Abstract
Acute hyperglycemia is common in critically ill patients. Strict control of blood glucose (BG) concentration has been considered important because hyperglycemia is associated independently with increased intensive care unit mortality. After intensive insulin therapy was reported to reduce mortality in selected surgical critically ill patients, lowering of BG levels was recommended as a means of improving patient outcomes. However, a large multicenter multination study has found that intensive insulin therapy increased mortality significantly. A difference in variability of BG control may be one possible explanation why the effect of intensive insulin therapy varied from beneficial to harmful. Several studies have confirmed significant associations between variability of BG levels and patient outcomes. Decreasing the variability of the BG concentration may be an important dimension of glucose management. If reducing swings in the BG concentration is a major biologic mechanism behind the putative benefits of glucose control, it may not be necessary to pursue lower glucose levels with their attendant risk of hypoglycemia.
Keywords: critical illness, glucose, glycemia, insulin, variability
Stress-Induced Acute Hyperglycemia and Normoglycemia in Intensive Care
Acute hyperglycemia is common in critically ill patients.1–4 Approximately 90% of all patients develop blood glucose (BG) concentrations higher than 110 mg/dl during critical illness.5 In critically ill patients, there is a hypermetabolic state,6 with the predominant cause being the intense activation of counterregulatory hormone and cytokine responses.4 This response to illness results in both hyperglycemia and central (increase in hepatic glucose production)7 and peripheral insulin resistance,8 often compounded by an excessive administration of dextrose-containing infusions,4 use of corticosteroids, and sympathomimetic drugs.9
Strict control of BG concentration has been considered important because hyperglycemia is associated independently with increased intensive care unit (ICU) mortality8,10–16 and because this finding has been interpreted to represent evidence of causation. Based on the aforementioned biologic rationale, two single center trials of intensive insulin therapy (IIT) (target glucose concentration of 4.4–6.1 mmol/liter) were performed. In the 2001 Leuven I trial, IIT was reported to reduce mortality in selected surgical patients compared with conventional glycemic control (p < 0.04) (mean BG level: 8.5 mmol/liter).5 In 2006, the Leuven II trial of medical critically ill patients failed to reduce mortality on an intention-to-treat analysis (p = 0.31) (Table 1).17 Using the pooled data set of these two randomized controlled trials, IIT was associated with a reduction in mortality from 23.6 to 20.4% (p = 0.04). Subsequent investigations suggested that metabolic control, as reflected by normoglycemia, rather than any other effect of insulin, was responsible for this effect.18 Lowering BG levels has been recommended in international consensus guidelines as a means of improving patient outcomes.19,20
Table 1.
Mean and Standard Deviation of Glycemia, Protocol Use and Mortality in Trials of Intensive Insulin Therapy.
| Glycemic control | Insulin protocol application | Mortality | |||||
|---|---|---|---|---|---|---|---|
| Conventional | IIT | Conventional | IIT | Conventional | IIT | p-value | |
| Leuven I trial5 | 8.5 ± 1.8 | 5.7 ± 1.1 | 307/783 (39.2%) | 755/765 (98.7%) | 63/783 (8.0%) | 35/765 (4.6%) | <0.04 |
| Leuven II trial17 | 8.5 ± 1.7 | 6.2 ± 1.6 | 426/605 (70%) | 580/595 (98%) | 162/605 (26.8%) | 144/595 (24.2%) | 0.31 |
| VISEP trial21 | 8.4 ± 1.8 | 6.2 ± 1.0 | 215/290 (74.1%) | 243/247 (98.4%) | 75/289 (26.0%) | 61/247 (24.7%) | 0.74 |
| NICE-SUGAR trial23 | 8.0 ± 1.3 | 6.4 ± 1.7 | 2080/3014 (69.0%) | 2931/3014 (97.2%) | 751/3012 (24.9%) | 829/3010 (27.5%) | 0.02 |
Controversy Surrounding Intensive Insulin Therapy
Despite initial encouraging results, several concerns and doubts have been raised by the international medical community as to the appropriateness of embracing tight glycemic control protocols as a standard of care worldwide. Three large multicenter randomized control studies [Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) trial,21 Glucontrol trial,22 and, more recently, the Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial23] have been conducted to confirm the benefit of IIT in critically ill patients.
The VISEP study was the first large prospective multicenter randomized trial to specifically investigate the role of IIT in patients with severe sepsis21 (Table 1). In this trial, IIT was associated with a significantly increased incidence of hypoglycemia, defined as less than 40 mg/dl (2.2 mmol/liter), compared to conventional treatment. This study found no significant difference in 28 days of mortality (24.7% vs 26.0%, IIT vs conventional, p = 0.74) and 90 days of mortality (39.7% vs 35.4%, IIT vs conventional, p = 0.31). Because the observed rate of hypoglycemia was considered unacceptably high, the data safety monitoring committee strongly recommended stopping the insulin arm of the trial. The Glucontrol trial, although presented in abstract form, has not yet been published.
The NICE-SUGAR trial is a large multicenter, multi-national trial involving 6022 critically ill patients in 42 hospitals. This study found that IIT increased 90-day mortality significantly (IIT vs conventional control: 27.5% vs 24.9%, p = 0.02) (Table 1).
Two published meta-analyses have shown that, in critically ill adult patients, IIT is not associated with significantly reduced hospital mortality, but is clearly associated with an increased risk of hypoglycemia.24,25
How and Why Different Results?
The mechanisms responsible for the different effect of IIT on mortality in the two studies5,25,26 can only be a matter of speculation. Some suggest that the different types and amounts of nutritional support (aggressive use of parenteral nutrition in Leuven studies),26 the different case mix (postcardiac surgery patients in the Leuven study),27 or the different follow-up periods (28 days in the Leuven studies and 90 days in the NICE-SUGAR trial) were responsible for the difference in outcome. However, another hidden and large difference between the two studies was the rate of insulin protocol application seen in conventional groups of the two trials (39.2% vs 69.0%, Leuven I trial vs NICE-SUGAR trial, p < 0.0001) (Table 1), a difference, which, as we will argue, is crucial.
Variability of Glycemia in Critically Ill Patients
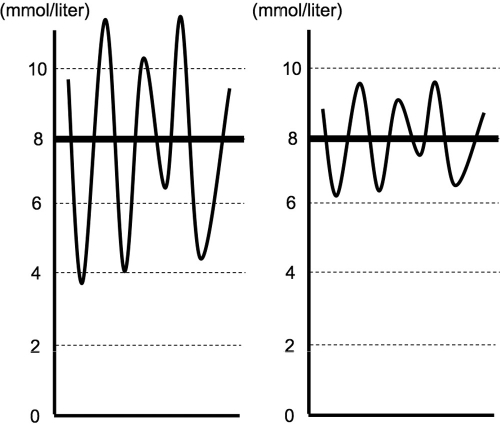
Blood glucose levels in critically ill patients swing markedly, even when using continuous feeding and insulin infusions.28 Remarkably, in the presence of the same mean value, glycemic control can be quite different based on the observed variability in BG (Figure 1).
Figure 1.
Graphic representation of glycemic control with a high mean glucose level and high variability (left) and with a high mean glucose level and low variability (right).
In four recently published IIT trials, an insulin protocol was applied to almost all patients in the IIT groups (about 98%, Table 1). If applied appropriately, such protocols should decrease both the mean BG concentration and its variability. Contrary to this, in the Leuven I trial,5 there was no specific insulin protocol for patients in the control groups of such trials until glycemia exceeded 11.1 or 11.9 mmol/liter. In this setting, 61% of patients in the conventional treatment arm did not appear to receive any glycemic control by protocol. Such a lack of protocol-based care may be logically expected to increase glycemic variability (Figure 1, left).28 The rest of the conventional group patients who received an insulin protocol targeting glycemia between 10 and 11.1 mmol/liter might have had a higher mean BG concentration but less glycemic variability (Figure 1, right). Importantly, in the Leuven II trial, VISEP trial, and NICE-SUGAR trial (all negative trials), an insulin protocol was applied to 70, 74%, and 69% of conventional arm patients, respectively. Therefore, it is possible that more Leuven I trial patients in the conventional treatment group had a greater degree of variability compared to the three later studies. This may explain why the dramatic results of the Leuven I study were not subsequently reproduced in other trials. In other words, the key is a protocol-dependent delivery of glycemic control with attendant decreases in variability. Larger glycemic variability may be pathophysiologically important, especially from a neurological perspective, and possibly as important as sustained hyperglycemia. However, until recently, there was little information regarding the meaning of this glycemic variability in critically ill patients.
Association of Glycemic Variability with Outcome
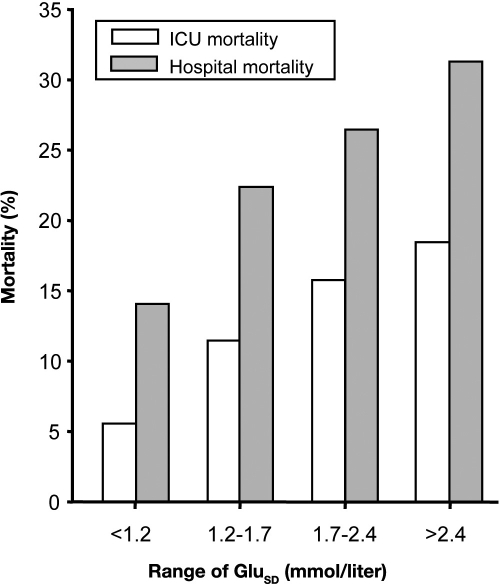
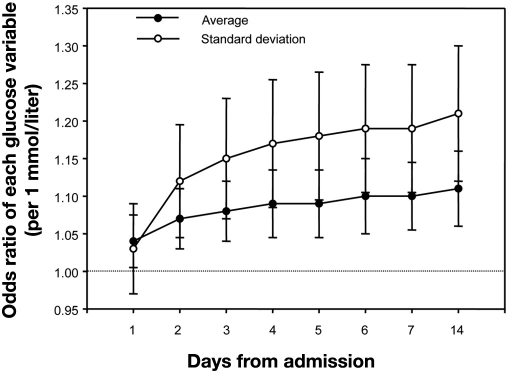
In 2006, we published the first study to assess the impact of variability of glycemia in critically ill patients. In this four center retrospective study of 7049 critically ill patients, we calculated the standard deviation (SD) of glycemia during ICU stay as a marker of variability. The mean glycemic variability in nonsurvivors was significantly higher compared with nonsurvivors (2.3mmol/liter vs 1.7 mmol/liter; p < 0.001). Greater glycemic variability was associated with significantly higher mortality (Figure 2). Using multivariable logistic regression analysis, glycemic variability was significantly associated with intensive care unit mortality [p < 0.001; odds ratios = 1.27(per 1 mmol/liter)] and hospital mortality [p = 0.013; odds ratios = 1.18 (per 1 mmol/liter)]. This independent association was greater when the ICU stay increased (Figure 3).
Figure 2.
Relationship between mortality and variability of BG control in critically ill patients. The SD of BG control during the ICU stay was used as a marker of variability of BG control. GluSD; SD of BG concentration.
Figure 3.
Time course of the predictive ability of average and SD of BG. Odds ratios (expressed with 95% confidential interval) for glucose indices indicate the risk change of ICU mortality per 1-mmol change in each index. For example, average of BG on 7 days from admission means average of entire glucose measurements during 7 days from admission. As time in the ICU increased, so did the ability of glucose control indices to predict the outcome.
Following our study, five other groups also assessed the possible effect of variability of glycemia. In a single center retrospective study of septic patients hospitalized for more than one day, Ali Na and colleagues29 found that patients with an increased glycemic lability index had an increased risk of hospital mortality (odds ratio = 4.73).
In a single center retrospective study, Hirshberg and colleagues30 also reported a significant association between variability of glycemia and increased mortality, nosocomial infection, and hospital length of stay. They defined variability of glycemia as occurring in patients who experienced both hyperglycemia (BG >8.4 mmol/liter) and hypoglycemia (BG <3.4 mmol/liter) during their ICU stay.
In a single center study, Dossett and associates31 showed that greater glycemic variability was significantly associated with increased mortality, whereas mean BG concentration was not. In this study, the SD, percentile values, successive changes in BG, and triangular index (calculated by dividing the maximum sample density distribution of each histogram by the total number of each measurement) for various glucose-related indices were used as markers of glycemic variability.
Waeschle and colleagues32 also showed a significant relationship between the SD of BG levels as a surrogate of glycemic variability and mortality in septic patients in a single center prospective study. In this study, a standard deviation of BG levels above 20 mg/dl was associated with a 9.6-fold increase in mortality compared with a deviation less than 20 mg/dl.
In a single center retrospective observational study, Krinsley33 found that mortality among patients with the lowest quartile of standard deviation of glucose levels, a surrogate of glycemic variability, was 12.1%, increasing to 19.9, 27.7, and 37.8% in the second, third, and fourth quartiles.
Thus, all of the aforementioned studies have so far confirmed that variability is associated with increased mortality and no studies have refuted this association.
Why Is Glycemic Variability Associated with Worse Outcomes?
There are at least four possible explanations for the association between glycemic variability and outcome that we and others have observed. First, less glycemic variability may reflect more attention to detail in medical and nursing care, which may be the real determinant of better outcomes. Second, less glycemic variability may be associated with less severe illness. Third, glycemic variability may have a true deleterious biological effect in critically ill patients. Fourth, any combination of the aforementioned factors may apply.
Studies support the hypothesis that swings in glucose levels may have biological toxicity. Quagliaro and associates34 have shown that, in umbilical vein cells, protein kinase Cβ, a surrogate of oxidative stress, was higher in the presence of fluctuations from hyperglycemia to normoglycemia when compared with sustained hyperglycemia. Monnier and colleagues35 have shown that glycemic variability may trigger adverse biologic events and oxidative stress in patients with type 2 diabetes. Such increased oxidative stress can result in endothelial dysfunction and contribute to vascular damages by triggering at least four major pathways: (1) enhanced polyol activity, causing sorbitol and fructose accumulation; (2) increased formation of advanced glycation end products; (3) activation of protein kinase C and nuclear factor κB; and (4) increased hexosamine pathway flux.36,37 Furthermore, it has been shown that large glycemic variability enhances monocyte adhesion to endothelial cells in rats.38,39 Risso and associates40 have shown that changing from hyperglycemia to normoglycemia rapidly causes increased apoptosis of human umbilical vein cells when compared with sustained hyperglycemia. Thus much evidence exists that glycemic variability may be more important than hyperglycemia at a biological and, perhaps, clinical level.
How to Reduce Glycemic Variability
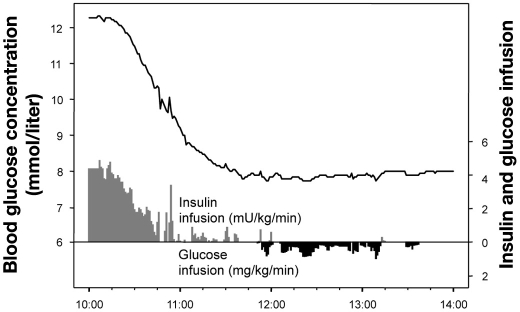
With regard to the aforementioned concern, new technologies are emerging that will allow continuous monitoring of glycemia and, thereby, superior control of BG variability. Figure 4 shows closed-loop glycemic control using STG-22™ (Nikkiso, Tokyo, Japan).41 The patient (body weight: 70 kg, male) had a postmitral valve replacement. He developed postoperative acute hyperglycemia (12.4 mmol/liter) upon admission to the ICU. Blood glucose monitoring was performed continuously using the STG-22 every 12 seconds by a dual lumen catheter blood sampling technique. The target BG level was 8 mmol/liter.42 His BG level was controlled by adjusting insulin (maximum dose: 20 IU/hour) and glucose (maximum dose; 8 g/hour) infusions. Once target BG levels (8 mmol/liter) were achieved, the SD of BG (surrogate of glycemic variability) was reduced to concentrations as low as 0.06 mmol/liter. With the availability of such technologies, studies should be able to better assess the impact of reductions in glycemic variability on outcomes in critically ill patients.
Figure 4.
Example of closed-loop glycemic control using the STG-22™ system (Nikkiso, Tokyo, Japan) in a postcardiac surgery patient (body weight: 70 kg, male). The patient developed postoperative acute hyperglycemia (12.4 mmol/liter) on admission to the ICU. Blood glucose monitoring using the STG-22 was performed continuously every 12 seconds by a dual lumen catheter blood sampling technique. The target BG level was 8 mmol/liter. The BG level of the patient was controlled by adjusted insulin infusion (gray bar) and glucose infusion (black bar). Once he achieved the target glucose level (8 mmol/liter), his SD of glycemia as a surrogate of glycemic variability was very small at 0.06 mmol/liter.
Conclusions
A difference in the variability of BG control (much higher in the control arm of the Leuven study, but equivalent in both arms of the NICE-SUGAR trial) may be one of the possible explanations why the effect of intensive insulin therapy varied from beneficial to harmful. Decreasing the variability of the BG concentration may be an important dimension of glucose management. If reducing swings in the BG concentration is a major biologic mechanism behind the putative benefits of glucose control, then it may not be necessary to pursue lower glucose levels with the attendant risks of hypoglycemia (safer glycemic control). The availability of continuous glucose monitoring technology, with semiclosed or closed-loop insulin delivery, should decrease BG variability markedly. Future studies may elucidate whether targeting glycemic variability is more important than targeting traditional measures of glycemia, such as the mean or median daily blood glucose.
Abbreviations
- BG
blood glucose
- ICU
intensive care unit
- IIT
intensive insulin therapy
- NICE-SUGAR
Normoglycemia in Intensive Care Evaluation—Survival Using Glucose Algorithm Regulation
- SD
standard deviation
- VISEP
Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis
References
- 1.Wolfe RR, Allsop JR, Burke JF. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]
- 2.Shangraw RE, Jahoor F, Miyoshi H, Neff WA, Stuart CA, Herndon DN, Wolfe RR. Differentiation between septic and postburn insulin resistance. Metabolism. 1989;38(10):983–989. doi: 10.1016/0026-0495(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 3.Cely CM, Arora P, Quartin AA, Kett DH, Schein RM. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness. Chest. 2004;126(3):879–887. doi: 10.1378/chest.126.3.879. [DOI] [PubMed] [Google Scholar]
- 4.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17(1):107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 6.Mizock BA. Alterations in carbohydrate metabolism during stress: a review of the literature. Am J Med. 1995;98(1):75–84. doi: 10.1016/S0002-9343(99)80083-7. [DOI] [PubMed] [Google Scholar]
- 7.Khaodhiar L, McCowen K, Bistrian B. Perioperative hyperglycemia, infection or risk? Curr Opin Clin Nutr Metab Care. 1999;2(1):79–82. doi: 10.1097/00075197-199901000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Robinson LE, van Soeren MH. Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues. 2004;15:45–62. doi: 10.1097/00044067-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Gearhart MM, Parbhoo SK. Hyperglycemia in the critically ill patient. AACN Clin Issues. 2006;17:50–55. doi: 10.1097/00044067-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Burkett E, Keijzers G, Lind J. The relationship between blood glucose level and QTc duration in the critically ill. Crit Care Resusc. 2009;11(1):8–13. [PubMed] [Google Scholar]
- 11.Mitchell I, Knight E, Gissane J, Tamhane R, Kolli R, Leditschke IA, Bellomo R, Finfer S Australian New Zealand Intensive Care Society Clinical Trials Group. A phase II randomised controlled trial of intensive insulin therapy in general intensive care patients. Crit Care Resusc. 2006;8(4):289–293. [PubMed] [Google Scholar]
- 12.Coursin DB, Connery LE, Ketzler JT. Perioperative diabetic and hyperglycemic management issues. Crit Care Med. 2004;32(4 Suppl):S116–S125. doi: 10.1097/01.ccm.0000115623.52021.c0. [DOI] [PubMed] [Google Scholar]
- 13.Malmberg K, Ryden L, Efendic S, Herlitz J, Nicol P, Waldenström A, Wedel H, Welin L. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 14.Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314(7093):1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orford N, Stow P, Green D, Corke C. Safety and feasibility of an insulin adjustment protocol to maintain blood glucose concentrations within a narrow range in critically ill patients in an Australian level III adult intensive care unit. Crit Care Resusc. 2004;6(2):92–98. [PubMed] [Google Scholar]
- 16.Orford NR. Intensive insulin therapy in septic shock. Crit Care Resusc. 2006;8(3):230–234. [PubMed] [Google Scholar]
- 17.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 18.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 19.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34(1):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 22. http://clinicaltrials.gov/ct2/show/NCT00107601?term=GLUCONTROL&rank=1.
- 23.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 24.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 26.Egi M, Morimatsu H, Toda Y, Matsusaki T, Suzuki S, Shimizu K, Iwasaki T, Takeuchi M, Bellomo R, Morita K. Hyperglycemia and the outcome of pediatric cardiac surgery patients requiring peritoneal dialysis. Int J Artif Organs. 2008;31(4):309–316. doi: 10.1177/039139880803100406. [DOI] [PubMed] [Google Scholar]
- 27.Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P, Li W, Bates S. Intensive insulin therapy in postoperative intensive care unit patients: a decision analysis. Am J Respir Crit Care Med. 2006;173(4):407–413. doi: 10.1164/rccm.200506-961OC. [DOI] [PubMed] [Google Scholar]
- 28.Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P. Circadian rhythm of blood glucose values in critically ill patients. Crit Care Med. 2007;35(2):416–421. doi: 10.1097/01.CCM.0000253814.78836.43. [DOI] [PubMed] [Google Scholar]
- 29.Ali NA, O'Brien JM, Jr., Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF, Jr., Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36(8):2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9(4):361–366. doi: 10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 31.Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM, Jr., May AK. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg. 2008;74(8):679–685. doi: 10.1177/000313480807400802. discussion 685. [DOI] [PubMed] [Google Scholar]
- 32.Waeschle RM, Moerer O, Hilgers R, Herrmann P, Neumann P, Quintel M. The impact of the severity of sepsis on the risk of hypoglycaemia and glycaemic variability. Crit Care. 2008;12(5):R129. doi: 10.1186/cc7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 34.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 35.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 36.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 37.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 38.Watada H, Azuma K, Kawamori R. Glucose fluctuation on the progression of diabetic macroangiopathy–new findings from monocyte adhesion to endothelial cells. Diabetes Res Clin Pract. 2007;77(Suppl 1):S58–S61. doi: 10.1016/j.diabres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 39.Azuma K, Kawamori R, Toyofuku Y, Kitahara Y, Sato F, Shimizu T, Miura K, Mine T, Tanaka Y, Mitsumata M, Watada H. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol. 2006;26(10):2275–2280. doi: 10.1161/01.ATV.0000239488.05069.03. [DOI] [PubMed] [Google Scholar]
- 40.Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281(5):E924–E930. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. The accuracy of a continuous blood glucose monitor during surgery. Anesth Analg. 2008;106(1):160–163. doi: 10.1213/01.ane.0000296461.26492.3c. table of contents. [DOI] [PubMed] [Google Scholar]
- 42.Bellomo R, Egi M. What is a NICE-SUGAR for patients in the intensive care unit? Mayo Clin Proc. 2009;84(5):400–402. doi: 10.1016/S0025-6196(11)60557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]