Abstract
Glycemic control with intensive insulin therapy (IIT) has received widespread adoption secondary to findings of improved clinical outcomes and survival in the burn population. Severe burn as a model for trauma is characterized by a hypermetabolic state, hyperglycemia, and insulin resistance. In this article, we review the findings of a burn center research facility in terms of understanding glucose management. The conferred benefits from IIT, our findings of poor outcomes associated with glycemic variability, advantages from preserved diurnal variation of glucose and insulin, and impacts of glucometer error and hematocrit correction factor are discussed. We conclude with direction for further study and the need for a reliable continuous glucose monitoring system. Such efforts will further the endeavor for achieving adequate glycemic control in order to assess the efficacy of target ranges and use of IIT.
Keywords: artificial pancreas, burn, computer decision support, continuous glucose monitor, diurnal variation, glucometer, glucose variability, hematocrit effect, hypoglycemia, intensive insulin therapy
Introduction
Widespread adoption of intensive insulin therapy (IIT) resulted from promising reports1–8 that tight glycemic control improves outcomes in critically ill patients. Despite findings9,10 that IIT in tight normal range (80 to 110 mg/dl) confers no benefit to most intensive care unit (ICU) patients and, in fact, increases risk of hypoglycemic events,2,10–14 the practice of moderate glycemic control (140 to 180 mg/dl) continues to be advocated.15,16 Unfortunately, consensus on population-specific target ranges is lacking.
Clinical practices guiding the treatment of the trauma population may be the cornerstone for management of the critically ill, but patients with major burns epitomize the hyperdynamic physiologic stress response.17,18 Burn trauma differs in severity and duration as compared to that of other critically ill patients.18 The exaggerated stress response following burn injury is characterized by alterations in endocrinologic and immunologic function,17,18 glucose intolerance or insulin resistance,19,20 negative nitrogen balance, catabolism, and an overall hyper-metabolic state.21,22 These patients have reliably long lengths of hospital stay, more complications, frequent septic events, and increased mortality compared to general ICU patients.
Although debate continues about the ideal glucose target range for optimal benefit, the current burn community practice is to achieve relatively tight control, with 73% of verified American Burn Association (ABA) centers reporting target glucose of less than 120 mg/dl in a survey.23 Furthermore, studies support glycemic control for improved skin graft survival,24 reduction of infection,25 and improved survival26 in the severely burned patient. In the large international study Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR, trauma patients did in fact demonstrate the most benefit from IIT from the observed trend toward improved survival (p = .07).9 Undoubtedly, injured patients differ from other critically ill patients. Thermal injuries represent an extreme model of trauma with prolonged recovery time, supporting the notion that IIT confers benefit to the burn population as well. Therefore, it is difficult to ascertain the effect of the NICE-SUGAR study on practice within the burn community. Certainly, prospective multicenter trials are critical to determining the ideal target glucose ranges to optimize burn management.
The purpose of this review is to describe the recent advances in our understanding of glucose management for the burned patient, specifically examining the effects of glycemic variability on outcome, diurnal rhythms of insulin and glucose, point-of-care (POC) glucometer error, and development of an artificial pancreas to optimize glucose control in the critically ill.
Intensive Insulin Therapy for the Burned Patient
Unique Aspects of Burn Management
Severe burn injury [greater than 40% of the total body surface area (TBSA)] is a devastating form of trauma that ultimately affects all metabolic processes. Underlying metabolism is accelerated, basal temperature is elevated, tachycardia persists, and stress hormones are released.27,28 Nutritional demands are dramatically increased, up to twice the normal requirements of traditional ICU patients.27 Because burn patients develop hepatic dysfunction and fail to appropriately metabolize lipids,27 enteral feeding formulas at our center are low in lipids (16%) and high in carbohydrates (63%) and proteins (21%).
Recent advances in the surgical management of burn wounds advocate total excision of all nonviable tissue and covering with autogolous, cadaver, or temporary biologic grafts.29 New wounds are created during harvest for donor grafts, further increasing nutritional requirements for wound healing. Serial surgical procedures are required to continue the process of wound closure, repeatedly inciting the stress response and prolonging the hypermetabolic state. Enteral feeds are frequently discontinued for surgery, delayed gastric emptying, vasoactive agent-dependent septic shock, and daily showers required for wound care. Hyperglycemia is common because of the stress of frequent interventions, sepsis, and high-volume carbohydrate feeds. Insulin resistance, elevated production of counter-regulatory hormones, and administration of exogenous corticosteroids for adrenal insufficiency further exacerbate glycemic imbalance. Importantly, there is a greater risk of clinically significant hypoglycemic events (<40 mg/dl) for burn patients. Significant loss of muscle mass due to catabolism, hepatic dysfunction, endocrine and hormonal derangement, frequent interruption of enteral feeds, and significant procedural metabolic stress amplify the frequency of hypoglycemic episodes. Combined, these factors contribute to the difficulty of maintaining euglycemia in the critically ill burn patient.
Hyperglycemia Is Associated with Poor Outcomes
Great debate surrounds current IIT practices, with disparate outcomes reported for various critical care populations. Surgical patients appear to benefit from tight control,4,8 yet medical ICU patients do not respond as favorably unless ICU stay is greater than 3 days.7 Meta-analysis of published reports reveals no improvement in overall outcomes for IIT practices,10 although some benefit may occur for surgical patients.2,30 However, trauma and burn patients have been shown to have high morbidity and mortality associated with hyperglycemia, supporting the practice of tight glycemic control for these patients.1,6,31,32 The NICE-SUGAR9 study reported a significant increase in 90-day mortality for ICU patients receiving IIT targeting glucose levels of 81–108 mg/dl compared to a conservative target of less than 180 mg/dl. Conversely, the only subgroups in this study that apparently benefited from IIT were the trauma population and patients requiring exogenous corticosteroid administration. As previously noted, severe burn is considered a representative model of injury, and thus the findings of the NICE-SUGAR study9 support IIT practice for burn and trauma populations. The benefit of glycemic control with exogenous insulin infusion for the burn patient may be linked to the reduction of infectious complications25,26,33 and multiorgan failure.34 Insulin therapy is associated with the attenuation of the hypermetabolic state,35,36 improvement in wound healing,37 and preservation of muscle mass,38,39 factors essential to the survival of patients with extensive burn injuries. In our burn center, we reviewed the chart of all adult patients with >20% TBSA burns treated with insulin infusions between 2002 and 2004. During the first 7 days of admission, patients who manifested better glucose control (n = 47, mean glucose 133 mg/dl) had a 45% reduction in mortality compared to patients in whom tight control was not obtained (n = 41, mean glucose 174 mg/dl). These findings have strengthened the resolve within our burn center to continue tight glycemic control for our ICU patients9 as we conduct further analysis to determine the ideal target range to maximize outcomes while minimizing exposure to hypoglycemic events.
Glycemic Variability Predicts Poor Outcomes
Not only has hyperglycemia been shown to correlate with increased mortality in critically ill patients, but variability in glucose has also been associated with poor outcomes. Several clinical studies have demonstrated an association of higher fluctuation in glucose levels and increased mortality in ICU patients.41–43 Within our burn center, we noted a similar pattern in adult burn patients with a TBSA greater than 20%. A review was conducted for patients admitted who had at least 100 recorded blood glucose (BG) measurements and had been treated with IIT.44 The purpose of this analysis was to describe the effect of glycemic variability on mortality, defined as being greater than 50% time out of the 80 to 110 mg/dl target range. The average of individual glucose measurements out of range was 50% ± 8% (range of 30% to 65%) for an average of 840 (range of 103 to 5314) values per patient. The percentage excursion for a high variability score was 56% (n = 26) compared to 46% for those with low variability (n = 23; p < .001). There was no difference in injury severity score, age, TBSA injured, or gender between groups. Despite similar days of ventilator support and hospital and ICU length of stay, the more variable group was noted to have over twice the mortality of the less variable group (50% versus 22%; p ≤ .05). The question remains whether glucose variability represents severity of illness or whether failure to control glucose results in a poor outcome in the burned ICU patient. We are currently evaluating a large database composed of multiple centers to begin to address this conundrum.
Diurnal Variation of Insulin and Glucose Levels Is Preserved
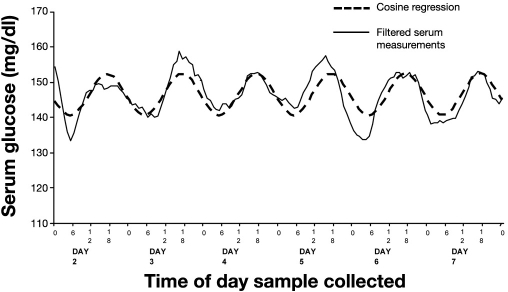
Many circadian patterns present in healthy individuals are lost during critical illness, such as those of cortisol and leptin;45 however, patterns of glucose and insulin have been noted to persist in general ICU patients.46 Research at our burn center was conducted to establish the presence or absence of diurnal patterns of glucose and insulin in the burn-injured patient. To this end, a review was conducted of patients receiving at least 7 days of IIT.47 Blood glucose values and insulin requirements were time-matched between patients hourly from time of admission. A frequency analysis of glucose values revealed a strong 24 h pattern present on day 2, with peaks in glucose occurring at 5 p.m. and troughs at 5 a.m. A paired t test between our data and a cosine wave reflecting 24 h periodicity revealed a correlation of 0.82 (Figure 1). However, when the correlation of survivors was compared to that of nonsurvivors, the cosine equation deteriorated, and the survivors demonstrated a better fit (r2 = 0.82) than patients who died (r2 = 0.50). Furthermore, the cosine amplitude of the nonsurvivor glucose curve was significantly less than that of the survivor curve (8.4 versus 16.5 mg/dl, p = .01), representing blunted variation in the diurnal pattern. In addition, patterns of exogenous insulin requirements revealed a peak at noon and a trough occurring at midnight, an offset of 5 from glucose patterns, reflecting the complex interplay of glucose metabolism and insulin sensitivity in the critically ill burned patient. Coupled with the diurnal pattern of insulin requirements in the burn ICU patient is the overall increase in insulin requirement during the first week of ICU stay despite constant glucose levels.47 A regression analysis of exogenous insulin requirements over time reveals a linear increase during the first 7 days of hospitalization (slope = 0.13, r2 = 0.57, p < .001). Such increases in insulin to maintain euglycemia represent a trend for increasing insulin resistance over time. This pattern challenges our presumption that routine ICU practices such as continuous feeding regimens and fixed glycemic target ranges are appropriate despite their failure to match underlying physiologic processes noted in the circadian rhythms present in the critically ill.
Figure 1.
Circadian rhythm of serum glucose levels during first week of ICU stay for burn patients. Reprinted from The American Journal of Surgery with permission from Elsevier.47
Bedside Glucose Measurement Error
Concurrent with the extensive adoption of IIT following the compelling results of Van den Berghe and associates published in 2001,8 further practice changes occurred within the critical care community. In 1999, Hébert and colleagues48 demonstrated improved outcomes in certain patients managed with a restrictive blood transfusion strategy, targeting hemoglobin of 7 mg/dl compared to a traditional value of 10 mg/dl. These positive findings were confirmed for the burn population by Kwan and coworkers,49 and the recent ABA survey showed that 51% of verified centers transfuse patients to hemoglobin levels of 7 mg/dl.23 Thus adoption of restrictive transfusion within the burn community is common, coupled with prevalent use of IIT to maintain tight glycemic control.
Independently, these events may lack profound clinical impact, but when combined, a storm is created when POC glucometer technology is used. These useful bedside devices certainly expedite the frequent glucose quantification required for safe implementation of IIT. However, POC technology was designed and approved for diabetes patients at home, targeting glucose levels under 200 mg/dl, and were never intended for use in the critical care environment where high precision and accuracy are required to avoid complications. Clinical practice changes advocating IIT at the same time that restrictive transfusion practices were adopted exceeded the capability of current POC technology. The margin of error noted in POC glucometer package inserts of 20%50 exceeds the recommendation of the Food and Drug Administration to achieve no more than 15% error,51 posing serious clinical risk when narrow target ranges of 80 to 110 mg/dl are routinely used.23 Such inaccuracy fails to meet the call by the American Diabetes Association (ADA) to limit POC error to less than 5%,52 thus conferring significant hypoglycemic risk for patients undergoing IIT.
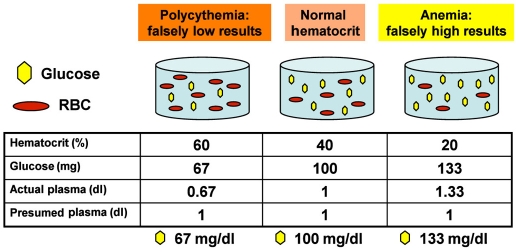
The problem with current single-channel POC glucometer technology resides with the use of whole-blood samples for glucose quantification, as these devices are programmed to assume a normal hematocrit (HCT) of 40% (Figure 2). Thus the internal calculation of BG assumes a constant displacement of plasma by red blood cells (RBCs) in the sample; however, an anemic sample contains fewer RBCs, so less displacement occurs and ratio concentration is erroneous. The denominator for HCT is fixed, resulting in systematic glucose overestimation for anemic samples and underestimation for polycythemic samples.53–55 When relatively normal hemoglobin targets (10 mg/dl) guide blood transfusion, a patient will likely have an HCT closer to normal (30%), and error may be attenuated. However, since permissive anemia strategies target an HCT closer to 20% (7 mg/dl), half of normal levels, significant overestimation of true glucose values results because of the increased amount of glucose available to the sensor; the bias is therefore toward undetected hypoglycemia.
Figure 2.
Effect of HCT on glucometer performance. Polycythemic samples result in underestimation of serum glucose by single-channel glucometers; anemic samples cause glucose overestimation. The original blood volume was 1.67 dl.
This problem of POC error is pervasive. According to the ABA survey of burn centers, 95% of verified centers routinely implement single-channel glucometers for POC glucose quantification to guide IIT therapy.23 The individual practice changes of tight glycemic control and permissive anemia combined with POC use have significantly increased the risk for occult hypoglycemia within the burn community.56 Although the HCT effect on glucometer performance is well described,53,55,57–59 no practical solutions have been proposed for popular single-channel devices.
Glucometer Error Can Be Corrected
Recognition of the effect of low HCT on POC glucometers within our burn center was the result of careful analysis. We observed a systematic overestimation of POC glucose values compared to laboratory values during a clinical study of high-dose insulin therapy. Potential factors affecting glucometer performance, including heat, humidity, age of test strips, chemical substances, altitude, condition of sample, condition of glucometer, and operator experience, were evaluated and eliminated. Additionally, use of capillary blood has been associated with glucometer inaccuracy.60–63 Severely burned patients tend to have injuries to the upper extremities, making capillary finger sticks impractical and peripheral intravenous access problematic. Frequent bouts of septic shock, requirements for vasoactive agents, and persistent generalized edema further complicate the accuracy of capillary sampling. Thus only arterial or venous samples are used for glucose quantification in our burn ICU.
Next, we assessed the type of laboratory specimen tube as a potential source of the systematic error. We routinely utilized additive-free serum separator BD Vacutainer® tubes (BD, Franklin Lakes, NJ) for chemistry analysis, including glucose quantification. Because erythrocytes continue to consume glucose ex vivo, the serum separator evacuation tube is not ideal for preserving glucose because of the lack of chemical additives to halt component depletion. However, a gray-top Vacutainer tube contains sodium fluoride, which is specifically designed to preserve glucose-containing specimens. We processed matched whole-blood samples to determine whether the type of evacuation tube could account for the discrepancy in the glucose measurements. Although moderate degradation of glucose occurred in the additive-free specimens, the difference was slight in comparison to that observed.56 Discovery of this effect has prompted a change in our standard of care for laboratory glucose quantification; currently, the sodium fluoride additive evacuator tube is used for all glucose samples and serves as the reference standard.
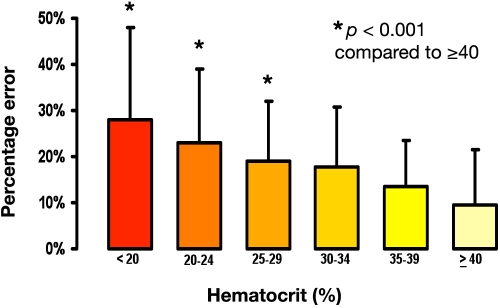
Finally, we evaluated the patient's HCT level relative to the degree of error (Figure 3) and found a linear correlation between the degree of anemia and the percentage error in the POC measurements. Thus HCT was deemed the most significant source of glucometer error in our burn ICU population. We performed a regression analysis of prospectively collected samples from hemodynamically stable subjects in our institution's burn, trauma, surgical, and medical ICUs. We compared glucose quantification by the SureStep™ Flexx (LifeScan, Milpitas, CA) single-channel POC glucometer with specimens collected in sodium fluoride evacuation tubes processed by the laboratory serum analyzer (Vitros Fusion, Ortho Clinical Diagnostics, Rochester, NY). The result was an error rate similar to that previously noted, 19% ± 7% for an average HCT of 25%. Use of a derived mathematical correction formula approximated the serum glucose value, reducing the mean error to -0.02% ± 4.78% (r2 = 0.97). The correction formula was as follows:
where LN is the natural log and HCT is the most recent for a hemodynamically stable patient.64 Validation of this formula and evaluation of three widely used POC glucometers used by ABA-verified burn centers were conducted on additional matched whole-blood specimens. Error after mathematical correction on the new samples using the SureStep Flexx resulted in a nonsignificant difference in POC measures from laboratory analysis. Using the same methodology for the original mathematical model, we performed a regression analysis for the other single-channel glucometers on prospectively collected and matched samples to develop device-specific correction formulas. Clinically acceptable correction to fulfill the requirement for IIT accuracy in the ICU was achieved for all devices tested (Table 1).64 Furthermore, use of the mathematical formulas achieved correlation with the reference standard that meets the ADA-recommended 5% error rate.52
Figure 3.
Glucometer error increases in a linearly as HCT decreases.
Table 1.
Comparison of Error Found with Uncorrected Single-Channel Glucometers and after Mathematical Correction for Level of Hematocrit
| Glucometer | Mean uncorrected error (SDa %) | Uncorrected versus reference mean (p value) | Mean corrected error (SD%) | Corrected versus reference mean (p value) |
|---|---|---|---|---|
| SureStep Flexx™ n = 196 | 16.0% (7.5)a | <0.0001 | −0.01%b (4.8) | NSa |
| Accu-Chek Inform™ n = 187 | 16.0% (6.7) | <0.0001 | −0.54%b (5.6) | NS |
| Accu-Chek Advantage™ n = 187 | 16.9% (6.7) | <0.0001 | −0.6%b (5.5) | NS |
| Precision PCx™ n = 108 | 18.7% (10.1) | <0.0001 | −0.2%b (8.0) | NS |
SD, standard deviation; NS, not significant.
p < 0.0001, uncorrected versus corrected % mean error.
The question of when to apply the correction formulas was answered with a large retrospective review of 12,800 glucose and HCT-matched measurements to determine the point at which a clinically significant error occurred (Figure 3; U.S. Army Institute of Surgical Research unpublished data). The critical level at which an error greater than 5% occurred was 34% HCT. Researchers described significant anemia in general ICU patients with hemoglobin levels of less than 10 mg/dl within 3 days of ICU admission.65,66 Because of the risk of sepsis associated with blood transfusion,67,68 burn providers now maintain patients' hemoglobin levels below 10mg/dl.23,69 Maintaining hemoglobin of 7 mg/dl proposed by Hébert and colleagues48 targets a HCT of approximately 21%. Thus the problem of anemia is prevalent, and associated glucometer error due to HCT can affect all critically ill populations.
Fortunately, technological advances have now eliminated the problem of HCT effect.70 Evaluation of a new four-channel glucometer (StatStrip™, Nova Biomedical, Waltham, MA) conducted within our center has demonstrated reliable accuracy of this technology for general ICU patients with significant anemia.71 Prospectively collected whole-blood samples were tested on the single-channel SureStep Flexx, the four-channel StatStrip, and the central chemistry analyzer (Vitros Fusion), and tests for equivalence were performed. The average HCT for samples from the burn, trauma, surgical, and medical ICUs was 26.6% ± 5.2% (range of 18.5% to 43.1%). With a zone of indifference set for ±5%, the difference between the four-channel glucometer and the reference standard (laboratory analyzer) was -0.67% (95% confidence interval: -1.79% to 0.45%). There was no difference between the mathematically corrected single-channel and four-channel POC meters compared with the reference value (p = .61 and .65, respectively); however, the uncorrected single-channel glucometer was significantly different from the reference value (p = .006). Thus we concluded that HCT is the most significant factor associated with glucometer error, because our mathematical formula only incorporates HCT in correction, and the four-channel device corrects for numerous potentially interfering substances.71
Correction of Hematocrit Effect Reduces Hypoglycemia
The clinical importance of the effect of HCT on glucometer performance was revealed when we analyzed the rates of hypoglycemia before and after our burn unit instituted routine corrections for the effect of HCT. The hypo-glycemic rates were compared for all patients admitted to the burn center for the 6 months prior to implementation of correction and the 6 months after the change. We found a significant reduction in glucose values of less than 60 mg/dl and less than 80 mg/dl after adjustment. In addition, correction improved our time in the moderate glycemic range of 80 to 150 g/dl, but curiously, time in the tight glycemic range of 80 to 110 mg/dl was reduced (p = .002). When a comparison was made between the burn ICU where correction was implemented and the surgical ICU where use of uncorrected glucometer measurements continued, a significant reduction in hypo-glycemic events of less than 80 mg/dl was noted only in the burn unit (p < .001). This reduction is attributed to reduced insulin dosing for normal glucose values that were previously artificially inflated by the systematic glucometer error.
Correction factors for single-channel glucometers are device specific; we developed mathematical formulas for the four devices widely used at the time of our study by using blood from patients in the surgical, medical, and burn ICUs.23 Although the development of these formulas was based on serum blood samples, the results are applicable to capillary sampling when used with caution, given the inherent shortcomings of samples subject to poor perfusion.62,63 Mathematical correction64 always shifts the BG value lower than that calculated by the POC device, reducing potential insulin doses and thus erring on the side of safer dosing. Newer devices can be tested in anemic critically ill patients in the same manner64 for development of device-specific correction formulas when identified error exceeds ADA recommendations.72 Use of capillary samples is routine in general ICUs, and care must be taken to recognize patient-specific characteristics, such as hypoperfusion, edema, and use of vasoactive agents associated with systematic error.62 Aside from sampling source, no other uniform differences73 exist between burn and other ICU patients; thus mathematical correction of single-channel devices may be appropriate for all anemic patients. However, the highly accurate Nova StatStrip four-channel glucometer ushers in a new age of technology, setting new performance standards for POC devices.
Glycemic Control Challenges
Current methods to maintain euglycemia in the critically ill are immature at best. Future success for optimal glycemic control rests in our ability to replicate the capability of the human pancreas.74,75 Endocrine regulation in the body is dependent on varied responses to multiple sources of stimuli; a mechanical correlate is a nonlinear feedback control mechanism. Development of a responsive system to regulate infusion of exogenous insulin to control serum glucose levels has become an achievable goal with technological advances. Several components are required to create a virtual “closed-loop” system: a continuous insulin infusion pump, an intravascular continuous glucose monitoring (CGM) system, and a computer decision support software (CDSS) controller.76 Infusion pump technology is advanced, and such devices can network with software applications. Development of CGM technology74,77 is underway to provide real-time near-continuous BG quantification, minimizing a concomitant increase in nursing workload requirements.78 Several CDSS applications for insulin management are currently commercially available,79–82 demonstrating reduced glycemic excursion and significantly fewer hypoglycemic events.
Currently available CGM monitoring systems are sub-cutaneous sensors designed for use by diabetes outpatients. Goldberg and associates77 tested a commercially available device, Medtronic MiniMed™ (Northridge, CA), in a medical ICU. After a 72 h period, CGM glucose values were compared retrospectively to values obtained from a glucometer using capillary blood. Accuracy was similar to that of published outpatient studies, unaffected by edema, hypotension, or vasopressor therapy. While systematic differences were not found in the study, the accuracy of the CGM increased with elevated BG but was limited at hypoglycemic levels. Although the device readings are comparable to capillary BG values, it may fail to meet performance expectations for real-time use in an ICU setting using serum samples.
Critically ill patients, burn patients in particular, tend to be quite edematous with substantial soft tissue involvement and frequently require vasopressor support for low mean arterial pressure, compromising glucose equilibration between the intravascular and the interstitial compartments. Persistent edema present in the burned patient or physiologic delay of glucose equilibration between compartments due to low perfusion states will interfere with the ability to treat using real-time subcutaneous CGM sensors.
Further work has been done at our burn center to evaluate the ability of a CDSS system to successfully and safely guide IIT in the critically injured burn patient. A CDSS system was compared in a randomized crossover design to our standard of care paper-based insulin titration protocol. EndoTool™ (Hospira, Lake Forest, IL), a commercially available computer software package, was selected for the analysis. This program provides individualized hourly insulin infusion recommendations based on multiple control mathematics algorithms by means of the patient's glucose trend. Available for use at the bedside, this program allows the nurse to enter the hourly glucose value with any supplemental caloric intake into the system, and within seconds, the recommended insulin infusion rate is displayed with the suggested time for subsequent glucose quantification. Clinical judgment is paramount when managing IIT for critically ill patients, and nurses are at liberty to override computer recommendations based on the clinical scenario. However, we found that nurses accepted the computer recommendations more often than they followed the traditional paper protocol. Preliminary data demonstrate that computerized decision support provides better glycemic control in the 80 to 110 mg/dl target range without increased hypoglycemic events than our conventional nurse-managed paper protocol. As a result, the EndoTool computer software system has become the standard of care for glucose control in our burn ICU.
As an added benefit, computerized controllers have the potential to increase uniformity among clinical trials,83 improving consistency among individual providers. However, despite the enhancement in achieving the glycemic targets that CDSS confers over traditional paper protocols, routinely achieving the desired glucose range greater than 50% of the time remains problematic.83–86 Further improvements in CDSS technology are required for successful evaluation of the benefit of IIT and determination of ideal target ranges for unique critically ill populations.9
Future Study
Additional investigation is underway to elucidate the pattern of insulin requirements and glucose levels in burn patients over the entire course of ICU management to determine differences between survivors and nonsurvivors. Additionally, no data exist comparing burned patients with and burned patients without diabetes or the effects of traumatic brain injury with regard to preservation of diurnal variability in glucose and insulin resistance patterns. Should differences be found related to diabetes status or concomitant traumatic brain injury, alteration of glycemic goals may be required to optimize care regimens.
Additional investigation is necessary to fully understand implications for the underlying diurnal variation in insulin resistance and glucose variability as related to feeding regimens and glucose targets in the ICU. Physiologic feeding such as bolus enteral feeds with nocturnal rest may more closely resemble natural rhythms and interrupt the trend for progressive insulin resistance over time associated with continuous enteral feeds. Furthermore, targeting more conservative glycemic goals during sleep, mimicking the natural diurnal pattern of glucose levels, may provide rest to cellular insulin receptors, again reducing the tendency of a progressively greater insulin requirement to maintain euglycemia during the ICU course. Implementation of computer decision support technology may facilitate such patient-specific therapies possessing the capability to target individual goals for glycemic control and cycle various glycemic ranges during a 24 h period. A reliable CGM system remains the barrier to emulating the basic function of the human pancreas. To that end, studies are underway to evaluate currently available Food and Drug Administration-cleared systems. Once a suitable device is validated, a large multicenter study is required to assess the effectiveness of the concept of the artificial pancreas in an open-loop model to initiate evaluation of IIT efficacy. However, understanding and ultimately manipulating the complex interplay of insulin sensitivity at the cellular level with dynamic hormonal fluctuation in the critically ill patient provides further challenges to optimizing glycemic control.
Conclusion
Although extensive research has focused on understanding the complex interplay of endocrine processes after burn injury to devise the optimum strategy for glucose regulation, many challenges and questions remain. Discovery of the effect of HCT on the accuracy and safety of widely used single-channel glucometers and, more importantly, development of an interim solution pending future technological advances in POC technology have the potential to improve the safety and clinical utility of these devices. Challenges related to sample source bias based on physiologic compromise must be addressed in future continuous monitoring technology, as use of capillary blood for POC glucose quantification for the patient in shock is unsuitable. Recognition of circadian rhythms in insulin and glucose regulation in the burn patient may alter traditional feeding practices and insulin targets. Finally, development of an artificial pancreas will benefit all critically ill patients with provision of optimal glycemic management. Until improvements are made to consistently reach and maintain patients within a target glucose range, the efficacy of IIT cannot be established.
Abbreviations
- ABA
American Burn Association
- ADA
American Diabetes Association
- BG
blood glucose
- CDSS
computer decision support software
- CGM
continuous glucose monitoring
- HCT
hematocrit
- ICU
intensive care unit
- IIT
intensive insulin therapy
- POC
point of care
- RBC
red blood cell
- TBSA
total body surface area
References
- 1.Gale SC, Sicoutris C, Reilly PM, Schwab CW, Gracias VH. Poor glycemic control is associated with increased mortality in critically ill trauma patients. Am Surg. 2007;73(5):454–460. doi: 10.1177/000313480707300507. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi GY, Murad MH, Flynn DN, Erwin PJ, Cavalcante AB, Bay Nielsen H, Capes SE, Thorlund K, Montori VM, Devereaux PJ. Effect of perioperative insulin infusion on surgical morbidity and mortality: systematic review and meta-analysis of randomized trials. Mayo Clin Proc. 2008;83(4):418–430. doi: 10.4065/83.4.418. [DOI] [PubMed] [Google Scholar]
- 3.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 4.Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004;164(19):2005–2011. doi: 10.1001/archinte.164.18.2005. [DOI] [PubMed] [Google Scholar]
- 5.Reed CC, Stewart RM, Sherman M, Myers JG, Corneille MG, Larson N, Gerhardt S, Beadle R, Gamboa C, Dent D, Cohn SM, Pruitt BA., Jr. Intensive insulin protocol improves glucose control and is associated with a reduction in intensive care unit mortality. J Am Coll Surg. 2007;204(5):1048–1055. doi: 10.1016/j.jamcollsurg.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Scalea TM, Bochicchio GV, Bichicchio KM, Johnson SB, Joshi M, Pyle A. Tight glycemic control in critically injured trauma patients. Ann Surg. 2007;246(4):605–612. doi: 10.1097/SLA.0b013e318155a789. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 9.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 10.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 11.Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, Kahoul SH, Britts RJ, Sakkijha MH. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36(12):3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 12.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 13.De La Rosa Gdel C, Donado JH, Restrepo AH, Quintero AM, González LG, Saldarriaga NE, Bedoya M, Toro JM, Velásquez JB, Valencia JC, Arango CM, Aleman PH, Vasquez EM, Chavarriaga JC, Yepes A, Pulido W, Cadavid CA. Grupo de Investigacion en Cuidado intensivo: GICI-HPTU. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intesive care unit: a randomised clinical trial. Crit Care. 2008;12(5):R120. doi: 10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P, Li W, Bates S. Intensive insulin therapy in postoperative intensive care unit patients: a decision analysis. Am J Respir Crit Care Med. 2006;173(4):407–413. doi: 10.1164/rccm.200506-961OC. [DOI] [PubMed] [Google Scholar]
- 15.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists, American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surviving Sepsis Campaign. Statement on glucose control in severe sepsis. 2009. http://www.survivingsepsis.org/About_the_Campaign/Documents/SSC. Accessed October 27.
- 17.Atiyeh BS, Gunn SW, Dibo SA. Metabolic implications of severe burn injuries and their management: a systematic review of the literature. World J Surg. 2008;32(8):1857–1869. doi: 10.1007/s00268-008-9587-8. [DOI] [PubMed] [Google Scholar]
- 18.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe RR, Durkot MJ, Allsop JR, Burke JF. Glucose metabolism in severely burned patients. Metabolism. 1979;28(10):1031–1039. doi: 10.1016/0026-0495(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 20.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94(5):1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 22.Wanek S, Wolf SE. Metabolic response to injury and role of anabolic hormones. Curr Opin Clin Nutr Metab Care. 2007;10(3):272–277. doi: 10.1097/MCO.0b013e3280f31b17. [DOI] [PubMed] [Google Scholar]
- 23.Mann EA, Pidcoke HF, Salinas J, Holcomb JB, Wolf SE, Wade CE. The impact of intensive insulin protocols and restrictive blood transfusion strategies on glucose measurement in American Burn Association (ABA) verified burn centers. J Burn Care Res. 2008;29(5):718–723. doi: 10.1097/BCR.0b013e3181848c74. [DOI] [PubMed] [Google Scholar]
- 24.Mowlavi A, Andrews K, Milner S, Herndon DN, Heggers JP. The effects of hyperglycemia on skin graft survival in the burn patient. Ann Plast Surg. 2000;45(6):629–632. doi: 10.1097/00000637-200045060-00010. [DOI] [PubMed] [Google Scholar]
- 25.Pham TN, Warren AJ, Phan HH, Molitor F, Greenhalgh DG, Palmieri TL. Impact of tight glycemic control in severely burned children. J Trauma. 2005;59(5):1148–1154. doi: 10.1097/01.ta.0000188933.16637.68. [DOI] [PubMed] [Google Scholar]
- 26.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51(3):540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 28.Gauglitz GG, Herndon DN, Jeschke MG. Insulin resistance postburn: underlying mechanisms and current therapeutic strategies. J Burn Care Res. 2008;29(5):683–694. doi: 10.1097/BCR.0b013e31818481ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeSanti L. Pathophysiology and current management of burn injury. Adv Skin Wound Care. 2005;18(6):323–332. doi: 10.1097/00129334-200507000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collier B, Diaz J, Jr., Forbes R, Morris J, Jr., May A, Guy J, Ozdas A, Dupont W, Miller R, Jensen G. The impact of a normoglycemic management protocol on clinical outcomes in the trauma intensive care unit. JPEN J Parenter Enteral Nutr. 2005;29(5):353–359. doi: 10.1177/0148607105029005353. [DOI] [PubMed] [Google Scholar]
- 32.Wahl WL, Taddonio M, Maggio PM, Arbabi S, Hemmila MR. Mean glucose values predict trauma patient mortality. J Trauma. 2008;65(1):42–48. doi: 10.1097/TA.0b013e318176c54e. [DOI] [PubMed] [Google Scholar]
- 33.Hemmila MR, Taddonio MA, Arbabi S, Maggio PM, Wahl WL. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2008;144(4):629–637. doi: 10.1016/j.surg.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeschke MG, Boehning DF, Finnerty CC, Herndon DN. Effect of insulin on the inflammatory and acute phase response after burn injury. Crit Care Med. 2007;35(9 Suppl):S519–S523. doi: 10.1097/01.CCM.0000282027.10288.10. [DOI] [PubMed] [Google Scholar]
- 35.Thomas SJ, Morimoto K, Herndon DN, Ferrando AA, Wolfe RR, Klein GL, Wolf SE. The effect of prolonged euglycemic hyper-insulinemia on lean body mass after severe burn. Surgery. 2002;132(2):341–347. doi: 10.1067/msy.2002.126871. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XJ, Chinkes DL, Wolf SE, Wolfe RR. Insulin but not growth hormone stimulates protein anabolism in skin wound and muscle. Am J Physiol. 1999;276(4 Pt 1):E712–E720. doi: 10.1152/ajpendo.1999.276.4.E712. [DOI] [PubMed] [Google Scholar]
- 37.Pierre EJ, Barrow RE, Hawkins HK, Nguyen TT, Sakurai Y, Desai M, Wolfe RR, Herndon DN. Effects of insulin on wound healing. J Trauma. 1998;44(2):342–345. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229(1):11–18. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gore DC, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Extremity hyperinsulinemia stimulates muscle protein synthesis in severely injured patients. Am J Physiol Endocrinol Metab. 2004;286(4):E529–E534. doi: 10.1152/ajpendo.00258.2003. [DOI] [PubMed] [Google Scholar]
- 40.Pidcoke HF, Wade CE, Wanek S, Wang JJ, Concannon M, Loo F, Wolf SE. Decreased mortality in adult burns with improved glucose control [Abstract]; 13th Congress International Society for Burn Injuries; Sept 25–29, 2006; Fortaleza, Brazil. 2006. [Google Scholar]
- 41.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105(2):244–252. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 43.Ali NA, O'Brien JM Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF, Jr., Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36(8):2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pidcoke HF, Rohleder L, Wanek SM, Wang J, Holcomb JB, Wolf SE, Wade CE. Glucose variability associated with mortality in burns [Abstract] J Trauma. 2007;62:272. doi: 10.1097/TA.0b013e3181baef4b. [DOI] [PubMed] [Google Scholar]
- 45.Hobson KG, Havel PJ, McMurtry AL, Lawless MB, Palmieri TL, Greenhalgh DD. Circulating leptin and cortisol after burn injury: loss of diurnal pattern. J Burn Care Rehab. 2004;25(6):491–499. doi: 10.1097/01.bcr.0000144532.02792.6e. [DOI] [PubMed] [Google Scholar]
- 46.Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P. Circadian rhythm of blood glucose values in critically ill patients. Crit Care Med. 2007;35(2):416–421. doi: 10.1097/01.CCM.0000253814.78836.43. [DOI] [PubMed] [Google Scholar]
- 47.Pidcoke HF, Salinas J, Wanek SM, Concannon M, Loo F, Wirfel KL, Holcomb JB, Wolf SE, Wade CE. Patterns of exogenous insulin requirement reflect insulin sensitivity changes in trauma. Am J Surg. 2007;194(6):798–803. doi: 10.1016/j.amjsurg.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 49.Kwan P, Gomez M, Cartotto R. Safe and successful restriction of transfusion of burn patients. J Burn Care Res. 2006;27(6):826–834. doi: 10.1097/01.BCR.0000245494.45125.3E. [DOI] [PubMed] [Google Scholar]
- 50.LifeScanHealthcare. SureStepPro Professional Blood Glucose Management System: test strips for glucose testing in whole blood. Milpitas: LifeScan, Johnson & Johnson; 2003. [Google Scholar]
- 51.1996. U.S. Food and Drug Administration. Review criteria assessment of portable blood glucose monitoring in vitro diagnostic devices using glucose oxidase, dehydrogenase or hexokinase methodology. U.S. Department of Health and Human Services.
- 52.American Diabetes Association. Position statement: self-monitoring of blood glucose. Diabetes Care. 1996;19:62S–66S. [Google Scholar]
- 53.Karon BS, Griesmann L, Scott R, Bryant SC, Dubois JA, Shirey TL, Presti S, Santrach PJ. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther. 2008;10(2):111–120. doi: 10.1089/dia.2007.0257. [DOI] [PubMed] [Google Scholar]
- 54.Louie RF, Tang Z, Sutton DV, Lee JH, Kost GJ. Point-of-care glucose testing: effects of critical care variables, influence of reference instruments, and a modular glucose meter design. Arch Pathol Lab Med. 2000;124(2):257–266. doi: 10.5858/2000-124-0257-POCGT. [DOI] [PubMed] [Google Scholar]
- 55.Tang Z, Lee JH, Louie RF, Kost GJ. Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med. 2000;124(8):1135–1140. doi: 10.5858/2000-124-1135-EODHLO. [DOI] [PubMed] [Google Scholar]
- 56.Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypo-glycemia in intensive care unit patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2009 Sep 28; doi: 10.1097/CCM.0b013e3181bc826f. epub ahead of print. DOI: 10.1097/CCM.0b013e3181bc826f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook A, Laughlin D, Moore M, North D, Wilkins K, Wong G, Wallace-Scroggs A, Halvorsen L. Differences in glucose values obtained from point-of-care glucose meters and laboratory analysis in critically ill patients. Am J Crit Care. 2009;18(1):65–72. doi: 10.4037/ajcc2009626. [DOI] [PubMed] [Google Scholar]
- 58.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Tang ZP, Louie RF, Kost GJ. The effect of hematocrit on six glucose meter systems for point-of-care testing [Abstract] New Orleans, LA: Medical Pathology and Clinical Chemistry, University of California Davis Health System, Sacramento, CA; 1999. AACC/ASCLS 1999 Annual Meeting; July 25–29, 1999. [Google Scholar]
- 60.Boyd R, Leigh B, Stuart P. Capillary versus venous bedside blood glucose estimations. Emerg Med J. 2005;22(3):177–179. doi: 10.1136/emj.2003.011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33(12):2079–2084. doi: 10.1007/s00134-007-0835-4. [DOI] [PubMed] [Google Scholar]
- 62.Desachy A, Vuagnat AC, Ghazali AD, Baudin OT, Longuet OH, Calvat SN, Gissot V. Accuracy of bedside glucometry in critically ill patients: influence of clinical charateristics and perfusion index. Mayo Clin Proc. 2008;83(4):400–405. doi: 10.4065/83.4.400. [DOI] [PubMed] [Google Scholar]
- 63.Slater-MacLean L, Cembrowski G, Chin D, Shalapay C, Binette T, Hegadoren K, Newburn-Cook C. Accuracy of glycemic measurements in the critically ill. Diabetes Technol Ther. 2008;10(3):169–177. doi: 10.1089/dia.2008.0263. [DOI] [PubMed] [Google Scholar]
- 64.Mann EA, Salinas J, Pidcoke HF, Wolf SE, Holcomb JB, Wade CE. Error rates resulting from anemia can be corrected in multiple commonly used point-of-care glucometers. J Trauma. 2008;64(1):15–21. doi: 10.1097/TA.0b013e318160b9e4. [DOI] [PubMed] [Google Scholar]
- 65.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med. 2004;32(1):39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 66.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D. ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 67.Jeschke MG, Chinkes DL, Finnerty CC, Przkora R, Pereira CT, Herndon DN. Blood transfusions are associated with increased risk for development of sepsis in severely burned pediatric patients. Crit Care Med. 2007;35(2):579–583. doi: 10.1097/01.CCM.0000253812.09236.98. [DOI] [PubMed] [Google Scholar]
- 68.Palmieri TL, Caruso DM, Foster KN, Cairns BA, Peck MD, Gamelli RL, Mozingo DW, Kagan RJ, Wahl W, Kemalyan NA, Fish JS, Gomez M, Sheridan RL, Faucher LD, Latenser BA, Gibran NS, Klein RL, Solem LD, Saffle JR, Morris SE, Jeng JC, Voigt D, Howard PA, Molitor F, Greenhalgh DG. American Burn Association Burn Multicenter Trials Group. Effect of blood transfusion on outcome after major burn injury: a multicenter study. Crit Care Med. 2006;34(6):1602–1607. doi: 10.1097/01.CCM.0000217472.97524.0E. [DOI] [PubMed] [Google Scholar]
- 69.Palmieri TL, Lee T, O'Mara MS, Greenhalgh DG. Effects of restrictive blood transfusion policy on outcomes in children with burn injury. J Burn Care Res. 2007;28(1):65–70. doi: 10.1097/BCR.0B013E31802C895E. [DOI] [PubMed] [Google Scholar]
- 70.Rao LV, Jakubiak F, Sidwell JS, Winkelman JW, Snyder ML. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clin Chim Acta. 2005;356(1–2):178–183. doi: 10.1016/j.cccn.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 71.Mann E, Pidcoke H, Salinas J, Holcomb J, Wade C, Wolf S. Hematocrit effect outweighs other sources of glucometer error in critical care. Crit Care Med. 2007;35(12):A140. [Google Scholar]
- 72.Thomas LE, Kane MP, Bakst G, Busch RS, Hamilton RA, Abelseth JM. A glucose meter accuracy and precision comparison: the Freestyle Flash versus the Accu-Chek Advantage, Accu-Chek Compact Plus, Ascensia Contour, and the BD Logic. Diabet Technol Ther. 2008;10(2):102–110. doi: 10.1089/dia.2007.0244. [DOI] [PubMed] [Google Scholar]
- 73.Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(2):403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 74.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 75.Steil GM, Clark B, Kanderian S, Rebrin K. Modeling insulin action for development of a closed-loop artificial pancreas. Diabetes Technol Ther. 2005;7(1):94–108. doi: 10.1089/dia.2005.7.94. [DOI] [PubMed] [Google Scholar]
- 76.Bequette BW. A critical assessment of algorithms and challenges in the development of a closed-loop artificial pancreas. Diabetes Technol Ther. 2005;7(1):28–47. doi: 10.1089/dia.2005.7.28. [DOI] [PubMed] [Google Scholar]
- 77.Goldberg PA, Siegel MD, Russell RR, Sherwin RS, Halickman JI, Cooper DA, Dziura JD, Inzucchi SE. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol Ther. 2004;6(3):339–347. doi: 10.1089/152091504774198034. [DOI] [PubMed] [Google Scholar]
- 78.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15(4):370–377. [PubMed] [Google Scholar]
- 79.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28(10):2418–2423. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 80.Juneja R, Roudebush C, Kumar N, Macy A, Golas A, Wall D, Wolverton C, Nelson D, Carroll J, Flanders SJ. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technol Ther. 2007;9(3):232–240. doi: 10.1089/dia.2006.0015. [DOI] [PubMed] [Google Scholar]
- 81.Saager L, Collins GL, Burnside B, Tymkew H, Zhang L, Jacobsohn E, Avidan M. A randomized study in diabetic patients undergoing cardiac surgery comparing computer-guided glucose management with a standard sliding scale protocol. J Cardiothorac Vasc Anesth. 2008;22(3):377–382. doi: 10.1053/j.jvca.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 82.Vogelzang M, Loef BG, Regtien JG, van der Horst IC, van Assen H, Zijlstra F, Nijsten MW. Computer-assisted glucose control in critically ill patients. Intensive Care Med. 2008;34(8):1421–1427. doi: 10.1007/s00134-008-1091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morris AH, Orme J, Jr., Truwit JD, Steingrub J, Grissom C, Lee KH, Li GL, Thompson BT, Brower R, Tidswell M, Bernard GR, Sorenson D, Sward K, Zheng H, Schoenfeld D, Warner H. A replicable method for blood glucose control in critically ill patients. Crit Care Med. 2008;36(6):1787–1795. doi: 10.1097/CCM.0b013e3181743a5a. [DOI] [PubMed] [Google Scholar]
- 84.Dortch MJ, Mowery NT, Ozdas A, Dossett L, Cao H, Collier B, Holder G, Miller RA, May AK. A computerized insulin infusion titration protocol improves glucose control with less hypoglycemia compared to a manual titration protocol in a trauma intensive care unit. JPEN J Parenter Enteral Nutr. 2008;32(1):18–27. doi: 10.1177/014860710803200118. [DOI] [PubMed] [Google Scholar]
- 85.Kaukonen KM, Rantala M, Pettilä V, Hynninen M. Severe hypo-glycemia during intensive insulin therapy. Acta Anaesthesiol Scand. 2009;53(1):61–65. doi: 10.1111/j.1399-6576.2008.01795.x. [DOI] [PubMed] [Google Scholar]
- 86.McMullin J, Brozek J, McDonald E, Clarke F, Jaeschke R, Heels-Ansdell D, Leppert R, Foss A, Cook D. Lowering of glucose in critical care: a randomized pilot trial. J Crit Care. 2007;22(2):112–119. doi: 10.1016/j.jcrc.2006.08.002. [DOI] [PubMed] [Google Scholar]