Abstract
Automation and standardization of the glucose measurement process have the potential to greatly improve glycemic control, clinical outcome, and safety while reducing cost. The resources required to monitor glycemia in hospitalized patients have thus far limited the implementation of intensive glucose management to patients in critical care units. Numerous available and up-and-coming technologies are targeted for the hospital patient population. Advantages and limitations of these devices are discussed herewith in.
Keywords: continuous glucose monitor, diabetes, glucose sensor, hospital
Introduction
Hyperglycemia and hypoglycemia commonly occur in the hospital despite significant nursing time and hospital resources devoted to glucose control.1–8 The majority of patients at risk for dysglycemia are monitored four to five times per day using fingerstick capillary blood and a point-of-care (POC) blood glucose (BG) meter with test strips. Manpower issues limit intensive insulin therapy (IIT) and frequent BG monitoring to a small number of patients managed in the operating rooms and critical care units.3,9,10 Caregivers must carefully interpret BG measurements and adjust insulin therapy in relation to meals, medications, illness, and insulin sensitivity to control the concentration of glucose safely and effectively.11–17 Titration of insulin in relation to the metabolic needs of the patient can be difficult. Twenty-one percent of all POC glucose measurements at our university hospital are greater than 200 mg/dl and 4% are less than 70 mg/dl (July 2009 RALS database).
Continuous glucose monitoring (CGM) systems developed for the hospital measure the concentration of glucose in blood, plasma, or interstitial fluid (ISF) every 1 to 15 minutes. Each type of CGM system has unique advantages and limitations when used to monitor patients in critical care and general floor environments.10,18–30 Advancements in chemistry, outer membrane biocompatibility, electronics, optics, miniaturization, signal processing, and manufacturing have improved CGM accuracy, stability, sensitivity, specificity, and robustness.10,31–35 Automation and standardization of the glucose measurement process have the potential to greatly improve BG control, clinical outcome, safety, and cost.
In the near future we envision that high-risk patients will be monitored continuously in real-time from hospital admission to discharge to facilitate the timely detection and prevention of hyper- and hypoglycemia. The CGM sensor and display will travel with the patient from the emergency department or operating room to the critical care unit, radiology, and general floor. Caregivers will observe the CGM display during each patient encounter to assess the (1) glucose concentration (mg/dl or mmol/liter), (2) direction of glucose change (increasing, decreasing, or stable) and (3) rate of glucose change (slow, fast, or stable).10,20,36–39 One, 3, or 6 hours of glucose trend data will be displayed at the bedside, central monitoring station, and personal digital assistant. The dose of intra-venous (IV) and subcutaneous insulin will be adjusted frequently according to glucose trend data using clinical protocols and computer algorithms.8,12,29,35,40–50
Vascular catheter blood glucose CGM systems may become the standard of care for the management of glucose levels in critical care environments of the hospital. The systems automatically transfer whole blood from a radial artery, peripheral vein, or central vein catheter to an external flow-through sensor. Standardization of blood sample acquisition, analysis, and calibration increase the accuracy and precision of glucose measurement,51 a major advantage of CGM systems compared to routine clinical methods.
Current and Future Technologies
The Biostator™ (Miles Laboratory), STG-22 (Nikkiso Inc.), and Glucostator (GmbH Inc.) remove blood through a dual lumen vascular catheter at a slow and constant rate. The blood is heparinized and transported to a flow-through glucose–oxidase electrochemical sensor. Glucose is measured every 1 to 5 minutes. Closed-loop algorithms direct the IV infusion of insulin and glucose.52–54 Safe and effective performance has been demonstrated during surgery and in intensive care unit (ICU) patients. Sample acquisition may be problematic, especially from a peripheral vein, and blood loss may exceed 100 ml/day. Those systems are still large, complex, difficult to maintain, and not currently approved for clinical use in the United States.47,52,54–58
The GlucoScout™ (International Biomedical Ltd.) has Food and Drug Administration (FDA)-approved labeling to sample blood from a radial artery, peripheral vein, or central venous catheter every 5 minutes for 72 hours. Real-time CGM measurements can be used to adjust the dose of insulin. Blood is transported to an external sterile flow-through glucose–oxidase electrochemical sensor and returned back into the bloodstream followed by a 6-ml infusion of a glucose–salt solution. The flush solution automatically recalibrates the sensor prior to each sample acquisition.30,59,60 Sample acquisition may be problematic, especially from a peripheral vein,20 whereas frequent sampling and flushing into the radial artery may cause hand edema. Blood sampled from the proximal port of a central venous catheter may also be contaminated from adjacent infusions.
The OptiScanner™ (OptiScan Inc.) transports blood from a vascular catheter to an external monitor, extracts a plasma sample, and measures the concentration of glucose using mid-infrared spectroscopy. A large and diverse library of plasma spectra is used to produce a universal calibration algorithm. The optical measurement of plasma has low scattering and a high signal-to-noise ratio. Clinical trial data demonstrated satisfactory accuracy, sensitivity, and specificity for continuous glucose monitoring in the critical care setting. Blood loss and flush volume were clinically acceptable.61
The intravenous blood glucose monitor (developed jointly by Edwards LifeSciences and DexCom Inc.) transports a small volume of blood into the lumen of an IV catheter, measures the concentration of glucose using a sterile enzyme-based electrochemical sensor, and returns the sample back into the bloodstream using a glucose–salt solution. The measurement process and calibration are automated and standardized to ensure accuracy and precision. The very small sample volume is optimal for sample acquisition from a peripheral vein and also minimizes flush volume.
The GlucCath™ (Glumetrics Inc.) is a small diameter optical fiber that is inserted into the lumen of a peripheral vein. After removal of the introducer, only the small fiber remains intravascular for several days. Glucose passes through a porous membrane to interact with boronic acid fluorescent chemistry. Accuracy, sensitivity, and specificity have been optimized for the euglycemic and hypoglycemic ranges. There is a large and rapid change in the fluorescent signal following a small change in the BG level. Long-term stability permits infrequent calibration using a reference BG measurement.
The DIRAMO™ System for Continuous Blood Glucose Monitoring (Flowsion Medical) is a small diameter dialysis catheter that is inserted into the lumen of a peripheral vein for up to 72 hours. Glucose molecules diffuse from the blood into the glucose-free dialysate solution through a microdialysis membrane. The glucose enriched perfusate then reacts with glucose oxidase in a microfluidic reaction chip. The concentration of glucose is continuously measured every second and detected optically by a chemiluminescent sensor. The glucose concentration is proportional to the chemiluminescence intensity.
The GlucoDay (Minarini Inc.) utilizes a small diameter dialysis catheter inserted in the subcutaneous tissue of the abdomen. Glucose molecules diffuse through the small-pore membrane from the ISF into the dialysate solution. An external flow-through glucose–oxidase electrochemical sensor measures the concentration of glucose every 3 minutes for 48 hours. Calibration requires a daily reference BG measurement.19,23 Other dialysis catheter CGM systems use near-infrared spectroscopy or a physical change to measure the concentration of glucose.62–64 Slow transport ensures complete equilibration but increases the time lag. Current dialysis catheters are fragile and prone to fracture and premature failure.10
The systems described so far require invasive methods for glucose sampling. Noninvasive CGM systems utilize near-infrared spectroscopy, electrical impedance, and physical changes to estimate the concentration of glucose in the skin and subcutaneous tissue. Noninvasive CGM systems have been limited by low sensitivity, specificity, signal-to-noise ratio, and difficulty maintaining a stable sensor–tissue interface.65
Interstitial fluid CGM systems may be optimal for patients managed on general floors where ambulation and ease of clinical use are most important. Three subcutaneous tissue CGM systems have FDA-approved labeling for measuring the concentration of glucose in the ISF of ambulatory patients with diabetes every 1 to 5 minutes for 3 to 7 days. These CGM systems remain an adjunctive device, as any adjustment in insulin therapy requires a confirmatory BG measurement using a reference meter.31,66
The Guardian RT (Medtronic Diabetes Inc.), DexCom Seven (DexCom Inc.), and Navigator (Abbott Diabetes) are inserted easily into the subcutaneous tissue of the abdomen, flank, thigh, buttocks, upper arm, and chest. Novel porous membranes and electrochemistry enhance accuracy, sensitivity, specificity, and stability while minimizing the influence of hypoxemia, oxidizing compounds, and biofouling.32,67
Current CGM systems require at least an hour of run-in time after insertion due to an unstable sensor–tissue interface. Sensitivity often decreases and fluctuates after insertion for several hours. Accuracy depends greatly on the calibration method. Reference BG measurements should be obtained for calibration only when the blood/tissue glucose level is stable (<0.5 mg/dl/min rate of change). Frequent recalibration may be required in some patients due to ongoing changes in the sensor–tissue interface, membrane, enzyme, or electrochemistry.68 A change in ISF CGM measurement may lag a BG change by 10 to 30 minutes (average 12 minutes).28,69 Reference BG measurements should be time shifted when used for calibration of an ISF sensor.70–72
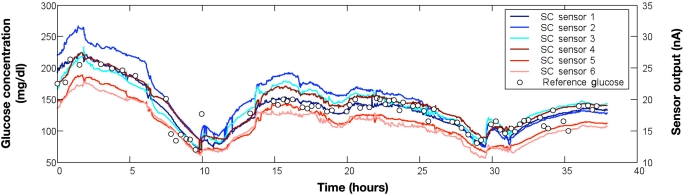
All CGM systems alarm when the glucose level exceeds a high or low threshold. Several systems have alarm algorithms that predict the onset of hyperglycemia and hypoglycemia 10 to 30 minutes into the future.35,73 Sensitivity and specificity for hypoglycemia detection are improved when the algorithm considers rate of change and threshold.66 False alarms, missed hypoglycemia events, and data loss can be decreased by averaging multiple CGM sensors (Figure 1). Two or more sensor output signals that move in parallel can be processed in real time to improve accuracy and robustness, whereas signals that deviate significantly from the mean can be used to detect sensor malfunction.74 There is a trade-off among the number of sensors, complexity, practicality, and cost.
Figure 1.
Glucose trend data measured simultaneously from six interstitial fluid continuous glucose monitors (modified research-only Guardian RT systems, Medtronic Diabetes, Northridge, CA) during and after major abdominal surgery. Patient had type 2 diabetes managed chronically with subcutaneous insulin and managed currently with an intravenous infusion of regular insulin. All CGM measurements moved in parallel (direction and rate of change) during the 38 hours of data collection. Several CGM sensors correlated closely with reference blood glucose measurements (blood sampled from a radial artery catheter and measured in duplicate using an Omni-9 analyzer; Roche Diagnostics). Several CGM sensors, however, demonstrated a consistent negative or positive bias relative to the reference BG measurements.
Clinical trials in the hospital typically compare CGM glucose data (every 5 minutes or 288 measurements/day) to intermittent BG data measured with a reference analyzer (every 20 to 240 minutes or 6 to 72 measurements/day).22,23,75,76 Blood CGM systems have shown excellent accuracy when evaluated in patients requiring surgery and intensive care. Methods to sample blood frequently from a vascular catheter have improved significantly.51,77,78
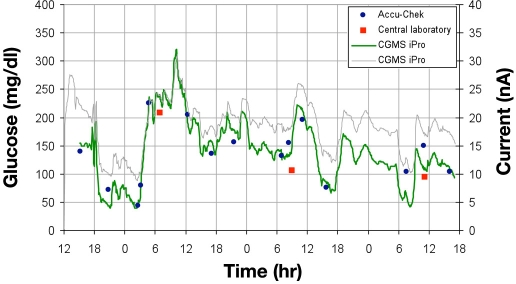
Interstitial fluid CGM systems are easy to utilize in the hospital environment and provide clinically useful real-time glucose information (Figure 2). The majority of ISF sensors correlate closely with reference BG measurements.79 The reason why some ISF sensors drift significantly over a short period of time or fail prematurely is not well understood.18,25,68,76 Larger and longer clinical trials are currently underway to evaluate CGM safety and performance in a variety of hospital patient populations.
Figure 2.
A continuous glucose monitor was inserted into the thigh of a hospitalized patient with type 2 diabetes managed with subcutaneous insulin. A CGMS iPro Recorder (Medtronic Diabetes, Northridge, CA) measured and recorded the concentration of interstitial fluid glucose every 5 minutes. Sensor output current (thin line) was calibrated to POC measurements from an Accu-Chek Inform meter (solid circles) and the hospital laboratory (solid squares) to produce an estimate of blood glucose (thick line). Recurrent hypoglycemia was recorded twice over the 72-hour study period.
Pitfalls of BG Monitoring in Hospitalized Patients
Hospitals have not standardized or optimized the methods of blood sample collection, handling, or POC laboratory analysis to ensure an accurate and precise glucose measurement. Preanalytical and analytical errors are additive.80–86 CGM accuracy depends on an accurate reference BG measurement.72
Capillary blood sampled from a fingertip may produce an erroneous glucose measurement due to tissue edema, low peripheral perfusion, or small sample size.87 Blood sampled from a vascular catheter and stopcock may be contaminated with glucose-free or glucose-containing solution traveling through IV tubing or a collateral vein. A hemolyzed specimen may cause a low glucose measurement due to dilution with intracellular fluid. Ongoing glycolysis will decrease the glucose concentration of a blood sample sent to a central laboratory for analysis. The glucose concentration of a centrifuged specimen is higher than a noncentrifuged specimen due to the movement of proteins out of the plasma.86,88
Glucose measurements from a POC meter, ICU analyzer, and central laboratory analyzer may be significantly different, depending on the method of sample application, type of enzyme, chemistry of assay, type of transducer, and method of calibration. The accuracy, precision, and sensitivity of each analyzer may differ when measuring samples of whole blood or plasma in the hypoglycemic, euglycemic, and hyperglycemic ranges.89 Some analyzers are affected by changes in hematocrit, oxygen, pH, temperature, acetaminophen, uric acid, ascorbic acid, and carbohydrates that resemble glucose (e.g., maltose).90–94
Blood sampled simultaneously from the fingertip, radial artery, peripheral vein, superior vena cava, right atrium, and pulmonary artery will have a different BG concentration.91,92,95,96 This difference is due to glucose uptake by the cells, mixing of blood from multiple tissue beds, hepatic glucose output, and changes in the plasma volume.
Sample type, method of sample collection, sample handling, and analyzer type should be standardized. Glucose measurements should be performed in duplicate or triplicate and averaged. In reality, nurses and technicians perform a single BG measurement every 1 to 6 hours with a POC meter and test strip. The outcome results of clinical trials performed in the ICU should be interpreted with caution due to variability in the type of blood sampled, blood sample handling, and type of analyzer used for glucose monitoring. A duplicate BG measurement is obtained only to confirm hypoglycemia or to double-check a measurement that does not fit the clinical situation.
Research studies have not reported whether the sample or analyzer type was similar for the treatment group and conventional group. For example, the Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation trial did not determine the influence of sample type and analyzer type on the glucose measurement. This influence is especially important because the glucose difference between the IIT group and the conventional treatment group was 23 mg/dl and more than 50% of the BG measurements overlapped.97,98
Initial CGM Calibration and Recalibration
CGM accuracy depends greatly on the process of calibration. Regulatory agencies will require an initial calibration and recalibration of all CGM systems using patient blood samples or reference glucose standards one to three times per day and whenever sensor drift is suspected. Ideally, blood should be sampled from the same anatomic site as the CGM sensor is located. Blood sample collection, handling, and measurement should be standardized using the most accurate glucose analyzer available in the hospital. An optimal CGM system will calibrate the sensor frequently and automatically using an external glucose standard rather than a patient blood sample.
Interstitial fluid CGM systems should be calibrated during a period of glucose stability (e.g., around 6 a.m. after an overnight fast or 6 hours after the dinner meal). CGM trend data can be used to determine trend stability (<0.5 mg/dl/min change) and to detect an outlier BG measurement.72 For example, a reference BG measurement that deviates significantly from the averaged, smoothed, and time-shifted CGM trend (International Organization for Standardization standard #15197; BG >20% above 75 mg/dl; >15 mg/dl below 75 mg/dl) should be considered an outlier and not used for patient management or CGM calibration.68
Frequency of BG Monitoring
Multiple hourly glucose measurements are required to accurately characterize the glucose waveform of a hospitalized patient. In reality, the concentration of BG is measured only four to five times per day in the majority of hospitalized patients with diabetes. A small number of hospitalized patients are treated aggressively with IIT and hourly glucose monitoring.3–8 The risk of severe and prolonged hypoglycemia is increased greatly due to the low frequency of monitoring, especially on general floors.
The average and maximum rates of BG change in hospitalized patients managed in the ICU and general floors have not been well studied. The concentration of BG changes slowly (<1 mg/dl/min) more than 90% of the time in an ambulatory patient with diabetes. The maximum rate of change is rarely greater than 3 mg/dl/min.10 In the hospital, however, the concentration of blood/ISF glucose can fall faster than 3 mg/dl/min following a large IV dose of insulin, an abrupt decrease in nutrient delivery, or an acute increase in insulin sensitivity.25,76,99 Thus, BG monitoring once per hour is insufficient to eliminate the risk of hypoglycemia.
Approximately 5 minutes of a caregiver's time is required to measure the concentration of glucose using a blood sample, glucose meter, and test strip. Hourly monitoring equates to more than 2 hours of a caregiver's time, dedicated solely to BG monitoring of an individual patient.9Manpower issues therefore limit frequent glucose monitoring to a small group of high-risk patients managed in operating rooms, critical care units, intermediate care units, and general floors.
Clinical Use of CGM and Hypoglycemia
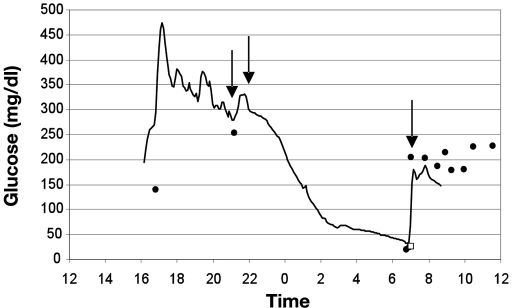
Automation of glucose monitoring is anticipated to save significant nursing time while improving glucose control and safety. Clinical use of CGM should also minimize the risk of mild to moderate hypoglycemia and eliminate the risk of severe and prolonged hypoglycemia (Figure 3). A caregiver will typically assess the risk for hypoglycemia during each patient encounter by glancing at the bedside display. A steep downward slope of glucose trend data is recognized easily as a high-risk clinical situation requiring increased vigilance.100 Intermittent visualization of the CGM trend will be the primary method for the detection of hypoglycemia in critical care and general floor environments. The CGM will alarm when hypoglycemia is detected or predicted.73 Timely information will allow the caregiver to intervene with oral or intravenous carbohydrates to prevent an adverse event. For CGM to become standard of care for all hospitalized patients at risk for hypoglycemia, clinical trials will need to demonstrate safe and effective performance in the ICU and general floor environments.
Figure 3.
Severe hypoglycemia recorded in a hospitalized patient with type 1 diabetes 1 day after orthopedic surgery. The interstitial fluid glucose concentration (solid line) was measured and recorded every 5 minutes using a CGMS iPro Continuous Glucose Recorder (Medtronic Diabetes, Northridge, CA). CGM data were calibrated using a retrospective method using POC glucose measurements (solid circles) from an Accu-Chek Inform meter (Roche Diagnostics USA, Indianapolis, IN), thus the clinical nurse was blinded to real-time CGM data. Capillary BG measurements at 1700 and 2100 hours were elevated. Peakless and rapid-acting insulin were injected into the subcutaneous tissue around 2100 hours. The patient was found unresponsive at 0700 hours with a POC glucose of 18 mg/dl and a central laboratory plasma glucose of 26 mg/dl (open square). A bolus dose of 50% glucose (25 grams) was delivered into the IV catheter at 0710 hours. The patient recovered without permanent injury. This adverse event could have been prevented if the nurse had access to real-time CGM data.
Hyperglycemia in the Hospital
Approximately 7.5% of the U.S. population currently has diabetes. Twenty-two million Americans have type 2 diabetes, 1 million have type 1 diabetes, and 135,000 have gestational diabetes. One-quarter to one-third of U.S. patients with diabetes and prediabetes are unaware and untreated. Patients who do not have diabetes commonly develop hyperglycemia during the stressful portion of a medical illness or surgical procedure, a condition known as hospital-related hyperglycemia.101,102 Insulin resistance of hepatic, renal, skeletal muscle, and adipose tissue is the hallmark feature of the stress response. The degree and duration of hyperglycemia correlate with the degree of tissue injury, autonomic nervous system activity, immune system activity, and the patient's ability to mount a stress response due to overall conditioning and nutrition.103–105 Hyperglycemia is amplified by the rapid administration of IV glucose solutions and the administration of catecholamine and steroid medications.106
The optimal range of BG control in a specific patient population remains controversial. The BG range of 100 to 180 mg/dl has traditionally been recommended to minimize the risk of hypoglycemia and the adverse effects of hyperglycemia. Blood glucose levels greater than 180 mg/dl often exceed the ability of the kidney to reabsorb glucose, leading to glycosuria, dehydration, and electrolyte abnormality.10 Observational studies have documented a lower risk for nosocomial infection, renal failure, and in-hospital mortality when the average BG level is maintained less than 200, 150, or 110 mg/dl.1–4,6,107,108
Several prospective randomized trials demonstrated a marked decrease in morbidity and mortality when hyperglycemia was controlled in the normal range (70 to 110 mg/dl) with hourly adjustments in IV insulin.8,41,42 Outcome data supporting IIT and tight BG control are most consistent in patients with and without diabetes undergoing cardiac surgery,1,108,109 whereas too aggressive insulin therapy may lead to increased morbidity and mortality.110,111
Intensive insulin therapy requires frequent BG monitoring and adjustments of the intravenous infusion dose of regular insulin (every 1 to 2 hours). Clinical algorithms consider the BG trend (direction and amount of change between measurements) to calculate each dose adjustment.8,43,112 Computer algorithms have been commercialized (Endotool, Glucommander) that bring the hyperglycemic patient into good glucose control safely and effectively with a low incidence of hypoglycemia. Glucose measurements are entered manually into a bedside computer every 1 to 2 hours. The nurse adjusts the rate of insulin delivery for the next hour based on the computer recommendation. The computer algorithm recommends very frequent monitoring (every 15 to 30 minutes) when the BG concentration changes rapidly near the hypoglycemia range.48–50
Glucose Sensor-Augmented Insulin Delivery
The Biostator and GlucoScout have been used successfully to monitor surgical and medical patients in the operating room and ICU. The dose of insulin has been adjusted safely based on real-time CGM measurements. Real-time BG monitoring (a measurement every 5 to 15 minutes) has great potential to increase the safety and effectiveness of clinical protocols and computer algorithms that recommend insulin dose adjustments.35,100
First-generation CGM systems display the BG trend and alarm for impending hypoglycemia/hyperglycemia. Second-generation CGM systems will recommend an insulin dose adjustment based on the BG trend.31,66 Future CGM systems will integrate infusion pumps that deliver IV insulin, IV glucose, and possibly IV parenteral nutrition and enteral tube feedings10,113 to optimize both nutrition and BG control.114–117 Algorithms will utilize pharmacodynamic models of regular insulin, glucose, total parenteral nutrition, partial parenteral nutrition, commercial nutrients, and meals to enhance insulin dose recommendations and alarms.118
Finally, computerized closed-loop systems will adjust the dose of IV insulin and IV glucose automatically every few minutes to clamp the BG level in the desired range. Closed-loop control will enhance safety and effectiveness when the clinical situation is changing rapidly.10,29,44,45,47,52,53,57,113,119 Lessons learned in the hospital will help advance research toward an artificial pancreas for the ambulatory patient with insulin-dependent diabetes.120,121
Abbreviations
- BG
blood glucose
- CGM
continuous glucose monitoring
- ICU
intensive care unit
- IIT
intensive insulin therapy
- ISF
interstitial fluid
- IV
intravenous
- POC
point of care
References
- 1.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 2.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 3.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–591. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of the critically ill? Diabetologia. 2006;49(8):1722–1725. doi: 10.1007/s00125-006-0306-4. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite SS, Buie MM, Thompson CL, Baldwin DF, Oertel MD, Robertson BA, Mehrotra HP. Hospital hypoglycemia: not only treatment but also prevention. Endocr Pract. 2004;10(Suppl 2):89–99. doi: 10.4158/EP.10.S2.89. [DOI] [PubMed] [Google Scholar]
- 6.Fischer KF, Lees JA, Newman JH. Hypoglycemia in hospitalized patients. Causes and outcomes. N Engl J Med. 1986;315(20):1245–1250. doi: 10.1056/NEJM198611133152002. [DOI] [PubMed] [Google Scholar]
- 7.Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367–369. doi: 10.2337/dc06-1715. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 9.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15(4):370–377. [PubMed] [Google Scholar]
- 10.Joseph JI. Management of the diabetic surgical patient. Textbook of type 2 diabetes. Martin Dunitz. 2007 [Google Scholar]
- 11.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355(18):1903–1911. doi: 10.1056/NEJMcp060094. [DOI] [PubMed] [Google Scholar]
- 12.Wilson M, Weinreb J, Hoo GW. Intensive insulin therapy in critical care: a review of 12 protocols. Diabetes Care. 2007;30(4):1005–1011. doi: 10.2337/dc06-1964. [DOI] [PubMed] [Google Scholar]
- 13.Lipshutz AK, Gropper MA. Perioperative glycemic control: an evidence-based review. Anesthesiology. 2009;110(2):408–421. doi: 10.1097/ALN.0b013e3181948a80. [DOI] [PubMed] [Google Scholar]
- 14.Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract. 2004;10(Suppl 2):71–80. doi: 10.4158/EP.10.S2.71. [DOI] [PubMed] [Google Scholar]
- 15.ACE/ADA Task Force on Inpatient Diabetes. American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Endocr Pract. 2006;12(4):458–468. doi: 10.4158/EP.12.4.458. [DOI] [PubMed] [Google Scholar]
- 16.Garber AJ, Moghissi ES, Bransome ED, Jr., Clark NG, Clement S, Cobin RH, Furnary AP, Hirsch IB, Levy P, Roberts R, Van den Berghe G, Zamudio V American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):77–82. doi: 10.4158/EP.10.1.77. [DOI] [PubMed] [Google Scholar]
- 17.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg PA, Siegel MD, Russell RR, Sherwin RS, Halickman JI, Cooper DA, Dziura JD, Inzucchi SE. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol Ther. 2004;6(3):339–347. doi: 10.1089/152091504774198034. [DOI] [PubMed] [Google Scholar]
- 19.Vriesendorp TM, DeVries JH, Holleman F, Dzoljic M, Hoekstra JB. The use of two continuous glucose sensors during and after surgery. Diabetes Technol Ther. 2005;7(2):315–322. doi: 10.1089/dia.2005.7.315. [DOI] [PubMed] [Google Scholar]
- 20.Ganesh A, Hipszer B, Loomba N, Torjman MC, Joseph J. Evaluation of the VIA blood chemistry monitor for glucose in healthy and diabetic volunteers. J Diabetes Sci Technol. 2008;2(2):182–193. doi: 10.1177/193229680800200203. 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hipszer B, Furlong KJ, Lessin JB, Grunwald Z, Joseph JI. Continuous glucose monitoring in the perioperative period. Anesthesiology. 2006;105:A583. [Google Scholar]
- 22.Hipszer B, Chervoneva I, Gratch DM, Heitz JW, Maguire DP, Yeo CJ, Grunwald Z, Joseph JI. The performance of subcutaneous glucose sensors in surgical patients. J Diabetes Sci Technol. 2008;2(2):A71. [Google Scholar]
- 23.De Block C, Manuel-Y-Keenoy B, Van Gaal L, Rogiers P. Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring. Diabetes Care. 2006;29(8):1750–1756. doi: 10.2337/dc05-2353. [DOI] [PubMed] [Google Scholar]
- 24.Murakami A, Gutierrez MA, Lage SH, Rebelo MF, Guiraldelli RH, Ramires JA. A continuous glucose monitoring system in critical cardiac patients in the intensive care unit. Comput Cardiol. 2006:233–236. [Google Scholar]
- 25.Corstjens AM, Ligtenberg JJ, van der Horst IC, Spanjersberg R, Lind JS, Tulleken JE, Meertens JH, Zijlstra JG. Accuracy and feasibility of point-of-care and continuous blood glucose analysis in critically ill ICU patients. Crit Care. 2006;10(5):R135. doi: 10.1186/cc5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bochicchio GV, Bochicchio KM, Lettich K, Lambert P, Herrera A, Lumpkins K, Scalea T, Magarian P. Cutting edge technology in tight glycemic control (TGC) Crit Care Med. 2007;35(Suppl):A142. [Google Scholar]
- 27.Monsod TP, Flanagan DE, Rife F, Saenz R, Caprio S, Sherwin RS, Tamborlane WV. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyper-insulinemia? Diabetes Care. 2002;25(5):889–893. doi: 10.2337/diacare.25.5.889. [DOI] [PubMed] [Google Scholar]
- 28.Aussedat B, Dupire-Angel M, Gifford R, Klein JC, Wilson GS, Reach G. Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metab. 2000;278(4):E716–E728. doi: 10.1152/ajpendo.2000.278.4.E716. [DOI] [PubMed] [Google Scholar]
- 29.Chee F, Fernando T, van Heerden PV. Closed-loop glucose control in critically ill patients using continuous glucose monitoring system (CGMS) in real time. IEEE Trans Inf Technol Biomed. 2003;7(1):43–53. doi: 10.1109/titb.2003.808509. [DOI] [PubMed] [Google Scholar]
- 30.Widness JA, Kulhavy JC, Johnson KJ, Cress GA, Kromer IJ, Acarregui MJ, Feld RD. Clinical performance of an in-line point-of-care monitor in neonates. Pediatrics. 2000;106(3):497–504. doi: 10.1542/peds.106.3.497. [DOI] [PubMed] [Google Scholar]
- 31.Garg SK. The future of continuous glucose monitoring. Diabetes Technol Ther. 2009;11(Suppl 1):S1–S3. doi: 10.1089/dia.2008.0105. [DOI] [PubMed] [Google Scholar]
- 32.McGarraugh G. The chemistry of commercial continuous glucose monitors. Diabetes Technol Ther. 2009;11(Suppl 1):S17–S24. doi: 10.1089/dia.2008.0133. [DOI] [PubMed] [Google Scholar]
- 33.Joseph JI, Goldstein BJ. Goldstein BJ, Muller-Wieland D, editors. New devices and drugs on the horizon. Informa Healthcare. 2003:447–456. Textbook of type 2 diabetes. [Google Scholar]
- 34.Rossetti P, Porcellati F, Fanelli CG, Bolli GB. Evaluation of the accuracy of a microdialysis-based glucose sensor during insulin-induced hypoglycemia, its recovery, and post-hypoglycemic hyper-glycemia in humans. Diabetes Technol Ther. 2006;8(3):326–337. doi: 10.1089/dia.2006.8.326. [DOI] [PubMed] [Google Scholar]
- 35.Allen HF, Rake A, Roy M, Brenner D, McKiernan CA. Prospective detection of hyperglycemia in critically ill children using continuous glucose monitoring. Pediatr Crit Care Med. 2008;9(2):153–158. doi: 10.1097/PCC.0b013e3181668b33. [DOI] [PubMed] [Google Scholar]
- 36.Reach G. Continuous glucose monitoring and diabetes health outcomes: a critical appraisal. Diabetes Technol Ther. 2008;10(2):69–80. doi: 10.1089/dia.2007.0261. [DOI] [PubMed] [Google Scholar]
- 37.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11(Suppl 1):S55–S67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 38.Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense freestyle navigator data. Diabetes Care. 2004;27(8):1922–1928. doi: 10.2337/diacare.27.8.1922. [DOI] [PubMed] [Google Scholar]
- 39.Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial) Diabetes Care. 2007;30(9):2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 40.Torjman MC, Dalal N, Goldberg ME. Glucose monitoring in acute care: technologies on the horizon. J Diabetes Sci Technol. 2008;2(2):178–181. doi: 10.1177/193229680800200202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 42.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 43.Plank J, Blaha J, Cordingley J, Wilinska ME, Chassin LJ, Morgan C, Squire S, Haluzik M, Kremen J, Svacina S, Toller W, Plasnik A, Ellmerer M, Hovorka R, Pieber TR. Multicentric, randomized, controlled trial to evaluate blood glucose control by the model predictive control algorithm versus routine glucose management protocols in intensive care unit patients: response to Ligtenberg etal. Diabetes Care. 2006;29(8):1987–1988. doi: 10.2337/dc06-0838. [DOI] [PubMed] [Google Scholar]
- 44.Chee F, Fernando T, van Heerden PV. Closed-loop control of blood glucose levels in critically ill patients. Anaesth Intensive Care. 2002;30(3):295–307. doi: 10.1177/0310057X0203000306. [DOI] [PubMed] [Google Scholar]
- 45.Chase JG, Shaw GM, Lin J, Doran CV, Hann C, Lotz T, Wake GC, Broughton B. Targeted glycemic reduction in critical care using closed-loop control. Diabetes Technol Ther. 2005;7(2):274–282. doi: 10.1089/dia.2005.7.274. [DOI] [PubMed] [Google Scholar]
- 46.Galletti PM, Kuntschen FR, Hahn C. Experimental and clinical studies with servo-controlled glucose and insulin administration during cardiopulmonary bypass. Mt Sinai J Med. 1985;52(7):500–507. [PubMed] [Google Scholar]
- 47.Schwartz SS, Horwitz DL, Zehfus B, Langer B, Moossa AR, Ribeiro G, Kaplan E, Rubenstein AH. Use of a glucose controlled insulin infusion system (artificial beta cell) to control diabetes during surgery. Diabetologia. 1979;16(3):157–164. doi: 10.1007/BF01219792. [DOI] [PubMed] [Google Scholar]
- 48.Steed RD, Davidson PC, Bode BW, Sivitz WI. Computer-controlled intravenous insulin infusion using intermittent bedside glucose monitoring: one year's experience. Diabetes. 1986;35(Suppl. 1):32A. [Google Scholar]
- 49.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120, 618 h of operation. Diabetes Care. 2005;28(10):2418–2423. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 50.Dunn K, Miller E, Cochran S, Burgess W. Optimized glucose management with EndoTool intravenous insulin (IVI) dosing. Diabetes. 2007;0463:P. [Google Scholar]
- 51.Schaller R, Feichtner F, Kohler H, Bodenlenz M, Plank J, Wutte A, Mader JK, Ellmerer M, Hellmich R, Wedig H, Hainisch R, Pieber TR, Schaupp L. A novel automated discontinuous venous blood monitoring system for ex vivo glucose determination in humans. Biosens Bioelectron. 2009;24(7):2239–2245. doi: 10.1016/j.bios.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 52.Kadish AH. A servomechanism for blood sugar control. Biomed Sci Instrum. 1963;1:171–176. [PubMed] [Google Scholar]
- 53.Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W. An artificial endocrine pancreas. Diabetes. 1974;23(5):389–396. doi: 10.2337/diab.23.5.389. [DOI] [PubMed] [Google Scholar]
- 54.Okabayashi T, Hnazaki K, Nishimori I, Sugimoto T, Maeda H, Yatabe T, Dabanaka K, Kobayashi M, Yamashita K. Continuous post-operative blood glucose monitoring and control using a closed-loop system in patients undergoing hepatic resection. Dig Dis Sci. 2008;53(5):1405–1410. doi: 10.1007/s10620-007-0010-3. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. The accuracy of a continuous blood glucose monitor during surgery. Anesth Analg. 2008;106(1):160–163. doi: 10.1213/01.ane.0000296461.26492.3c. table of contents. [DOI] [PubMed] [Google Scholar]
- 56.Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand. 2009;53(1):66–71. doi: 10.1111/j.1399-6576.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 57.Ozyol MB, Herfarth C, Kerner W, Pfeiffer EF. Postoperative blood-sugar regulation in pancreatectomized patients using the artificial “beta cell”. Chirurg. 1979;50(4):227–232. [PubMed] [Google Scholar]
- 58.Gin H, Catargi B, Rigalleau V, Rullier E, Roger P, Tabarin A. Experience with the Biostator for diagnosis and assisted surgery of 21 insulinomas. Eur J Endocrinol. 1998;139(4):371–377. doi: 10.1530/eje.0.1390371. [DOI] [PubMed] [Google Scholar]
- 59.Lucisano JY, Edelman SV, Quinto BD, Wong DK. Development of a biosensor-based, patient-attached blood glucose monitoring system. Proc Am Chem Soc. 1997;76:256. [Google Scholar]
- 60.VIA blood chemistry monitor for glucose VIA V-GLU 1 operator's manual. San Diego, CA: VIA Medical Corporation; 1998. [Google Scholar]
- 61.Krinsley J, Zheng P, Hall D, Magarian P. ICU validation of the Optiscanner, a continuous glucose monitoring device. Crit Care Med. 2006;34(12):A67. Available from: http://journals.lww.com/ccmjournal/Fulltext/2006/12002/Icu_Validation_of_the_Optiscanner,_A_Continuous.236.aspx. [Google Scholar]
- 62.Plank J, Schaller R, Ellmerer M, Koller D, Eberhardt R, Kohler G, Shoemaker M, Obermaier K, Toller W, Pieber T, Schaupp L. Continuous glucose monitoring using the SCGM1 system in postcardiothoracic surgery patients. Crit Care. 2006;10(Suppl 1):P254. Available from: http://ccforum.com/supplements/10/S1. [Google Scholar]
- 63.Heise HM, Kondepati VR, Damm U, Licht M, Feichtner F, Mader JM, Ellmerer M. Microdialysis based monitoring of subcutaneous interstitial and venous blood glucose in type 1 diabetic subjects by mid-infrared spectrometry for intensive insulin therapy. Optical Diagnostics Sensing VIII. 2008 Jan [Google Scholar]
- 64.Heise HM, Damm U, Bodenlenz M, Kondepati VR, Kohler G, Ellmerer M. Bedside monitoring of subcutaneous interstitial glucose in healthy individuals using microdialysis and infrared spectrometry. J Biomed Opt. 2007;12(2):024004. doi: 10.1117/1.2714907. [DOI] [PubMed] [Google Scholar]
- 65.Chuang H, Trieu M, Hurley J, Taylor EJ, England MR, Nasraway , Stanley A., Jr. Pilot studies of transdermal continuous glucose measurement in outpatient diabetic patients and in patients during and after cardiac surgery. J Diabetes Sci Technol 2008. 2008;2(4):595–602. doi: 10.1177/193229680800200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brauker J. Continuous glucose sensing: future technology developments. Diabetes Technol Ther. 2009;11(Suppl 1):S25–S36. doi: 10.1089/dia.2008.0137. [DOI] [PubMed] [Google Scholar]
- 67.Joseph J, Torjman MC. Implantable glucose sensors. Encyclopedia of Biomaterials. Marcel-Dekker Publisher. 2004 [Google Scholar]
- 68.Klonoff D, Bernhardt P, Ginsberg BH, Joseph JI, Mastrototaro JJ, Parker DR, Vesper H, Vigersky R. Performance metrics for continuous interstitial glucose monitoring; approved guideline. CSLI. 2008;28(33) [Google Scholar]
- 69.Rebrin K, Steil GM. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther. 2000;2(3):461–472. doi: 10.1089/15209150050194332. [DOI] [PubMed] [Google Scholar]
- 70.Hipszer B, Joseph JI. Lag associated with interstitial glucose sensors used in a diabetic surgical patient. Anesthesiology. 2006. 2006;105:A584. [Google Scholar]
- 71.Simon B, Treat V, Marco C, Rosenberg D, Joseph J, Hipszer B, Li Y, Chervoneva I, Padron-Massara L, Jabbour S. A comparison of glycaemic variability in CSII vs. MDI treated type 1 diabetic patients using CGMS. Int J Clin Pract. 2008;62(12):1858–1863. doi: 10.1111/j.1742-1241.2008.01932.x. [DOI] [PubMed] [Google Scholar]
- 72.Lodwig V, Heinemann L Glucose Monitoring Study Group. Continuous glucose monitoring with glucose sensors: calibration and assessment criteria. Diabetes Technol Ther. 2003;5(4):572–586. doi: 10.1089/152091503322250596. [DOI] [PubMed] [Google Scholar]
- 73.McGarraugh G, Bergenstal R. Detection of hypoglycemia with continuous interstitial and traditional blood glucose monitoring using the FreeStyle navigator continuous glucose monitoring system. Diabetes Technol Ther. 2009;11(3):145–150. doi: 10.1089/dia.2008.0047. [DOI] [PubMed] [Google Scholar]
- 74.Ward WK, Casey HM, Quinn MJ, Federiuk IF, Wood MD. A fully implantable subcutaneous glucose sensor array: enhanced accuracy from multiple sensing units and a median-based algorithm. Diabetes Technol Ther. 2003;5(6):943–952. doi: 10.1089/152091503322640980. [DOI] [PubMed] [Google Scholar]
- 75.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, Jaksic T, Agus MS. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006;118(3):1176–1184. doi: 10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]
- 76.Logtenberg SJ, Kleefstra N, Snellen FT, Groenier KH, Slingerland RJ, Nierich AP, Bilo HJ. Pre- and postoperative accuracy and safety of a real-time continuous glucose monitoring system in cardiac surgical patients: a randomized pilot study. Diabetes Technol Ther. 2009;11(1):31–37. doi: 10.1089/dia.2008.0028. [DOI] [PubMed] [Google Scholar]
- 77.Bailey PL, McJames SW, Cluff ML, Wells DT, Orr JA, Westenskow DR, Kern SE. Evaluation in volunteers of the VIA V-ABG automated bedside blood gas, chemistry, and hematocrit monitor. J Clin Monit Comput. 1998;14(5):339–346. doi: 10.1023/a:1009991527491. [DOI] [PubMed] [Google Scholar]
- 78.Kunjan K, Lloyd FP., Jr. Automated blood sampling and glucose sensing in critical care settings. J Diabetes Sci Technol. 2008;2(2):194–200. doi: 10.1177/193229680800200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sacks DB, Bernhardt P, Dunka LJ, Goldstein DE, Hortin GL, Mueller P. Point-of-care blood glucose testing in acute and chronic care facilities; approved guideline. NCCLS. 2002;22(17) [Google Scholar]
- 81.Desachy A, Vuagnat AC, Ghazali AD, Baudin OT, Longuet OH, Calvat SN, Gissot V. Accuracy of bedside glucometry in critically ill patients: influence of clinical characteristics and perfusion index. Mayo Clin Proc. 2008;83(4):400–405. doi: 10.4065/83.4.400. [DOI] [PubMed] [Google Scholar]
- 82.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 83.Fahy BG, Coursin DB. Critical glucose control: the devil is in the details. Mayo Clin Proc. 2008;83(4):394–397. doi: 10.4065/83.4.394. [DOI] [PubMed] [Google Scholar]
- 84.Finkielman JD, Oyen LJ, Afessa B. Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest. 2005;127(5):1749–1751. doi: 10.1378/chest.127.5.1749. [DOI] [PubMed] [Google Scholar]
- 85.Soo Hoo GW. Tight blood glucose control in the ICU: how best to measure glucose control? Chest. 2008;133(1):316–317. doi: 10.1378/chest.07-2214. author reply 317. [DOI] [PubMed] [Google Scholar]
- 86.Nichols JH. What is accuracy and how close must the agreement be? Diabetes Technol Ther. 2005;7(3):558–562. doi: 10.1089/dia.2005.7.558. [DOI] [PubMed] [Google Scholar]
- 87.Sylvain HF, Pokorny ME, English SM, Benson NH, Whitley TW, Ferenczy CJ, Harrison JG. Accuracy of fingerstick glucose values in shock patients. Am J Crit Care. 1995;4(1):44–48. [PubMed] [Google Scholar]
- 88.Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(2):403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 89.Torjman MC, Jahn L, Joseph JI, Crothall K. Accuracy of the hemocue portable glucose analyzer in a large nonhomogeneous population. Diabetes Technol Ther. 2001;3(4):591–600. doi: 10.1089/15209150152811216. [DOI] [PubMed] [Google Scholar]
- 90.Slater-MacLean L, Cembrowski G, Chin D, Shalapay C, Binette T, Hegadoren K, Newburn-Cook C. Accuracy of glycemic measure-ments in the critically ill. Diabetes Technol Ther. 2008;10(3):169–177. doi: 10.1089/dia.2008.0263. [DOI] [PubMed] [Google Scholar]
- 91.Karon BS, Griesmann L, Scott R, Bryant SC, Dubois JA, Shirey TL, Presti S, Santrach PJ. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther. 2008;10(2):111–120. doi: 10.1089/dia.2007.0257. [DOI] [PubMed] [Google Scholar]
- 92.Karon BS, Gandhi GY, Nuttall GA, Bryant SC, Schaff HV, McMahon MM, Santrach PJ. Accuracy of roche accu-chek inform whole blood capillary, arterial, and venous glucose values in patients receiving intensive intravenous insulin therapy after cardiac surgery. Am J Clin Pathol. 2007;127(6):919–926. doi: 10.1309/6RFQCKAAJGKWB8M4. [DOI] [PubMed] [Google Scholar]
- 93.Boyd JC, Bruns DE. Quality specifications for glucose meters: assessment by simulation modeling of errors in insulin dose. Clin Chem. 2001;47(2):209–214. [PubMed] [Google Scholar]
- 94.Mahoney JJ, Ellison JM. Assessing glucose monitor performance–a standardized approach. Diabetes Technol Ther. 2007;9(6):545–552. doi: 10.1089/dia.2007.0245. [DOI] [PubMed] [Google Scholar]
- 95.Nayak PP, Morris K, Lang H, Laker S, Stickley J, Davies P, Barrett T, Gao F, Gough S, Narendran P. Lack of agreement between arterial and central venous blood glucose measurement in critically ill children. Intensive Care Med. 2009;35(4):762–763. doi: 10.1007/s00134-008-1282-6. [DOI] [PubMed] [Google Scholar]
- 96.Flood J, Joseph J, Kim L, McNulty S, Chervoneva I. Glucose levels in blood simultaneously sampled from the radial artery, vena cava, and fingertip. Anesthesiology. 2007;107:A1123. [Google Scholar]
- 97.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 98.Mraovic B. Analysis: continuous glucose monitoring during intensive insulin therapy. J Diabetes Sci Technol. 2009;3(4):960–963. doi: 10.1177/193229680900300444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, Lee SL, Dziura JD, Inzucchi SE. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care. 2004;27(2):461–467. doi: 10.2337/diacare.27.2.461. [DOI] [PubMed] [Google Scholar]
- 100.Ryan M, Saverese V, Hipszer B, Dizdarevic I, Joseph M, Shively N, Joseph JI. Continuous glucose monitor (CGM) shows potential for early hypoglycemia detection in hospitalized patients. Diabetes Technol Ther. 2009. In press. [DOI] [PubMed]
- 101.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyper-glycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 102.Mizock BA. Alterations in carbohydrate metabolism during stress: a review of the literature. Am J Med. 1995;98(1):75–84. doi: 10.1016/S0002-9343(99)80083-7. [DOI] [PubMed] [Google Scholar]
- 103.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 104.Yu WK, Li WQ, Li N, Li JS. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol. 2003;9(8):1824–1827. doi: 10.3748/wjg.v9.i8.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turina M, Fry DE, Polk HC., Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33(7):1624–1633. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 106.Lukins MB, Manninen PH. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth Analg. 2005;100(4):1129–1133. doi: 10.1213/01.ANE.0000146943.45445.55. [DOI] [PubMed] [Google Scholar]
- 107.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290(15):2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 108.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 109.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–360. doi: 10.1016/s0003-4975(99)00014-4. discussion 360-2. [DOI] [PubMed] [Google Scholar]
- 110.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O'Brien PC, Johnson MG, Williams AR, Cutshall SM, Mundy LM, Rizza RA, McMahon MM. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146(4):233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 111.Preiser JC. Restoring normoglycaemia: not so harmless. Crit Care. 2008;12(1):116. doi: 10.1186/cc6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meijering S, Corstjens AM, Tulleken JE, Meertens JH, Zijlstra JG, Ligtenberg JJ. Towards a feasible algorithm for tight glycaemic control in critically ill patients: a systematic review of the literature. Crit Care. 2006;10(1):R19. doi: 10.1186/cc3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 114.Plank LD, Hill GL. Energy balance in critical illness. Proc Nutr Soc. 2003;62(2):545–552. doi: 10.1079/pns2003259. [DOI] [PubMed] [Google Scholar]
- 115.De Beaux, Chapman M, Fraser R, Finnis M, De Keulenaer B, Liberalli D, Satanek M. Enteral nutrition in the critically ill: a prospective survey in an Australian intensive care unit. Anaesth Intensive Care. 2001;29(6):619–622. doi: 10.1177/0310057X0102900611. [DOI] [PubMed] [Google Scholar]
- 116.Heyland DK, MacDonald S, Keefe L, Drover JW. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA. 1998;280(23):2013–2019. doi: 10.1001/jama.280.23.2013. [DOI] [PubMed] [Google Scholar]
- 117.McMahon MM, Miles JM. Glycemic control and nutrition in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2006;9(2):120–123. doi: 10.1097/01.mco.0000214570.90309.27. [DOI] [PubMed] [Google Scholar]
- 118.Hipszer B, Joseph J, Kam M. Pharmacokinetics of intravenous insulin delivery in humans with type 1 diabetes. Diabetes Technol Ther. 2005;7(1):83–93. doi: 10.1089/dia.2005.7.83. [DOI] [PubMed] [Google Scholar]
- 119.Watson BG, Elliott MJ, Pay DA, Williamson M. Diabetes mellitus and open heart surgery. A simple, practical closed-loop insulin infusion system for blood glucose control. Anaesthesia. 1986;41(3):250–257. doi: 10.1111/j.1365-2044.1986.tb12783.x. [DOI] [PubMed] [Google Scholar]
- 120.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 121.Renard E, Costalat G, Chevassus H, Bringer J. Closed loop insulin delivery using implanted insulin pumps and sensors in type 1 diabetic patients. Diabetes Res Clin Pract. 2006;12(Suppl 2)(74):S173–S177. [Google Scholar]