Abstract
The observation that elevated glucose occurs frequently in the setting of acute myocardial infarction was made decades ago. Since then numerous studies have documented that hyperglycemia is a powerful risk factor for increased mortality and in-hospital complications in patients with acute coronary syndromes. While some questions in this field have been answered in prior investigations, many critical gaps in knowledge continue to exist and remain subjects of intense debate. This review summarizes what is known about the relationship between hyperglycemia, glucose control, and outcomes in critically ill patients with acute coronary syndromes, addresses the gaps in knowledge and controversies, and offers general recommendations regarding glucose management in the coronary care unit.
Keywords: diabetes, glucose, insulin, myocardial infarction, outcomes, review
Relationship between Glucose Levels and Outcomes in Patients with Acute Myocardial Infarction
Among various cardiovascular disorders, the relationship between glucose levels and outcomes has been studied most extensively among patients with acute myocardial infarction (AMI). Numerous studies have demonstrated that hyperglycemia is common and is associated with a higher risk of mortality and in-hospital complications in this patient group.1–26 While the definition of hyperglycemia varies across different studies, several large observational investigations have demonstrated that elevated admission glucose (random glucose value >140 mg/dl—the definition of hyperglycemia used by the American Heart Association27) occurs in 51–58% of patients presenting with AMI.2,28 Although some patients with AMI may experience resolution of hyperglycemia during hospitalization, in most cases, hyperglycemia persists throughout the hospital course. Recent analysis of nearly 17,000 patients hospitalized with AMI showed that 41% of AMI patients have persistent hyperglycemia (mean hospitalization glucose >140 mg/dl) and about 14% have persistent severe hyperglycemia (mean hospitalization glucose >200 mg/dl).28
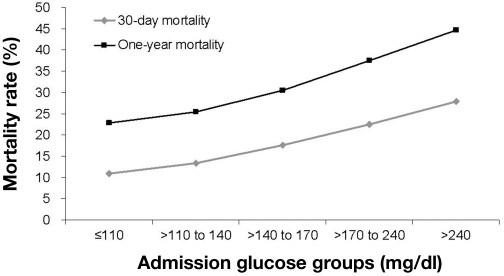
The relationship between higher glucose levels and increased mortality risk in AMI has been shown across various glucose metrics. Among these, admission glucose has been evaluated the most thoroughly. The largest epidemiologic study that evaluated the relationship between admission glucose levels and mortality in AMI patients was the analysis from the Cooperative Cardiovascular Project.2 In a cohort of 141,680 elderly patients, a clear, linear relationship was demonstrated between admission glucose levels and both 30-day and 1-year mortality (Figure 1). Other studies have confirmed these findings, showing a significant increase in the risk of short- and long-term mortality in hyperglycemic AMI patients,15, 21 and extended it to the entire range of acute coronary syndromes, including ST elevation myocardial infarction, non-ST elevation myocardial infarction, and unstable angina.5,29 A similar relationship between elevated glucose and increased risk of death has also been demonstrated with other glucose metrics, such as fasting glucose.25,30–32 Moreover, elevated admission glucose was shown to have an association with other adverse outcomes, such as “no-reflow” phenomenon after percutaneous coronary intervention, worse left ventricular systolic function, and higher rates of heart failure.5,6,22
Figure 1.
Relationship between admission glucose levels and mortality at 30 days and 1 year in patients with AMI. Reproduced with permission from Circulation.2
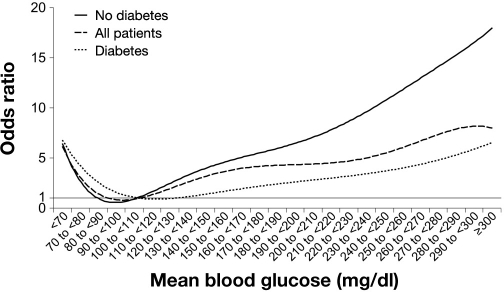
Importantly, the association between hyperglycemia and increased risk of death is not limited to the initial stages of AMI hospitalization. To the contrary, persistent hyperglycemia during hospitalization is even more prognostically important than hyperglycemia on admission. Clear evidence for this concept comes from analysis of nearly 17,000 patients hospitalized with AMI.28 Results from this study have clearly demonstrated a powerful relationship between average glucose during hospitalization and in-hospital mortality (Figure 2). Epidemiologic analyses from randomized clinical trials of glucose–insulin–potassium (GIK) therapy [Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation–Estudios Cardiologicos Latin America (CREATE-ECLA)]33 and studies of intensive glucose control in AMI [Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5)] 34 also confirmed the relationship between persistent hyperglycemia and increased mortality risk.
Figure 2.
Relationship between average glucose levels during hospitalization and in-hospital mortality in patients with AMI after multivariable adjustment (reference: mean blood glucose 100 to <100). Reproduced with permission from Circulation.28
The relationship between higher admission and average glucose levels and mortality is not limited to patients with diabetes. In fact, studies show that it is even more pronounced among AMI patients without established diabetes as compared with those who have known diabetes.2, 28 Specifically, mortality rises steeply in patients without recognized diabetes above a mean hospitalization glucose level of 120 mg/dl. However, among patients with known diabetes, only those patients with severe, sustained hyperglycemia (mean hospitalization glucose ≥200 mg/dl) experience a significantly higher risk of death compared with those whose mean glucose is <110 mg/dl (Figure 2).28
Relationship between Dynamic Changes in Glucose Levels during AMI Hospitalization and Patient Outcomes
Several observational studies have examined whether changes in glucose levels during hospitalization are associated with mortality in the setting of AMI, whether specific therapies used for glucose lowering during hospital stay (such as insulin) can impact patient outcomes, and which specific glucose levels achieved during hospitalization are associated with the best outcomes.
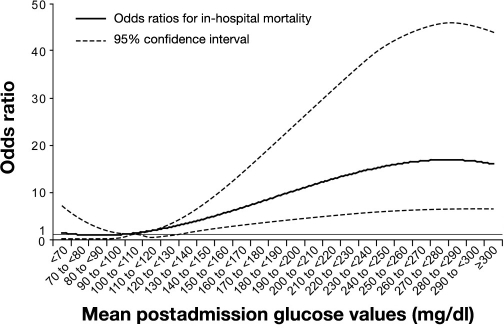
In the retrospective analysis of 1469 AMI patients with a baseline glucose of ≥140 mg/dl enrolled in the Complement and Reduction of Infarct Size after Angioplasty or Lytics (CARDIAL) trial, a drop in glucose of ≥30 mg/dl during the first 24 hours of hospitalization was associated with a lower risk of 30-day mortality, while no change or increase in glucose values was associated with a higher risk of death.35 These results were confirmed and expanded in a large observational study of nearly 8000 initially hyperglycemic patients hospitalized with AMI in the United States. Results from this investigation demonstrated that glucose normalization following admission to a hospital was associated with a significant reduction in hospital mortality, even after adjusting for numerous potential confounders (Figure 3).36 Specifically, patients with postadmission glucose levels of 80–130 mg/dl experienced the lowest mortality.
Figure 3.
Association between glucose normalization and risk of death during AMI hospitalization. Reproduced with permission from Archives of Internal Medicine.36
Relationship between Insulin Therapy and Outcomes in Hyperglycemic Patients with AMI
Few observational studies have evaluated the impact of in-hospital insulin therapy on mortality in hyperglycemic AMI patients. Insulin remains the most effective method of glucose lowering in the inpatient setting. Small, mechanistic studies suggested that insulin may have anti-inflammatory, profibrinolytic, and antiapoptotic properties and can inhibit the generation of reactive oxygen species and improve myocardial blood flow.37–45 However, whether insulin therapy is associated with any clinical benefit in AMI above and beyond its associated glucose-lowering effect is unclear. One observational study showed that patients with severe hyperglycemia on admission [≥11 mmol/liter (∼200 mg/dl)] and no prior history of diabetes had a 56% relative risk increase in mortality at 7 days if they did not receive insulin during hospitalization as compared with similar patients who received insulin therapy.46 However, this study was unable to determine whether patients that received insulin actually had better glucose control during hospitalization.
The largest and most methodologically rigorous study to address this issue evaluated nearly 8000 hyperglycemic patients with AMI and used propensity matching to minimize confounding associated with the decision to initiate insulin therapy during hospitalization.36 While glucose normalization was associated with better survival, there was no significant association between insulin therapy per se and mortality. In fact, patients whose glucose levels normalized after hospital admission did equally well regardless of whether glucose normalized spontaneously or after initiation of insulin therapy. Similarly, there was no significant difference in mortality among patients who remained hyperglycemic following insulin administration and those who remained hyperglycemic without receiving treatment. These findings suggest that any possible benefits of insulin therapy are likely mediated through the control of blood glucose and that it is the glucose value, rather than insulin treatment per se, that appears to be a more important predictor of patient outcomes.
Clinical Trials of Glucose Control in AMI
Although prior observational studies have demonstrated a powerful relationship between hyperglycemia and mortality in patients with AMI, these data do not answer the critical question: is hyperglycemia directly harmful or is it simply a marker of illness severity in this patient group? Prior physiologic studies show that elevated glucose is associated with microvascular dysfunction,47,48 vascular inflammation,49–51 prothrombotic state,52–58 endothelial dysfunction,59 and generation of reactive oxygen species.60,61 In addition, hyperglycemia has been linked with higher free fatty acid concentrations (which could have a proarrhythmic effect) and impaired myocardial glucose use, increasing the consumption of oxygen and potentially worsening ischemia.62,63 All of these mechanisms may increase myocardial injury in a setting of AMI and may explain the relationship between poor glucose control and adverse outcomes in this patient group. However, to definitively determine whether hyperglycemia is directly harmful, well-designed randomized clinical trials of target-driven intensive glucose control in hospitalized AMI patients are needed. Due to the lack of such trials, this question cannot be currently answered with certainty. Nevertheless, some conclusions can be drawn from the smaller clinical trials performed to date.
The original Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study64 randomized hyperglycemic patients (average glucose at randomization >275 mg/dl) to either standard therapy or intravenous glucose–insulin infusion titrated to achieve a prespecified target of 126–196 mg/dl during the acute phase, followed by multidose subcutaneous insulin injections for 3 months. Following randomization, clinically and statistically lower glucose levels were achieved in the glucose control patients (≈173 mg/dl) versus control (≈211 mg/dl) at 24 hours, and a significant difference was maintained through discharge. Lower mortality was observed in the glucose control patients (vs control) at discharge and at 3 months, but this mortality difference only reached statistical significance after 1 year of follow-up. Whether this mortality reduction was due to acute glucose lowering in-hospital, chronic glucose control postdischarge, or both could not be determined.
The HI-5 study used a similar design to DIGAMI (target was 72–180 mg/dl in the intensive arm), but failed to achieve a statistically significant difference in glucose values between intensive and conventional glucose groups (149 mg/dl vs 162 mg/dl postrandomization, p = not significant).34 Nevertheless, while mortality rates were not significantly different between the groups, the prespecified end points of reinfarction and heart failure were markedly lower in patients who received intensive glucose control. The DIGAMI-2 study also failed to achieve a significant glucose contrast between the groups and was similarly mortality neutral.65 Finally, a small, but elegant, randomized trial demonstrated that intensive glucose control with the Yale protocol (achieved glucose 128 mg/dl) improved left ventricular ejection fraction and reduced inflammation and apoptosis markedly (as measured by caspase-3 levels) in peri-infarction areas as compared to conventional control (achieved glucose 193 mg/dl).66 However, this study was designed only to evaluate surrogate end points.
The remaining studies in AMI have predominantly tested the GIK hypothesis and were not designed to study target-driven glucose control. Studies such as the Polish Glucose-Insulin-Potassium (POL-GIK)67 or the much larger CREATE-ECLA33 assigned patients to a fixed-dose GIK infusion regardless of their initial glucose values and had no prespecified glucose targets. As an example, in the POL-GIK study,67 the initial glucose at randomization was ≈124 mg/dl. In CREATE-ECLA,33 postrandomization glucose values were actually higher in the GIK group than in the control group. Thus, the GIK studies were never designed to evaluate targeted glucose control with insulin and their findings should not be used in guiding decisions about glucose management in AMI.
Thus, while clinical trial data for glucose control in AMI are scarce and inconclusive, the overall balance of evidence points toward a possible clinical benefit associated with intensive glucose control. Although one might be tempted to look for more definitive answers in the critical care field, this can also be problematic. First, findings from patients hospitalized with surgical illness, trauma, and sepsis cannot be simply extrapolated to those with AMI. The pathophysiology of these conditions is different, and the treatment thresholds and targets may be distinct as well. Prior studies have suggested that because the relationship between glucose values and mortality can change across various cardiovascular conditions,2,68 it may also vary substantially between cardiac and noncardiac disease states.
Second, data from randomized trials in critically ill patients are conflicting. The original (single-center) Leuven study showed dramatic reductions in mortality and complications in the surgical intensive care unit population with intensive glucose control as compared to usual care.69 However, these findings have not been reproduced in several subsequent and larger studies, most of which have been mortality neutral.70–73 The Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) clinical trial actually showed higher mortality in the intensive glucose control arm, which raised an alarm in the critical care community.74 However, results of NICE-SUGAR need to be interpreted in the context of the study design. It is important to understand that NICE-SUGAR compared “very intensive” glucose control to “good” glucose control, not to usual care. Specifically, an intravenous insulin protocol was used when needed in the control arm, producing an average glucose of ≈142 mg/dl in those patients. This glucose value was lower than what was achieved in the control groups of other critical care studies, lower than what was achieved in the intensive arm of most AMI studies, and lower than what is typically seen in routine clinical care. Thus, the most appropriate conclusion from the NICE-SUGAR study is that good glucose control (with values somewhere between 140 and 180 mg/dl) is “good enough” and more aggressive control provides no additive benefit (and could possibly be harmful) in the critical care setting.
Current Patterns of Hyperglycemia Management in Hospitalized AMI Patients
While specific glucose levels at which treatment should be initiated remain highly debatable, current consensus is that treatment with insulin should be considered in AMI patients with severe hyperglycemia (>180 mg/dl).27,75 A large study of AMI patients hospitalized in the United States from 2000 to 2005 showed that among patients with severe, sustained hyperglycemia (mean hospitalization glucose ≥200 mg/dl), nearly 40% of patients did not receive any insulin therapy.76 Moreover, there was substantial variability in insulin treatment rates across the 40 participating medical centers.77 A study performed in the United Kingdom also showed that 64% of patients without diabetes with admission glucose ≥11 mmol/liter (∼200 mg/dl) received no glucose-lowering treatments during hospitalization.46 The key reasons for poor treatment rates among AMI patients with severe hyperglycemia include lack of convincing evidence from prior clinical trials that target-driven glucose control improves outcomes in AMI, fear of hypoglycemia, unfamiliarity with effective and safe tools of glucose control, and clinical inertia. Addressing the first of these barriers will need to await the conduct of definitive large randomized trials in AMI; the other barriers are discussed briefly here.
The Prognostic Importance of Hypoglycemia in Patients with AMI
Since therapy of hyperglycemia in the AMI setting necessitates the use of insulin, concern about the short–term and long–term impact of hypoglycemia persists. Several prior studies have shown that glucose values in the hypoglycemic range may adversely impact mortality in AMI. Studies by Svensson and colleagues and Pinto and associates demonstrated that random glucose values ≤55 and <81 mg/dl, respectively, were associated with a marked increase in mortality (93% increase in the adjusted relative risk of 2-year mortality in the study by Svensson and colleagues).23,78 One of the larger observational studies also demonstrated a J-shaped relationship between average glucose values during hospitalization and in-hospital mortality (Figure 2).28 Specifically, patients with a mean hospitalization glucose of <70 mg/dl experienced a marked increase in the odds of mortality (6.4, p = 0.01) when compared to patients with a mean glucose of 100 to <110 mg/dl. Whether hypoglycemia is directly harmful in patients with AMI or whether it is simply a marker for the most critically ill patients (with liver insufficiency, sepsis, or shock) was evaluated in a large observational study.79 The authors demonstrated that while hypoglycemia was associated with increased short-term mortality in patients hospitalized with AMI, this risk was confined to those who developed hypoglycemia spontaneously as the result of severe illness. In contrast, hypoglycemia that occurred after insulin initiation was not associated with a higher mortality risk. Retrospective analysis of the DIGAMI 2 study also showed no significant association between hypoglycemia and mortality after adjustment for confounders.80 These findings suggest that hypoglycemia is a marker of severe illness rather than a direct cause of adverse outcomes. While continuous efforts to avoid hypoglycemia are certainly warranted, these studies offer some degree of reassurance to clinicians in their efforts to control glucose in the setting of AMI.
Can Glucose Control Be Implemented Safely and Effectively in the Cardiac Care Unit?
Numerous glucose-control protocols have been developed, and several of these have been extensively tested and demonstrated to be effective and safe.81,82 Typically, dynamic protocols (those that take into account not only the current glucose value, but the direction and magnitude of change in glucose over time, as well as patients' insulin sensitivity) are preferred in the critical care setting. While experience with these protocols in patients with AMI is relatively limited, data from the Mid America Heart Institute show that such protocols can be adapted easily to the AMI patient population with few modifications.83 Specifically, a modification of the Yale protocol was highly effective in rapidly achieving and maintaining glucose control. Mean 24-hour glucose was reduced markedly in initially hyperglycemic patients treated with the protocol as compared to similar patients prior to protocol implementation (135 mg/dl vs 181 mg/dl, p < 0.001). The rate of severe hypoglycemia was low. These results suggest that the use of evidence-based, effective, and safe protocols is highly preferable when and if clinicians choose to implement glucose control in their coronary care units. Several such protocols (including the Yale protocol) are freely available in the public domain. 65
Relationship between Glucose Levels and Outcomes in Acute Cardiovascular Conditions Other Than AMI
Studies of patients undergoing cardiothoracic surgery are reviewed in a separate publication in this symposium. Aside from patients with AMI and those undergoing cardiothoracic surgery, data regarding the relationship between glucose levels and mortality in patients with other cardiovascular conditions (aside from AMI) are much more limited.
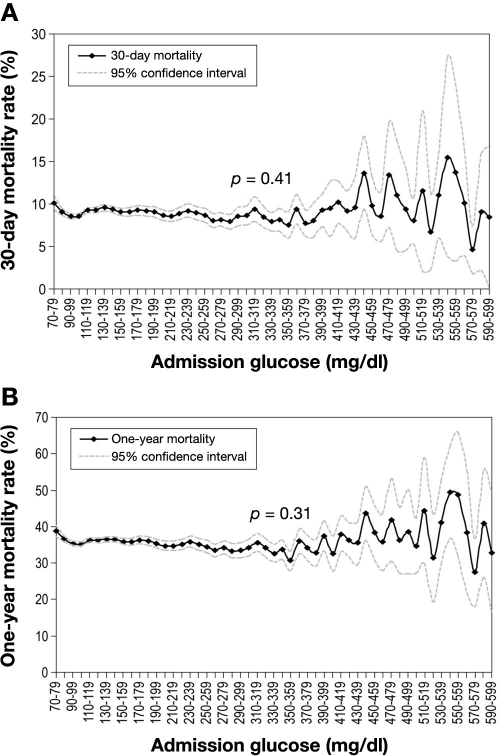
Other than AMI, perhaps the most commonly encountered reason for hospitalization in the coronary care unit is decompensated heart failure (HF). Several initial, smaller studies suggested that higher glucose is associated with a greater mortality risk in hospitalized patients with HF.84–86 However, the largest observational study to address this issue did not confirm these findings. Using data from the nationally representative cohort of over 50,000 elderly patients hospitalized with HF, the authors demonstrated that hyperglycemia occurs in nearly half of patients hospitalized with HF. However, they found no significant, graded association between glucose values on admission and short- and long-term mortality (Figure 4).68 The lack of relationship between glucose levels and risk of death was seen despite the greater severity of HF on presentation observed in hyperglycemic patients.
Figure 4.
Association between admission glucose and mortality at 30 days (A) and 1 year (B) in patients hospitalized with HF. Reproduced with permission from Circulation.68
These findings show that a strong association between glucose values and increased mortality risk observed in AMI cannot be simply extended to patients hospitalized with other acute cardiovascular conditions. They also imply that the implementation of intensive glucose control protocols in hyperglycemic patients with HF may be premature.
Summary and Recommendations
In summary, numerous studies have definitively established that hyperglycemia is highly prevalent and associated with an increased risk of death and in-hospital complications and that resolution of on-arrival hyperglycemia is associated with improved survival in patients hospitalized with AMI. However, due to the lack of appropriately designed randomized trials, the definitive answer in regards to glucose management in patients with AMI, including treatment thresholds and glucose targets, is lacking. A large, multicenter randomized trial of target-driven glucose control in AMI is clearly needed and is the next necessary and logical step. Until such a trial is completed, continuing a strategy of “good, but not too aggressive” glucose control in AMI continues to be a sound approach. Such an approach is supported by the American Heart Association's position statement on hyperglycemia in acute coronary syndromes,27 as well as by other professional societies.75
Such glucose management strategy should include the following components:
Glucose levels should be assessed and closely monitored in AMI patients hospitalized in a coronary care unit.
Glucose treatment initiation should be considered at a threshold of >180 mg/dl.
When glucose control strategy is pursued, glucose treatment targets should be within the conservative range of 140–180 mg/dl. While lower treatment targets (between 110 and140 mg/dl) may be reasonable in some patients, glucose levels <110 mg/dl are discouraged, as there is no evidence that more aggressive glucose lowering is beneficial.
Evidence-based protocols should be used if and when glucose control strategy is implemented. In general, protocols based on intravenous insulin infusion are preferred in the critical care setting. Such protocols should have the following properties: (a) be previously published in peer-reviewed journals and demonstrated to be effective and safe; (b) be tested in a variety of clinical settings and patient populations; (c) take into consideration not just the current glucose level, but the rate of change in glucose values, as well as insulin sensitivity; (d) provide explicit directions on the frequency of glucose testing depending on the clinical conditions; and (e) provide specific instructions on hypoglycemia management.
Implementation of glucose-control protocols in coronary care units is best accomplished through a multi-disciplinary team effort, which includes collaboration among cardiologists, endocrinologists, and nursing staff, as well as administrative support. Collection of unit-specific data regarding glucose control and rates of hypoglycemia both before and after protocol implementation is recommended to document institution-specific effectiveness and safety.
Abbreviations
- AMI
acute myocardial infarction
- CREATE-ECLA
Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation–Estudios Cardiologicos Latin America
- DIGAMI
Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction
- GIK
glucose–insulin–potassium
- HF
heart failure
- NICE-SUGAR
Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation
- POL-GIK
Polish Glucose-Insulin-Potassium
References
- 1.Datey K, Nanda N. Hyperglycemia after acute myocardial infarction: its relation to diabetes mellitus. N Engl J Med. 1967;276(5):262–265. doi: 10.1056/NEJM196702022760504. [DOI] [PubMed] [Google Scholar]
- 2.Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, Krumholz HM. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111(23):3078–3086. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 3.Bellodi G, Manicardi V, Malavasi V, Veneri L, Bernini G, Bossini P, Distefano S, Magnanini G, Muratori L, Rossi G, et al. Hyperglycemia and prognosis of acute myocardial infarction in patients without diabetes mellitus. Am J Cardiol. 1989;64(14):885–888. doi: 10.1016/0002-9149(89)90836-9. [DOI] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyper-glycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 5.Foo K, Cooper J, Deaner A, Knight C, Suliman A, Ranjadayalan K, Timmis AD. A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Heart. 2003;89(5):512–516. doi: 10.1136/heart.89.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K, Kuroda T, Tanaka K, Masuyama T, Hori M, Fujii K. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol. 2003;41(1):1–7. doi: 10.1016/s0735-1097(02)02626-8. [DOI] [PubMed] [Google Scholar]
- 7.Leor J, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Cardiogenic shock complicating acute myocardial infarction in patients without heart failure on admission: incidence, risk factors, and outcome. SPRINT Study Group. Am J Med. 1993;94(3):265–273. doi: 10.1016/0002-9343(93)90058-w. [DOI] [PubMed] [Google Scholar]
- 8.Madsen JK, Haunsøe S, Helquist S, Hommel E, Malthe I, Pedersen NT, Sengeløv H, Rønnow-Jessen D, Telmer S, Parving HH. Prevalence of hyperglycaemia and undiagnosed diabetes mellitus in patients with acute myocardial infarction. Acta Med Scand. 1986;220(4):329–332. doi: 10.1111/j.0954-6820.1986.tb02773.x. [DOI] [PubMed] [Google Scholar]
- 9.Mak KH, Mah PK, Tey BH, Sin FL, Chia G. Fasting blood sugar level: a determinant for in-hospital outcome in patients with first myocardial infarction and without glucose intolerance. Ann Acad Med Singapore. 1993;22(3):291–295. [PubMed] [Google Scholar]
- 10.O'Sullivan JJ, Conroy RM, Robinson K, Hickey N, Mulcahy R. In-hospital prognosis of patients with fasting hyperglycemia after first myocardial infarction. Diabetes Care. 1991;14(8):758–760. doi: 10.2337/diacare.14.8.758. [DOI] [PubMed] [Google Scholar]
- 11.Oswald GA, Corcoran S, Yudkin JS. Prevalence and risks of hyperglycaemia and undiagnosed diabetes in patients with acute myocardial infarction. Lancet. 1984;1(8389):1264–1267. doi: 10.1016/s0140-6736(84)92447-4. [DOI] [PubMed] [Google Scholar]
- 12.Oswald GA, Smith CC, Betteridge DJ, Yudkin JS. Determinants and importance of stress hyperglycaemia in non-diabetic patients with myocardial infarction. Br Med J (Clin Res Ed) 1986;293(6552):917–922. doi: 10.1136/bmj.293.6552.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sala J, Masia R, Gonzalez de Molina FJ, Fernandez-Real JM, Gil M, Bosch D, Ricart W, Sentí M, Marrugat J. REGICOR Investigators. Short-term mortality of myocardial infarction patients with diabetes or hyperglycaemia during admission. J Epidemiol Community Health. 2002;56(9):707–712. doi: 10.1136/jech.56.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sewdarsen M, Jialal I, Vythilingum S, Govender G, Rajput MC. Stress hyperglycaemia is a predictor of abnormal glucose tolerance in Indian patients with acute myocardial infarction. Diabetes Res. 1987;6(1):47–49. [PubMed] [Google Scholar]
- 15.Wahab NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40(10):1748–1754. doi: 10.1016/s0735-1097(02)02483-x. [DOI] [PubMed] [Google Scholar]
- 16.Yudkin JS, Oswald GA. Stress hyperglycemia and cause of death in non-diabetic patients with myocardial infarction. Br Med J (Clin Res Ed) 1987;294(6574):773. doi: 10.1136/bmj.294.6574.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolk J, van der Ploeg T, Cornel JH, Arnold AE, Sepers J, Umans VA. Impaired glucose metabolism predicts mortality after a myocardial infarction. Int J Cardiol. 2001;79(2–3):207–214. doi: 10.1016/s0167-5273(01)00422-3. [DOI] [PubMed] [Google Scholar]
- 18.Oswald GA, Yudkin JS. Hyperglycaemia following acute myocardial infarction: the contribution of undiagnosed diabetes. Diabet Med. 1987;4(1):68–70. doi: 10.1111/j.1464-5491.1987.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 19.Wong VW, Ross DL, Park K, Boyages SC, Cheung NW. Hyperglycemia: still an important predictor of adverse outcomes following AMI in the reperfusion era. Diabetes Res Clin Pract. 2004;64(2):85–91. doi: 10.1016/j.diabres.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Hadjadj S, Coisne D, Mauco G, Ragot S, Duengler F, Sosner P, Torremocha F, Herpin D, Marechaud R. Prognostic value of admission plasma glucose and HbA in acute myocardial infarction. Diabet Med. 2004;21(4):305–310. doi: 10.1111/j.1464-5491.2004.01112.x. [DOI] [PubMed] [Google Scholar]
- 21.Stranders I, Diamant M, van Gelder RE, Spruijt HJ, Twisk JW, Heine RJ, Visser FC. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164(9):982–988. doi: 10.1001/archinte.164.9.982. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Umemura T, Nakamura S, Yoshida M. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. Am J Heart. 2003;146(4):674–678. doi: 10.1016/S0002-8703(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 23.Svensson AM, McGuire DK, Abrahamsson P, Dellborg M. Association between hyper- and hypoglycaemia and 2 year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J. 2005;26(13):1255–1261. doi: 10.1093/eurheartj/ehi230. [DOI] [PubMed] [Google Scholar]
- 24.Kadri Z, Danchin N, Vaur L, Cottin Y, Gueret P, Zeller M, Lablanche JM, Blanchard D, Hanania G, Genès N, Cambou JP. USIC 2000 Investigators. Major impact of admission glycaemia on 30-day and one-year mortality in non-diabetic patients admitted for myocardial infarction: results from the nationwide French USIC 2000 study. Heart. 2006;92(7):910–915. doi: 10.1136/hrt.2005.073791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suleiman M, Hammerman H, Boulos M, Kapeliovich MR, Suleiman A, Agmon Y, Markiewicz W, Aronson D. Fasting glucose is an important independent risk factor for 30-day mortality in patients with acute myocardial infarction: a prospective study. Circulation. 2005;111(6):754–760. doi: 10.1161/01.CIR.0000155235.48601.2A. [DOI] [PubMed] [Google Scholar]
- 26.Meier JJ, Deifuss S, Klamann A, Launhardt V, Schmiegel WH, Nauck MA. Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the Langendreer Myocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA) Diabetes Care. 2005;28(10):2551–2553. doi: 10.2337/diacare.28.10.2551. [DOI] [PubMed] [Google Scholar]
- 27.Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, Raskin P. American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117(12):1610–1619. doi: 10.1161/CIRCULATIONAHA.107.188629. [DOI] [PubMed] [Google Scholar]
- 28.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus JA. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117(8):1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 29.Sinnaeve PR, Steg PG, Fox KA, Van de Werf F, Montalescot G, Granger CB, Knobel E, Anderson FA, Dabbous OH, Avezum A. GRACE Investigators. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med. 2009;169(4):402–409. doi: 10.1001/archinternmed.2008.572. [DOI] [PubMed] [Google Scholar]
- 30.Aronson D, Hammerman H, Kapeliovich MR, Suleiman A, Agmon Y, Beyar R, Markiewicz W, Suleiman M. Fasting glucose in acute myocardial infarction: incremental value for long-term mortality and relationship with left ventricular systolic function. Diabetes Care. 2007;30(4):960–966. doi: 10.2337/dc06-1735. [DOI] [PubMed] [Google Scholar]
- 31.Porter A, Assali AR, Zahalka A, Iakobishvili Z, Brosh D, Lev EI, Mager A, Battler A, Kornowski R, Hasdai D. Impaired fasting glucose and outcomes of ST-elevation acute coronary syndrome treated with primary percutaneous intervention among patients without previously known diabetes mellitus. Am Heart J. 2008;155(2):284–289. doi: 10.1016/j.ahj.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Verges B, Zeller M, Dentan G, Beer JC, Laurent Y, Janin-Manificat L, Makki H, Wolf JE, Cottin Y. Impact of fasting glycemia on short-term prognosis after acute myocardial infarction. J Clin Endocrinol Metab. 2007;92(6):2136–2140. doi: 10.1210/jc.2006-2584. [DOI] [PubMed] [Google Scholar]
- 33.Diaz R, Goyal A, Mehta SR, Afzal R, Xavier D, Pais P, Chrolavicius S, Zhu J, Kazmi K, Liu L, Budaj A, Zubaid M, Avezum A, Ruda M, Yusuf S. Glucose-insulin-potassium therapy in patients with ST-segment elevation myocardial infarction. JAMA. 2007;298(20):2399–2405. doi: 10.1001/jama.298.20.2399. [DOI] [PubMed] [Google Scholar]
- 34.Cheung NW, Wong VW, McLean M. The Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care. 2006;29(4):765–770. doi: 10.2337/diacare.29.04.06.dc05-1894. [DOI] [PubMed] [Google Scholar]
- 35.Goyal A, Mahaffey KW, Garg J, Nicolau JC, Hochman JS, Weaver WD, Theroux P, Oliveira GB, Todaro TG, Mojcik CF, Armstrong PW, Granger CB. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. 2006;27(11):1289–1297. doi: 10.1093/eurheartj/ehi884. [DOI] [PubMed] [Google Scholar]
- 36.Kosiborod M, Inzucchi SE, Krumholz HM, Masoudi FA, Goyal A, Xiao L, Jones PG, Fiske S, Spertus JA. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med. 2009;169(5):438–446. doi: 10.1001/archinternmed.2008.593. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhuri A, Janicke D, Wilson MF, Tripathy D, Garg R, Bandyopadhyay A, Calieri J, Hoffmeyer D, Syed T, Ghanim H, Aljada A, Dandona P. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109(7):849–854. doi: 10.1161/01.CIR.0000116762.77804.FC. [DOI] [PubMed] [Google Scholar]
- 38.Koskenkari JK, Kaukoranta PK, Rimpiläinen J, Vainionpää V, Ohtonen PP, Surcel HM, Juvonen T, Ala-Kokko TI. Anti-inflammatory effect of high-dose insulin treatment after urgent coronary revascularization surgery. Acta Anaesthesiol Scand. 2006;50(8):962–969. doi: 10.1111/j.1399-6576.2006.01100.x. [DOI] [PubMed] [Google Scholar]
- 39.Visser L, Zuurbier CJ, Hoek FJ, Opmeer BC, de Jonge E, de Mol BA, van Wezel HB. Glucose, insulin and potassium applied as perioperative hyperinsulinaemic normoglycaemic clamp: effects on inflammatory response during coronary artery surgery. Br J Anaesth. 2005;95(4):448–457. doi: 10.1093/bja/aei220. [DOI] [PubMed] [Google Scholar]
- 40.Wong VW, McLean M, Boyages SC, Cheung NW. C-reactive protein levels following acute myocardial infarction: effect of insulin infusion and tight glycemic control. Diabetes Care. 2004;27(12):2971–2973. doi: 10.2337/diacare.27.12.2971. [DOI] [PubMed] [Google Scholar]
- 41.Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, Ma XL. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105(12):1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- 42.Jonassen AK, Aasum E, Riemersma RA, Mjos OD, Larsen TS. Glucose-insulin-potassium reduces infarct size when administered during reperfusion. Cardiovasc Drugs Ther. 2000;14(6):615–623. doi: 10.1023/a:1007802630604. [DOI] [PubMed] [Google Scholar]
- 43.Jonassen AK, Brar BK, Mjos OD, Sack MN, Latchman DS, Yellon DM. Insulin administered at reoxygenation exerts a cardioprotective effect in myocytes by a possible anti-apoptotic mechanism. J Mol Cell Cardiol. 2000;32(5):757–764. doi: 10.1006/jmcc.2000.1118. [DOI] [PubMed] [Google Scholar]
- 44.Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89(12):1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 45.Lautamaki R, Airaksinen KE, Seppanen M, Toikka J, Harkonen R, Luotolahti M, Borra R, Sundell J, Knuuti J, Nuutila P. Insulin improves myocardial blood flow in patients with type 2 diabetes and coronary artery disease. Diabetes. 2006;55(2):511–516. doi: 10.2337/diabetes.55.02.06.db05-1023. [DOI] [PubMed] [Google Scholar]
- 46.Weston C, Walker L, Birkhead J. Early impact of insulin treatment on mortality for hyperglycaemic patients without known diabetes who present with an acute coronary syndrome. Heart. 2007;93(12):1542–1546. doi: 10.1136/hrt.2006.108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scognamiglio R, Negut C, De Kreutzenberg SV, Tiengo A, Avogaro A. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation. 2005;112(2):179–184. doi: 10.1161/CIRCULATIONAHA.104.495127. [DOI] [PubMed] [Google Scholar]
- 48.Scognamiglio R, Negut C, de Kreutzenberg SV, Tiengo A, Avogaro A. Effects of different insulin regimes on postprandial myocardial perfusion defects in type 2 diabetic patients. Diabetes Care. 2006;29(1):95–100. [PubMed] [Google Scholar]
- 49.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101(9):1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A, Dandona P. Glucose ingestion induces an increase in intranuclear nuclear factor kappa B, a fall in cellular inhibitor kappa B, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism. 2006;55(9):1177–1185. doi: 10.1016/j.metabol.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr. 2004;80(1):51–57. doi: 10.1093/ajcn/80.1.51. [DOI] [PubMed] [Google Scholar]
- 52.Pandolfi A, Giaccari A, Cilli C, Alberta MM, Morviducci L, De Filippis EA, Buongiorno A, Pellegrini G, Capani F, Consoli A. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol. 2001;38(2):71–76. doi: 10.1007/s005920170016. [DOI] [PubMed] [Google Scholar]
- 53.Gresele P, Guglielmini G, De Angelis M, Ciferri S, Ciofetta M, Falcinelli E, Lalli C, Ciabattoni G, Davì G, Bolli GB. Acute, short-term hyperglycemia enhances shear stress-induced platelet activation in patients with type II diabetes mellitus. J Am Coll Cardiol. 2003;41(6):1013–1020. doi: 10.1016/s0735-1097(02)02972-8. [DOI] [PubMed] [Google Scholar]
- 54.Ceriello A, Giacomello R, Stel G, Motz E, Taboga C, Tonutti L, Pirisi M, Falleti E, Bartoli E. Hyperglycemia-induced thrombin formation in diabetes. The possible role of oxidative stress. 1995;44(8):924–928. doi: 10.2337/diab.44.8.924. Diabetes. [DOI] [PubMed] [Google Scholar]
- 55.Ceriello A, Giugliano D, Quatraro A, Dello Russo P, Marchi E, Torella R. Hyperglycemia may determine fibrinopeptide A plasma level increase in humans. Metabolism. 1989;38(12):1162–1163. doi: 10.1016/0026-0495(89)90152-2. [DOI] [PubMed] [Google Scholar]
- 56.Ceriello A, Giugliano D, Quatraro A, Dello Russo P, Torella R. Blood glucose may condition factor VII levels in diabetic and normal subjects. Diabetologia. 1988;31(12):889–891. doi: 10.1007/BF00265372. [DOI] [PubMed] [Google Scholar]
- 57.Jones RL, Peterson CM. Reduced fibrinogen survival in diabetes mellitus. A reversible phenomenon. J Clin Invest. 1979;63(3):485–493. doi: 10.1172/JCI109326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakamoto T, Ogawa H, Kawano H, Hirai N, Miyamoto S, Takazoe K, Soejima H, Kugiyama K, Yoshimura M, Yasue H. Rapid change of platelet aggregability in acute hyperglycemia. Detection by a novel laser-light scattering method. Thromb Haemost. 2000;83(3):475–479. [PubMed] [Google Scholar]
- 59.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vaso-dilation of brachial artery. J Am Coll Cardiol. 1999;34(1):146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 60.Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem. 2000;275(23):17728–17739. doi: 10.1074/jbc.275.23.17728. [DOI] [PubMed] [Google Scholar]
- 61.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85(8):2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 62.Oliver MF. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am J Med. 2002;112(4):305–311. doi: 10.1016/s0002-9343(01)01104-4. [DOI] [PubMed] [Google Scholar]
- 63.Tansey MJ, Opie LH. Relation between plasma free fatty acids and arrhythmias within the first twelve hours of acute myocardial infarction. Lancet. 1983;2(8347):419–422. doi: 10.1016/s0140-6736(83)90388-4. [DOI] [PubMed] [Google Scholar]
- 64.Malmberg K, Rydén L, Efendic S, Herlitz J, Nicol P, Waldenström A, Wedel H, Welin L. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 65.Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, Hildebrandt P, MacLeod K, Laakso M, Torp-Pedersen C, Waldenström A. DIGAMI 2 Investigators. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26(7):650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 66.Marfella R, Di Filippo C, Portoghese M, Ferraraccio F, Rizzo MR, Siniscalchi M, Musacchio E, D'Amico M, Rossi F, Paolisso G. Tight glycemic control reduces heart inflammation and remodeling during acute myocardial infarction in hyperglycemic patients. J Am Coll Cardiol. 2009;53(16):1425–1436. doi: 10.1016/j.jacc.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 67.Ceremuzynski L, Budaj A, Czepiel A, Burzykowski T, Achremczyk P, Smielak-Korombel W, Maciejewicz J, Dziubinska J, Nartowicz E, Kawka-Urbanek T, Piotrowski W, Hanzlik J, Cieslinski A, Kawecka-Jaszcz K, Gessek J, Wrabec K. Low-dose glucose-insulin-potassium is ineffective in acute myocardial infarction: results of a randomized multicenter Pol-GIK trial. Cardiovasc Drugs Ther. 1999;13(3):191–200. doi: 10.1023/a:1007787924085. [DOI] [PubMed] [Google Scholar]
- 68.Kosiborod M, Inzucchi SE, Spertus JA, Wang Y, Masoudi FA, Havranek EP, Krumholz HM. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. 2009;119(14):1899–1907. doi: 10.1161/CIRCULATIONAHA.108.821843. [DOI] [PubMed] [Google Scholar]
- 69.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 70.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 71.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O'Brien PC, Johnson MG, Williams AR, Cutshall SM, Mundy LM, Rizza RA, McMahon MM. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146(4):233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 72.Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG. GIST Trialists Collaboration. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6(5):397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 73.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 74.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 75.Moghissi ES, Korytkowski MT, Dinardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1191–1231. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kosiborod M, Inzucchi S, Clark B, Krumholz H, Jones P, O'Keefe J, Spertus JA. National patterns of glucose control among patients hospitalized with acute myocardial infarction. J Am Coll Cardiol. 2007;49(9):1018–1183. 283A. [Google Scholar]
- 77.Kosiborod M, Inzucchi S, Clark B, Krumholz H, Jones P, Spertus J. Variability in the hospital use of insulin to control sustained hyperglycemia among acute myocardial infarction patients. J Am Coll Cardiol. 2007;49(9):1018–1186. 284A. [Google Scholar]
- 78.Pinto DS, Skolnick AH, Kirtane AJ, Murphy SA, Barron HV, Giugliano RP, Cannon CP, Braunwald E, Gibson CM. TIMI Study Group. U-shaped relationship of blood glucose with adverse outcomes among patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2005;46(1):178–180. doi: 10.1016/j.jacc.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 79.Kosiborod M, Inzucchi SE, Goyal A, Krumholz HM, Masoudi FA, Xiao L, Spertus JA. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301(15):1556–1564. doi: 10.1001/jama.2009.496. [DOI] [PubMed] [Google Scholar]
- 80.Mellbin LG, Malmberg K, Waldenström A, Wedel H, Rydén L. DIGAMI 2 investigators. Prognostic implications of hypoglycaemic episodes during hospitalisation for myocardial infarction in patients with type 2 diabetes: a report from the DIGAMI 2 trial. Heart. 2009;95(9):721–727. doi: 10.1136/hrt.2008.152835. [DOI] [PubMed] [Google Scholar]
- 81.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 82.Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, Lee SL, Dziura JD, Inzucchi SE. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care. 2004;27(2):461–467. doi: 10.2337/diacare.27.2.461. [DOI] [PubMed] [Google Scholar]
- 83.Kosiborod M, Inzucchi S, Hamburg M, Riggs L, Koshy S, Krumholz H, Xiao L, Khalid A, Spertus JA. Feasibility, effectiveness and safety of intensive glucose control in critically ill hyperglycemic patients hospitalized with acute coronary syndromes. Circulation. 2007;116(16):3529. II-799. [Google Scholar]
- 84.Barsheshet A, Garty M, Grossman E, Sandach A, Lewis BS, Gottlieb S, Shotan A, Behar S, Caspi A, Schwartz R, Tenenbaum A, Leor J. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med. 2006;166(15):1613–1619. doi: 10.1001/archinte.166.15.1613. [DOI] [PubMed] [Google Scholar]
- 85.Berry C, Brett M, Stevenson K, McMurray JJ, Norrie J. Nature and prognostic importance of abnormal glucose tolerance and diabetes in acute heart failure. Heart. 2008;94(3):296–304. doi: 10.1136/hrt.2006.110999. [DOI] [PubMed] [Google Scholar]
- 86.Newton JD, Squire IB. Glucose and haemoglobin in the assessment of prognosis after first hospitalisation for heart failure. Heart. 2006;92(10):1441–1446. doi: 10.1136/hrt.2005.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]