers the technology behind glucose measurement and the evidence questioning the use of many POC devices for perioperative glucose management.
Keywords: glucometer, glucose measurement, intense insulin therapy, intraoperative, laboratory, perioperative, point-of-care device
Introduction
Since the publication of the landmark paper by Van den Berghe and colleagues showing improved outcomes in critical care patients treated with an intensive regime to maintain blood glucose between 80 and 110 mg/dl,1 the deleterious effects of hyperglycemia and the potential impact of aggressive maintenance of normoglycemia have been an area of intense study. The recent international, randomized multicenter Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial demonstrating increased mortality from intensive blood sugar control in critically ill adults2 draws attention to the requirement for accurate perioperative glucose measurement. This study reported increased mortality among adult intensive care patients in whom blood glucose was targeted to 81–108 mg/dl, compared with a more traditional goal of less than 180 mg/dl. Furthermore, severe hypoglycemia was reported in 6.8% of patients in the intensive control group compared to only 0.5% in the conventional group. In this trial, glucose levels were measured using either point-of-care (POC) devices or central laboratory devices (CLD), with samples obtained from either arterial catheters or capillary sites. Out of 288 episodes of presumed severe hypoglycemia, defined as a blood glucose value less than 40 mg/dl, only 173 (60.1%) were subsequently confirmed by laboratory measurement.2
Reliably accurate measurement of blood glucose in the perioperative and critical care environment is essential. This article reviews indications, regulation, requirements, technology, and accuracy of currently available blood glucose measurement systems in use in the perioperative arena, which includes critical care units, with emphasis on the importance of accuracy at hypoglycemic (less than 70 mg/dl) values. Included in POC devices are self-monitoring of blood glucose (SMBG) devices, originally marketed and regulated for patient home use, as well as the i-Stat® and HemoCue® devices. Because most intensive care units (ICUs) rely on POC devices for glucose measurement,3 it is critical that the clinician appreciates that a faster bedside result has a distinct drawback—a reduction in accuracy.
Indications for Perioperative Blood Glucose Testing
Intraoperative Testing
Blood glucose is measured intraoperatively with either a POC device or as part of an array of assays performed by a CLD. The injurious effects of hyperglycemia in the operating room have been highlighted by many studies in general surgery,4,5 liver transplantation,6 vascular,7 and cardiac surgery.8–11 Although an optimal range for intraoperative blood glucose in both adults and children undergoing major surgery has not yet been defined, it seems that extremes of blood glucose are associated with increased morbidity and mortality in the perioperative setting. Although accuracy at all glucose concentrations is important, it is vital in the hypoglycemic range.
Testing in the ICU
Abnormalities of blood glucose, particularly hyperglycemia, are common in the adult ICU population, even in patients without a diagnosis of diabetes. Critical illness hyperglycemia is commonly regarded as an extreme form of “stress hyperglycemia” typically attributed to insulin resistance caused by endogenous and exogenous catecholamines and glucocorticoids.12,13 In addition, high levels of circulating free fatty acids inhibit peripheral glucose uptake and utilization,14 causing hepatic steatosis, which impairs liver glucose regulation in the critically ill.15
A prospective trial of 1548 adult surgical ICU patients showed that intensive insulin therapy to maintain a blood glucose level between 80 and 110 mg/dl reduced ICU mortality by 32%.1 The greatest effect was seen on deaths from multiorgan failure due to sepsis. Following this and other publications linking hyperglycemia with worse outcomes in the perioperative setting, the American Diabetes Association (ADA) published guidelines suggesting that blood glucose in critically ill patients be kept as close to 110 mg/dl as possible and generally below 140 mg/dl.16 Many professional societies made similar recommendations.17,18 Concerns over iatrogenic hypoglycemia and the applicability of the findings to other ICU populations have inhibited the universal adoption of these guidelines. A subsequent meta-analysis of 26 randomized controlled trials of tight glucose control in 13,567 adult critically ill patients showed no mortality benefit despite an increased risk of hypoglycemia.19 Since the publication of the NICE-SUGAR trial, the American Association of Clinical Endocrinologists and the ADA have revised their original guidelines. They now advise a more modest goal for glycemic control in the ICU patient of 140–180 mg/dl.20
Factors Affecting Accuracy of Glucose Measurement
Terminology
A potential error in current practice arises from the use of blood and plasma glucose as interchangeable terms, with the consequent risk of misinterpretation.21 The glucose concentration in plasma is approximately 11% higher than that in whole blood due to the higher water content in plasma (93%) compared with erythrocytes (73%), and therefore a multiplier of 1.11 for the conversion of glucose in blood to plasma has been recommended.22 Use of plasma glucose concentration is suggested because the physiological activity of glucose corresponds more closely with plasma concentration than whole blood glucose concentration, which varies considerably with hematocrit.23,24 Although POC devices measure glucose in whole blood, they almost all self-correct internally, reporting results as plasma glucose. In the United States, plasma glucose levels are reported most commonly in milligrams per deciliter, but in many other countries are reported in Système International units of millimoles per liter, with 18 mg/dl = 1 mmol/liter.
Sampling Site
The ADA and World Health Organization recommend the use of venous plasma glucose for measuring and reporting, but recognize the widespread use of capillary blood sampling25 (fingertip blood samples are commonly expressed as capillary blood samples), despite evidence that this may lead to measurement error.26 The difference between capillary and venous glucose is typically small in nonhypotensive fasting subjects, but can be up to 8% higher in capillary blood after meals or glucose challenge.20,27 In some studies, arterial blood glucose has been shown to be significantly higher than both capillary and venous blood glucose,28,29 whereas the difference was clinically unimportant in another study.30 Compared to capillary blood, arterial sampling is generally accepted to be a more accurate measurement.31,32
Patient and Environmental Factors
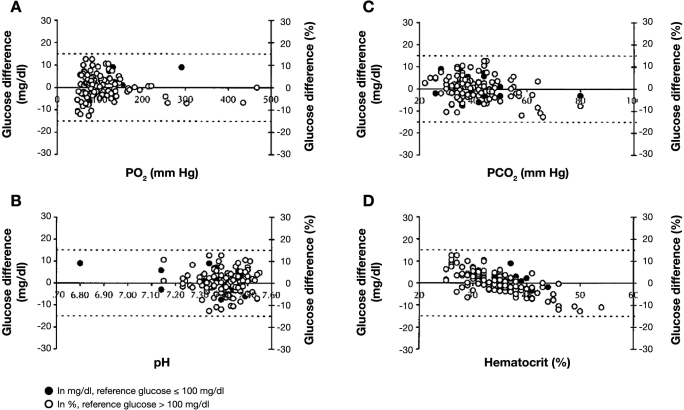
Poor peripheral perfusion (e.g., circulatory shock) results in increased tissue glucose extraction and a lower glucose value in capillary than venous blood. Capillary blood glucose specimens from severely hypotensive patients are more likely to underestimate arterial and central venous blood glucose, resulting in an incorrect diagnosis of systemic hypoglycemia compared to normotensive patients.33 Similar results have been obtained in intensive care patients.32,34–36 Anemia decreases and polycythemia increases the difference between whole blood and plasma glucose not only for the aforementioned reasons, but also impedance of plasma diffusion into the test strip by the higher viscosity (increased hematocrit) may alter results obtained by some POC devices37,38 (see Figure 1, graph D).39 This effect has particular relevance in critically ill neonates in whom the hematocrit may vary widely, and rapid detection of hypoglycemia is essential. Many POC devices have been shown to be inadequate in this setting.40–43
Figure 1.
Plots of paired differences of SureStep Pro glucose measurements minus primary reference glucose measurements as a function of critical care variables (n = 129). Paired differences versus (A) PO2, (B) pH, (C) PCO2, and (D) hematocrit. The PO2 range was 47–467 mm Hg; PCO2, 22–80 mm Hg; pH, 6.80–7.55; and hematocrit, 25–54%. Dashed lines represent error tolerances, ± 15 mg/dl for glucose ≤ 100 mg/dl, and ≤ 15% for glucose >100 mg/dl. Reprinted with permission from Archives of Pathology and Laboratory Medicine.39
Point-of-care device test strips using glucose oxidase (see later) are prone to errors caused by oxygen effects. Tang and colleagues showed that errors of 15% or more could occur with highly oxygenated blood samples (i.e., PaO2 >100 mm Hg),44 and variances of up to 46% from reference values have been observed under hyperbaric conditions.45–48 These devices have also been shown to underestimate blood glucose at altitude (i.e., low ambient PO2) by 1–2% per 1000 feet of elevation,49,50 and errors of more than 15% have been shown when analyzing hypoxic blood (PO2 < 44 mm Hg).51 The severity of errors with low PO2 is highly dependent on the type of test strip (electrochemical vs photometric) and type of enzyme employed in different test strips. The effect of temperature on the test strip reaction rate has been shown to cause clinically relevant reductions in the accuracy of some devices, with low temperatures typically causing underestimation and high temperatures causing overestimation of true blood glucose,52,53 even within the limits specified by the manufacturers. Glucose concentrations also follow a circadian rhythm.54 Patient and environmental factors affecting accuracy are summarized in Table 1.38
Table 1.
Confounding Variables in Glucose Measurementa
| Variable | Methodology affectedb | |
|---|---|---|
| GO | GD | |
| Whole blood | ↓ | ↓ |
| Arterial | ↑ | ↑ |
| Capillary | ↑ | ↑ |
| Postprandial state | ↑ | ↑ |
| Hematocrit | ||
| Anemia | ↑ | ↑ |
| Polycythemia | ↓ | ↓ |
| Oxygen concentration | ||
| Hypoxia | ↑ | – |
| Oxygen therapy | ↓ | – |
| pH (6.8–7.55) | ||
| Low pH | – / ↓ | – |
| High pH | – / ↑ | – |
| Hypothermia | ↑ | ↓ / ↑ |
| Hypotension | ↑ | ↓ / ↑ |
| Drugs | ||
| Ascorbic acid | ↓ | ↓ / - |
| Acetaminophen | ↓ | ↑ |
| Dopamine | – | ↓ |
| Icodextrin | – | ↑ |
| Mannitol | ↑ | – |
Copyright © 2007 American Diabetes Association. From Diabetes Care®, Vol. 30, 2007: 403-409. Reprinted with permission from The American Diabetes Association.38
Changes relative to venous plasma measured as central laboratory. GO, glucose oxidase; GD, glucose dehydrogenase.
U.S. Regulatory Guidelines
The Food and Drug Administration (FDA) exerts regulatory control over all glucose measurement devices, including CLD and POC devices. Manufacturers must show acceptable accuracy and documentation of potential confounding interferences (e.g., hematocrit). Self-monitoring of blood glucose (SMBG) devices must also demonstrate satisfactory human factor performance prior to FDA regulatory clearance.55 Central laboratory devices have reported accuracies of 2.2–2.8% coefficient of variation (CV)56 (Table 2) and must meet Clinical Laboratory Improvement Amendment (CLIA) requirements.57 The CLIA of 1988 is the set of federal regulations that set forth the conditions that all laboratories must meet to be certified to perform testing on human specimens. Coefficient of variation is defined as the ratio of the standard deviation (SD) to the mean. Regulation of SMBGs has been a compromise between accuracy and the need to assist industry in encouraging the establishment of home glucose monitoring, with its overwhelming long-term beneficial effects. In 1996, the FDA assembled a panel58 sponsored by the ADA, FDA, National Institutes of Health, and Center for Disease Control to review accuracy standards for SMBG devices. The consensus document published called for results within 10% total error (bias plus imprecision) of reference values, with future devices within 5%.59 This has not been achieved for any of the SMBG devices in the intervening years.60 Current guidance from the FDA61 has not changed from 1996, and the FDA target [based on International Organization for Standardization (ISO) 15197:2003] for SMBG accuracy specifies 95% of readings within 15 mg/dl for glucose values of 75 mg/dl or less and within 20% for others. Furthermore, the FDA admonishes manufacturers that “you should clarify that critically ill patients (e.g., those with severe hypotension or shock, hyperglycemic-hyperosmolar state, hypoxia, severe dehydration, diabetic ketoacidosis) should not be tested with blood glucose meters because inaccurate results may occur.” This has not prevented the unanticipated stealth migration of SMBG devices to the hospital environment, where their inherent inaccuracy compared with CLDs is not widely appreciated and is therefore potentially dangerous when used for tight glucose control.
Table 2.
| Method | Number | Percentage of total | Mean (mg/dl) | CV (%)c |
|---|---|---|---|---|
| Hexokinase | ||||
| Photometric (visible) 2.8 | 107 | 4 | 155.1 | |
| Photometric (ultraviolet) | 2668 | 49 | 154.2 | 2.4 |
| Glucose oxidase | ||||
| Photometric | 1351 | 25 | ||
| Automated (1327) | 154.5 | 2.6 | ||
| Manual (24) | 158.3 | 4.5 | ||
| Oxygen electrode | 1148 | 21 | 157.5 | 2.2 |
| Glucose dehydrogenase | 35 | 1 | 154.17 | 2.5 |
Results are based on 2003 Cap Survey, Set C-A, Specimen C-11 “Number,” and numbers in parentheses indicate how manylaboratories used the indicated method type.
Reproduced with permission from Elsevier.56
Coefficient of variation for all results by all methods of the indicated method type from all manufacturers. It may include a component of variations attributable to differences in calibrators and to matrix effects.
Measurement of Glucose
The small and colorless glucose molecule is very difficult to measure directly. Therefore, all marketed glucose measurement devices use indirect measurement methods.
Glucose Measurement Methodologies
All techniques are enzymatic, with measurement of by-products by optical (reflectometric) or electrochemical (amperometric) methods (details of these methodologies have been published previously38,56)
The following three enzyme reactions are used.
Hexokinase (used most commonly in CLD) phosphorylates glucose to glucose-6-phosphate, which is then oxidized by glucose-6-phosphate dehydrogenase to form NADH from NAD. The NADH formed is directly proportional to the amount of glucose present and is either measured at 340 nm or by other methods.
Glucose oxidase (the traditional enzyme in SMBG devices) catalyzes the oxidation of glucose to gluconic acid and hydrogen peroxide (H2O2). There are various means of measuring H2O2, the concentration of which is proportional to the glucose present.
Glucose-1-dehydrogenase (GDH) catalyzes the oxidation of glucose to gluconolactone, which converts NADH from NAD. The NADH formed is directly proportional to the amount of glucose present and is either measured at 340 nm or by other methods. A second GDH-based measurement system with steadily increasing market share, glucose-1-dehydrogenase pyrroloquinolinequinone, ismore nonspecific for glucose. Measurement devices usingthis enzyme system may yield falsely high glucose readings in the presence of maltose, xylose, or galactose. This can be a problem with patients receiving peritoneal dialysis when icodextrin is metabolized to maltose.62
In a typical reflectometric system, blood is placed on the disposable strip and is impregnated with the enzymes glucose oxidase and peroxidase and with specific color indicators. When whole blood is placed on the strip, hydrogen peroxide is formed in the presence of peroxidase to cause a color change, the intensity of which is directly proportional to the glucose concentration.
Amperometric monitors quantify glucose by measuring the current produced when glucose dehydrogenase catalyzes the oxidation of glucose to gluconolactone or when glucose oxidase catalyzes the oxidation of glucose to gluconic acid. The resultant current is proportional to the concentration of glucose.
Accuracy Assessment of Glucose Measurement Devices
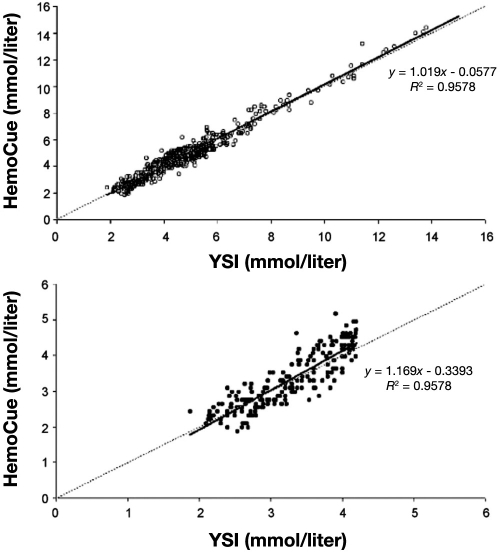
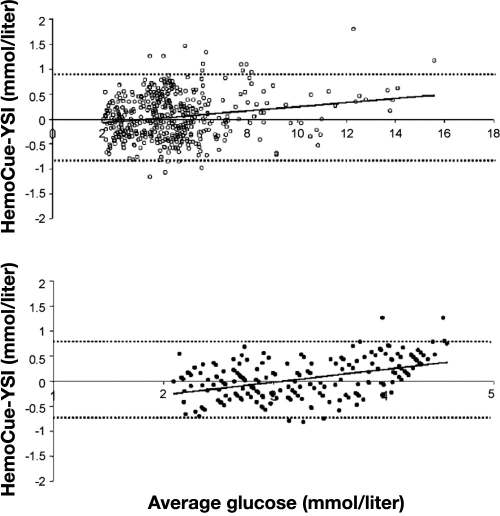
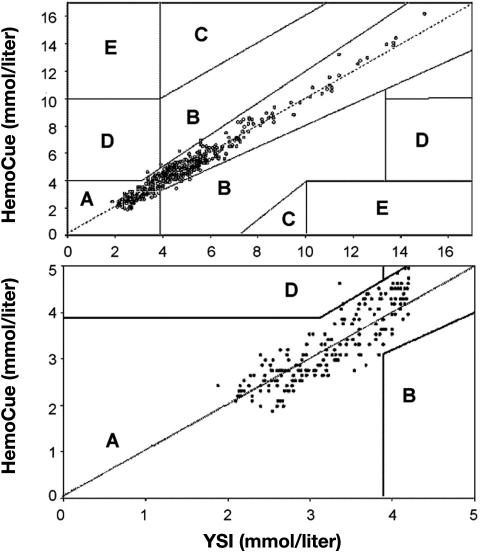
There are a number of methods to express device accuracy, as illustrated with the following examples. Stork and associates63 compared the accuracy of the HemoCue® analyzer, a POC device that uses a glucose dehydrogenase reaction with a disposable microcuvette and a dual wavelength photometer, with a Yellow Springs Instrument (YSI), a CLD with very high accuracy, in volunteers with a wide range of glucose values induced with insulin clamping. These were controlled experiments, with hypoglycemia induced with insulin under tightly monitored conditions. Figure 2 shows data expressed as regression with the HemoCue plotted against reference measurements (YSI). Despite the inappropriate use of correlation coefficients by the authors,64 it is clear that there is less agreement between the techniques at low glucose values. This illustrates well the danger in considering only the overall accuracy of a device without looking specifically at hypoglycemic values. Figure 3 shows Bland–Altman plots of the absolute difference between HemoCue and YSI. Finally, Figure 4 shows the same data plotted on Clarke error grids.65 A Clarke grid is a plot of clinical accuracy, comparing a glucose meter to a reference measurement. The section of the graph with the higher letter (A to E) signifies the more dangerous error. The FDA has assigned considerable weight to the accuracy of SMBG devices plotted on Clarke grids. However, note the expansive box “A” (lowest danger zone) in the bottom of Figure 4, where it is readily apparent that a grave error may occur between measurement with a POC device and a reference. With the movement of these SMBG devices into the perioperative environment, the possibility of a fatal error may exist, especially at low glucose values.
Figure 2.
Regression line and regression equation (after 10% correction for the difference between whole blood and plasma glucose concentration) for all values in the upper panel (open circles) and in the lower panel for hypoglycemic values separately (YSI ≤ 4.2 mmol/liter; solid circles). Reprinted with permission from European Journal of Endocrinology.63
Figure 3.
Altman's residual plot (mean of paired values plotted against the absolute difference between paired values), with 95% limits of agreement (means ± 1.96 SD; broken lines) and regression line. (Top) Altman's residual plot of all values (open circles) is shown. For 18 (3.6%) paired values, the difference was outside the 95% limits of agreement (−0.82 to 0.89 mmol/liter). (Bottom) Plot of hypoglycemic values separately (YSI ≤4.2 mmol/liter; solid circles). Six (2.9%) were outside the 95% limits of agreement (−0.71 to 0.70 mmol/liter). Reprinted with permission from European Journal of Endocrinology.63
Figure 4.
Error grid analysis of HemoCue plasma glucose concentrations (Y axes) with the YSI as reference method (X axes). All values are plotted in the upper panel (open circles); 98.8% were within the clinically accurate zone A. One value was in zone D (0.2%), possibly leading to dangerous failure to detect and treat, and five values were in zone B, the zone with benign estimate errors. Error grid analyses for hypoglycemic values separately (YSI ≤ 4.2 mmol/liter; solid circles) are plotted in the lower panel. All hypoglycemic HemoCue values except one (99.5%) were within zone A; 1.4% of the values were in zone D. Reprinted with permission from European Journal of Endocrinology.63
Point-of-Care Devices
The POC devices include such marketed systems as the HemoCue (see earlier discussion), the iStat®, and SMBG devices introduced in the late 1970s. These SMBG devices have been marketed into the hospital environment either as the identical product or as the same technology packaged as a hospital-specific product. The POC devices have three major advantages over CLD.
Cost—It is considerably less expensive to obtain a bedside glucose measurement than a CLD result.
Time—The turnaround time is much faster than sending a specimen to a laboratory.66
Minimal sample volume needed—The required volume is 2–10 μl of blood compared to much larger volumes for a CLD.67
The distinct disadvantage of most of the POC devices is a reduction in accuracy, which is especially relevant in the hypoglycemic range.
iStat
The iStat (Abbott Point of Care, Princeton, NJ) system uses glucose oxidase and measures glucose amperometrically in a cartridge. Company literature reports accuracy equal to that of CLD, although peer-reviewed literature is sparse.
HemoCue
The HemoCue analyzer (Lake Forest, CA) was discussed earlier. It should be noted that Stork and colleagues63 concluded that “…these methods (i.e., HemoCue and YSI) can be used interchangeably for research and clinical purposes in adults.” An additional advantage of this device is that it does not show significant change with hematocrit compared to other POC devices.68
Self-Monitoring of Blood Glucose Devices Used in the Hospital Setting
With the use of more strict glucose protocols and the need for faster results, there is a push to increase the perioperative use of POC devices. Table 367 is a partial list, along with their specific enzyme and test method. It is not the intent of the authors to mention specifically marketed devices. Table 3 includes a number of devices currently used in the hospital environment, but any SMBG device granted FDA regulatory clearance may be used in a hospital with an appropriate continuous quality assurance (CQI) protocol. The usual minimum CQI requirement is that three different control solutions (low, mid, and high glucose) be used. Most manufacturers only supply those for a few meters because of the increased overhead of making the solutions.
Table 3.
Characteristics of Glucose Meters Useda
| Test method | Enzyme | Specimen | Sample volume (μl) | Linearity (mg/dl) | Analysis time (s) | |
|---|---|---|---|---|---|---|
| Reference method YSI 2300 | Amperometric | Glucose oxidase | Whole bloodb | 25 | 0–900 | 90c |
| LifeScan | ||||||
| One Touch II Hospital | Photometric | Glucose oxidase | Whole blood | 10 | 0–600 | 15–30 |
| SureStep Pro/Flexx | Photometric | Glucose oxidase | Whole Blood | 10 | 0–500 | 15–30 |
| Abbott/MediSense | ||||||
| Precision QID | Amperometric | Glucose oxidase | Whole blood | 3.5 | 20–600 | 20 |
| Precision PCx | Amperometric | Glucose oxidase | Whole blood | 3.5 | 20–600 | 20 |
| Roche | ||||||
| Accu-Chek Advantage | Amperometric | Glucose dehydrogenase | Whole blood | 4 | 10–600 | 26 |
| Accu-Chek Comfort Curve | Amperometric | Glucose dehydrogenase | Whole blood | 4 | 10–600 | 26 |
| Bayer | ||||||
| Elite XL | Amperometric | Glucose oxidase | Whole blood | 2 | 20–600 | 30 |
Reprinted with permission from Archives of Pathology & Laboratory Medicine. Copyright 2006. College of American Pathologists.67
Can also use plasma and serum.
Sample to sample
Hypoglycemic Accuracy Is Really All That Matters
There is not much hypoglycemic accuracy data on POC glucose devices for two reasons. First, there has traditionally not been much interest, although this may be changing. Second, it is difficult to obtain large datasets from hypo-glycemic patients. Clamp studies (see earlier discussion) are ideal but expensive.
The difficulty of examining POC devices is illustrated by Slater-Maclean and colleagues.31 A comparison of 1227 glucose measurements in critical care patients was made among three POC devices, a POC blood gas and glucose analyzer (Bayer Chiron 865®) and a CLD (YSI). The authors stated that “the majority of the measurements exceeded 75 mg/dl and none was less than 40 mg/dl.” However, on close examination, it appears that no measurements were below 70 mg/dl. Not one! It is hard to agree with the authors' recommendation for use of an instrument for measurement of glucose in the critical care environment with not one data point in the hypoglycemic range. To be fair, there are few data regarding any POC devices in the low glucose range.
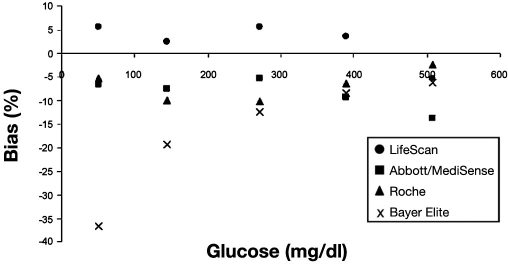
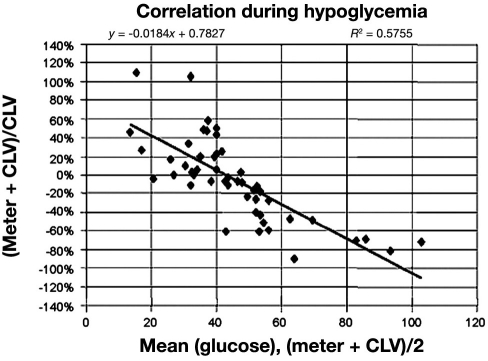
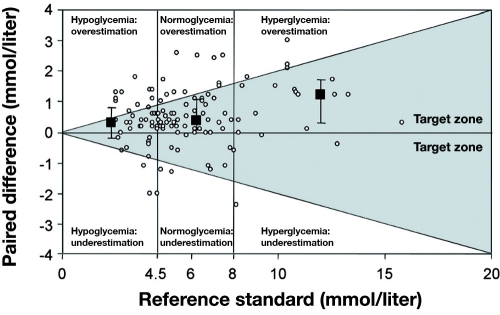
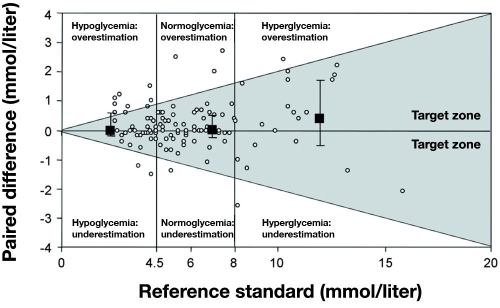
Khan and colleagues67 compared seven POC devices (see Table 3) at four different glucose levels. Figure 5 is a plot of percentage bias for each device compared to reference measurements. All meters (by manufacturer) had similar accuracy expressed as percentage bias at hypoglycemia, except the Bayer Elite®, which was very inaccurate. Illustrating how poorly percentage bias predicts performance during actual measurements, Figure 6 is a Bland–Altman plot of glucose measurements made with the Accu-Chek Comfort® (Roche) compared to a CLD, with 51% of the readings in the hypoglycemic range disagreeing by more than 20%,67 clearly showing a high degree of inaccuracy with this device with hypoglycemic measurements. In 30 critically ill patients, Kanji and colleagues32 estimated clinical agreement (said to exist when the result led to correct adjustment in insulin infusion titration) in capillary and arterial samples, comparing the Accu-Chek Inform® with a CLD. Clinical agreement was better with arterial (69.9%) than capillary measurement (56.8%) compared to the CLD for the entire glucose range. During hypoglycemia, capillary measurement (Figure 7) resulted in clinical agreement only 26.3% of the time. Arterial sampling led to better agreement with the CLD, but still only 55.6% of samples led to the correct clinical decision (Figure 8). In a retrospective study of 197 ICU patients, Finkielman and colleagues69 made 816 simultaneous blood glucose measurements with a POC device (SureStep® Flexx®, LifeScan, Milpitas, CA) and a CLD. The mean difference between the two measurement techniques was 7.9 ± 17.6 mg/dl, which appears to be fair agreement. However, on 18 occasions, the POC glucose was reported as <50 mg/dl, but the mean CLD measurement was 66.9 mg/dl, with a range of 13 to 198 mg/dl! The authors concluded, “On average, bGlu (POC measurement) provides a reasonable estimate for pGlu (CLD measurement). However, for the individual patient, bGlu gives an unreliable estimate of pGlu.”
Figure 5.
Percentage bias for each point-of-care testing glucose meter compared with the YSI 2300 reference method. Reprinted with permission from Archives of Pathology and Laboratory Medicine.67
Figure 6.
Difference plot of the Accu-Chek Comfort (Roche) and a CLD (the Bland—Altman plot) at hypoglycemic values. Meter, glucose meter readings; CLV, central laboratory value. Reprinted with permission from Archives of Pathology and Laboratory Medicine.67
Figure 7.
Paired differences (n = 118) between glucose meter analysis of capillary blood and reference standard for all 30 patients. Paired differences were calculated by subtracting reference standard values from capillary sample values. Medians and interquartile ranges are presented for each blood glucose stratum. Data represent 118 paired observations for 30 patients. Data points reflecting a paired difference >0 indicate an underestimation. Reprinted with permission from Critical Care Medicine.32
Figure 8.
Paired differences (n = 113) between glucose meter analysis of arterial blood and reference standard for all 30 patients. Paired differences were calculated by subtracting reference standard values from capillary sample values. Medians and interquartile ranges are presented for each blood glucose stratum. Data represent 113 paired observations for 30 patients. Data points reflecting a paired difference >0 indicate an overestimation of the true glucose value, whereas a paired difference <0 indicates an underestimation. Reprinted with permission from Critical Care Medicine.32
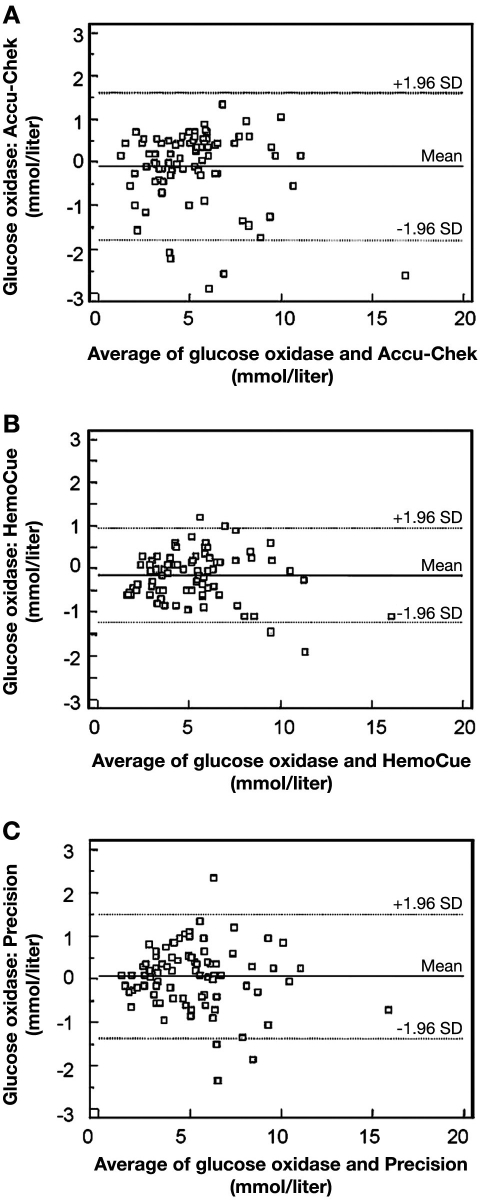
Hoedemaekers and colleagues70 randomly sampled arterial blood in ICU and non-ICU patients, all under the Van den Berghe protocol.1 Three different POC devices (Accu-Chek sensor, HemoCue, and Abbott Precision®) were compared to CLD. Accuracy was compared to ISO standards, which require the glucose measurements >4.1 mmol/liter (74 mg/dl) to be within 20% of reference values and for glucose measurements <4.1 mmol/liter (74 mg/dl) to be within 0.8 mmol/liter (14.4 mg/dl) of reference values; in both cases 95% of the time. Paired samples from the ICU failed to meet ISO criteria from the Accu-Chek sensor, HemoCue, and Precision in 9 of 82 (11.0%), 4 of 82 (4.9%), and 11 of 82 (13.4%) of the cases, respectively (Figure 9). The authors concluded that “… glucose results from three point-of-care testing devices were inaccurate in both the ICU and non-ICU patients. Among ICU patients, inaccurate glucose readings were most frequently falsely elevated, resulting in misinterpretation of high glucose values with subsequent inappropriate insulin administration or masking true hypoglycemia.” They concluded that “… these POC devices seem attractive because of simple handling and rapid results, they should not be used in ICU patients.”70
Figure 9.
Bland—Altman plot of agreement between simultaneous glucose measurements by the Accu-Chek and glucose oxidase method (A), HemoCue method (B), and Precision method (C) in 82 arterial samples obtained from 53 random intensive care patients. Reprinted with permission from Critical Care Medicine.70
Summary
There is little dispute that the advent of home glucose monitoring using POC devices has greatly facilitated good glycemic control for patients with diabetes, but the increasing weight of evidence that effective glycemic control influences outcome in a range of acute medical and surgical conditions has propelled their adoption within the hospital setting without the regulatory scrutiny that might be expected of a critical hospital diagnostic monitor. Tight glycemic control mandates precise measurement of blood glucose, yet the lack of accuracy of POC devices, especially in the potentially harmful hypoglycemic range, has escaped widespread recognition among health care professionals who understandably assume that POC device readings can be safely substituted for CLD results. Further, the lag between product release, assessment, and publication means that very few, if any, devices evaluated in the open literature are still marketed to hospitals; their successors (which often share a similar trade name) may have very different performance characteristics, adding to the uncertainty surrounding the reliability of POC glucose results.
A range of patient and environmental factors compound the intrinsic inaccuracy of many POC devices; these are especially likely to be encountered within the perioperative and critical care environment with patients in whom errors in glycemic measurement are most likely to cause harm. Guidance from the FDA specifically warns against the use of POC devices in this context, yet they continue to be widely used for patients in intensive care and major surgery, without appropriate evaluation and regulation. In an era where glycemic control is the subject of so much research interest, the challenge for the perioperative clinician is to understand the limitations placed on glucose measurement accuracy by factors both intrinsic and extrinsic to the measurement device. Carefully controlled clinical trials are needed to examine these variables more closely and elucidate best practice for perioperative glucose measurement.
Acknowledgments
The authors thank Nik Gravenstein, M.D., and John L. Smith, Ph.D., for reading the manuscript and offering thoughtful suggestions.
Abbreviations
- ADA
American Diabetes Association
- CLD
central laboratory devices
- CLIA
Clinical Laboratory Improvement Amendment
- CQI
continuous quality assurance
- CV
coefficient of variation
- FDA
Food and Drug Administration
- GDH
glucose-1-dehydrogenase
- ICU
intensive care unit
- ISO
International Organization for Standardization
- NICE-SUGAR
Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation
- POC
point of care
- SD
standard deviation
- SMBG
self-monitoring of blood glucose
- YSI
Yellow Springs Instrument
References
- 1.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Varon J. Intensive insulin therapy in the ICU: is it now time to jump off the bandwagon? Resuscitation. 2007;74(1):191–193. doi: 10.1016/j.resuscitation.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, Rogers SO. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248(4):585–591. doi: 10.1097/SLA.0b013e31818990d1. [DOI] [PubMed] [Google Scholar]
- 5.Vilar-Compte D, Alvarez de Iturbe I, Martin-Onraet A, Perez-Amador M, Sanchez-Hernandez C, Volkow P. Hyperglycemia as a risk factor for surgical site infections in patients undergoing mastectomy. Am J Infect Control. 2008;36(3):192–198. doi: 10.1016/j.ajic.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Park C, Hsu C, Neelakanta G, Nourmand H, Braunfeld M, Wray C, Steadman RH, Hu KQ, Cheng RT, Xia VW. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. 2009;87(7):1031–1036. doi: 10.1097/TP.0b013e31819cc3e6. [DOI] [PubMed] [Google Scholar]
- 7.McGirt MJ, Woodworth GF, Brooke BS, Coon AL, Jain S, Buck D, Huang J, Clatterbuck RE, Tamargo RJ, Perler BA. Hyperglycemia independently increases the risk of perioperative stroke, myocardial infarction, and death after carotid endarterectomy. Neurosurgery. 2006;58(6):1066–1073. doi: 10.1227/01.NEU.0000215887.59922.36. [DOI] [PubMed] [Google Scholar]
- 8.Guvener M, Pasaoglu I, Demircin M, Oc M. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J. 2002;49(5):531–537. doi: 10.1507/endocrj.49.531. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, Schrader LM, Rizza RA, McMahon MM. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80(7):862–866. doi: 10.4065/80.7.862. [DOI] [PubMed] [Google Scholar]
- 10.Furnary AP, Wu Y. Eliminating the diabetic disadvantage: the Portland Diabetic Project. Semin Thorac Cardiovasc Surg. 2006;18(4):302–308. doi: 10.1053/j.semtcvs.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, Bridges CR, Haan CK, Svedjeholm R, Taegtmeyer H, Shemin RJ. Society of Thoracic Surgeons Blood Glucose Guideline Task Force. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87(2):663–669. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Andreelli F, Jacquier D, Troy S. Molecular aspects of insulin therapy in critically ill patients. Curr Opin Clin Nutr Metab Care. 2006;9(2):124–130. doi: 10.1097/01.mco.0000214571.97933.0a. [DOI] [PubMed] [Google Scholar]
- 13.Akhtar S, Barash PG, Inzucchi SE. Scientific principles and clinical implications of perioperative glucose regulation and control. Anesth Analg. 2009 doi: 10.1213/ANE.0b013e3181c6be63. In press. [DOI] [PubMed] [Google Scholar]
- 14.Shuldiner AR, McLenithan JC. Genes and pathophysiology of type 2 diabetes: more than just the Randle cycle all over again. J Clin Invest. 2004;114(10):1414–1417. doi: 10.1172/JCI23586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114(2):147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes–2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 17.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 19.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. Can Med Assoc J. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association Consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 21.Burrin JM, Alberti KG. What is blood glucose: can it be measured? Diabet Med. 1990;7(3):199–206. doi: 10.1111/j.1464-5491.1990.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 22.D'Orazio P, Burnett RW, Fogh-Andersen N, Jacobs E, Kuwa K, Kulpmann WR, Larsson L, Lewenstam A, Maas AH, Mager G, Naskalski JW, Okorodudu AO. IFCC-SD-WG-SEPOCT. Approved IFCC recommendation on reporting results for blood glucose: International Federation of Clinical Chemistry and Laboratory Medicine Scientific Division, Working Group on Selective Electrodes and Point-of-Care Testing (IFCC-SD-WG-SEPOCT) Clin Chem Lab Med. 2006;44(12):1486–1490. doi: 10.1515/CCLM.2006.275. [DOI] [PubMed] [Google Scholar]
- 23.Neely RD, Kiwanuka JB, Hadden DR. Influence of sample type on the interpretation of the oral glucose tolerance test for gestational diabetes mellitus. Diabet Med. 1991;8(2):129–134. doi: 10.1111/j.1464-5491.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 24.D'Orazio P, Burnett RW, Fogh-Andersen N, Jacobs E, Kuwa K, Kulpmann WR, Larsson L, Lewenstam A, Maas AH, Mager G, Naskalski JW, Okorodudu AO. International Federation of Clinical Chemistry Scientific Division Working Group on Selective Electrodes and Point of Care Testing. Approved IFCC recommendation on reporting results for blood glucose (abbreviated) Clin Chem. 2005;51(9):1573–1576. doi: 10.1373/clinchem.2005.051979. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Geneva: World Health Organization; 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. [Google Scholar]
- 26.Stahl M, Brandslund I, Jorgensen LG, Hyltoft Petersen P, Borch-Johnsen K, de Fine Olivarius N. Can capillary whole blood glucose and venous plasma glucose measurements be used interchangeably in diagnosis of diabetes mellitus? Scand J Clin Lab Invest. 2002;62(2):159–166. doi: 10.1080/003655102753611799. [DOI] [PubMed] [Google Scholar]
- 27.Carstensen B, Lindstrom J, Sundvall J, Borch-Johnsen K, Tuomilehto J. Measurement of blood glucose: comparison between different types of specimens. Ann Clin Biochem. 2008;45(Pt 2):140–148. doi: 10.1258/acb.2007.006212. [DOI] [PubMed] [Google Scholar]
- 28.Karon BS, Gandhi GY, Nuttall GA, Bryant SC, Schaff HV, McMahon MM, Santrach PJ. Accuracy of roche accu-chek inform whole blood capillary, arterial, and venous glucose values in patients receiving intensive intravenous insulin therapy after cardiac surgery. Am J Clin Pathol. 2007;127(6):919–926. doi: 10.1309/6RFQCKAAJGKWB8M4. [DOI] [PubMed] [Google Scholar]
- 29.Maser RE, Butler MA, DeCherney GS. Use of arterial blood with bedside glucose reflectance meters in an intensive care unit: are they accurate? Crit Care Med. 1994;22(4):595–599. doi: 10.1097/00003246-199404000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Petersen JR, Graves DF, Tacker DH, Okorodudu AO, Mohammad AA, Cardenas VJ., Jr. Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clin Chim Acta. 2008;396(1–2):10–13. doi: 10.1016/j.cca.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Slater-MacLean L, Cembrowski G, Chin D, Shalapay C, Binette T, Hegadoren K, Newburn-Cook C. Accuracy of glycemic measurements in the critically ill. Diabetes Technol Ther. 2008;10(3):169–177. doi: 10.1089/dia.2008.0263. [DOI] [PubMed] [Google Scholar]
- 32.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 33.Atkin SH, Dasmahapatra A, Jaker MA, Chorost MI, Reddy S. Fingerstick glucose determination in shock. Ann Intern Med. 1991;114(12):1020–1024. doi: 10.7326/0003-4819-114-12-1020. [DOI] [PubMed] [Google Scholar]
- 34.Sylvain HF, Pokorny ME, English SM, Benson NH, Whitley TW, Ferenczy CJ, Harrison JG. Accuracy of fingerstick glucose values in shock patients. Am J Crit Care. 1995;4(1):44–48. [PubMed] [Google Scholar]
- 35.Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33(12):2079–2084. doi: 10.1007/s00134-007-0835-4. [DOI] [PubMed] [Google Scholar]
- 36.Desachy A, Vuagnat AC, Ghazali AD, Baudin OT, Longuet OH, Calvat SN, Gissot V. Accuracy of bedside glucometry in critically ill patients: influence of clinical characteristics and perfusion index. Mayo Clin Proc. 2008;83(4):400–405. doi: 10.4065/83.4.400. [DOI] [PubMed] [Google Scholar]
- 37.Dacombe CM, Dalton RG, Goldie DJ, Osborne JP. Effect of packed cell volume on blood glucose estimations. Arch Dis Child. 1981;56(10):789–791. doi: 10.1136/adc.56.10.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(2):403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 39.Louie RF, Tang Z, Sutton DV, Lee JH, Kost GJ. Point-of-care glucose testing: effects of critical care variables, influence of reference instruments, and a modular glucose meter design. Arch Pathol Lab Med. 2000;124(2):257–266. doi: 10.5858/2000-124-0257-POCGT. [DOI] [PubMed] [Google Scholar]
- 40.Dahlberg M, Whitelaw A. Evaluation of HemoCue Blood Glucose Analyzer for the instant diagnosis of hypoglycaemia in newborns. Scand J Clin Lab Invest. 1997;57(8):719–724. doi: 10.3109/00365519709105234. [DOI] [PubMed] [Google Scholar]
- 41.Ho HT, Yeung WK, Young BW. Evaluation of “point of care” devices in the measurement of low blood glucose in neonatal practice. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F356–F359. doi: 10.1136/adc.2003.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenthal M, Ugele B, Lipowsky G, Kuster H. The Accutrend sensor glucose analyzer may not be adequate in bedside testing for neonatal hypoglycemia. Eur J Pediatr. 2006;165(2):99–103. doi: 10.1007/s00431-005-0013-z. [DOI] [PubMed] [Google Scholar]
- 43.Balion C, Grey V, Ismaila A, Blatz S, Seidlitz W. Screening for hypoglycemia at the bedside in the neonatal intensive care unit (NICU) with the Abbott PCx glucose meter. BMC Pediatr. 2006;6:28. doi: 10.1186/1471-2431-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Z, Louie RF, Payes M, Chang KC, Kost GJ. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2(3):349–362. doi: 10.1089/15209150050194215. [DOI] [PubMed] [Google Scholar]
- 45.Shafer MR. 1991 Federal Nursing Service Award recipient. The effect of increased atmospheric pressure on glucose reagent strip accuracy. Mil Med. 1992;157(4):162–165. [PubMed] [Google Scholar]
- 46.Price ME, Jr., Hammett-Stabler C, Kemper GB, Davis MG, Piepmeier EH., Jr. Evaluation of glucose monitoring devices in the hyperbaric chamber. Mil Med. 1995;160(3):143–146. [PubMed] [Google Scholar]
- 47.Edge CJ, Grieve AP, Gibbins N, O'Sullivan F, Bryson P. Effects of pressure on whole blood glucose measurements using the Bayer Glucometer 4 blood glucose meter. Undersea Hyperb Med. 1996;23(4):221–224. [PubMed] [Google Scholar]
- 48.Vote DA, Doar O, Moon RE, Toffaletti JG. Blood glucose meter performance under hyperbaric oxygen conditions. Clin Chim Acta. 2001;305(1–2):81–87. doi: 10.1016/s0009-8981(00)00418-6. [DOI] [PubMed] [Google Scholar]
- 49.Giordano BP, Thrash W, Hollenbaugh L, Dube WP, Hodges C, Swain A, Banion CR, Klingensmith GJ. Performance of seven blood glucose testing systems at high altitude. Diabetes Educ. 1989;15(5):444–448. doi: 10.1177/014572178901500515. [DOI] [PubMed] [Google Scholar]
- 50.Fink KS, Christensen DB, Ellsworth A. Effect of high altitude on blood glucose meter performance. Diabetes Technol Ther. 2002;4(5):627–635. doi: 10.1089/152091502320798259. [DOI] [PubMed] [Google Scholar]
- 51.Kilpatrick ES, Rumley AG, Smith EA. Variations in sample pH and pO2 affect ExacTech meter glucose measurements. Diabet Med. 1994;11(5):506–509. doi: 10.1111/j.1464-5491.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 52.King JM, Eigenmann CA, Colagiuri S. Effect of ambient temperature and humidity on performance of blood glucose meters. Diabet Med. 1995;12(4):337–340. doi: 10.1111/j.1464-5491.1995.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 53.Nawawi H, Sazali BS, Kamaruzaman BH, Yazid TN, Jemain AA, Ismail F, Khalid BA. Effect of ambient temperature on analytical and clinical performance of a blood glucose monitoring system: Omnitest Sensor glucose meter. Ann Clin Biochem. 2001;38(Pt 6):676–683. doi: 10.1258/0004563011901091. [DOI] [PubMed] [Google Scholar]
- 54.Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P. Circadian rhythm of blood glucose values in critically ill patients. Crit Care Med. 2007;35(2):416–421. doi: 10.1097/01.CCM.0000253814.78836.43. [DOI] [PubMed] [Google Scholar]
- 55.U.S. Department of Health and Human Services; Food and Drug Administration. Medical device use-safety: incorporating human factors engineering into risk management. 2000. Jul 18, [cited 2009 Jun 14]. Available from: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm094461.pdf.
- 56.Burtis CA, Ashwood ER, Bruns DE, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 4th ed. St. Louis, MO: Elsevier-Saunders; 2006. p. 868. [Google Scholar]
- 57.U.S. Department of Health and Human Services. Food and Drug Administration; Center for Disease Control and Prevention. Current CLIA regulations. 2004. Jan 24, [cited 2009 Aug 11]. Available from: http://wwwn.cdc.gov/clia/regs/toc.aspx.
- 58.U.S. Department of Health and Human Services. Food and Drug Administration. Review criteria assessment of portable blood glucose monitoring in vitro diagnostic devices using glucose oxidase, dehydrogenase or hexokinase methodology [cited 2009 Jun 14]. Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm094134.htm.
- 59.American Diabetes Association. Consensus statement on self-monitoring of blood glucose. Diabetes Care. 1996;19:S62–S66. [Google Scholar]
- 60.Skeie S, Thue G, Nerhus K, Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin Chem. 2002;48(7):994–1003. [PubMed] [Google Scholar]
- 61.U.S. Department of Health and Human Services. Food and Drug Administration. Draft guidance for industry and FDA staff–total product life cycle for portable invasive blood glucose monitoring systems [cited 2009 Jun 14]. Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm079138.htm.
- 62.Flore KMJ, Delanghe JR. Analytical interferences in point-of-care testing glucometers by icodextran and its metabolites: an overview. Perit Dial Int. 2009;29(4):377–383. [PubMed] [Google Scholar]
- 63.Stork AD, Kemperman H, Erkelens DW, Veneman TF. Comparison of the accuracy of the HemoCue glucose analyzer with the Yellow Springs Instrument glucose oxidase analyzer, particularly in hypoglycemia. Eur J Endocrinol. 2005;153(2):275–281. doi: 10.1530/eje.1.01952. [DOI] [PubMed] [Google Scholar]
- 64.Bland JM, Altman DG. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. 2003;22(1):85–93. doi: 10.1002/uog.122. [DOI] [PubMed] [Google Scholar]
- 65.Clarke WL. The original Clarke error grid analysis (EGA) Diabetes Technol Ther. 2005;7(5):776–779. doi: 10.1089/dia.2005.7.776. [DOI] [PubMed] [Google Scholar]
- 66.Winkelman JW, Wybenga DR, Tanasijevic MJ. The fiscal consequences of central vs distributed testing of glucose. Clin Chem. 1994;40(8):1628–1630. [PubMed] [Google Scholar]
- 67.Khan AI, Vasquez Y, Gray J, Wians FH, Jr., Kroll MH. The variability of results between point-of-care testing glucose meters and the central laboratory analyzer. Arch Pathol Lab Med. 2006;130(10):1527–1532. doi: 10.5858/2006-130-1527-TVORBP. [DOI] [PubMed] [Google Scholar]
- 68.Wiener K. An assessment of the effect of haematocrit on the HemoCue blood glucose analyser. Ann Clin Biochem. 1993;30(Pt 1):90–93. doi: 10.1177/000456329303000116. [DOI] [PubMed] [Google Scholar]
- 69.Finkielman JD, Oyen LJ, Afessa B. Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest. 2005;127(5):49–51. doi: 10.1378/chest.127.5.1749. [DOI] [PubMed] [Google Scholar]
- 70.Hoedemaekers CW, Klein Gunnewiek JM, Prinsen MA, Willems JL, Van der Hoeven JG. Accuracy of bedside glucose measurement from three glucometers in critically ill patients. Crit Care Med. 2008;36(11):3062–3066. doi: 10.1097/CCM.0b013e318186ffe6. [DOI] [PubMed] [Google Scholar]