Abstract
Background
Glycemic variability (GV) has recently been associated with mortality in critically ill patients. The impact of diabetes or its absence on GV as a risk factor for mortality is unknown.
Methods
A total of 4084 adult intensive care unit (ICU) patients admitted between October 15, 1999, and June 30, 2009, with at least three central laboratory measurements of venous glucose samples during ICU stay were studied retrospectively. The patients were analyzed according to treatment era and presence or absence of diabetes: 1460 admitted before February 1, 2003, when there was no specific treatment protocol for hyperglycemia (“PRE”) and 2624 patients admitted after a glycemic control protocol was instituted (“GC”). 3142 were patients without diabetes (“NON”), and 942 were patients with diabetes (“DM”). The coefficient of variation (CV) [standard deviation (SD)/mean glucose level (MGL)] of each patient was used as a measure of GV. Patients were grouped by MGL (mg/dl) during ICU stay (70–99, 100–119, 120–139, 140–179, and 180+) as well as by CV (<15%, 15–30%, 30–50%, and 50%+).
Results
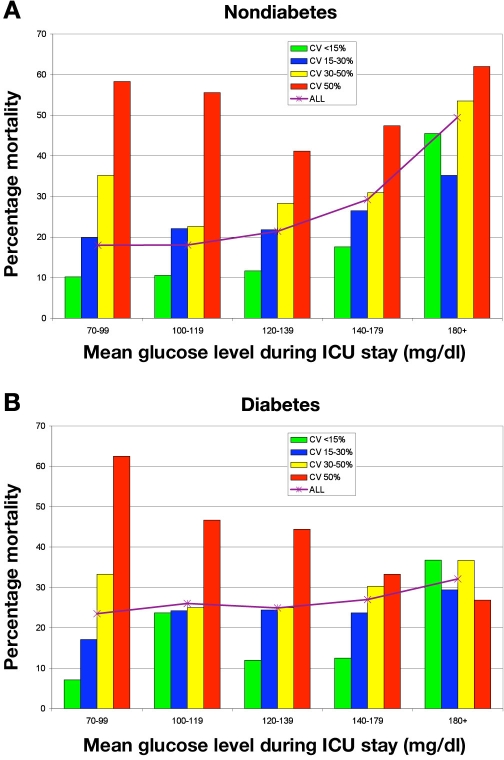
Patients with diabetes had higher MGL, SD, and CV than did NON (p < .0001 for all comparisons). Mean glucose level was lower among both GC groups compared to their corresponding PRE groups (p < .0001), but CV did not change significantly between eras. Multivariable logistic regression analysis demonstrated that low CV was independently associated with decreased risk of mortality and high CV was independently associated with increased risk of mortality among NON PRE and GC patients, even after exclusion of patients with severe (<40 mg/dl) or moderate (40–59 mg/dl) hypoglycemia. There was no association between CV and mortality among DM using the same multivariable model. Mortality among NON from the entire cohort, with MGL 70–99 mg/dl during ICU stay, was 10.2% for patients with CV < 15% versus 58.3% for those with CV 50%+; for NON with MGL 100–119 mg/dl, corresponding rates were 10.6% and 55.6%.
Conclusions
Low GV during ICU stay was associated with increased survival among NON, and high GV was associated with increased mortality, even after adjustment for severity of illness. There was no independent association of GV with mortality among DM. Attempts to minimize GV may have a significant beneficial impact on outcomes of critically ill patients without diabetes.
Keywords: diabetes, glucose, intensive care unit, mortality, variability
Introduction
Hyperglycemia has been well described as a risk factor for adverse outcomes in different populations of acutely ill patients, including patients with medical and neurosurgical diagnoses,1,2 coronary artery disease,3–5 postoperative cardiovascular surgery patients,6,7 and heterogeneous populations of adult intensive care unit (ICU) patients.8–10 Following the publication of a single-center interventional study11 targeting euglycemia in a surgical ICU setting among predominantly cardiovascular postoperative patients, all of whom required mechanical ventilation, “tight glycemic control” (TGC) was adopted as a standard of care by professional societies12 and implemented in ICUs around the world.
However, there has been limited corroboration of the strikingly positive findings seen in the 2001 Leuven study.13–16 The second Leuven trial, performed in a medical ICU,17 demonstrated improved survival among the prespecified cohort of patients requiring more than three days of ICU care, but not in the entire intention-to-treat group; the investigators of this study confirmed that the high rate of severe hypoglycemia (SH) sustained in the interventional group mitigated the benefit of TGC.18 Two subsequent multicenter European trials were terminated prematurely, in part because of excessive rates of SH.19,20 The multicenter Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (i.e., NICE-SUGAR) trial reported a modest increase in 90-day, but not 28-day, mortality among patients in the interventional arm.21 However, these results must be interpreted in the context of a significantly higher rate of SH (blood glucose <40 mg/dl) in the interventional arm (6.8% versus 0.5%, odds ratio [95% confidence interval (CI)], 14.7 [9.0–25.9], p < .001), a low rate of glucose values achieving the target range of 81–108 mg/dl, and significant overlap of glycemic values with the control arm.22
An additional factor may have contributed to the negative results of other interventional studies. Egi and colleagues performed a retrospective evaluation of over 7000 patients admitted to five different Australian hospitals and identified glycemic variability (GV), defined as the standard deviation (SD) of each patient's mean glucose level (MGL) during ICU stay, as a stronger predictor of mortality than was MGL.23 Glycemic variability has also recently been independently associated with mortality in cohorts of septic24 and ventilated surgical ICU patients.25
Published work from this institution confirmed a strong independent association between GV and mortality in a large single-center retrospectively analyzed cohort of mixed medical–surgical ICU patients, especially among patients with good glycemic control, reflected by their MGL.26 Indeed, among patients with MGL 70–99 mg/dl in this study, there was a five-fold increase in mortality comparing those with the lowest and highest quartile of GV. Emerging literature suggests that hyperglycemia might exert an even more deleterious effect on those patients without diabetes than among patients with diabetes during acute illness.4,5,9,10,27–29 This investigation expands the cohort described in the earlier publication26 to determine specifically whether GV exerts the same impact on the outcomes of critically ill patients with or without diabetes, as well as to assess the impact of a glycemic control program on GV.
Materials and Methods
Stamford Hospital is a 305-bed community hospital that serves as a major teaching affiliate of Columbia University College of Physicians and Surgeons. The 14-bed ICU treats a mixed population of adult medical, surgical, cardiac, and trauma patients; cardiovascular surgery was not performed at the institution during the period of this study. Medical and surgical house staff, closely supervised by a group of intensivists, write all orders in the unit, which is highly protocol and data driven.
A total of 8889 patients were admitted to the ICU between October 15, 1999, and June 30, 2009. The study cohort consists of 4084 patients who had three or more central lab glucose values obtained during ICU stay. Point-of-care data obtained from capillary blood obtained by finger stick were not available for the entire duration of the study and were not included in this investigation. Excluded patients included 42 (0.5%) with an admitting diagnosis of diabetic ketoacidosis and 8 (0.1%) with an admitting diagnosis of hyperglycemic hyperosmolar non-ketotic coma. The author determined prospectively the presence of diabetes, based on all clinical information available at the time of ICU admission. Formal evaluation of potential diabetes, such as oral glucose tolerance testing, could not be performed due to the critical and unstable nature of the patient population; hemoglobin A1c status was not routinely assessed.
Data were abstracted from the ICU's comprehensive clinical database created by the director of critical care. Mortality is defined throughout as hospital mortality; discharge status of every patient was confirmed by linking the hospital's administrative database to the ICU database. During the period of this study, nutritional therapy was guided by a standardized protocol emphasizing early institution of enteral feeding. Total parenteral nutrition was used in approximately 10–15% of the population during at least a portion of their ICU stay. Details of each patient's nutritional support are not stored in the ICU database and therefore cannot be reported in the aggregate.
Before February 1, 2003, there was no protocol used to evaluate or treat hyperglycemia in the ICU. After February 1, 2003, a glycemic control protocol was in place, targeting a blood sugar of 80–140 mg/dl. Continuous intravenous insulin was instituted if blood glucose levels exceeded 200 mg/dl on two successive occasions; subcutaneous insulin was used for milder degrees of hyperglycemia. After January 11, 2005, the target range was 80–125 mg/dl, with the trigger for continuous insulin infusion lowered to 180 mg/dl. Insulin dosing decisions were made by the bedside nurse using a paper-based dosing guideline.10,13 Severe hypoglycemia was defined as blood glucose <40 mg/dl. Moderate hypoglycemia (MH) was defined as blood glucose 40–59 mg/dl.
Blood glucose monitoring was performed routinely every three hours; patients receiving continuous intravenous insulin were monitored hourly. Most testing was performed using bedside glucometers; capillary blood was the most frequently used source. Approximately 15% of testing consisted of scheduled venous samples processed on a central laboratory analyzer. The ICU database does not contain details about insulin dosing and duration for individual patients; therefore, this information is not included in the current analysis. A total of 1460 patients were admitted before the glycemic control was implemented (“PRE”) and 2624 after (“GC”).
The SD of each patient's MGL was calculated using Microsoft Excel (Redmond, Washington). Coefficient of variation (CV) of glucose (SD/MGL, [%]) was derived for each patient. Patients were grouped by increments of MGL (mg/dl) (70–99, 100–119, 120–139, 140–179, and 180+) as well as by increments of CV (<15%, 15% <30%, 30% >50%, and >50%).
Continuous variables were analyzed using the Mann–Whitney rank sum test for nonparametric values and reported as median (interquartile range [IQR]). Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were calculated without the age component (APACHE II [mod]) in order to separately analyze the effect of age and severity of illness on mortality. These corrections were two points for age 45–54; three points for age 55–64; five points for age 65–74; and six points for age >75. Categorical variables were analyzed using the Chi square test. Multivariable logistic regression models were created using parameters found to be statistically significant at the p < .10 level on univariable analysis. These models were optimized using forward stepwise analysis. Statistical testing was performed using the MedCalc statistical package (V9.5.1.0; http://www.medcalc.be).
The study was approved by the Stamford Hospital Institutional Review Board, which waived the requirement for informed consent.
Results
Table 1 details important clinical characteristics and outcomes of the patients. Patients with diabetes (“DM”) were older and had a higher severity of illness in both eras. They were more likely to have been admitted with medical rather than surgical diagnoses and had a higher mortality rate than did patients without diabetes (“NON”). Intensive care unit length of stay was similar between the two groups. Mortality decreased during GC among NON (absolute decrease 4.1%, p = .0097); the 2.9% absolute decrease among DM during GC did not reach statistical significance. Intensive care unit length of stay decreased significantly among NON during GC, but not among DM.
Table 1.
Baseline Characteristics and Selected Outcomes, Grouped by Treatment Era and Diabetes Status a
| PRE NON | PRE DM | PRE NON versus DM | GC NON | GC DM | GC NON versus DM | Pre versus GC NON | Pre versus GC DM | |
|---|---|---|---|---|---|---|---|---|
| Number | 1140 | 320 | 2002 | 622 | ||||
| Age | 71 (54–80) | 72 (64–79) | 0.0374 | 71 (54–81) | 72 (62–80) | 0.0007 | 0.5763 | 0.5112 |
| APACHE II (mod) | 15 (9–20) | 16 (10–21) | 0.0366 | 14 (9–20) | 15.5 (11–23) | <0.0001 | 0.4863 | 0.4364 |
| Distribution of patients | ||||||||
| Medical | 780 (68.4) | 264 (82.5) | <0.0001 | 1229 (61.4) | 436 (70.1) | 0.0001 | 0.0001 | 0.0001 |
| Surgical | 287 (25.2) | 53 (16.6) | 0.0017 | 585 (29.2) | 170 (27.3) | 0.3906 | 0.0167 | 0.0003 |
| Trauma | 73 (6.4) | 3 (0.9) | 0.0002 | 188 (9.4) | 16 (2.6) | <0.0001 | 0.0044 | 0.1482 |
| Vent | 607 (53.2) | 181 (56.6) | 0.3229 | 1007 (50.3) | 324 (52.1) | 0.4628 | 0.1208 | 0.2170 |
| ICU length of stay | 3.7 (2.2–7.0) | 3.5 (2.3–5.8) | 0.5639 | 3.3 (2.0–6.3) | 3.2 (1.8–6.7) | 0.5643 | 0.0047 | 0.1551 |
| Mortality | 285 (25.0) | 94 (29.4) | 0.1323 | 419 (20.9) | 165 (26.5) | 0.0040 | 0.0097 | 0.3953 |
Results expressed as number (%) or median (IQR). Statistical testing by Chi square test for comparisons of percentages and Wilcoxon rank-sum test for comparisons of continuous variables.
Table 2 reports details of glycemic control, where DM had higher MGL, SD, CV, and occurrences of both SH and MH (SH <40 mg/dl and MH = 40–59 mg/dl) than did NON during both eras. Standard deviation was highly correlated with MGL among both NON (R2 = 0.448, p < .0001 during PRE and R2 = 0.470, p < .0001 during GC) and DM (R2 = 0.384, p < .0001 during PRE and R2 = 0.493, p < .0001 during GC). Mean glucose level and SD were significantly lower among NON than among DM during both eras and decreased significantly for each group during GC (p < .0001 for all comparisons). Coefficient of variation was lower among NON than among DM in each era but did not decrease significantly during GC.
Table 2.
Glycemic Controla
| PRE NON | PRE DM | PRE NON versus DM | GC NON | GC DM | GC NON versus DM | PRE versus GC NON | PRE versus GC DM | |
|---|---|---|---|---|---|---|---|---|
| Glucose tests/patient | 5 (3–10) | 6 (4–10) | 0.2019 | 5 (4–10) | 6 (4–10) | 0.4132 | 0.1351 | 0.8370 |
| Mean (mg/dl) | 126.3 (109.7–149.6) | 184.3 (146.8–227.0) | <0.0001 | 114.8 (103.7–128.2) | 127.3 (111.1–148.8) | <0.0001 | <0.0001 | <0.0001 |
| SD (mg/dl) | 25.6 (15.5–43.3) | 55.37 (32.5–83.6) | <0.0001 | 22.7 (14.5–33.9) | 36.37 (23.7–56.1) | <0.0001 | <0.0001 | <0.0001 |
| CV (%) | 20.4 (13.0–30.1) | 31.1 (19.8–43.4) | <0.0001 | 19.6 (13.3–28.6) | 28.8 (19.8–41.5) | <0.0001 | 0.2817 | 0.5184 |
| SHb (% patients) | 18 (1.6) | 17 (5.3) | 0.0003 | 46 (2.3) | 42 (6.8) | <0.0001 | 0.2150 | 0.4704 |
| MHc (% patients) | 35 (3.1) | 23 (7.2) | 0.0015 | 150 (7.5) | 69 (11.1) | 0.0059 | <0.0001 | 0.0724 |
Glucose tests/patient, mean, SD, and CV expres sed as median (IQR). These values represent the median of each patient's values. Results expressed as number (%) or median (IQR). Statistical testing by Chi square test for comparisons of percentages and Wilcoxon rank-sum test for comparisons of continuous variables.
SH = blood glucose <40 mg/dl.
MH = blood glucose 40–59 mg/dl.
Episodes of SH and MH increased during GC among DM and NON. The occurrence of one or more episodes of SH was associated with increased risk of mortality among PRE NON [OR (95% CI) 7.61 (2.06–28.06), p < .0023], TGC NON [OR (95%CI) 2.68 (1.37–5.24), p = .0039], and TGC DM [OR (95%CI) 2.91 (1.40–6.05), p = 0.0041], adjusted for age, modified APACHE II, and mechanical ventilation, but not among PRE DM. There was no independent association of increased risk of mortality with MH among any group using the same multivariable model.
Table 3 displays statistically significant associations between various clinical factors and CV among the different groups as well as the entire cohort of PRE and GC. Severity of illness, based on the modified APACHE II, was moderately associated with CV; age and the number of glucose values per patient were more inconsistently associated, reflected by their lower regression coefficients.
Table 3.
Determinants of Coefficient of Variation: Multivariable Logistic Regression Model to Calculate “r” a
| Age | p value | Modified APACHE II score | p value | Tests | p value | DM | p value | |
|---|---|---|---|---|---|---|---|---|
| PRE NON | 0.033 | .2070 | 0.328 | <.0001 | 0.235 | <.0001 | n/a | |
| PRE DM | 0.040 | .1380 | 0.154 | .0077 | 0.095 | .1495 | n/a | |
| GC NON | 0.150 | <.0001 | 0.285 | <.0001 | 0.104 | .0186 | n/a | |
| GC DM | 0.009 | .6689 | 0.220 | <.0001 | 0.006 | .2906 | n/a | |
| All PRE | 0.056 | .0633 | 0.291 | <.0001 | 0.201 | <.0001 | 0.218 | <.0001 |
| All GC | 0.142 | <.0001 | 0.277 | <.0001 | 0.076 | .2109 | 0.259 | <.0001 |
| All NON | 0.103 | <.0001 | 0.301 | <.0001 | 0.151 | <.0001 | n/a | |
| All DM | 0.019 | .2872 | 0.196 | <.0001 | 0.033 | .8338 | n/a | |
| All | 0.108 | <.0001 | 0.281 | <.0001 | 0.119 | <.0001 | 0.242 | <.0001 |
Age, modified APACHE II score (age component deleted from the total score), tests (the number of glucose tests obtained during ICU stay), and presence of DM were found to be significantly associated with CV using univariable logistic regression analysis. This table displays the regression coefficients and associated p values for these factors using a multivariable logistic regression analysis. Different subgroups were created on the basis of admission date (before or after the implementation of glycemic control protocol) and presence or absence of DM.
Figure 1A illustrates the strong relationship between CV and mortality among NON, grouped by MGL during ICU stay. For example, among patients with MGL 70–99 mg/dl, mortality was 10.2% and 58.3%, comparing patients with the lowest and highest CV. Figure 1B details these data for DM. Table 4 details results of regression analysis of the association between different increments of CV and mortality. Among NON, low CV was associated with survival while high CV was associated with increased risk of mortality during both eras. This finding was not significantly altered even after exclusion of all patients with SH or MH. Among DM, there was no association between any of the four increments of CV and mortality during either era.
Figure 1.
Glycemic variability, mortality, and their relation to mean glucose level during ICU stay: entire cohort. Mortality grouped by increments of MGL during ICU stay for NON (A) and DM (B).
Table 4.
Multivariable Logistic Regression Analysis of Mortality and Glycemic Variability, Reflected by Coefficient of Variationa
| Nondiabetes | Diabetes | |||
|---|---|---|---|---|
| CV (%) | OR (95% CI) | p value | OR (95% CI) | p value |
| PRE | ||||
| 0–15 | 0.48 (0.32–0.68) | .0001 | 1.23 (0.57–2.68) | .5997 |
| 15–30 | 1.30 (0.96–1.75) | .856 | 0.62 (0.35–1.10) | .1015 |
| 30–50 | 1.07 (0.75–1.53) | .7083 | 1.09 (0.63–1.88) | .7660 |
| 50+ | 2.12 (1.23–3.65) | .0066 | 1.51 (0.77–2.91) | .2303 |
| GC | ||||
| 0–15 | 0.66 (0.49–0.90) | .0096 | 0.64 (0.32–1.31) | .2251 |
| 15–30 | 0.83 (0.64–1.07) | .1579 | 1.12 (0.75–1.67) | .5828 |
| 30–50 | 1.44 (1.06–1.96) | .0207 | 1.00 (0.62–1.51) | .9981 |
| 50+ | 2.39 (1.48–3.86) | .0004 | 1.08 (0.62–1.90) | .7798 |
Multivariable model includes age, modified APACHE II score (age component deleted), and mechanical ventilation.
Table 5 illustrates the results of multivariable logistic regression analysis of mortality. Increasing age, modified APACHE II score, and mechanical ventilation each were associated independently with increased risk of mortality. The presence of diabetes on admission did not have any independent association with mortality for the entire cohort or the entire PRE and GC subpopulations. Treatment in the GC era was associated with decreased risk of mortality. Finally, increasing CV was independently associated with increased risk of mortality among NON in both eras; this association remained highly significant (p < .0001) even after exclusion of patients with SH. In contrast, increasing CV was not associated with increased mortality among DM in either era using this multivariable model.
Table 5.
Determinants of Mortality
| Age | p value | Mod AP | p value | Vent | p value | DM | p value | GC | p value | CV | p value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE NON | 1.03 (1.03–1.04) | <.0001 | 1.09 (1.07–1.11) | <.0001 | 1.84 (1.32–2.57) | .0003 | n/a | n/a | 1.02 (1.01–1.02) | .00082 | ||
| PRE DM | 1.03 (1.01–1.06) | .0088 | 1.09 (1.05–1.13) | <.0001 | 1.69 (0.96–2.98) | .686 | n/a | n/a | 1.01 (0.99–1.02) | .3755 | ||
| GC NON | 1.04 (1.03–1.04) | <.0001 | 1.13 (1.11–1.15) | <.0001 | 2.10 (1.57–2.80) | <.0001 | n/a | n/a | 1.02 (1.01–1.03) | <.0001 | ||
| GC DM | 1.02 (1.01–1.04) | .0047 | 1.10 (1.08–1.13) | <.0001 | 1.86 (1.21–2.85) | .0045 | n/a | n/a | 1.00 (0.99–1.02) | .2724 | ||
| All PRE | 1.03 (1.02–1.04) | <.0001 | 1.09 (1.07–1.11) | <.0001 | 1.82 (1.36–2.42) | <.0001 | 0.98 (0.72–1.33) | .8895 | n/a | 1.01 (1.11–1.02) | .0006 | |
| All GC | 1.03 (1.03–1.04) | <.0001 | 1.12 (1.11–1.14) | <.0001 | 2.00 (1.58–2.54) | <.0001 | 0.96 (0.75–1.23) | .7443 | n/a | 1.02 (1.10–1.02) | <.0001 | |
| All NON | 1.03 (1.03–1.04) | <.0001 | 1.11 (1.10–1.13) | <.0001 | 1.98 (1.59–2.46) | <.0001 | n/a | 0.77 (0.63–0.94) | .0092 | 1.02 (1.11–1.03) | <.0001 | |
| All DM | 1.03 (1.11–1.04) | .0001 | 1.10 (1.08–1.12) | <.0001 | 1.79 (1.28–2.52) | .0008 | n/a | 0.80 (0.58–1.11) | .1801 | 1.01 (1.00a–1.02) | .1477 | |
| All | 1.03 (1.03–1.04) | <.0001 | 1.11 (1.10–1.12) | <.0001 | 1.93 (1.61–2.32) | <.0001 | 0.97 (0.80–1.18) | .7936 | 0.77 (0.65–0.92) | .0029 | 1.02 (1.01–1.02) | <.0001 |
Actual value is 0.9977.
Discussion
The salient finding of this investigation is that GV, as reflected in the CV (%, calculated for each patient as SD/MGL) of each patient's glucose level during ICU stay, had a strong, independent association with mortality among critically ill NON patients, but not among DM patients. From both eras (before and after institution of a glycemic control program in the ICU), NON with CV <15% had increased survival, after adjustment for age, severity of illness (APACHE II [mod]) and mechanical ventilation, while patients with CV 50%+ had increased mortality. These results were not significantly changed if patients with severe (<40 mg/dl) or moderate (40–59 mg/dl) hypoglycemia were excluded, and the results were most dramatic among patients with ostensibly very-well-controlled glucose levels. Indeed, among NON with MGL 70–99 mg/dl during ICU stay, mortality increased from 10.2% for those with CV <15% to 55.8% for those with CV 50%+. Finally, while NON had lower MGL, SD, and CV than did DM during both eras, MGL and SD, but not CV, decreased when comparing PRE and GC NON as well as PRE and GC DM.
Emerging literature since 2006 has begun to define the association of GV with mortality in different populations of critically ill patients.23–26 Egi and associates reviewed glycemic data from 7049 Australian patients admitted to five different ICUs over four years.23 Nonsurvivors had higher SD and CV; multivariable analysis demonstrated that both SD and CV were independently associated with mortality. Ali and coworkers investigated the association between GV and mortality in a cohort of 1246 septic patients.25 The Glycemic Lability Index (GLI) was determined to have a stronger association with mortality than did SD in this study.30 Patients were stratified into high and low MGL and GLI subgroups (above and below the median values for each). Multivariable modeling demonstrated that only patients with elevated GLI and low MGL had increased odds of mortality during hospitalization [OR 4.73 (95% CI 2.6–8.7)]. Dossett and colleagues investigated the impact of GV in a cohort of ventilated surgical ICU patients receiving intensive insulin therapy to maintain euglycemia.26 Although survivors and nonsurvivors had similar MGL during ICU stay, several measures of GV were elevated among the nonsurvivors. Finally, work from Stamford Hospital has confirmed these findings in a large cohort of mixed medical–surgical patients and, moreover, confirmed the findings of Ali and associates25 that the independent effect of GV was most pronounced among patients with apparently well-controlled glucose levels, based on MGL.24
Increasing GV as a risk factor for adverse outcomes in critically ill patients has biologic plausibility, supported by clinical studies conducted in outpatient patients with diabetes31 as well as by basic science investigations.32–34 Induced fluctuation in glycemic levels was more likely to produce apoptosis than was sustained hyperglycemia in a study involving human umbilical endothelial cells.32 Another investigation demonstrated that markers of oxidative stress were produced in much higher concentrations by alternations in glycemic levels than by sustained hyperglycemia.33 Oxidative stress may be one of the unifying mechanisms underpinning the vasoconstriction, microvascular thrombosis, and inflammation associated with hyperglycemia and GV.34
Emerging literature has described worsened outcomes in acutely ill patients without diabetes compared to patients with diabetes experiencing equivalent degrees of hyperglycemia, establishing hyperglycemia as a much more potent risk factor for mortality and morbidity among the acutely ill patients without diabetes than among those with diabetes. An underappreciated finding in the landmark Leuven 1 trial, for example, is that the survival advantage of intensive insulin therapy was seen mainly among the patients without diabetes.11 The mortality rates in the conventionally versus the intensively treated groups were 8.4% versus 4.7% and 5.8% versus 4.0% for those without diabetes or with diabetes, respectively.11 Umpierrez and coworkers found that newly diagnosed hyperglycemia (admission or fasting glucose >125 mg/dl or random glucose >200 mg/dl) was associated with a 16% mortality rate among a mixed population of patients admitted to a community hospital, compared to mortality rates of 3% among known patients with diabetes and 1.7% among patients without hyperglycemia.27 Three large cohorts of heterogeneous ICU patients identified hyper-glycemia during ICU admission as having a more significant impact on mortality risk among patients without diabetes than among patients with diabetes.9,10,29 The same finding has been described in patients admitted with myocardial infarction4,5 and in several series of patients presenting with acute neurologic injury.28
The reason for the difference in risk comparing those with diabetes and those without who sustain equivalent degrees of hyperglycemia is not known with certainty. One may speculate that an established patient with diabetes may develop a relative tolerance to the cellular and microvascular complications associated with moderate degrees of hyperglycemia. In contrast, the same degree of hyperglycemia may reflect a “storm” of counter-regulatory hormone and cytokine release in the critically ill patient without diabetes. Nevertheless, while a much greater degree of stress may be required to yield equivalent degrees of moderate hyperglycemia in the patient without diabetes, adjustment for severity of illness in the current cohort and earlier studies10 indicates that the hyperglycemia per se, and not just severity of illness, contributed independently to the risk of mortality. Finally, while NON and DM groups each demonstrated significant reductions in MGL comparing PRE and GC, there was a significant decrease in mortality only among NON during the GC era (OR [95% CI] for mortality 0.77 [0.63–0.94], p = .0092).
The strengths of this study include its large sample size, the heterogeneous nature of the critically ill patient population, increasing the possibility of generalizing the findings, and the prospective and comprehensive nature of the data collection. One potential limitation relates to the assignment of the presence of diabetes for the individual patient. This determination was made by the director of critical care at the time of admission to the ICU based on all available information at the time of presentation. It is likely that a certain percentage of patients admitted as those without diabetes represent as of yet undiagnosed diabetes. An additional limitation is the relative paucity of the data points used to determine the primary measure. Because glucose values included only those measured in the central laboratory, rather than point-of-care values, many patients had only one or two values obtained per day. On the other hand, exclusive use of central lab values obviated any potential inaccuracies, and resulting spurious variability, engendered by the pre-analytical variances that may be present when using capillary blood glucose measurements.35–37 Finally, this is a single-center investigation. While the patient population is heterogeneous, and likely representative of adult ICU patients in other institutions, corroboration of these findings using a multicenter database is warranted.
Other investigations of the impact of GV on mortality of critically ill patients have used SD as a primary surrogate for GV; Egi and colleagues evaluated both SD and CV, finding that both were independently associated with mortality.23–26 Standard deviation is strongly correlated with MGL (p < .0001 for NON in this investigation). The use of CV “normalizes” this relationship, allowing clearer comparison of the effect of GV between groups of patients with different levels of overall glycemic control. Nevertheless, there are several reasons why neither CV nor SD may be the perfect measure of GV. Most notably, these measures do not account for the temporal sequence in which the glucose values were obtained. For example, the following two sequences of glucose values reflect very different degrees of glycemic control: 200–180–160–130–110–100 mg/dl versus 110–180–100–200–130–160 mg/dl, yet they have the same SD. Moreover, there is no accounting for timing of the values; a time-averaged measure would likely reflect more accurately the status of the patient's glycemic control. Finally, equivalent degrees of GV occurring at different times during ICU stay may have different clinical implications. Compare, for example, the initial period of glycemic stabilization of a critically ill patient when values may decline sequentially from 200 to 100 mg/dl to an excursion in the opposite direction that may herald a septic complication three days later. The evaluation of other potential “candidate” measures, such as the GLI or the mean amplitude of glycemic excursion, was beyond the scope of the current study.30,38
The findings of this, and other investigations regarding GV,23–26 have important clinical implications. Glycemic variability is identified here as a strong independent contributor to the risk of mortality in critically ill patients without diabetes. Patients who do not have diabetes and had low levels of GV had statistically significantly improved survival, even after adjustment for severity of illness, while high levels of GV were independently associated with increased risk of mortality. This is, then, a potentially modifiable risk factor for adverse outcomes in these patients. The challenge for ICU clinicians is to design programs to minimize GV. Efforts to ensure safe and effective glycemic control include, in part, optimal implementation of glycemic management protocols, the use of frequent monitoring in an effort to avoid glycemic excursions, and the availability of data outcome tools to monitor the progress of the program and provide continual feedback to its drivers.39,40 Future efforts should include the identification of more accurate and clinically useful metrics to express, and ultimately manage, GV.
Conclusions
Low GV, reflected by CV of glucose, during ICU stay was associated with increased survival among those without known diabetes, and high GV was associated with increased mortality, even after adjustment for severity of illness and MGL. There was no independent association of CV with mortality among patients with diabetes. Ultimately, this retrospective analysis is hypothesis generating. Future randomized interventional trials targeting GV as an endpoint will be needed to confirm that minimizing GV reduces mortality in critically ill patients.
Abbreviations
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- CI
confidence interval
- CV
coefficient of variation
- DM
patients with diabetes
- GC
patients admitted after a glycemic control protocol was instituted
- GLI
Glycemic Lability Index
- GV
glycemic variability
- ICU
intensive care unit
- IQR
interquartile range
- MGL
mean glucose level
- MH
moderate hypoglycemia
- NON
patients without diabetes
- OR
odds ratio
- PRE
patients admitted before a specific treatment protocol for hyperglycemia was instituted
- SD
standard deviation
- SH
severe hypoglycemia
- TGC
tight glycemic control
References
- 1.Wass CT, Lanier WL. Glucose modulation of ischemic brain injury: review and clinical recommendations. Mayo Clin Proc. 1996;71(8):801–812. doi: 10.1016/S0025-6196(11)64847-7. [DOI] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 3.Foo K, Cooper J, Deaner A, Knight C, Suliman A, Ranjadayalan K, Timmis AD. A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Heart. 2003;89(5):512–516. doi: 10.1136/heart.89.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyper-glycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 5.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus JA. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117(8):1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 6.Furnary AP, Wu Y. Eliminating the diabetic disadvantage: the Portland Diabetic Project. Semin Thorac Cardiovasc Surg. 2006;18(4):302–308. doi: 10.1053/j.semtcvs.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Smith CE, Styn NR, Kalhan S, Pinchak AC, Gill IS, Kramer RP, Sidhu T. Intraoperative glucose control in diabetic and nondiabetic patients during cardiac surgery. J Cardiothorac Vasc Anesth. 2005;19(2):201–208. doi: 10.1053/j.jvca.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 9.Rady MY, Johnson DJ, Patel BM, Larson JS, Helmers RA. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80(12):1558–1567. doi: 10.4065/80.12.1558. [DOI] [PubMed] [Google Scholar]
- 10.Krinsley JS. Glycemic control, diabetic status and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: six and one-half years experience at a university-affiliated community hospital. Semin Thorac Cardiovasc Surg. 2006;18(4):317–325. doi: 10.1053/j.semtcvs.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasslaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 12.American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endoc Prac. 2004;10:77–82. doi: 10.4158/EP.10.1.77. [DOI] [PubMed] [Google Scholar]
- 13.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 14.Scalea TM, Bochicchio GV, Bochicchio KM, Johnson SB, Joshi M, Pyle A. Tight glycemic control in critically injured trauma patients. Ann Surg. 2007;246(4):605–610. doi: 10.1097/SLA.0b013e318155a789. [DOI] [PubMed] [Google Scholar]
- 15.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109(12):1497–1502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- 16.Reed CC, Stewart RM, Sherman M, Myers JG, Corneille MG, Larson N, Gerhardt S, Beadle R, Gamboa C, Dent D, Cohn SM, Pruitt BA., Jr. Intensive insulin protocol improves glucose control and is associated with a reduction in intensive care unit mortality. J Am Coll Surg. 2007;204(5):1048–1054. doi: 10.1016/j.jamcollsurg.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 18.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 19.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Eng J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 20.Devos P, Preiser JC, Melot C. Impact of tight glycemic control by intensive insulin therapy on ICU mortality and the rate of hypoglycaemia: final results of the Glucontrol study. Intensive Care Med. 2007;33(Suppl 2):S189. [Google Scholar]
- 21.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 22.Van den Berghe G, Bouillon R, Mesotten D. Glucose control in critically ill patients. N Engl J Med. 2009;361(1):89. doi: 10.1056/NEJMc090812. letter. [DOI] [PubMed] [Google Scholar]
- 23.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality of critically ill patients. Anesthesiology. 2006;105(2):244–252. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Ali NA, O'Brien JM, Jr., Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF, Jr., Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36(8):2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM, Jr., May AK. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg. 2008;74(8):679–685. doi: 10.1177/000313480807400802. [DOI] [PubMed] [Google Scholar]
- 26.Krinsley JS. Glycemic variability: a strong, independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 27.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 28.Conner TM, Flesner-Gurley KR, Barner JC. Hyperglycemia in the hospital setting: the case for improved control among non-diabetics. Ann Pharmacother. 2005;39(3):492–501. doi: 10.1345/aph.1E308. [DOI] [PubMed] [Google Scholar]
- 29.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36(8):2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 30.Ryan EA, Shandro T, Green K, Paty BW, Senior PA, Bigam D, Shapiro AM, Vantyghem MC. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53(4):955–962. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- 31.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 32.Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281(5):E924–E930. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 33.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch IB. Glycemic variability: it's not just about A1C anymore! Diabetes Technol Ther. 2005;7(5):780–783. doi: 10.1089/dia.2005.7.780. [DOI] [PubMed] [Google Scholar]
- 35.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 36.Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33(12):2079–2084. doi: 10.1007/s00134-007-0835-4. [DOI] [PubMed] [Google Scholar]
- 37.Desachy A, Vuagnat AC, Ghazali AD, Baudin OT, Longuet OH, Calvat SN, Gissot V. Accuracy of bedside glucometry in critically ill patients: influence of clinical characteristics and perfusion index. Mayo Clin Proc. 2008;83(4):400–405. doi: 10.4065/83.4.400. [DOI] [PubMed] [Google Scholar]
- 38.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 39.De Block C, Manuel-Y-Keenoy B, Rogiers P, Jorens P, Van Gaal L. Glucose control and use of continuous glucose monitoring in the intensive care unit: a critical review. Curr Diabetes Rev. 2008;4(3):234–244. doi: 10.2174/157339908785294460. [DOI] [PubMed] [Google Scholar]
- 40.Krinsley JS, Preiser JC. Moving beyond tight glycemic control to safe effective glucose control. Crit Care. 2008;12(3):149. doi: 10.1186/cc6889. [DOI] [PMC free article] [PubMed] [Google Scholar]